Abstract
Nociceptive sensitization involves an increase in responsiveness of pain sensing neurons to sensory stimuli, typically through the lowering of their nociceptive threshold. Nociceptive sensitization is common following tissue damage, inflammation, and disease and serves to protect the affected area while it heals. Organisms can become sensitized to a range of noxious and innocuous stimuli, including thermal stimuli. The basic mechanisms underlying sensitization to warm or painfully hot stimuli have begun to be elucidated, however, sensitization to cold is not well understood. Here, we develop a Drosophila assay to study cold sensitization after UV-induced epidermal damage in larvae. Larvae respond to acute cold stimuli with a set of unique behaviors that include a contraction of the head and tail (CT) or a raising of the head and tail into a U-Shape (US). Under baseline, non-injured conditions larvae primarily produce a CT response to an acute cold (10°C) stimulus, however, we show that cold-evoked responses shift following tissue damage: CT responses decrease, US responses increase and some larvae exhibit a lateral body roll (BR) that is typically only observed in response to high temperature and noxious mechanical stimuli. At the cellular level, class III neurons are required for the decrease in CT, chordotonal neurons are required for the increase in US, and chordotonal and class IV neurons are required for the appearance of BR responses after UV. At the molecular level, we found that the transient receptor potential (TRP) channel brivido-1 (brv1) is required for these behavioral shifts. Our Drosophila model will allow us to precisely identify the genes and circuits involved in cold nociceptive sensitization.
Introduction
Nociceptive sensitization is an exaggerated behavioral or biological response to a normal stimulus due to a lowered nociceptive threshold. It is typically observed after tissue damage or injury. Nociceptive sensitization commonly develops near the site of injury, where the local sensory neurons are temporarily hypersensitized until the wound effectively heals [1]. Nociceptive sensitization is thought to foster protective behavioral mechanisms to prevent further tissue damage and aid the healing process [2]. However, when the pain extends beyond the time necessary for wound healing, it becomes maladaptive and more difficult to treat. There are a number of clinical conditions that are associated with maladaptive nociceptive sensitization, which greatly reduce the quality of life in patients and remain difficult to treat. Thermal sensitivity to cool-cold temperatures can be experienced as innocuous cool temperatures being perceived as painful (cold allodynia), or harsh cold temperatures perceived as more painful (cold hyperalgesia). While cold allodynia is common in patients with multiple sclerosis [3], fibromyalgia [4], stroke [5, 6], and chemotherapy-induced neuropathy [7, 8], the mechanisms that underlie cold sensitization in these conditions are largely unknown.
A wide array of nociceptive assays and tools have been used in vertebrates to investigate cold nociception and nociceptive sensitization, including: a cold plate [9], tail-flick [10], exposure to acetone [11], or dry ice assays [12]. Under non-injured conditions vertebrate cool-sensing neurons (peripheral C and Aδ fibers) are activated between 20–37°C with increased firing upon cooling down to 20–17°C, while noxious cold-sensing neurons are activated between 10–20°C with increased firing down to 0°C [13]. These neurons are also required for observed sensitized responses to cold [13], however neuronal activation and hence nociceptive thresholds are prone to shift under the context of injury or damage [14] and the mechanisms underlying these shifts are largely unknown.
At the molecular level, a handful of cold-sensitive channels have been proposed as cool or noxious cold receptors. A large focus has been on transient receptor potential (TRP) channels, which have known functions in nociception, thermosensation, and nociceptive sensitization [15, 16]. TRP channels are variably selective cation channels containing multiple subunits and six transmembrane domains that fall into several different gene families including: TRPC, TRPM, TRPV, TRPA, TRPP and TRPML. While TRPV channels have been characterized as warmth and heat activated [17], TRPM8 and TRPA1 are most notably implicated in cold sensing [18, 19] and inflammatory or damage-induced cold hypersensitivity in vertebrates [20, 21]. Experiments in TRPM8 null mice, however, have shown that a small population of dorsal root ganglion (DRG) neurons lacking TRPM8 are still able to respond to cold or menthol [22]. Likewise mice mutant for TRPM8, while exhibiting significant impairment of cold sensing, will still avoid painfully cold temperatures (16–20°C) [22]. Similar studies in TRPA1 null mice show that although these mice show severe defects in responding to cold, the mutation does not completely abolish cold-evoked behaviors [23]. Lastly, additional studies of cold-responsive DRG (as well as superior cervical ganglia) neurons did not respond to TRPM8 or TRPA1 agonists [24]. Together these studies suggest that cold receptor(s) beyond TRPM8 and TRPA1 exist.
Studies in noxious cold detection and cold hypersensitivity in vertebrates to date suggest a complex process, likely involving multiple receptors and/or modulators. Genetically tractable organisms such as the fruit fly could greatly aid this work by offering a simpler platform for study that offers a sophisticated genetic toolkit. Identifying the cells and channels involved in cold-sensing in invertebrates is relatively recent, including studies in flies [25, 26], worms [25, 27], and the leech [28]. The majority of these studies (other than [26]) however, focused on fairly innocuous ‘cool’ ranges (> 12°C) and measured thermal preference, or avoidance of temperatures just outside preferred ranges. Drosophila utilize cool-sensing neurons in the head [29, 30], and chordotonal neurons in the larval body wall [31], as well as the TRP channels trp, trpl [32], inactive [31], and the TRPP type brivido genes [30], to avoid mildly cool temperatures outside their preferred temperature range.
Recently, it was shown that Drosophila larvae also respond to acute noxious cold stimuli with distinct behaviors, including a contraction (CT) of the anterior and posterior segments towards the center of the body, and a raising of these anterior and posterior segments into the air to create a U-Shape (US) [26]. Both of these behaviors are distinct from normal locomotion, most gentle touch behaviors [33–36] as well as the stereotyped 360° lateral body roll (BR) behavior observed in response to high heat [37, 38] and noxious mechanical stimuli [37, 39–41]. The primary cold-evoked behavior, CT, requires class III multidendritic (md) sensory neurons, which innervate the barrier epidermis and are directly activated by cold temperatures [26]. Cold-evoked CT behavior is also mediated by class III expression of the TRP channels Polycystic kidney disease gene 2 (Pkd2, a TRPP channel), NompC (a TRPN channel) and Trpm [26].
Although invertebrate models including C. elegans [42], Aplysia [43], and Drosophila [38, 44, 45] have been used to study nociceptive sensitization, none have looked at injury-induced cold hypersensitization. In Drosophila, larvae sensitize to mildly warm and hot stimuli following UV-induced tissue damage [38, 45]. Larvae normally respond to acute high temperature stimuli (≥ 45°C) with a 360° lateral BR behavior [38]. After UV damage however, larvae exhibit BR in response to subthreshold temperatures (thermal allodynia), and show a robust increase in the number of BR responders to noxious heat with a concomitant decrease in response latency (thermal hyperalgesia) [38]. Sensitization to warm and hot stimuli require class IV md sensory neurons, which are also required for baseline responses to high temperature in the absence of tissue damage [38]. UV exposure results in a rapid apoptotic breakdown of the larval epidermis between 16–24 hours after UV administration; the underlying sensory neurons, however, remain intact [38, 46]. Further, class IV md neurons expressing the TRP channel painless (a TRPA channel) are required for baseline responses and UV-induced sensitized responses to heat [45].
Currently, it is unknown if Drosophila larvae sensitize to cold stimuli following tissue damage, and if so, whether they utilize known or distinct sensory neurons and receptors for this sensitization. Here we characterize a marked shift in behavioral responses to cold temperatures after epidermal injury. We have found that class III and class IV md sensory neurons, as well as peripheral chordotonal neurons, are required for this observed shift in cold-evoked behavior. Lastly, we identify a role for the TRP channel Brv1 in injury-induced shifts in behavioral responses to cold temperatures. This work establishes a platform for future studies on the cellular and molecular bases of cold nociception and injury-induced cold sensitization.
Materials and methods
Fly stocks and genetics
Drosophila melanogaster larvae were raised on cornmeal media at 25°C. w1118 was used as a control strain. Mutant brv1L563>STOP [30] and deficiency allele brv1-Df (Df(3L)Exel9007) were obtained from Marco Gallio. Gal4 lines: 19-12-Gal4 (class III[47]), ppk1.9-Gal4 (class IV [48]), and iav-Gal4 (chordotonal [49, 50]). UAS transgenes: UAS-TeTxLC (active tetanus toxin [51]), UAS-IMP TNT VI-A (inactive tetanus toxin [51]), UAS-mCD8::GFP [52] and UAS-GCaMP6m [53]. UAS-RNAi line from Bloomington Drosophila Stock Center [54]: 31496 (UAS-brv1RNAi).
Local cold probe assay
Details on the custom-built Peltier cold probe (TE Technologies, Inc.) used in this study can be found here [26]. For behavioral analysis, mid 3rd instar larvae were placed on a stage under a brightfield stereomicroscope (Zeiss Stemi 2000). As previously described [26], the probe tip was gently placed on the dorsal midline (segments A4-A6) for up to 10 s or the initiation of a behavioral response, while larvae moved freely under the microscope. Larvae that did not exhibit a behavioral response within 10 s were recorded as non-responders. Behavioral responses were characterized as either: 1. A contraction (CT); 2. A 45–90° simultaneous head and posterior raise (US for U-shape) (as described previously [26]); or 3. A 360° lateral body roll (BR), which was observed under sensitized conditions. For all data, only larvae that initially exhibited normal locomotion were tested and each larva was stimulated only once. Lastly, in Gal4/UAS experiments, transgenes were heterozygous and no balancers or markers were present in the larvae used for behavioral testing. Experimenter was blind to genoype being tested.
UV-induced tissue damage
To determine effects of UV-induced tissue damage on larval cold nociception and sensitization, larvae were irradiated by exposure to 10–14 mJ/cm2 UV-C (as previously described [38]) and then allowed to recover on food in a 25°C incubator before being tested in the nociceptive assays 4, 8, 16 or 24 hours later. For this, 3rd instar larvae of similar size were selected 4–5 days after egg laying and irradiated, then tested in the cold assay after recovery from UV. Before irradiation, larvae were immobilized on a cold slide for a few minutes, then fine-tipped forceps were used to position the larvae dorsal side up in a row along the length of the slide. The spectrolinker XL-1000 UV crosslinker (Spectroline) was warmed up, and the UV dose was measured with a hand-held UV spectrophotometer (AccuMAX XS-254, Spectroline) just prior to exposure to get an accurate reading. All UV doses fell within 10–14 mJ/cm2, which have been shown to induce epidermal cell death [55] while sparing the peripheral sensory neurons from significant morphological changes [46, 56]. Larvae were then administered UV (actual dose recorded), and carefully placed into a vial of food with a paintbrush to recover for a variable amount of time before being tested in nociceptive assays.
Neuronal morphology: Live imaging and confocal microscopy
Live confocal imaging of neuronal morphology was performed as previously described [26, 57]. Briefly, third instar larvae were mounted on microscope slides using 1:5 (v/v) diethyl ether:halocarbon oil and imaged using Zeiss LSM780 laser confocal microscope. For neurometric analyses of class III md neurons, maximum intensity projections of z-stacks were analyzed using ImageJ as previously described [58]. For chordotonal neurons, maximum projections were analyzed using Zeiss Zen Blue software, where mean fluorescence intensity (total fluorescence intensity normalized to area) was analyzed for regions of interest (cell body and dendrites). In this study mean fluorescence intensity was used as a measure of potentially altered cellular integrity or potential changes in protein translation, trafficking or recycling that could impact fluorescence intensity.
in vivo calcium imaging
For in vivo GCaMP analysis to visualize CIII, CIV, and Ch neurons, 19-12-Gal4 (Class III) and UAS-GCaMP6m were used in combination with ppk1.9-Gal4 (Class IV) or iav-Gal4 (Chordotonal) respectively. in vivo calcium imaging was performed as previously described [26, 59]. Briefly, third instar larvae were mounted on a microscope slide with minimal water to prevent desiccation and placed on a Peltier stage (Linkam PE120) for time lapse imaging. The following temperature regimen was used during time lapse imaging: 1 minute at 25°C, ramp down to 6°C at 20°C/minute, hold at 6°C for 10 seconds, ramp up to 25°C at 20°C/minute, and hold at 25°C for one minute. Images were recorded at 212.55 μm x 212.55 μm resolution and 307.2 ms per frame. Raw time-lapse files were motion corrected in Fiji using the Stack Reg function. A region of interest was manually drawn around the cell body and mean fluorescence intensity across time was collected. ΔF/F0 was calculated as previously described [26].
Results
Drosophila larvae are sensitized to cold after tissue damage
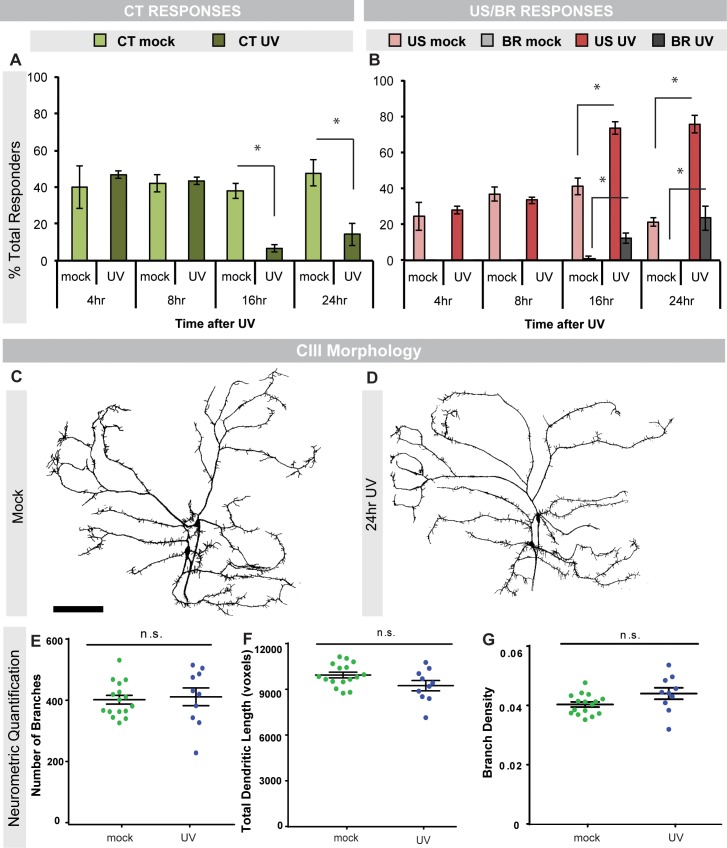
Given that Drosophila larvae sensitize to noxious heat after tissue damage [38], and respond to noxious cold in the absence of injury [26], we wanted to determine if larvae also sensitize to noxious cold after tissue damage. We used UV irradiation to damage the dorsal epidermis [38] and then allowed mock-treated or irradiated larvae to recover for different amounts of time before testing for changes in cold nociception. Intriguingly, the primary cold behavior at 10°C, contraction (CT) [26], was significantly decreased at 16 and 24 hours after UV damage compared to mock-treated controls (Fig 1A, S1A–S1C Fig). Interestingly, the decrease in CT response comes primarily from the number of fast responders (within 3 seconds) rather than slow responders (between 4–10 seconds) at both 16 (S1B Fig) and 24 hrs (S1C Fig) after UV. Despite the observed decrease in CT behavior, upon analyzing cold nociceptive class III (CIII) sensory neurons after mock or UV irradiation, we found they exhibit normal dendritic morphology after UV irradiation (Fig 1C-1G). In contrast to the CT response, the percent of U-Shape (US) responders was increased 16 and 24 hours after UV (Fig 1B, S1A–S1C Fig). Once again, the change in the response was derived primarily from fast responders (S1B and S1C Fig). Interestingly, we also observed a significant number of larvae that responded to the cold probe with a nocifensive body roll (BR) at these time points (Fig 1B, S1A–S1C Fig). BR is normally only seen in response to high temperature and harsh touch and is not observed in response to a cold stimulus under baseline conditions [26]. These data suggest that epidermal tissue damage results in a shift of behavioral responses to acute noxious cold stimuli despite the lack of damage to the underlying sensory neurons. In this case, this shift appears as a switch from the dominant cold-responsive behavior at 10°C, a CT, to US and the normally heat-evoked BR response.
Fig 1. Cold-evoked behavioral switches and neuronal morphology after UV.
(A) Percent of CT responders or (B) percent of US and BR responders to cold probe (10°C stimulation) at indicated times after UV irradiation in mock- or UV-treated larvae. CT = Contraction; US = U-Shape; BR = Body roll, n = 90. (C-D) in vivo confocal images of larval CIII sensory neuron dendrite morphology in mock-treated larvae (C) and larvae 24 hours post UV treatment (D). Neurons were visualized via 19-12-Gal4>UAS-mCD8::GFP. Scale bar: 100 μm (E-G) Neurometric quantification of CIII sensory neurons. (E) Number of branches, (F) total dendritic length, and (G) branch density (number of branches/total dendritic length), n = 10–16 neurons. Data are presented as mean ± s.e.m (A-B, E-G). Stats: (A-B) Two-tailed Fisher’s Exact test, * = p < 0.005, comparisons were made between UV and mock control at each time point. (E-G) Two-tailed t-test, n.s. = not significant, comparisons were between UV and mock control of each neurometric measure.
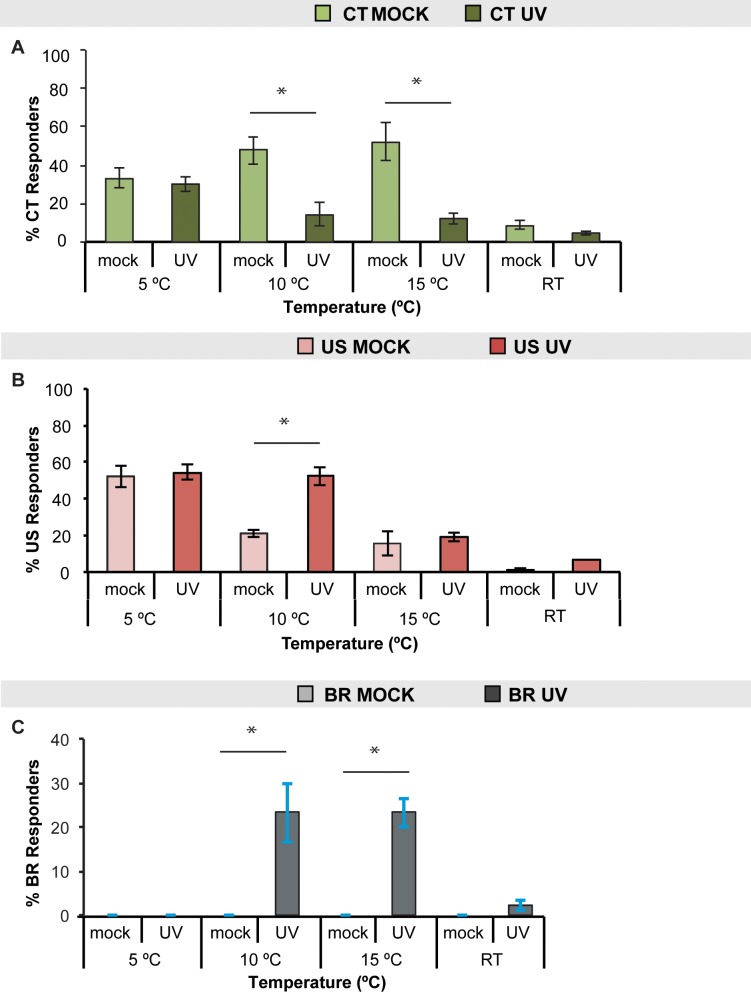
To determine the temperature range(s) over which UV-induced changes in behavioral responses to cold are observed, we tested larvae 24 hours after UV (peak sensitization response) at multiple cold to cool temperatures. While decreases in CT and the emergence of BR were observed at 10°C and 15°C (Fig 2A and 2C respectively, and S1D Fig), increases in US were only observed at 10°C (Fig 2B and S1D Fig). Therefore, 10°C was the only temperature where all three changes were observed: decrease in CT, increase in US, and emergence of BR (Fig 2, S1D Fig). No significant changes in cold-evoked behaviors were seen with a room temperature (RT) probe, eliminating gentle touch as a contributor to the observed responses (Fig 2).
Fig 2. Dependence of UV-Induced cold-evoked behaviors on temperature.
Larvae were tested 24 hours after UV exposure or mock treatment with the cold probe either at 5°C, 10°C, 15°C, or room temperature (RT) and the percent responders for (A) CT, (B) US, or (C) BR, were averaged, n = 90. Data are presented as the average ± s.e.m.. Stats: two-tailed Fisher’s Exact test, * = p < 0.05, comparisons were made between UV and mock control at each temperature.
Since the received UV dose can vary slightly with each administration (10–14 mJ/cm2), we wanted to determine if variations in the UV intensity could impact cold sensitization after UV. Larvae were immobilized (see methods) and subjected to 10–14 mJ/cm2 UV to the dorsal side. Larvae were grouped based on the actual measured dose of UV and then allowed to recover for 24 hours before their cold responses were assessed. We observed some changes in CT and BR responses with 13 mJ/cm2 UV, however the majority of responses did not differ significantly between doses (S2 Fig). Although the UV dose required to cause apoptosis of the larval epidermis is greater than 12 mJ/cm2 [55], our data suggests the degree of apoptosis in the epidermis is not associated with cold-evoked responses after UV.
Peripheral sensory neurons are required for UV-induced change in cold responses
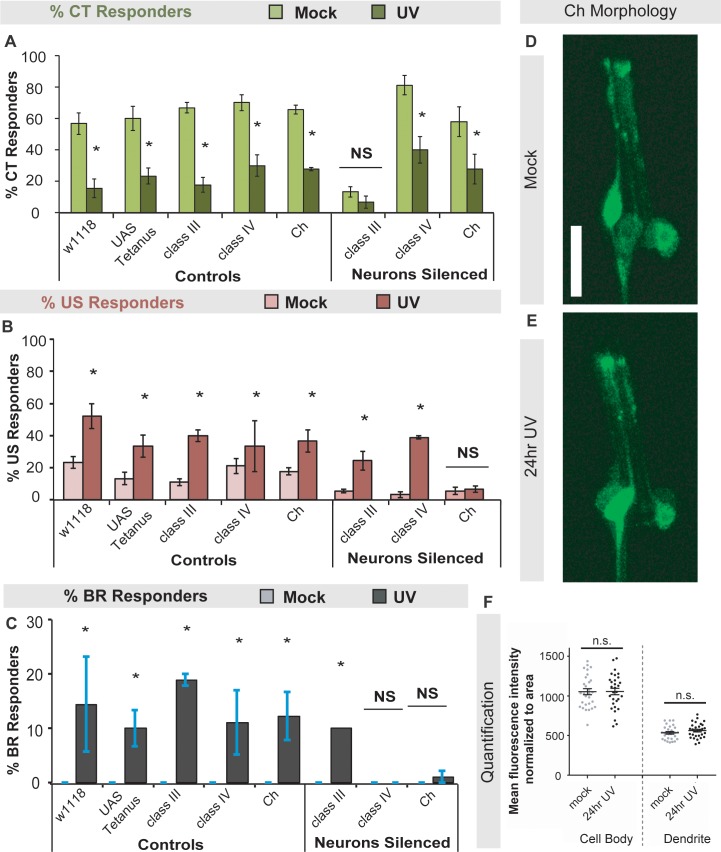
Since baseline CT responses to cold require CIII md sensory neurons [26] and BR responses to heat and mechanical stimuli are mediated by class IV (CIV) md neurons [37, 38], we next determined if either of these neuron classes are required for UV-induced changes in cold responses. We also tested bipolar chordotonal (Ch) neurons which were initially described as stretch receptors [60], but recently found to be important for avoiding cool temperatures in larvae [29, 31]. We utilized the Drosophila Gal4/UAS system [61] to target the expression of a tetanus toxin transgene (UAS-TNT-E [51]) in specific classes of sensory neurons using class-specific drivers (see materials and methods). Briefly, the expression of tetanus toxin essentially prevents neurotransmission, effectively silencing the neurons of interest [51]. We UV-irradiated larvae in which CIII, CIV or Ch neurons were silenced and tested their cold-evoked responses 24 hours after UV exposure. Silencing CIII neurons blocked CT responses in both mock (as shown previously [26]) and UV-treated larvae (Fig 3A). US responses were blocked when Ch neurons were silenced, but not when CIII or CIV neurons were silenced compared to genetic and mock-treated controls (Fig 3B). Consistent with previous studies, increased BR responses after UV were blocked when CIV and Ch neurons were silenced (Fig 3C and [39]). Exposure to UV, however, does not alter CIV neuron morphology [46]. Likewise, analysis of Ch neuron morphology after UV irradiation revealed that were no significant differences in mean fluorescence intensity normalized to area for the cell body or the dendrite (Fig 3D-3F). These data suggest a novel and important role for CIV (BR) and Ch neurons (US, BR) in UV-induced changes in cold responses.
Fig 3. The UV-induced switch of cold-evoked behaviors requires class IV and Ch peripheral sensory neurons.
(A-C) Larvae expressing an active or inactive (control) form of the tetanus toxin transgene (see materials and methods) in class III (CIII), class IV (CIV) or chordotonal neurons (Ch) were tested for cold-evoked behaviors 24 hours after UV exposure. Percent of (A) CT, (B) US, or (C) BR responders were averaged and UV- versus mock-treated responses were compared. n = 90. (D-E) Representative in vivo confocal microscopy images of (D) mock treated larvae and (E) larvae 24 hours post UV treatment. (D-E) Larval chordotonal morphology was visualized via iav-Gal4>UAS-mCD8::GFP. Scale bar: 20 μm. (F) Mean fluorescence intensity normalized to area for cell body and dendrites, where n = 29–30 neurons. Data are presented as the average ± s.e.m (A-C,F). Stats: (A-C) two-tailed Fisher’s Exact test * = p < 0.05. (F) Two-tailed t-test, n.s. = not significant, comparisons were between UV and mock control.
UV irradiation does not alter cold-evoked calcium responses
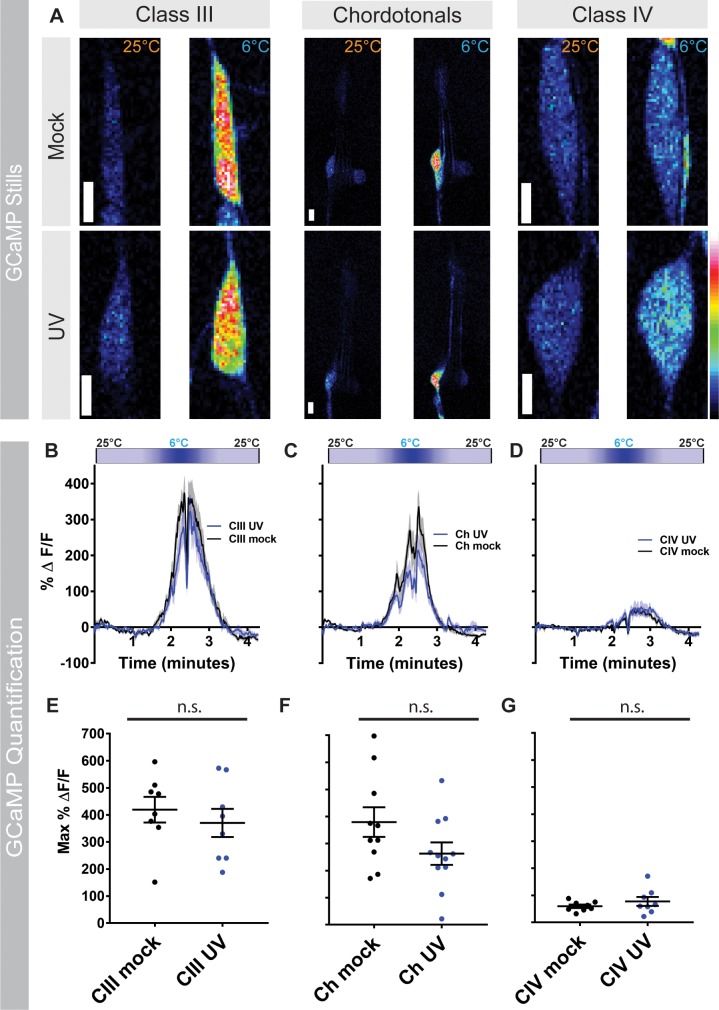
To investigate whether there are any calcium changes at the sensory neuron level after UV, we expressed GCaMP in relevant sensory neuron subtypes in mock and UV-irradiated third instar larvae and exposed these larvae to noxious cold. Consistent with previous findings [26], in mock treated larvae, CIII sensory neurons displayed a robust calcium response to noxious cold (6°C) compared to baseline levels (Fig 4A, 4B and 4E). Although we found that 6°C is the temperature where we observe a peak calcium response, we also analyzed responses at 10°C to match the previously observed peak of CT behavior [26] and found similar increases in calcium responses at that temperature (S3 Fig). After UV irradiation, CIII sensory neurons still exhibited strong cold-evoked calcium responses, albeit with a slight, but non significant decrease in GCaMP response relative to mock CIII controls (Fig 4A, 4B and 4E and S3A Fig). Interestingly, in mock-treated larvae, Ch neurons showed similar magnitude calcium responses to noxious cold as observed in CIII neurons (Fig 4A, 4C and 4F and S3B Fig). As with CIII sensory neurons, Ch neurons still had robust cold-evoked calcium reponses after UV irradiation and a slight, but non significant, reduction in max ΔF compared to mock-treated Ch neurons (Fig 4A, 4C and 4F, and S3B Fig). Lastly, we investigated calcium responses in mock-treated CIV sensory neurons. Consistent with previous results [26], the CIV calcium response to noxious cold is significantly lower than in CIII and Ch sensory neurons (p<0.0001; two-way ANOVA with Sidak mulitple correction test) (Fig 4G). In UV-irradiated larvae, CIV sensory neurons have a slightly higher, but non significant, calcium response compared to mock treated neurons (Fig 4A, 4D and 4G and S3C Fig). Together, these results suggest that the switches in noxious cold-evoked behavioral output following UV irradiation appear independent of alterations in calcium physiology in sensory neuron somata.
Fig 4. UV irradiation does not alter cold-evoked calcium responses.
(A) in vivo confocal stills of larval CIII, Ch, and CIV sensory neurons expressing GCaMP6m at 25°C and 6°C in mock treated larvae and 24 hours post UV treatment. Scale bar: 5 μm. (B-D) Change in GCaMP6m fluorescence (ΔeltaF) over time for (B) CIII, (C) Ch, and (D) CIV sensory neurons. Cold-map on top of each graph represents stimulus temperature. Data represented as mean GCaMP6 response (black and blue trace lines) ± s.e.m (grey). (E-G) Max change in GCaMP6m fluorescence for mock- and UV-treated larvae 24 hours post-irradiation for (E) CIII, (F) Ch, and (G) CIV sensory neurons, Data are presented as mean ± s.e.m., n = 8–11 larvae. Stats: Two-tailed t-test (E-G), where the comparisons are between mock and UV treated conditions. n.s. = not significant.
TRP channel Brv1 is required for UV-induced changes in responses to cold in a class specific manner
TRP channels mediate a multitude of thermosensory responses in Drosophila and other animals [15]. We therefore hypothesized that changes in cold responses after UV may depend on TRP channels expressed in specific sensory neurons involved in sensitization to cold. TRP channels are expressed in CIII, CIV and Ch neurons [26, 31, 58] and function in these cells to mediate detection of sensory stimuli. In particular, we examined the TRPP channel gene Brv1. Brv channels have been implicated in cold avoidance in adult flies [30] and in larvae [62].
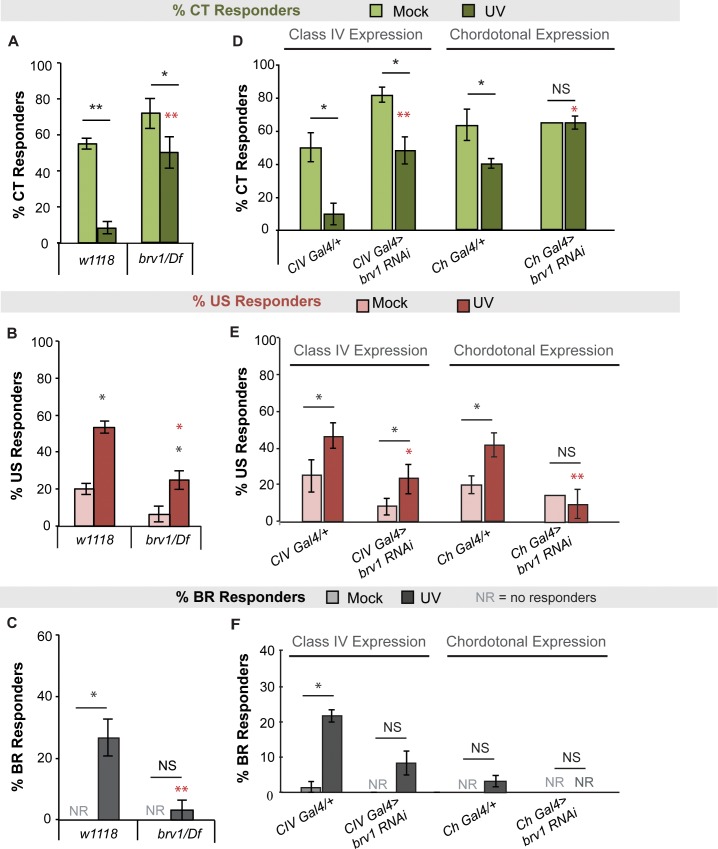
To test whether Brv1 is required for UV-induced changes in cold responses, we assayed mutants of brv1 over a relevant deficiency for cold responses 24 hours after UV. Larvae mutant for brv1 exhibited decreases in CT responders (Fig 5A and S4A Fig), and increases in US responders (Fig 5B and S4B Fig) to the cold probe after UV compared to mock controls. The mutant UV responses, however, are significantly altered when compared to irradiated control larvae for both CT (Fig 5A, red asterisks, see also S4A Fig) and US responses (Fig 5B, red asterisks, see also S4B Fig). Larvae mutant for brv1 also did not exhibit cold-evoked BR responses after UV as seen in wildtype controls (Fig 5C).
Fig 5. Brv1 is involved in the UV-induced shift of behavioral responses to cold.
(A-C) Percent of (A) CT, (B) US or (C) BR responders to the cold probe (10°C) 24 hours after UV in TRP channel mutant for Brv1 over a relevant deficiency (Df). (D-F) Percent of (D) CT, (E) US, or (F) BR responders to the cold probe 24 hours after UV in larvae expressing brv1-RNAi transgenes in class IV or chordotonal neurons. (A-F) n = 3 sets of 20 larvae averaged ± s.e.m., except for (E) where Ch-Gal4>brv1-RNAi was an n = 20 larvae (therefore, SD not shown). Stats: two-tailed Fisher’s exact test, * = p < 0.05, ** = p < 0.001. Black asterisks indicate statistical analysis between mock and UV groups for a given genotype. Red asterisks indicate statistical analysis between (A-C) UV-treated w1118 and mutant, or (D-F) UV-treated Gal4 alone and RNAi. NS = no significance between UV and mock of same genotype.
To determine where brv1 may be required for UV-induced shifts in cold behaviors, we examined larvae expressing a brv1-specific UAS-RNAi transgene in CIV or Ch neurons. Interestingly, while brv1RNAi still exhibited a UV-induced decrease of CT responders when expressed in CIV neurons, this decrease was completely blocked when brv1RNAi was expressed in Ch neurons (Fig 5D and S4C Fig). Similarly, irradiated larvae expressing brv1RNAi in CIV neurons still showed an increase in US responders compared to mock controls (Fig 5E [p = 0.04] and S4D Fig) but larvae expressing brv1RNAi in Ch neurons showed a blocked increase in US responders after UV compared to mock controls (Fig 5E and S4D Fig). For BR responses however, larvae expressing brv1RNAi in CIV neurons had blocked increases in responses after UV compared to mock controls (Fig 5F). It is more difficult to discern the contributions of Brv1 in Ch neurons however, since although no increase in BR responses was observed upon expression of brv1RNAi, it was also quite low in the Gal4 alone control (Fig 5F). Collectively, these data suggest that Brv1 likely acts in Ch neurons to mediate increases in US responses and the decreases in CT responses to cold after UV. Further, Brv1 likely acts in CIV neurons to evoke emergent BR responses to cold after UV. It may be possible that Brv1 also acts in CIII neurons to contribute to the shift in CT responders however, given that CIII neurons are required for the baseline CT response (see Fig 3A), any shift in behavior or sensitization we observed would be difficult to parse out from baseline defects.
Discussion
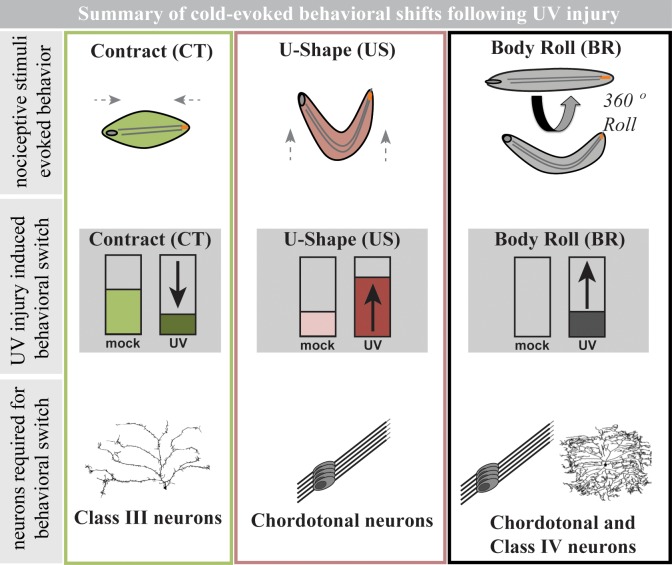
We show that after UV-induced epidermal tissue damage Drosophila larvae exhibit altered responses to cold stimuli. They do so in a complex manner involving a shift in behavioral output away from CT and towards US and BR responses. The increase in US responses appears to require Ch neurons while the emergence of BR responses to cold requires both CIV and Ch neurons (see summary, Fig 6). Molecularly, the TRPP type channel, Brv1, is required for the observed UV-induced behavioral shifts in response to noxious cold acting in CIV and/or Ch neurons.
Fig 6. Summary of cold-evoked behavioral shifts following UV injury in Drosophila larvae.
The figure is divided into three columns; the first row illustrates baseline nociceptive stimuli-evoked behaviors: a full body contraction (CT), a raising of the head and tail into a “U-Shape” (US), and a 360° lateral body roll (BR). The middle row indicates the UV injury-induced shifts in each of these behaviors observed in larvae upon cold exposure relative to mock treatment. The bottom row indicates the sensory neuron types required for the UV-induced behavioral shifts.
Morphological alterations following UV-induced injury could potentially contribute to cold sensitization behavioral shifts; however, we demonstrate that neither CIII nor Ch neurons exhibit morphological changes when comparing mock- and UV-irradiated larvae. Previous studies using a similar irradiation paradigm reveal no UV-induced change in CIV dendritic morphology [46].
Physiologically, cold-evoked calcium analysis in mock- versus UV-treated larvae revealed that the UV-induced behavioral switch appears to occur independently of calcium dynamics in CIII, Ch or CIV sensory neurons. These analyses did, however, reveal that Ch neurons, like CIII neurons, robustly respond to noxious cold under native conditions (in the absence of injury). As we did not observe any significant changes in mean calcium dynamics with UV treatment at the level of the primary sensory neurons, the observed shifts in behavioral output in response to noxious cold following injury may be an effect mediated at the synapse or downstream circuit(s). For example, previous studies have demonstrated that co-activation of Ch neurons and CIV neurons via vibration and noxious heat, respectively, synergistically increases the BR response, suggestive of an interaction between these sensory circuits [63]. Moreover, CIII and CIV axons terminate in adjacent regions of the neuropil [64] and recent studies have documented interneuron targets shared by CIII and CIV neurons that can serve as integrators of somatosensory behavioral responses [65, 66]. UV exposure could lead to alterations in interneuron targets or affect motor neuron activity, either of which could switch the observed motor program/behavioral output to cold. Another possibility, less likely, could be a weakening of CIII synapses to yet unknown first order interneurons, thus leading to alternate behaviors.
brv1, discovered as a cold sensor in adult flies [30] and in larvae [62], here appears to function in CIV neurons to contribute to the emergence of BR responses, and in Ch neurons to mediate a decrease in CT responders and increase in US responses to cold after UV damage. The fact that we see an overall decrease in cold-evoked CT in the irradiated brv1 mutant, yet when brv1-RNAi is expressed in Ch neurons this decrease is blocked, suggests that perhaps opposing roles of brv1 in different neuronal subtypes balances the ultimate behavioral response produced.
Why does this shift from the dominant cold response (CT) towards two alternative behaviors (US and BR) following UV-induced tissue damage occur? Given that UV exposure causes epidermal damage and apoptosis [38], and that the CT response requires a significant change in body length [26], it may be that a full-body contraction is physically more “painful”, or exacerbates epidermal damage, more than US or BR, making it advantageous for the larva to avoid CT in favor of other cold-evoked behaviors. We have previously shown that thermogenetic activation of CIII neurons, via expression of TRPA1 and application of heat (45°C), and CIV neurons (by the heat alone), results in an approximate balance of CT and BR responses [26], suggesting that crosstalk between these neurons may be present. Mechanistically, the behavioral shift from CT to US and BR could result from UV altering the sensitivity, localization, and/or expression level of Brv1 in CIV and Ch neurons. While many TRP channels are expressed on CIII neurons [26, 58], specific changes in TRP channel sensitivity or expression could presumably alter neuronal function, allowing the CIV neuron behavior (BR) to be observed at the expense of other behaviors mediated by other (CIII and Ch) neurons. For Ch neurons, it remains unclear which channels may be required for the observed emergence of BR responses to cold, since brv1 only appears to be required for US responses as well as the decrease in CT responses after UV. Currently, it is unknown whether brv1 is expressed in Ch neurons.
In all, this work establishes that Drosophila can be used to study nociceptive cold sensitization and to identify key players in the process. Utilizing Drosophila, and the tool and assay described here, will complement approaches in other systems aimed at identifying novel genetic players and mechanisms underlying cold nociception and nociceptive sensitization. Further investigation into the mechanisms of cold sensitization using these tools and assays will aid our understanding of the mediators that may be involved in clinically relevant cold hypersensitization.
Supporting information
(A) Proportion of US (red), CT (green), or BR (grey) responses to cold probe at different time points after UV, data shown as a stacked graph. For each behavior, the average over three sets of 30 larvae are shown. (B,C) Proportion of CT, US or BR fast (dark grey), slow (light grey) or non-responders (nr, white) in mock or UV-treated larvae to the cold probe (10°C) (B) 16 hours (C) or 24 hours after UV. Fast = response less than 4 seconds, slow = response between 4–10 seconds, nr = no response within 10 seconds. (D) Proportion of US, CT, or BR responses to the cold probe at different temperatures in mock or UV-treated larvae, data shown as a stacked graph. For each behavior, the average over three sets of 30 larvae are shown. (A-D) n = 3 sets of 30. Data are presented as mean ± s.e.m percentage of categorical responders. Stats: Two-tailed Fisher’s Exact test, * = p < 0.05, * = p < 0.001, comparisons were made between UV and mock control at each time point.
(PDF)
Percent of responders to cold probe (10°C) 24 hours after UV with varying dose (10–14 mJ/cm2). Bars represent average responders ± s.e.m.. * = p < 0.05 by two-tailed Fisher’s Exact test, comparing percent responders of each behavior between each UV dose, both US and BR were significantly different at 13 mJ/cm2 when compared to other UV-doses n = 3 sets of 30.
(PDF)
(A-C) Percent change in GCaMP6m fluorescence at 10°C for mock- and UV-treated larvae 24 hours post-irradiation for CIII (A), Ch (B), and CIV sensory neurons (C), where the middle line is mean ± s.e.m. and n = 8–11 larvae. Stats: Two-tailed t-test (A-C), where the comparisons are between mock and UV treated conditions. n.s. = not significant.
(PDF)
(A) CT or (B) US, responses in wildtype (w1118) or Brv1 mutant larvae, shown as a percent change in response after UV. (C) CT or (D) US responses in larvae expressing Brv-RNAi in class IV (CIV) or Chordotonal (Ch) neurons, compared to genetic controls (w1118 and Gal4 alone). (A-C) Data is calculated as (% UV responders—% mock responders)/ % mock responders. Therefore all bars that are above 1 indicate that the UV response was less than the mock response, and all bars below 1 indicate the UV response was more than the mock response, as indicated by arrows.
(PDF)
Acknowledgments
We acknowledge the VDRC stock center for UAS-RNAi transgenes and Marco Gallio, Teri Watnick, Kartik Venkatachalam, and Yuh Nung Jan for fly stocks. Stocks obtained from the Bloomington Drosophila stock center (NIH P40ODO18537) were used in this study. We thank Shatabdi Bhattacharjee for assistance with UV irradiation. We also thank members of the Cox and Galko labs for critical evaluation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the National Institute of Neurological Disorders and Stroke (R01 NS086082 to DNC) and a NIH Predoctoral Kirschstein National Research Service Award Fellowship (NINDS F31 NS083306 to HNT). This work was also supported by the National Institute of General Medical Sciences (R35 GM126929 to MJG), the Marilyn and Frederick R. Lummis, Jr. MD Fellowship (HNT); and Georgia State University (the 2CI Neurogenomics Fellowship and Kenneth W. and Georgeanne F. Honeycutt Fellowship to AAP; and a GSU Brains and Behavior grant to DNC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nature medicine. 2010;16(11):1248–57. 10.1038/nm.2235 ; PubMed Central PMCID: PMC5022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crook RJ, Dickson K, Hanlon RT, Walters ET. Nociceptive sensitization reduces predation risk. Curr Biol. 2014;24(10):1121–5. 10.1016/j.cub.2014.03.043 . [DOI] [PubMed] [Google Scholar]
- 3.Khan N, Smith MT. Multiple sclerosis-induced neuropathic pain: pharmacological management and pathophysiological insights from rodent EAE models. Inflammopharmacology. 2014;22(1):1–22. 10.1007/s10787-013-0195-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berglund B, Harju EL, Kosek E, Lindblom U. Quantitative and qualitative perceptual analysis of cold dysesthesia and hyperalgesia in fibromyalgia. Pain. 2002;96(1–2):177–87. . [DOI] [PubMed] [Google Scholar]
- 5.Wanklyn P, Ilsley DW, Greenstein D, Hampton IF, Roper TA, Kester RC, et al. The cold hemiplegic arm. Stroke. 1994;25(9):1765–70. . [DOI] [PubMed] [Google Scholar]
- 6.Greenspan JD, Ohara S, Sarlani E, Lenz FA. Allodynia in patients with post-stroke central pain (CPSP) studied by statistical quantitative sensory testing within individuals. Pain. 2004;109(3):357–66. 10.1016/j.pain.2004.02.002 . [DOI] [PubMed] [Google Scholar]
- 7.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249(1):9–17. . [DOI] [PubMed] [Google Scholar]
- 8.Extra JM, Marty M, Brienza S, Misset JL. Pharmacokinetics and safety profile of oxaliplatin. Semin Oncol. 1998;25(2 Suppl 5):13–22. . [PubMed] [Google Scholar]
- 9.Jasmin L, Kohan L, Franssen M, Janni G, Goff JR. The cold plate as a test of nociceptive behaviors: description and application to the study of chronic neuropathic and inflammatory pain models. Pain. 1998;75(2–3):367–82. . [DOI] [PubMed] [Google Scholar]
- 10.Pizziketti RJ, Pressman NS, Geller EB, Cowan A, Adler MW. Rat cold water tail-flick: a novel analgesic test that distinguishes opioid agonists from mixed agonist-antagonists. Eur J Pharmacol. 1985;119(1–2):23–9. . [DOI] [PubMed] [Google Scholar]
- 11.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59(3):369–76. . [DOI] [PubMed] [Google Scholar]
- 12.Brenner DS, Golden JP, Gereau RWt. A novel behavioral assay for measuring cold sensation in mice. PLoS One. 2012;7(6):e39765 10.1371/journal.pone.0039765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vriens J, Nilius B, Voets T. Peripheral thermosensation in mammals. Nat Rev Neurosci. 2014;15(9):573–89. 10.1038/nrn3784 . [DOI] [PubMed] [Google Scholar]
- 14.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. 10.1146/annurev.neuro.051508.135531 ; PubMed Central PMCID: PMC2768555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. 10.1146/annurev.biochem.75.103004.142819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moran MM, Szallasi A. Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. British journal of pharmacology. 2017;175(12):2185–203. 10.1111/bph.14044 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–24. 10.1038/39807 . [DOI] [PubMed] [Google Scholar]
- 18.Sarria I, Ling J, Xu GY, Gu JG. Sensory discrimination between innocuous and noxious cold by TRPM8-expressing DRG neurons of rats. Mol Pain. 2012;8:79 10.1186/1744-8069-8-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112(6):819–29. . [DOI] [PubMed] [Google Scholar]
- 20.Lippoldt EK, Elmes RR, McCoy DD, Knowlton WM, McKemy DD. Artemin, a glial cell line-derived neurotrophic factor family member, induces TRPM8-dependent cold pain. J Neurosci. 2013;33(30):12543–52. 10.1523/JNEUROSCI.5765-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iftinca M, Flynn R, Basso L, Melo H, Aboushousha R, Taylor L, et al. The stress protein heat shock cognate 70 (Hsc70) inhibits the Transient Receptor Potential Vanilloid type 1 (TRPV1) channel. Mol Pain. 2016;12(pii: 1744806916663945). 10.1177/1744806916663945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54(3):371–8. 10.1016/j.neuron.2007.02.024 . [DOI] [PubMed] [Google Scholar]
- 23.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50(2):277–89. 10.1016/j.neuron.2006.03.042 . [DOI] [PubMed] [Google Scholar]
- 24.Munns C, AlQatari M, Koltzenburg M. Many cold sensitive peripheral neurons of the mouse do not express TRPM8 or TRPA1. Cell Calcium. 2007;41(4):331–42. 10.1016/j.ceca.2006.07.008 . [DOI] [PubMed] [Google Scholar]
- 25.Garrity PA, Goodman MB, Samuel AD, Sengupta P. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev. 2010;24(21):2365–82. 10.1101/gad.1953710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner HN, Armengol K, Patel AA, Himmel NJ, Sullivan L, Iyer SC, et al. The TRP Channels Pkd2, NompC, and Trpm Act in Cold-Sensing Neurons to Mediate Unique Aversive Behaviors to Noxious Cold in Drosophila. Curr Biol. 2016;26(23):3116–28. Epub 2016/11/08. 10.1016/j.cub.2016.09.038 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatzigeorgiou M, Yoo S, Watson JD, Lee WH, Spencer WC, Kindt KS, et al. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat Neurosci. 2010;13(7):861–8. 10.1038/nn.2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Summers T, Wang Y, Hanten B, Burrell BD. Physiological, pharmacological and behavioral evidence for a TRPA1 channel that can elicit defensive responses in the medicinal leech. J Exp Biol. 2015;218(Pt 19):3023–31. 10.1242/jeb.120600 . [DOI] [PubMed] [Google Scholar]
- 29.Klein M, Afonso B, Vonner AJ, Hernandez-Nunez L, Berck M, Tabone CJ, et al. Sensory determinants of behavioral dynamics in Drosophila thermotaxis. Proc Natl Acad Sci U S A. 2015;112(2):E220–9. 10.1073/pnas.1416212112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallio M, Ofstad TA, Macpherson LJ, Wang JW, Zuker CS. The coding of temperature in the Drosophila brain. Cell. 2011;144(4):614–24. 10.1016/j.cell.2011.01.028 ; PubMed Central PMCID: PMC3336488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon Y, Shen WL, Shim HS, Montell C. Fine thermotactic discrimination between the optimal and slightly cooler temperatures via a TRPV channel in chordotonal neurons. J Neurosci. 2010;30(31):10465–71. 10.1523/JNEUROSCI.1631-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenzweig M, Kang K, Garrity PA. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2008;105(38):14668–73. 10.1073/pnas.0805041105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12(6):1195–206. . [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Cameron S, Chang WT, Rao Y. Control of directional change after mechanical stimulation in Drosophila. Mol Brain. 2012;5:39 10.1186/1756-6606-5-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, et al. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013;493(7431):221–5. 10.1038/nature11685 ; PubMed Central PMCID: PMC3917554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsubouchi A, Caldwell JC, Tracey WD. Dendritic filopodia, Ripped Pocket, NOMPC, and NMDARs contribute to the sense of touch in Drosophila larvae. Curr Biol. 2012;22(22):2124–34. 10.1016/j.cub.2012.09.019 ; PubMed Central PMCID: PMC3511824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tracey WD Jr., Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113(2):261–73. . [DOI] [PubMed] [Google Scholar]
- 38.Babcock DT, Landry C, Galko MJ. Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr Biol. 2009;19(10):799–806. 10.1016/j.cub.2009.03.062 ; PubMed Central PMCID: PMC4017352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, et al. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol. 2007;17(24):2105–16. 10.1016/j.cub.2007.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong L, Hwang RY, Tracey WD. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr Biol. 2010;20(5):429–34. 10.1016/j.cub.2009.12.057 ; PubMed Central PMCID: PMC2995491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483(7388):209–12. 10.1038/nature10801 ; PubMed Central PMCID: PMC3297676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rose JK, Rankin CH. Analyses of habituation in Caenorhabditis elegans. Learn Mem. 2001;8(2):63–9. 10.1101/lm.37801 . [DOI] [PubMed] [Google Scholar]
- 43.Illich PA, Walters ET. Mechanosensory neurons innervating Aplysia siphon encode noxious stimuli and display nociceptive sensitization. J Neurosci. 1997;17(1):459–69. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Babcock DT, Shi S, Jo J, Shaw M, Gutstein HB, Galko MJ. Hedgehog signaling regulates nociceptive sensitization. Curr Biol. 2011;21(18):1525–33. 10.1016/j.cub.2011.08.020 ; PubMed Central PMCID: PMC3262399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Im SH, Takle K, Jo J, Babcock DT, Ma Z, Xiang Y, et al. Tachykinin acts upstream of autocrine Hedgehog signaling during nociceptive sensitization in Drosophila. Elife. 2015;4:e10735 10.7554/eLife.10735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Im SH, Patel AA, Cox DN, Galko MJ. Drosophila Insulin receptor regulates the persistence of injury-induced nociceptive sensitization. Disease models & mechanisms. 2018;11(5). 10.1242/dmm.034231 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468(7326):921–6. 10.1038/nature09576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ainsley JA, Pettus JM, Bosenko D, Gerstein CE, Zinkevich N, Anderson MG, et al. Enhanced locomotion caused by loss of the Drosophila DEG/ENaC protein Pickpocket1. Curr Biol. 2003;13(17):1557–63. . [DOI] [PubMed] [Google Scholar]
- 49.Petersen LK, Stowers RS. A Gateway MultiSite recombination cloning toolkit. PLoS One. 2011;6(9):e24531 10.1371/journal.pone.0024531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448(7150):151–6. 10.1038/nature05954 . [DOI] [PubMed] [Google Scholar]
- 51.Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14(2):341–51. . [DOI] [PubMed] [Google Scholar]
- 52.Pfeiffer BD, Ngo TT, Hibbard KL, Murphy C, Jenett A, Truman JW, et al. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186(2):735–55. 10.1534/genetics.110.119917 ; PubMed Central PMCID: PMC2942869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32(40):13819–40. 10.1523/JNEUROSCI.2601-12.2012 ; PubMed Central PMCID: PMC3482105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ni JQ, Markstein M, Binari R, Pfeiffer B, Liu LP, Villalta C, et al. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat Methods. 2008;5(1):49–51. 10.1038/nmeth1146 ; PubMed Central PMCID: PMC2290002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jo J, Im SH, Babcock DT, Iyer SC, Gunawan F, Cox DN, et al. Drosophila caspase activity is required independently of apoptosis to produce active TNF/Eiger during nociceptive sensitization. Cell Death Dis. 2017;8(5):e2786 10.1038/cddis.2016.474 ; PubMed Central PMCID: PMC5520682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Babcock DT. Damage-induced inflammation and nociceptive hypersensitivity in Drosophila larvae [Dissertation] Texas Medical Center Library: University of Texas Graduate School of Biomedical Science at Houston; 2010. [Google Scholar]
- 57.Das R, Bhattacharjee S, Patel AA, Harris JM, Bhattacharya S, Letcher JM, et al. Dendritic Cytoskeletal Architecture Is Modulated by Combinatorial Transcriptional Regulation in Drosophila melanogaster. Genetics. 2017;207(4):1401–21. 10.1534/genetics.117.300393 ; PubMed Central PMCID: PMC5714456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iyer EP, Iyer SC, Sullivan L, Wang D, Meduri R, Graybeal LL, et al. Functional genomic analyses of two morphologically distinct classes of Drosophila sensory neurons: post-mitotic roles of transcription factors in dendritic patterning. PLoS One. 2013;8(8):e72434 10.1371/journal.pone.0072434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel AA, Cox DN. Behavioral and Functional Assays for Investigating Mechanisms of Noxious Cold Detection and Multimodal Sensory Processing in Drosophila Larvae. Bio-protocol. 2017;7(13). 10.21769/BioProtoc.2388 ; PubMed Central PMCID: PMC5564679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghysen A, Dambly-Chaudiere C, Aceves E, Jan L, Jan Y. Sensory neurons and peripheral pathways in Drosophila embryos. Roux's archives of developmental biology: the official organ of the EDBO. 1986;195(5):281–9. 10.1007/BF00376060 . [DOI] [PubMed] [Google Scholar]
- 61.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–15. . [DOI] [PubMed] [Google Scholar]
- 62.Ni L, Klein M, Svec KV, Budelli G, Chang EC, Ferrer AJ, et al. The Ionotropic Receptors IR21a and IR25a mediate cool sensing in Drosophila. Elife. 2016;5 10.7554/eLife.13254 ; PubMed Central PMCID: PMC4851551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohyama T, Schneider-Mizell CM, Fetter RD, Aleman JV, Franconville R, Rivera-Alba M, et al. A multilevel multimodal circuit enhances action selection in Drosophila. Nature. 2015;520(7549):633–9. 10.1038/nature14297 . [DOI] [PubMed] [Google Scholar]
- 64.Grueber WB, Ye B, Yang CH, Younger S, Borden K, Jan LY, et al. Projections of Drosophila multidendritic neurons in the central nervous system: links with peripheral dendrite morphology. Development. 2007;134(1):55–64. 10.1242/dev.02666 . [DOI] [PubMed] [Google Scholar]
- 65.Burgos A, Honjo K, Ohyama T, Qian CS, Shin GJ, Gohl DM, et al. Nociceptive interneurons control modular motor pathways to promote escape behavior in Drosophila. Elife. 2018;7 10.7554/eLife.26016 ; PubMed Central PMCID: PMC5869015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu C, Petersen M, Hoyer N, Spitzweck B, Tenedini F, Wang D, et al. Sensory integration and neuromodulatory feedback facilitate Drosophila mechanonociceptive behavior. Nat Neurosci. 2017;20(8):1085–95. 10.1038/nn.4580 ; PubMed Central PMCID: PMC5931224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Proportion of US (red), CT (green), or BR (grey) responses to cold probe at different time points after UV, data shown as a stacked graph. For each behavior, the average over three sets of 30 larvae are shown. (B,C) Proportion of CT, US or BR fast (dark grey), slow (light grey) or non-responders (nr, white) in mock or UV-treated larvae to the cold probe (10°C) (B) 16 hours (C) or 24 hours after UV. Fast = response less than 4 seconds, slow = response between 4–10 seconds, nr = no response within 10 seconds. (D) Proportion of US, CT, or BR responses to the cold probe at different temperatures in mock or UV-treated larvae, data shown as a stacked graph. For each behavior, the average over three sets of 30 larvae are shown. (A-D) n = 3 sets of 30. Data are presented as mean ± s.e.m percentage of categorical responders. Stats: Two-tailed Fisher’s Exact test, * = p < 0.05, * = p < 0.001, comparisons were made between UV and mock control at each time point.
(PDF)
Percent of responders to cold probe (10°C) 24 hours after UV with varying dose (10–14 mJ/cm2). Bars represent average responders ± s.e.m.. * = p < 0.05 by two-tailed Fisher’s Exact test, comparing percent responders of each behavior between each UV dose, both US and BR were significantly different at 13 mJ/cm2 when compared to other UV-doses n = 3 sets of 30.
(PDF)
(A-C) Percent change in GCaMP6m fluorescence at 10°C for mock- and UV-treated larvae 24 hours post-irradiation for CIII (A), Ch (B), and CIV sensory neurons (C), where the middle line is mean ± s.e.m. and n = 8–11 larvae. Stats: Two-tailed t-test (A-C), where the comparisons are between mock and UV treated conditions. n.s. = not significant.
(PDF)
(A) CT or (B) US, responses in wildtype (w1118) or Brv1 mutant larvae, shown as a percent change in response after UV. (C) CT or (D) US responses in larvae expressing Brv-RNAi in class IV (CIV) or Chordotonal (Ch) neurons, compared to genetic controls (w1118 and Gal4 alone). (A-C) Data is calculated as (% UV responders—% mock responders)/ % mock responders. Therefore all bars that are above 1 indicate that the UV response was less than the mock response, and all bars below 1 indicate the UV response was more than the mock response, as indicated by arrows.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.