Abstract
Introduction
Tenofovir (TDF)-containing PrEP is effective for HIV prevention, but its effect on health-related quality of life (QOL) is unknown. Using data from HPTN 069/ACTG A5305, a randomized study of potential PrEP regimens comparing maraviroc alone, or together with TDF or emtricitabine (FTC), to TDF + FTC (control), we evaluated the impact of these regimens on QOL in at-risk HIV-uninfected U.S. women and men.
Methods
QOL was measured at baseline (before starting medications) and every 8 weeks through week 48 using the EQ-5D-3L. Responses were converted to a scale from 0.0 (death) to 1.0 (perfect health), using published valuation weights. Mean scores were compared between groups at each time point using nonparametric testing. Multivariable linear regression was used to adjust for potential confounders.
Results
We analyzed 186 women (median age 35 years, 65% black, 17% Hispanic) and 405 men (median age 30 years, 28% black, 22% Hispanic), including 9 transgender participants analyzed based on sex-at-birth. Mean baseline QOL was 0.91 for women and 0.95 for men. There were minimal changes in mean QOL over time for any regimen (women: p = 0.29; men: p = 0.14). There were no significant differences between participants who continued the regimen compared to participants who discontinued early (women: p = 0.61; men: p = 0.1). Mean QOL did not differ significantly by regimen at any time point, both unadjusted and after adjustment for age, race/ethnicity, adherence, and use of alcohol, marijuana, opiates, and other substances.
Conclusions
QOL in at-risk individuals starting candidate PrEP regimens in a clinical trial is similar to the general population and maintained over time. This finding did not vary among regimens or when adjusted for demographics, adherence, and substance use. Our findings are the first to show that starting a candidate PrEP regimen in at-risk HIV-uninfected U.S. women and men was not associated with significant changes in QOL.
Trial registration
Clinicaltrials.gov NCT01505114.
Introduction
Tenofovir disoproxil fumarate (TDF)-containing antiretroviral (ARV) regimens are safe and effective as pre-exposure prophylaxis (PrEP) to prevent HIV infection in at-risk individuals [1–3] and are recommended in current guidelines [4–7]. However, daily ARV for PrEP use may have an effect on health-related quality of life (QOL).
While there is a large body of literature on QOL in people living with HIV taking ARVs, the potential QOL effect of ARV administration for prevention on HIV-uninfected individuals is not known. Results from qualitative and survey studies suggest that medication toxicity, the burden of daily pill-taking, and stigma associated with ARV use may decrease QOL, which has contributed to patient and provider concerns about PrEP [8, 9]. Conversely, receipt of PrEP may reduce the anxiety and fear associated with HIV acquisition, and in that way positively affect QOL [9, 10]. No clinical studies of PrEP have reported the QOL impact of uninfected individuals taking ARV regimens for HIV prevention.
Using data from HPTN 069/ACTG A5305, a randomized phase 2 safety trial comparing 3 candidate PrEP regimens to a control regimen with TDF and emtricitabine (FTC), we evaluated the impact of ARV administration on QOL in U.S. individuals at-risk for HIV acquisition, assessed for differences between candidate PrEP regimens, and investigated factors associated with QOL in this population.
Methods
Design
HPTN 069/ACTG A5305 was a phase 2 randomized, double-blinded controlled safety and tolerability trial comparing maraviroc (MVC), MVC with FTC, MVC with TDF, and TDF with FTC (control) for HIV prevention.[11, 12] The study was approved by the institutional review boards at each participating site, and all participants provided written informed consent. The names of the individual institutional review boards that approved the study are listed in the supplementary information files (S1 Table).
Participant selection
Study participants were enrolled between July 2012 and December 2014, with follow-up concluding in November 2015. The study population included HIV-uninfected women, men who have sex with men (MSM), and transgender individuals in the United States who were at risk for HIV acquisition based on self-reported condomless intercourse in the past 90 days with a male partner who was either HIV positive or of unknown serostatus [11, 12].
Measurement of QOL
QOL was measured with the EQ-5D-3L, a validated instrument for QOL [13]. Survey responses were converted to health utilities, a preference-weighted measure of health status, on a scale from 0.0 (death) to 1.0 (perfect health) by using U.S. population valuation weights [14]. We also evaluated the EQ-5D Visual Analogue Scale (VAS), respondents’ self-rated perception of their health status on a 0 to 100 scale, with 0 as the worst health the respondent can imagine, and 100 as the best health the respondent can imagine. QOL was assessed at baseline (prior to initiating PrEP study drugs) and every 8 weeks through week 48. Participants who discontinued study drugs but remained in study also completed QOL assessments.
Statistical analysis
The clinical trials recruited, randomized, and analyzed men and women in separate groups. In this manuscript, to be consistent with those trials, we analyze and report QOL results separately based on sex-at-birth.[11, 12] Mean scores were compared among regimens using nonparametric testing. Multivariable linear regression was used to adjust for demographics, substance use, and participants’ self-reported ability to take the regimen as prescribed. We used a linear regression model based on previous literature suggesting that these models perform similarly to other modeling approaches.[15] Analyses were conducted in SAS Version 9.4.
Results
A total of 594 participants (188 women and 406 men) were enrolled in the study. Two women and one man were excluded from the QOL analysis due to failure to complete the baseline QOL assessment. We analyzed data from 186 women (median age 35 years, age range 18–61 years, 65% black, 17% Hispanic) and 405 men (median age 30 years, age range 18–70 years, 28% black, 22% Hispanic). Seven transgender women and two transgender men were grouped according to sex-at-birth. Baseline characteristics of study participants are shown in Table 1, stratified by treatment group. Among the women, 160 (86%) completed follow-up and 115 (62%) remained on their study regimens for all 48 weeks. Among the men, 343 (84%) completed study follow up, and 281 (69%) remained on study regimens. Table 2 shows the number of participants that discontinued study medications or missed the QOL assessment at each time point.
Table 1. Baseline characteristics stratified by sex and randomized regimen.
| Women (n = 186) | Men (n = 405) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MVC (n = 45) | MVC+TDF (n = 49) | MVC+FTC (n = 45) | TDF+FTC (n = 47) | Total (n = 186) | MVC (n = 101) | MVC+TDF (n = 99) | MVC+FTC (n = 105) | TDF+FTC (n = 100) | Total (n = 405) | |
| Median age (range) | 39 (18–61) | 35 (22–60) | 35 (19–57) | 35 (18–60) | 35 (18–61) | 30 (18–65) | 30 (18–70) | 29 (18–62) | 31 (18–60) | 30 (18–70) |
| Black Race: (%) | 29 (64.4) | 32 (65.3) | 31 (68.9) | 29 (61.7) | 121 (65.1) | 31 (30.7) | 30 (30.3) | 33 (31.4) | 21 (21.0) | 115 (28.4) |
| Alcohol Use: (%) | ||||||||||
| Daily | 3 (6.7) | 2 (4.2) | 3 (6.8) | 1 (2.1) | 9 (4.9) | 11 (10.9) | 13 (13.1) | 18 (17.1) | 26 (26.0) | 68 (16.8) |
| Yes, but not daily | 28 (62.2) | 33 (68.8) | 30 (68.2) | 35 (74.5) | 126 (68.5) | 78 (77.2) | 77 (77.8) | 72 (68.6) | 59 (59.0) | 286 (70.6) |
| No | 14 (31.1) | 13 (27.1) | 11 (25.0) | 11 (23.4) | 49 (26.6) | 12 (11.9) | 9 (9.1) | 15 (14.3) | 15 (15.0) | 51 (12.6) |
| Marijuana Use: (%) | ||||||||||
| Daily | 3 (6.8) | 3 (6.1) | 7 (15.9) | 4 (8.9) | 17 (9.3) | 21 (20.8) | 14 (14.1) | 7 (6.7) | 13 (13.0) | 55 (13.6) |
| Yes, but not daily | 6 (13.6) | 17 (34.7) | 11 (25.0) | 12 (26.7) | 46 (25.3) | 30 (29.7) | 33 (33.3) | 50 (47.6) | 27 (27.0) | 140 (34.6) |
| No | 35 (79.5) | 29 (59.2) | 26 (59.1) | 29 (64.4) | 119 (65.4) | 50 (49.5) | 52 (52.5) | 48 (45.7) | 60 (60.0) | 210 (51.9) |
| Opiate Use: (%) | ||||||||||
| Ever | 4 (8.9) | 2 (4.1) | 3 (7.0) | 6 (12.8) | 15 (8.2) | 11 (10.9) | 4 (4.0) | 10 (9.5) | 10 (10.0) | 35 (8.6) |
| Never | 41 (91.1) | 47 (95.9) | 40 (93.0) | 41 (87.2) | 169 (91.8) | 90 (89.1) | 95 (96.0) | 95 (90.5) | 90 (90.0) | 370 (91.4) |
| Other substance use: (%) | ||||||||||
| Ever | 9 (20.5) | 8 (16.3) | 12 (27.9) | 13 (27.7) | 42 (23.0) | 57 (56.4) | 45 (45.5) | 52 (49.5) | 57 (57.0) | 211 (52.1) |
| Never | 35 (79.5) | 41 (83.7) | 31 (72.1) | 34 (72.3) | 141 (77.0) | 44 (43.6) | 54 (54.5) | 53 (50.5) | 43 (43.0) | 194 (47.9) |
MVC = maraviroc, TDF = tenofovir disaproxil fumarate, FTC = emtricitabine
Table 2. Number of participants that discontinued regimens or missed quality of life assessment.
| Women | |||
| On Medications, Provided QOL | Off Medications, Provided QOL | Off Medications, Did not provide QOL | |
| Week 0 | 186 | 0 | 0 |
| Week 8 | 169 | 10 | 7 |
| Week 16 | 152 | 13 | 21 |
| Week 24 | 144 | 19 | 23 |
| Week 32 | 132 | 23 | 31 |
| Week 40 | 130 | 28 | 28 |
| Week 48 | 130 | 30 | 26 |
| Men | |||
| On Medications, Provided QOL | Off Medications, Provided QOL | Off Medications, Did not provide QOL | |
| Week 0 | 405 | 0 | 0 |
| Week 8 | 379 | 5 | 20 |
| Week 16 | 366 | 11 | 28 |
| Week 24 | 350 | 17 | 38 |
| Week 32 | 336 | 17 | 52 |
| Week 40 | 326 | 23 | 56 |
| Week 48 | 319 | 24 | 62 |
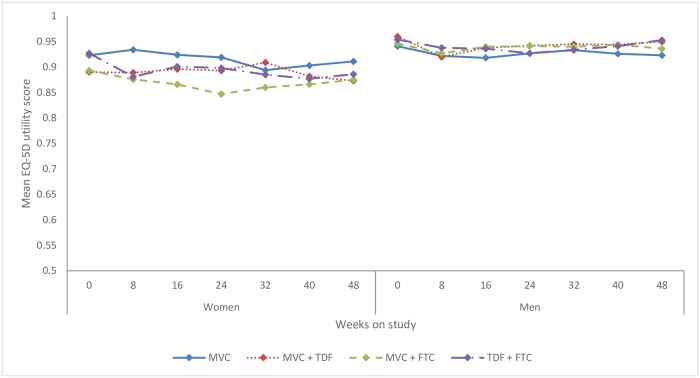
There was no significant change in QOL score between the baseline assessment and any time during or at the end of the study. The mean QOL score for women was 0.91 (95%CI: 0.89–0.93) at pre-PrEP baseline and 0.89 (95%CI: 0.86–0.91) at week 48 (p = 0.29). The mean score for men was 0.95 (95%CI: 0.94–0.96) at pre-PrEP baseline and 0.94 (95%CI: 0.93–0.95) at week 48 (p = 0.14). Pre-PrEP baseline QOL scores were similar across the four ARV PrEP regimens, and there were minimal changes over time for any regimen. (Fig 1).
Fig 1. Mean EQ-5D utility (Quality of life) score by PrEP study regimen over time.
Legend: Mean utility measured by EQ-5D, converted to health utility using U.S. valuation weights, where 0 is equivalent to death and 1 to perfect health. MVC = maraviroc, TDF = tenofovir disoproxil fumarate, FTC = emtricitabine.
There was no significant difference in QOL at the end of the study among participants who stayed on the study regimen, participants who discontinued and restarted the regimen during the trial, and participants who discontinued the regimen early but continued study follow-up (women: p = 0.61; men: p = 0.1) (Table 3).
Table 3. Mean EQ-5D utility (Quality of life) score at week 48 by continuation of study regimen.
| Women | Men | |||||
|---|---|---|---|---|---|---|
| N | Mean Utility | 95% CI | N | Mean Utility | 95% CI | |
| Stayed on regimen | 113 | 0.88 | 0.85–0.91 | 281 | 0.95 | 0.93–0.96 |
| Discontinued and restarted regimen | 15 | 0.90 | 0.82–0.98 | 38 | 0.95 | 0.91–0.98 |
| Off regimen | 30 | 0.91 | 0.86–0.96 | 24 | 0.87 | 0.78–0.95 |
Mean Utility measured by EQ-5D, converted to health utility using U.S. valuation weights. Treatment status measured at week 48.
In multivariate analyses, there was no difference in mean QOL among regimens at any time point when adjusted for age, race/ethnicity, alcohol use, marijuana use, opiate use, other substance use, or the most recent self-reported adherence assessment. Higher QOL was associated with self-reported greater ability to take the regimen as prescribed in both women and men at most time points. At week 48, women with a high ability to take the regimen had a higher QOL than those without high ability (β = 0.08, 95% CI [0.01, 0.15], p = 0.04). For men at week 48, there was a higher QOL associated with high ability (β = 0.03, 95% CI [-0.01, 0.07], p = 0.1). Each year of increased age was associated with lower baseline QOL in women (β = -0.002, 95% CI [-0.004, -0.001], p = 0.007), but not in men.
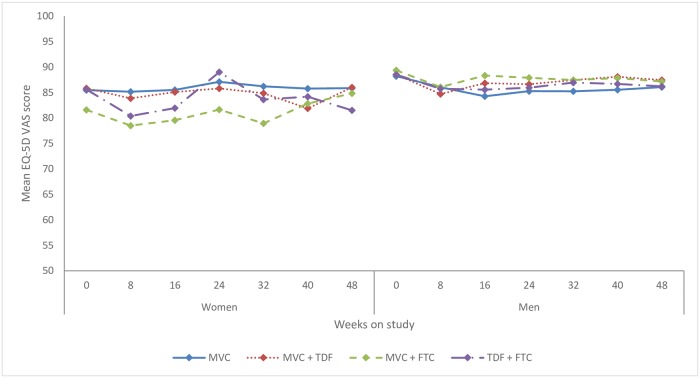
Results using the EQ-5D Visual Analog Scale (VAS) were similar to those using the EQ-5D utility scores. Mean scores for women changed from 84.7 (95%CI: 82.5–86.9) at pre-PrEP baseline to 84.5 (95%CI: 82.2–86.9) at week 48. For men, mean VAS score was 88.7 (95%CI: 87.8–89.5) at baseline and 86.7 (95%CI: 85.5–87.9) at the end of study. Fig 2 shows changes in mean EQ-5D VAS over time for each treatment group and sex.
Fig 2. Mean EQ-5D visual analogue scale (VAS) score by PrEP study regimen over time.
Mean utility measured by EQ-5D VAS, where 0 is the worst health the respondent can imagine, and 100 is the best. MVC = maraviroc, TDF = tenofovir disoproxil fumarate, FTC = emtricitabine.
Discussion
Our findings show that QOL in at-risk individuals prior to starting candidate HIV PrEP regimens in a clinical trial was similar to published values for the U.S. population of comparable age [16]. QOL was maintained at a high level during the period of candidate PrEP administration for all regimens. Higher baseline QOL was associated with younger age in women. During the study, higher QOL was associated with a high self-reported ability to take medication as prescribed. While the clinical significance of the differences in QOL as a result of these factors is uncertain, previous studies have indicated that the minimally important difference for EQ-5D scores using US valuation weights is 0.04.[17]
The stability of QOL over time was maintained for each of the maraviroc-containing regimens and also for TDF/FTC, which is currently the US Food and Drug Administration-approved PrEP standard of care. Our findings show that QOL as a global construct is not impacted by PrEP administration, a message important for both clinicians and at-risk individuals. Also, this finding may have implications for cost-effectiveness evaluation of PrEP regimens that include health utility as an outcome. However, specific domains of well-being, such as sexual well-being and anxiety related to HIV acquisition, which may be positively impacted by PrEP administration, were not specifically measured in the current analysis.
This study had several limitations. The study regimen comprised 3 pills (vs. 1 pill for the U.S. FDA approved TDF/FTC HIV PrEP regimen); the efficacy of MVC-containing regimens for HIV-prevention is not known and these regimens are not approved for HIV PrEP. Results in our study population may not be fully generalizable to the population of individuals who take PrEP. The EQ-5D may not be sensitive to small differences in QOL and may have ceiling effects for individuals in good health. [15] Our modeling approach may not account for regression to the mean. Associations between QOL and adherence or demographic factors may not be causal, as unmeasured confounders may affect these relationships. Missing observations from participants who did not appear for study follow-up may be different from those that remained in follow-up. Per the design of HPTN 069/ACTG A5305, at-risk heterosexual men were not included in the study; and a low number of transgender participants precluded analysis of that subgroup.
Conclusions
Our findings are the first to show that starting a candidate PrEP regimen in a randomized clinical trial of at-risk HIV-uninfected U.S. women and men was not associated with significant changes in QOL.
Supporting information
(DOCX)
(ZIP)
Acknowledgments
We would like to recognize the participants, their partners and families, other members of the HPTN 069/ACTG A5305 study team, FHI 360, the pharmaceutical sponsors who provided study drugs (Gilead Sciences Inc, and Viiv Healthcare), and the study staff at the participating sites: Case Western Reserve, Cleveland, Ohio (grant UM1-AI-069501); Fenway/Harvard Medical School, Boston, Massachusetts (UM1-AI-069412); George Washington University, Washington, DC (UM1-AI-069503; AI-117970 [DC CFAR]); Johns Hopkins University, Baltimore, Maryland (UM1-AI-069465); Rutgers New Jersey Medical School (UM1-AI-069466); San Francisco Health Department/University of California, San Francisco (UM1-AI-069496); University of California, Los Angeles (UM1-AI-069424); University of North Carolina, Chapel Hill (UM1-AI-069423, UL1-TR-001111, P30-AI-50410); University of Pennsylvania, Philadelphia (UM1-AI-069534 and P30-AI-045008); University of Pittsburgh, Pennsylvania (UM1-AI-069494, UL1-RR-024153, UL1-TR-000005); University of Puerto Rico, San Juan (UM1-AI-069415); University of Washington, Seattle (UM1-AI-69481); and Weill Cornell Medicine, New York, New York (UM1-AI-069419 and UL1-RR-024996). We would also like to acknowledge Krista Yuhas and Jing Wang at the Statistical Center for HIV/AIDS Research and Prevention at the Fred Hutchinson Cancer Research Center for assistance with analyses.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by DAIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health through the HPTN (grants UM1-AI068619, UM1-AI068613, and UM1-AI068617) and the AIDS Clinical Trials Group (grant UM1-AI-068636). Gilead Sciences and ViiV Healthcare provided study drugs.
References
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. 10.1056/NEJMoa1011205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med. 2015;373(23):2237–46. 10.1056/NEJMoa1506273 [DOI] [PubMed] [Google Scholar]
- 3.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. 10.1016/S0140-6736(15)00056-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunthard HF, Saag MS, Benson CA, del Rio C, Eron JJ, Gallant JE, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2016 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316(2):191–210. 10.1001/jama.2016.8900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs For Treating and Preventing HIV Infection: Recommendations for a Public Health Approach - 2nd ed [Internet]. 2016 [8/2017]. http://www.who.int/hiv/pub/arv/arv-2016/en/. [PubMed]
- 6.Centers for Disease Control and Prevention. U.S. Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 Update: a clinical practice guideline. [Internet]. 2018 [7/2018]. https://www.cdc.gov/hiv/pdf/guidelines/cdc-hiv-prep-guidelines-2017.pdf.
- 7.Saag MS, Benson CA, Gandhi RT, Hoy JF, Landovitz RJ, Mugavero MJ, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2018 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320(4):379–96. 10.1001/jama.2018.8431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krakower DS, Oldenburg CE, Mitty JA, Wilson IB, Kurth AE, Maloney KM, et al. Knowledge, Beliefs and Practices Regarding Antiretroviral Medications for HIV Prevention: Results from a Survey of Healthcare Providers in New England. PLoS One. 2015;10(7):e0132398 10.1371/journal.pone.0132398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng P, Su S, Fairley CK, Chu M, Jiang S, Zhuang X, et al. A Global Estimate of the Acceptability of Pre-exposure Prophylaxis for HIV Among Men Who have Sex with Men: A Systematic Review and Meta-analysis. AIDS Behav. 2017. February 7 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10.Storholm ED, Volk JE, Marcus JL, Silverberg MJ, Satre DD. Risk Perception, Sexual Behaviors, and PrEP Adherence Among Substance-Using Men Who Have Sex with Men: a Qualitative Study. Prev Sci. 2017;18(6):737–47. 10.1007/s11121-017-0799-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulick RM, Wilkin TJ, Chen YQ, Landovitz RJ, Amico KR, Young AM, et al. Safety and tolerability of maraviroc-containing regimens to prevent hiv infection in women: A phase 2 randomized trial. Annals of Internal Medicine. 2017;167(6):384–93. 10.7326/M17-0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulick RM, Wilkin TJ, Chen YQ, Landovitz RJ, Amico KR, Young AM, et al. Phase 2 Study of the Safety and Tolerability of Maraviroc-Containing Regimens to Prevent HIV Infection in Men Who Have Sex With Men (HPTN 069/ACTG A5305). J Infect Dis. 2017;215(2):238–46. 10.1093/infdis/jiw525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.EuroQol G. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. [DOI] [PubMed] [Google Scholar]
- 14.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–20. [DOI] [PubMed] [Google Scholar]
- 15.Pullenayegum EM, Tarride JE, Xie F, Goeree R, Gerstein HC, O’Reilly D. Analysis of health utility data when some subjects attain the upper bound of 1: are Tobit and CLAD models appropriate? Value Health. 2010;13(4):487–94. 10.1111/j.1524-4733.2010.00695.x [DOI] [PubMed] [Google Scholar]
- 16.Fryback DG, Dunham NC, Palta M, Hanmer J, Buechner J, Cherepanov D, et al. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care. 2007;45(12):1162–70. 10.1097/MLR.0b013e31814848f1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo N, Johnson J, Coons SJ. Using instrument-defined health state transitions to estimate minimally important differences for four preference-based health-related quality of life instruments. Med Care. 2010;48(4):365–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.