Abstract
Drosophila bithorax complex (BX-C) is one of the best model systems for studying the role of boundaries (insulators) in gene regulation. Expression of three homeotic genes, Ubx, abd-A, and Abd-B, is orchestrated by nine parasegment-specific regulatory domains. These domains are flanked by boundary elements, which function to block crosstalk between adjacent domains, ensuring that they can act autonomously. Paradoxically, seven of the BX-C regulatory domains are separated from their gene target by at least one boundary, and must “jump over” the intervening boundaries. To understand the jumping mechanism, the Mcp boundary was replaced with Fab-7 and Fab-8. Mcp is located between the iab-4 and iab-5 domains, and defines the border between the set of regulatory domains controlling abd-A and Abd-B. When Mcp is replaced by Fab-7 or Fab-8, they direct the iab-4 domain (which regulates abd-A) to inappropriately activate Abd-B in abdominal segment A4. For the Fab-8 replacement, ectopic induction was only observed when it was inserted in the same orientation as the endogenous Fab-8 boundary. A similar orientation dependence for bypass activity was observed when Fab-7 was replaced by Fab-8. Thus, boundaries perform two opposite functions in the context of BX-C–they block crosstalk between neighboring regulatory domains, but at the same time actively facilitate long distance communication between the regulatory domains and their respective target genes.
Author summary
Drosophila bithorax complex (BX-C) is one of a few examples demonstrating in vivo role of boundary/insulator elements in organization of independent chromatin domains. BX-C contains three HOX genes, whose parasegment-specific pattern is controlled by cis-regulatory domains flanked by boundary/insulator elements. Since the boundaries ensure autonomy of adjacent domains, the presence of these elements poses a paradox: how do the domains bypass the intervening boundaries and contact their proper regulatory targets? According to the textbook model, BX-C regulatory domains are able to bypass boundaries because they harbor special promoter targeting sequences. However, contrary to this model, we show here that the boundaries themselves play an active role in directing regulatory domains to their appropriate HOX gene promoter.
Introduction
The three homeotic (HOX) genes in the Drosophila Bithorax complex (BX-C), Ultrabithorax (Ubx), abdominal-A (abd-A) and Abdominal-B (Abd-B), are responsible for specifying cell identity in parasegments (PS) 5–14, which form the posterior half of the thorax and all of the abdominal segments of the adult fly [1–3]. Parasegment identity is determined by the precise expression pattern of the relevant HOX gene and this depends upon a large cis-regulatory region that spans 300 kb and is subdivided into nine PS domains that are aligned in the same order as the body segments in which they operate [4–6]. Ubx expression in PS5 and PS6 is directed by abx/bx and bxd/pbx, while abd-A expression in PS7, PS8, and PS9 is controlled by iab-2, iab-3, and iab-4 [7–10]. Abd-B is regulated by four domains, iab-5, iab-6, iab-7 and iab-8, which control expression in PS10, PS11, PS12 and PS13 respectively [6,11,12].
Each regulatory domain contains an initiator element, a set of tissue-specific enhancers and Polycomb Response Elements (PREs) and is flanked by boundary/insulator elements (Fig 1A;[13]. BX-C regulation is divided into two phases, initiation and maintenance [14,15]. During the initiation phase, a combination of gap and pair-rule proteins interact with initiation elements in each regulatory domain, setting the domain in the on or off state. In PS10, for example, the iab-5 domain, which regulates Abd-B, is activated by its initiator element, while the more distal Abd-B domains, iab-6 to iab-8 are set in the off state (Fig 1B). In PS11, the iab-6 initiator activates the domain, while the adjacent iab-7 and iab-8 domains are set in the off state. Once the gap and pair-rule gene proteins disappear during gastrulation, the on and off states of the regulatory domains are maintained by Trithorax (Trx) and Polycomb (PcG) group proteins, respectively [16,17].
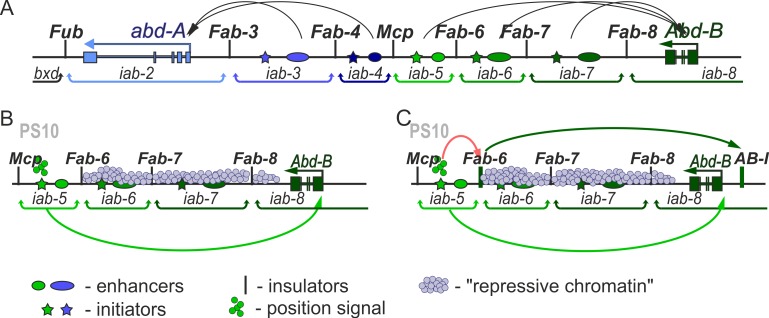
Fig 1. Models of an enhancer–promoter interactions in BX-C.
(A) Scheme of the regulatory region of the distal part of the BX-C. Horizontal arrows represent transcripts for abd-A (blue) and Abd-B (green). iab enhancers are shown as ovals color-coded with respect to the gene they control (darker shades of color indicate higher expression levels). The black arc arrows are a graphical illustration of the targeting of each cis-regulatory domain to the abd-A or Abd-Bm promoter. Vertical lines mark boundaries (Fub, Fab-3, Fab-4, Mcp, Fab-6, Fab-7, and Fab-8) of regulatory iab domains which are delimited by brackets behind the map. (B) and (C) Schematic representation of the models explaining interaction of the iab enhancers with the Abd-B promoter. (B) After stimulation of the initiator (colored star) by a positional signal (small circles with the same color as initiator), the iab enhancer directly interacts with the Abd-B promoter. This process is shown as light green arc arrow. Domains without positional signal are repressed (shown as light blue small circles across the domain, indicating "repressive chromatin"). (C) Boundary is directly involved in organization of the enhancer-promoter interactions. There is also a boundary-like element AB-I upstream of the Abd-B promoter that has communicator activity in bypass assays. Proteins of the positional signal bind with initiator of iab domain, and its active status modifies the boundary activity. This process is shown as red arc arrow. Boundary becomes able to bind proteins responsible for communication with AB-I. Interaction is shown as dark green arc arrow. As a result, the iab enhancer is localized in close proximity to the Abd-B promoter (shown as light green arc arrow).
In order to select and then maintain their activity states independent of outside influence, adjacent regulatory domains are separated from each other by boundary elements or insulators [18–24]. Mutations that impair boundary function permit crosstalk between positive and negative regulatory elements in adjacent domains and this leads to the misspecification of parasegment identity. This has been observed for deletions that remove five of the BX-C boundaries (Front-ultraabdominal (Fub), Miscadestral pigmentation (Mcp), Frontadominal-6 (Fab-6), Frontadominal-7 (Fab-7), and Frontadominal-8 (Fab-8)) [6,17,19,20,22,23,25,26].
While these findings indicate that boundaries are needed to ensure the functional autonomy of the regulatory domains, their presence also poses a paradox [27,28]. Seven of the nine BX-C regulatory domains are separated from their target HOX gene by at least one intervening boundary element. For example, the iab-6 regulatory domain must “jump over” or “bypass” Fab-7 and Fab-8 in order to interact with the Abd-B promoter (Fig 1A). That the blocking function of boundaries could pose a significant problem has been demonstrated by experiments in which Fab-7 is replaced by heterologous elements such as scs, gypsy or multimerized binding sites for the architectural proteins dCTCF, Pita or Su(Hw) [25,29–31]. In these replacements, the heterologous boundary blocked crosstalk between iab-6 and iab-7 just like the endogenous boundary, Fab-7. However, the boundaries were not permissive for bypass, preventing iab-6 from regulating Abd-B.
A number of models have been proposed to account for this paradox. One is that BX-C boundaries must have unique properties that distinguish them from generic fly boundaries. Since they function to block crosstalk between enhancers and silencers in adjacent domains, an appealing idea is that they would be permissive for enhancer/silencer interactions with promoters (Fig 1B). However, several findings argue against this notion. For one, BX-C boundaries resemble those elsewhere in the genome in that they contain binding sites for architectural proteins such as Pita, dCTCF, and Su(Hw) [23,31–35]. Consistent with their utilization of these generic architectural proteins, when placed between enhancers (or silencers) and a reporter gene, BX-C boundaries block regulatory interactions just like boundaries from elsewhere in the genome [19,36–42]. Similarly, there is no indication in these transgene assays that the blocking activity of BX-C boundaries are subject to parasegmental regulation. Also arguing against the idea that BX-C boundaries have unique properties, the Mcp boundary, which is located between iab-4 and iab-5, is unable to replace Fab-7 [31]. Like the heterologous boundaries, it blocks crosstalk, but it is not permissive for bypass. A second model is that there are special sequences, called promoter targeting sequence (PTS), located in each regulatory domain that actively mediate bypass [43–45]. While the PTS sequences that have been identified in iab-6 and iab-7 enable enhancers to “jump over” an intervening boundary in transgene assays, they do not have a required function in the context of BX-C and are completely dispensable for Abd-B regulation [18,30].
A third model (Fig 1C) is suggested by transgene “insulator bypass” assays [46,47]. In one version of this assay, two boundaries instead of one are placed in between an enhancer and the reporter. When the two boundaries pair with each other, the enhancer is brought in close proximity to the reporter, thereby activating rather than blocking expression. Consistent with a possible role in BX-C bypass, these pairing interactions can occur over large distances and even skip over many intervening boundaries [48–51]. The transgene assays point to two important features of boundary pairing interactions that are likely to be relevant in BX-C. First, pairing interactions are specific. For this reason boundaries must be properly matched with their neighborhood in order to function appropriately. A requirement for matching is illustrated in transgene bypass experiments in which multimerized binding sites for specific architectural proteins are paired with themselves or with each other[52]. Bypass was observed when multimerized dCTCF, Zw5 or Su(Hw) binding sites were paired with themselves; however, heterologous combinations (e.g. dCTCF sites with Su(Hw) sites) did not support bypass. A second feature is that pairing interactions between boundaries are typically orientation dependent For example, scs pairs with itself head-to-head, not head-to-tail [52].
If both blocking and bypass activities are intrinsic properties of fly boundaries then the BX-C boundaries themselves may facilitate contacts between the regulatory domains and their target genes (Fig 1C). Moreover, the fact that both blocking and bypass activity are non-autonomous (in that they depend on partner pairing) could potentially explain why heterologous Fab-7 replacements like gypsy and Mcp behave anomalously while Fab-8 functions appropriately. Several observations fit with this idea. There is an extensive region upstream of the Abd-B promoter that has been implicated in tethering the Abd-B regulatory domains to the promoter [53–56] and this region could play an important role in mediating bypass by boundaries associated with the distal Abd-B regulatory domains (iab-5, iab-6, iab-7). Included in this region is a promoter tethering element (PTE) that facilitates interactions between iab enhancers and the Abd-B promoter in transgene assays [57,58]. Just beyond the PTE is a boundary-like element, AB-I. In transgene assays AB-I mediates bypass when combined with either Fab-7 or Fab-8. In contrast, a combination between AB-I and Mcp fails to support bypass [59,60]. The ability of both Fab-7 and Fab-8 to pair with AB-I is recapitulated in Fab-7 replacement experiments. Unlike Mcp, Fab-8 has both blocking and bypass activity when inserted in place of Fab-7 [30]. Moreover, its’ bypass but not blocking activity is orientation-dependent. When inserted in the same orientation as the endogenous Fab-8 boundary, it mediates blocking and bypass, while it does not support bypass when inserted in the opposite orientation.
In the studies reported here we have tested this model by replacing the endogenous Mcp boundary with heterologous boundaries. Mcp defines the border between the set of regulatory domains that control abd-A and those that control Abd-B expression (Fig 1A). Unlike the boundaries that are within the Abd-B regulatory domain (e.g. Fab-7 or Fab-8), Mcp is not located between a regulatory domain and its target gene. Instead, it defines the boundary between regulatory domains that target abd-A and those that target Abd-B. For this reason, we expected that it does not need bypass activity. Consistent with this expectation, we find that multimerized dCTCF binding sites fully substitute for Mcp. A different result is obtained for the Abd-B-associated boundaries, Fab-7 and Fab-8. Both boundaries are (for the most part) able to block crosstalk between the abd-A regulatory domain iab-4, which specifies A4 (PS9) and the Abd-B regulatory domain iab-5, which specifies A5 (PS10). Their blocking activity is orientation independent. However, in spite of blocking crosstalk, these replacements still inappropriately induce Abd-B expression in A4 (PS9), causing the misspecification of this segment. For the Fab-7 replacements, this occurred in both orientations, while for the Fab-8 replacement ectopic induction was only observed when it was inserted in the same orientation as the endogenous Fab-8 boundary. We present evidence showing that the boundary replacements activate the Abd-B gene in A4 (PS9) by inappropriately targeting the iab-4 domain to the Abd-B promoter. In addition to altering the specification of A4 (PS9), the Fab-7 replacements induce novel transformations of A5 and A6. These findings indicate that when Fab-7 is inserted into the BX-C in place of Mcp, it perturbs Abd-B regulation in several segments besides PS9.
Results
Substitution of Mcp by an attP integration site in the BX-C
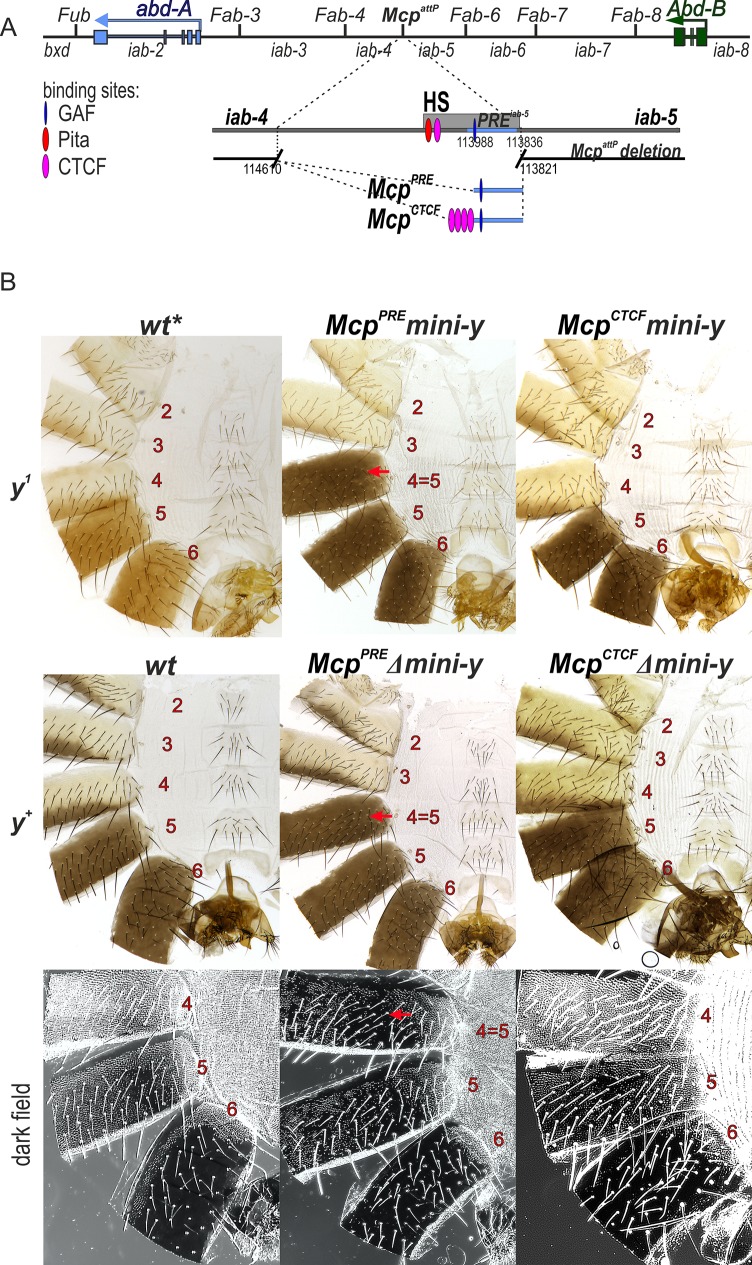
The Mcp boundary is defined by 340 bp core sequence that has enhancer blocking activity in transgene assays [36] and blocks crosstalk between iab-6 and iab-7 when substituted for Fab-7 [31]. Located just distal to the boundary is a PRE that negatively regulates the activity of the iab-5 enhancers [61]. We used CRISPR to delete a 789 bp DNA segment including the Mcp boundary and the PRE and replace it with an eGFP reporter flanked by two attP sites (McpattP) (S1 Fig). The presence of two attP sites in opposite orientation allows integration of different DNA fragments by recombination mediated cassette exchange (RMCE; [62]) using the phiC31 integration system [63].
Multimerized dCTCF sites substitute for Mcp
The Mcp boundary marks the division between the set of regulatory domains that control the abd-A and Abd-B genes (Fig 1A). The iab-4 domain is just proximal to Mcp, and it directs abd-A expression in PS9. The iab-5 domain is on the distal side and it regulates Abd-B in PS10. A boundary in this position would be needed to block crosstalk between iab-4 and iab-5; however, neither of these domains would require the intervening boundary to have bypass activity. On the proximal side, iab-4 must bypass the putative Fab-3 and Fab-4 boundaries in order to activate the abd-A promoter, while on the distal side, iab-5 must bypass Fab-6, Fab-7 and Fab-8 in order to activate Abd-B. If this expectation is correct, a generic boundary that has blocking activity but is unable to direct iab-4 to the abd-A promoter or iab-5 to the Abd-B promoter should be able to substitute for Mcp. To test this prediction (Fig 2), we introduced either the iab-5 PRE itself (McpPRE) or the PRE in combination with four dCTCF sites (McpCTCF). In Fab-7 replacement experiments four dCTCF sites in combination with the iab-7 PRE blocked crosstalk between the iab-6 and iab-7 domains, but did not allow the iab-6 domain to regulate Abd-B expression in PS11 [30].
Fig 2. The CTCF sites block crosstalk between the iab-4 and iab-5 domains.
(A) Molecular maps of the Mcp boundary. The coordinates of the McpattP deletion and McpPRE McpCTCF replacement fragments according to complete sequence of BX-C in SEQ89E numbering [4] are shown below. DNAse hypersensitive site is shown as a light gray box above the coordinate bar. Binding sites for GAF, Pita and dCTCF are indicated by blue, red and purple ovals, respectively. PRE element from iab-5 is marked as a blue stripe. Replacement fragments are shown below. wt* indicate wt with respect to Mcp. (B) The cuticle preparations of wt, McpPRE and McpCTCF males. The morphology of the 2nd to 6th abdominal segments is shown. Abnormalities in segment phenotype are shown by the red arrows. The localization of trichomes on the 4th to 6th abdominal tergites are shown in dark field.
Abd-B is a master regulator of pigmentation in the male abdominal A5 and A6 segments and it controls the expression of the yellow and tan genes which are involved in melanin synthesis [64–66]. In flies carrying the null y1 allele, the tan gene is still expressed and the pigmentation in A5 and A6 is light brown-yellow not black [66,67]. In order to be able to recover recombinants and also to monitor the blocking activity of the replacement sequence and the on/off state of the iab-5 domain, we used a y1 genetic background and included a minimal yellow (mini-y) reporter in our Mcp replacement construct (S1 Fig). The mini-y reporter consists of the cDNA and the 340 bp yellow promoter and lacks the wing, body and bristle enhancers of the endogenous yellow gene. As a result, activity of the mini-y reporter depends upon proximity to nearby enhancers. Expression of the mini-y reporter was examined in the y1 background.
Based on previous studies [5,21,68], the expression of this reporter should be determined by the activity state of the iab-5 domain. When iab-5 is shutoff by Pc-G dependent silencing in PS9 and more anterior parasegments, the mini-y reporter will also be silenced. When iab-5 is turned on in PS10 and more posterior parasegments, the mini-y reporter will be expressed. This parasegment-specific regulation of the reporter activity will be reflected in the segmental pattern of black melanin pigmentation in the adult cuticle. In replacements in which blocking activity is compromised, mini-y will be expressed in PS9 and in adults the A4 tergite will be black, just like the A5 and A6 tergites. In contrast, in replacements that have blocking activity mini-y will be silenced in PS9, but active in PS10 and PS11. In this case, A5 and A6 will have black pigmentation, while the stripe of pigmentation along the posterior edge of the A4 tergite will be light yellow brown, as only the tan gene will contribute to pigmentation in this segment.
When we replaced the Mcp deletion by the iab-5 PRE alone (McpPRE) the mini-y reporter was active not only in A5 (PS10) and more posterior segments, but also in A4 (PS9).
As shown in Fig 2, the pigmentation in A4 is black like that in A5 indicating that the reporter is expressed in both segments (Fig 2). This finding shows that, similar to classical Mcp deletions, the McpPRE replacement does not have blocking activity. In these Mcp deletions iab-5 is ectopically activated in PS9 by the iab-4 initiator. It then drives Abd-B expression in PS9 resulting in a gain-of-function (GOF) transformation of parasegment identity from PS9 to PS10. We used two approaches to test whether this was true for the McpPRE replacement. In the first, we excised the mini-y reporter and introduced an X chromosome with a wild type yellow (y+) gene. Since Abd-B directly regulates y+ expression in the abdomen [65,67], a transformation of PS9 into PS10 should be accompanied by a PS10-like pattern of pigmentation. Fig 2 shows that this is the case. We also examined the pattern of Abd-B protein expression in the embryonic CNS. In wild type embryos Abd-B is not expressed is PS9, while it is expressed at low levels in PS10. As shown in Fig 3A, similar levels of Abd-B protein are detected in PS9 and PS10 in the McpPRE replacement.
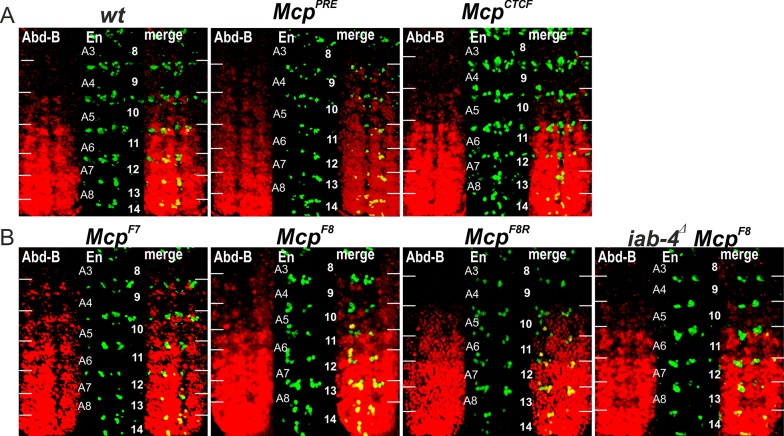
Fig 3. Expression of Abd-B in Mcp replacement embryos.
(A) Abd-B expression in wt, McpPRE and McpCTCF embryos. (B) Abd-B expression in McpF7, McpF8, McpF8R and iab-4Δ McpF8 embryos. Each panel shows an image of the embryonic CNS of stage 14 embryos stained with antibodies to Abd-B (red) and Engrailed (En, green). En is used to mark the anterior boundary of each parasegment. White horizontal bars approximately delimit parasegment/segment boundaries. Parasegments numbered from 9 to 14 on the right side of the panels; approximate positions of segments are shown on the left side of the wild type (wt) panel and marked A4 to A8. The wild type expression pattern of Abd-B in the embryonic CNS is characterized by a stepwise gradient of increasing protein level from PS10 to PS14. The McpF8 or McpF7 embryos have similar low Abd-B expression in PS9 and PS10. The Abd-B expression in PS9 is absent in iab-4Δ McpF8 and McpF8R embryos.
As predicted, a quite different result is obtained when we combined the iab-5 PRE with multimerized dCTCF sites. Expression of the mini-y reporter in the McpCTCF replacement was restricted to A5 (PS10) and A6 (PS11) as would be expected if the multimerized dCTCF sites block crosstalk between the iab-4 and iab-5 domains so that iab-5 is silenced by Pc-G factors in PS9 (Fig 2). The same pigmentation pattern is observed for the endogenous yellow in the Δmini-y derivative of McpCTCF, indicating that Abd-B is not turned on ectopically in PS9. This conclusion is confirmed by antibody staining experiments (Fig 3A). Thus, unlike replacements of Fab-7, a generic boundary can fully substitute for Mcp.
Substitution of Mcp for Fab-7 disrupts Abd-B regulation in parasegments PS9, PS10 and PS11
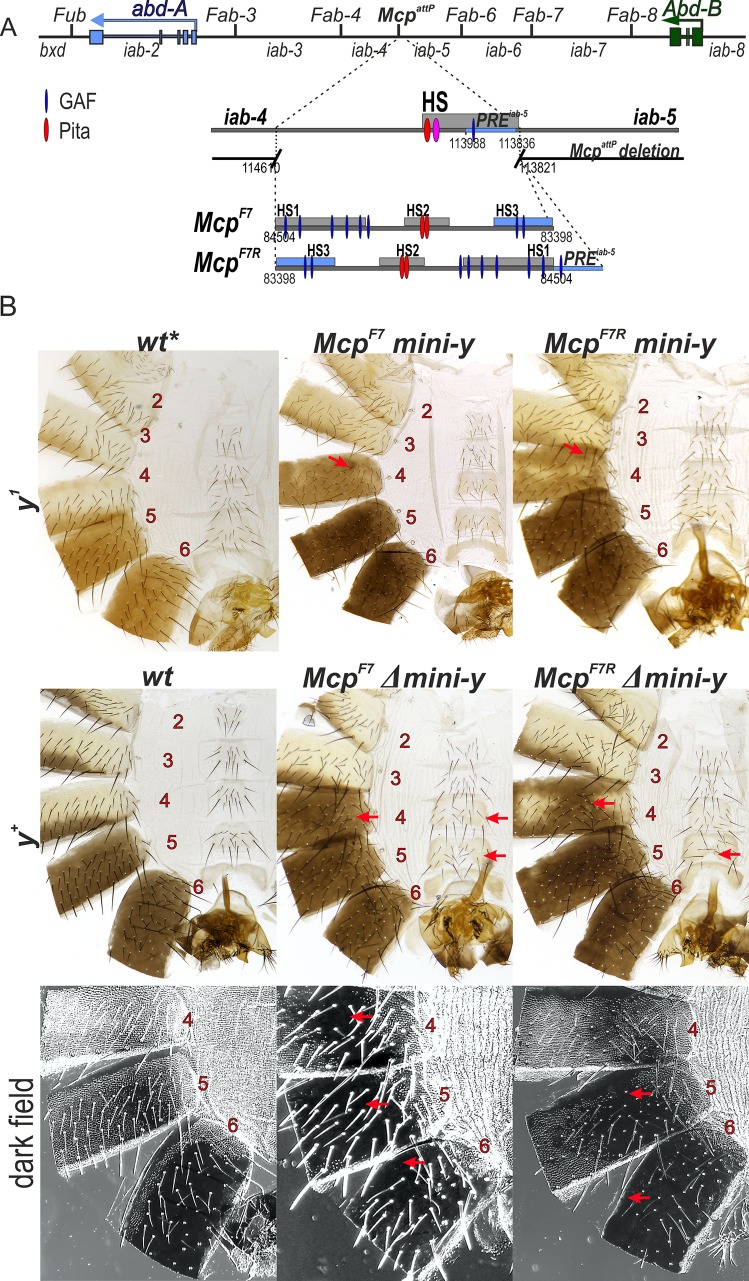
We next tested whether the Fab-7 boundary can substitute for Mcp. The Fab-7 region consists of a minor (HS*) and three major (HS1, HS2 and HS3) nuclease hypersensitive sequences [17,21,22,41,42]. Unlike Mcp or other known or suspected boundaries in BX-C, dCTCF does not bind to Fab-7 [33,69]. Instead, Fab-7 boundary function depends upon two BEN domain protein complexes, Elba and Insensitive, the C2H2 zinc finger protein Pita, and a large multiprotein complex, called the LBC [31,70–73]. In addition to a boundary function, the HS3 sequence can also function as a PRE (iab-7 PRE; [73,74]. In previous studies, we found that a combination of HS1+HS2+HS3 can functionally substitute for the complete Fab-7 boundary in vivo and we used this sequence (named for simplicity F7) for the Mcp replacements (Fig 4). Although Fab-7 has only limited orientation dependence in its endogenous context [30,73], we inserted the HS1+HS2+HS3 sequence in both the forward (same as endogenous Fab-7) and reverse orientations in the Mcp replacement platform as indicated in Fig 4. The phenotypic effects of the Fab-7 replacement inserted in the forward orientation, McpF7, are considered first.
Fig 4. McpF7 and McpF7R support Abd-B activation in the A4 segment.
(A) Schematic representation of the Fab-7 boundary. The 1.1 kb Fab-7 replacement consists of HS1, HS2 and HS3 (iab-7 PRE) regions (shown as blue box). (B) Morphology of the 2nd to 6th abdominal segments in McpF7 and McpF7R males. Other designations are as in Fig 2.
Like the McpCTCF replacement, the mini-y reporter is turned on in A5 (PS10) and A6 (PS11) in McpF7 males, and the tergites in both of these segments are black. However, McpF7 differs in two respects from McpCTCF. First, there are one or two small patches of darkly pigmented cuticle in the A4 tergite (marked by the arrow). These patches are variable and appear to be clonal in origin. This finding indicates that the blocking activity of McpF7 is incomplete, and that the mini-y reporter and thus the iab-5 domain is ectopically activated by the iab-4 domain in a small number of PS9 cells. Second, instead of a stripe of yellow-brown pigmentation along the posterior margin, nearly the entire A4 tergite is covered in yellow-brown pigmentation. This pattern of pigmentation is not observed in A4 in y1 males carrying the McpCTCF replacement and the mini-y reporter (Fig 2) or for that matter in control wild type y1 males (see Fig 4). The presence of the yellow-brown pigmentation throughout most of the A4 tergite suggests that cells in this segment (PS9) are not properly specified. This is the case. When the mini-y reporter was excised and replaced by the endogenous X-linked y+ gene, the A4 tergite has a black pigmentation like A5 and A6 (Fig 4). Since expression of the yellow gene is controlled by Abd-B, this observation indicates that Abd-B must be ectopically activated throughout A4. Antibody staining experiments of the CNS in McpF7 embryos indicate that this inference is correct (Fig 3B).
A simple interpretation of these findings is that McpF7 is unable to block crosstalk between iab-4 and iab-5 and, as a result, iab-5 is ectopically activated in PS9 cells and inappropriately drives Abd-B expression. However, such an interpretation is inconsistent with the expression pattern of the mini-y reporter; it is only activated in small clones in the A4 tergite and not in the entire A4 tergite. By way of comparison, the dark black pigmentation generated by the reporter in McpPRE, which has no boundary activity, is clearly quite different from the yellow-brown pigmentation observed for the reporter in McpF7. In this respect, McpF7 resembles McpCTCF in that the iab-5 domain must be shut off by Pc-G silencing in (most) PS9 cells. This would imply that the iab-4 regulatory domain (or one of the other abd-A domains that is turned on in PS9 cells) must be responsible for ectopically activating Abd-B expression in PS9. Moreover, this would mean that the mechanism underlying the misspecification of A4 (PS9) in the McpF7 replacement differs from that in McpPRE or Mcp1 where iab-5 is not properly silenced in PS9 cells.
There are other abnormalities in McpF7 replacement indicating that it has complicated and novel effect on Abd-B expression. In wild type males, the A6 sternite has a banana shape and no bristles, while the A5 and A4 sternites resemble isosceles trapezoids and are covered with bristles. While the A4 and A5 sternites in McpF7 males still have bristles, they are split into two connected lobes that resemble the banana shape of the A6 sternite. These morphological abnormalities indicate that the Fab-7 replacement induces a weak GOF transformation of both A4 (PS9) and A5 (PS10) towards an A6 (PS11) identity. This type of transformation is not observed in Mcp boundary deletions, nor it is observed in the McpPRE replacement.
Further evidence of A4/A5→A6 transformation can be seen in the pattern of trichome hairs in the tergites. In wild type flies, the A4 and A5 tergites are covered with trichomes, while trichomes are only found along the anterior and ventral margins of the A6 tergite (see darkfield image in Fig 4). In the McpF7 replacement, there are large regions of the A4 and A5 tergite that are devoid of trichomes. There are even anomalies in A6: the band of trichomes along the anterior margin is absent. Similar alterations in cuticular phenotypes are observed in McpF7 females (S2 Fig). These findings indicate that the normal regulation of Abd-B is disrupted in several parasegments when Mcp is replaced by the Fab-7 boundary.
In its endogenous context, the functioning of Fab-7 is weakly orientation dependent. For this reason, we anticipated that the reverse Mcp replacement, McpF7R, would give a similar though milder spectrum of phenotypic effects. Fig 4 shows that this is the case. In y+ background, large regions of the A4 tergite have a black pigmentation like A5 and A6. The ectopic activation appears to be weaker than in the McpF7 replacement as there are regions in A4 in which the endogenous yellow gene is not turned on. Also, and unlike McpF7, there are no bald patches in the A4 trichomes, while the sternite appears to have a normal isosceles trapezoid shape. However, the novel transformations seen in McpF7 in the more posterior segments A5 (PS10) and A6 (PS11) are still evident. The A5 tergite is not completely covered with trichomes, while the trichomes along the anterior margin of A6 are absent. The A5 sternite is also misshapen. Thus, like McpF7, introducing a reversed Fab-7 boundary in place of Mcp disrupts Abd-B regulation in PS9 and also in other parasegments. Since the pattern of mini-y expression in McpF7R indicates that the iab-5 domain is silenced in (most) PS9 cells, the iab-5 regulatory domain can’t be driving Abd-B expression in this parasegment. Instead, misexpression of Abd-B in PS9 is likely driven by the iab-4 domain. This possibility will be considered further below.
The Fab-8 boundary displays orientation-dependent effects on ectopic activation of Abd-B in the A4 abdominal segment
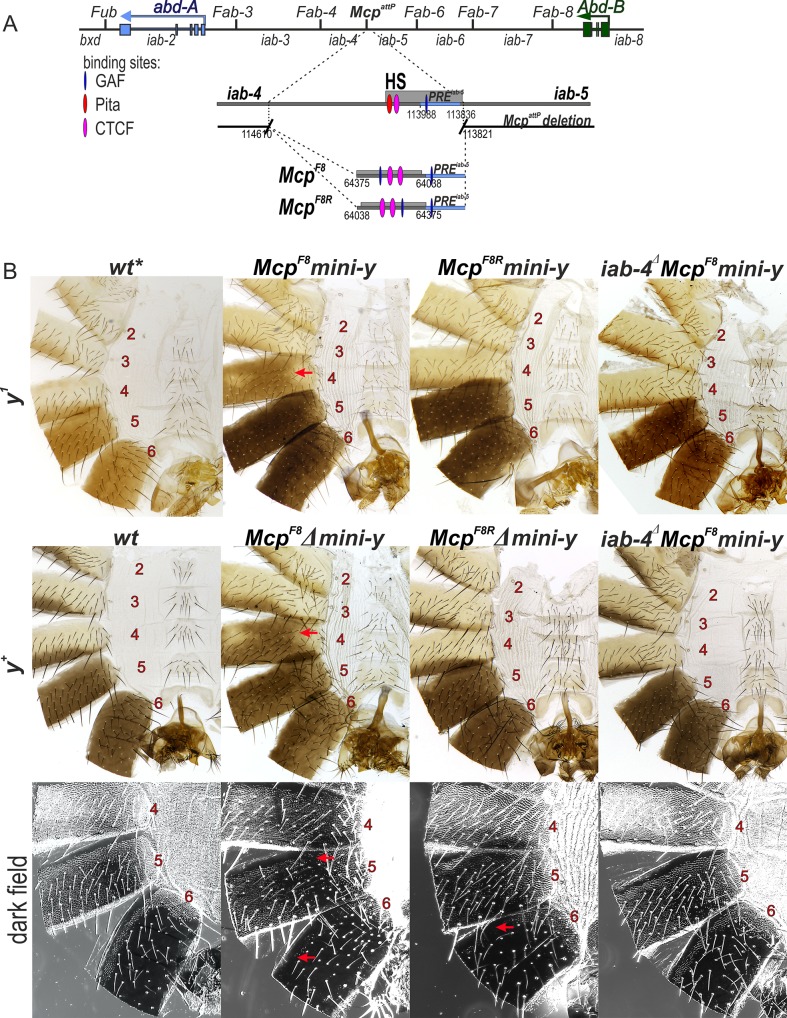
In previous Fab-7 replacement experiments we found that a 337 bp fragment (F8337) spanning the Fab-8 boundary nuclease hypersensitive site is sufficient to fully rescue a Fab-7 boundary deletion [30]. In the direct (forward) orientation this fragment not only blocks crosstalk but also supports bypass. However, when the orientation of the Fab-8 boundary is reversed, bypass activity is lost, while blocking is unaffected. Since F8337 appears to have full boundary function, we inserted this fragment in both orientations next to the iab-5 PRE in the Mcp deletion (McpF8 and McpF8R).
The effects of the Fab-8 replacement in the reverse orientation, McpF8R, will be considered first. Like the McpCTCF replacement, McpF8R blocks crosstalk between iab-4 and iab-5 and the mini-y reporter is off in A4 (Fig 5). The fact that mini-y is not expressed in PS9 also means that iab-5 is silenced as it should be in PS9 cells. After the deletion of the mini-y reporter and introducing a wild type y+ allele, the pigmentation in the adult male abdomen is equivalent to that in wild type flies. The morphological features of McpF8R tergites and sternites also resemble those in wild type flies or the McpCTCF replacement and there is no indication of the other abdominal transformations seen in the Fab-7 replacements. Consistent with the phenotype of the adult cuticle, the pattern of Abd-B expression in the embryonic CNS resembles wild type (Fig 3B). Thus, the McpF8R replacement fully substitutes for the endogenous Mcp boundary.
Fig 5. Activation of Abd-B by the iab-4 enhancer depends on the orientation of the Fab-8 insulator in McpF8 and McpF8R mutants.
(A) Molecular maps of the Fab-8 boundary and F8337. The Fab-8 insulator is shown as a horizontal bar. The proximal and distal endpoints of the Fab-8 fragments are shown below. For other designations see Fig 2. (B) Morphology of the 2nd to 6th abdominal segments in insulator in McpF8, McpF8R and iab-4Δ McpF8 males. Other designations are as in Fig 2.
A different result is obtained when the F8337 sequence is inserted in its normal forward orientation. Like the reverse orientation McpF8R, McpF8 efficiently blocks crosstalk between iab-4 and iab-5 and the mini-y reporter is not activated in A4 (PS9). On the other hand, like the Fab-7 replacements (McpF7 and McpF7R) most of the A4 tergite is covered in a yellow brown pigmentation instead of the normal stripe of yellow brown pigmentation along the posterior margin of the tergite that is seen in y1 males. Moreover, when the reporter is excised and the y1 allele replaced by the wild type y+ gene, nearly the entire A4 tergite is black. Consistent with the induction of y+ expression in A4, Abd-B is active in PS9 in the embryonic CNS (Fig 3B). The GOF transformation of A4 (PS9)→A5 (PS10) is not the only anomaly in McpF8 flies. While there does not seem to be any misspecification of the tergite or sternites in A5 (PS10), the line of trichomes along the anterior margin of the A6 tergite is disrupted or absent altogether indicating that there are some abnormalities in the temporal and/or special pattern of Abd-B expression in PS11.
Ectopic expression of Abd-B in A4 (PS9) requires a functional iab-4 domain
In the Fab-7 replacement experiments, the relative orientation of the Fab-8 boundary was thought to be important because it determined whether the chromatin loops formed between the replacement boundary and the AB-I element and/or the PTE sequence upstream of the Abd-B transcription start site were circle loops or stem loops [30,75]. In the forward orientation circle loops are expected to be formed and in this configuration, the downstream iab-5 regulatory domain is brought into close proximity with the Abd-B promoter. In the reverse orientation, iab-6 and iab-7 are predicted to form stem loops, and this configuration would tend to isolate the iab-5 regulatory domain from the Abd-B promoter.
It seemed possible that a similar mechanism might be in play in the Fab-8 replacements of Mcp. In the forward orientation (McpF8), the iab-4 regulatory domain would be brought into close proximity to the Abd-B gene, activating its ectopic expression in A4 (PS9). In the opposite orientation, the spatial relationship between the iab-4 domain and the Abd-B promoter would not be conducive for activation. In this case, Abd-B would be off in A4 (PS9). A strong prediction of this model is that the inappropriate activation of Abd-B in PS9 in the McpF8 replacement should depend on a functional iab-4 domain.
To test this prediction, we used CRISPR (see S3 Fig) to delete a 4,401 bp sequence (iab-4Δ) that spans the putative iab-4 initiation element in flies carrying the McpF8 replacement. The iab-4Δ sequence was selected based on the clustering of multiple binding sites for transcription factors controlling segmentation of the embryo. [76]. Fig 5 shows that the ectopic activation of y+ in A4 in McpF8 flies was eliminated by the iab-4Δ deletion. Moreover, Abd-B was not activated in A4 (PS9) in the embryonic CNS of iab-4Δ McpF8 embryos (Fig 3B). Interestingly, the loss of trichomes along the anterior margin of the A6 tergite in McpF8 also seemed to depend on a functional iab-4 domain. As can be seen in Fig 5, the trichome pattern in the A6 tergite of iab-4Δ McpF8 flies resembled that of wild type.
Discussion
Boundaries flanking the Abd-B regulatory domains must block crosstalk between adjacent regulatory domains but at the same time allow more distal domains to jump over one or more intervening boundaries and activate Abd-B expression. While several models have been advanced to account for these two paradoxical activities, replacement experiments argued that both must be intrinsic properties of the Abd-B boundaries. Thus Fab-7 and Fab-8 have blocking and bypass activities in Fab-7 replacement experiments, while heterologous boundaries including multimerized dCTCF sites and Mcp from BX-C do not. One idea is that Fab-7 and Fab-8 are simply “permissive” for bypass. They allow bypass to occur, while boundaries like multimerized dCTCF or Mcp are not permissive in the context of Fab-7. Another is that they actively facilitate bypass by directing the distal Abd-B regulatory domains to the Abd-B promoter. Potentially consistent with an “active” mechanism that involves boundary pairing interactions, the bypass activity of Fab-8 and to a lesser extent Fab-7 is orientation dependent.
In the studies reported here we have tested these two models further. For this purpose we used the Mcp boundary for in situ replacement experiments. Mcp defines the border between the regulatory domains that control expression of abd-A and Abd-B. In this location, it is required to block crosstalk between the flanking domains iab-4 and iab-5, but it does not need to mediate bypass. In this respect, it differs from the boundaries that are located within the set of regulatory domains that control either abd-A or Abd-B, as these boundaries must have both activities. If bypass were simply passive, insertion of a “permissive” Fab-7 or Fab-8 boundary in either orientation in place of Mcp would be no different from insertion of a generic “non-permissive” boundary such as multimerized dCTCF sites. Assuming that Fab-7 and Fab-8 can block crosstalk out of context, they should fully substitute for Mcp. In contrast, if bypass in the normal context involves an active mechanism in which more distal regulatory domains are brought to the Abd-B promoter, then Fab-7 and Fab-8 replacements might also be able to bring iab-4 to the Abd-B promoter in a configuration that activates transcription. If they do so, then this process would be expected to show the same orientation dependence as is observed for bypass of the Abd-B regulatory domains in Fab-7 replacements.
Consistent with the idea that a boundary located at the border between the domains that regulate abd-A and Abd-B need not have bypass activity, we found that multimerized binding sites for the dCTCF protein fully substitute for Mcp. Like the multimerized dCTCF sites, Fab-7 and Fab-8 are also able to block crosstalk between iab-4 and iab-5. In the case of Fab-7, its’ blocking activity is incomplete and there are small clones of cells in which the mini-y reporter is activated in A4. In contrast, the blocking activity of Fab-8 is comparable to the multimerized dCTCF sites and the mini-y reporter is off throughout A4. One plausible reason for this difference is that Mcp and the boundaries flanking Mcp (Fab-4 and Fab-6) utilize dCTCF as does Fab-8, while this architectural protein does not bind to Fab-7 [33].
Importantly, in spite of their normal (or near normal) ability to block crosstalk, both boundaries still perturb Abd-B regulation. In the case of Fab-8, the misregulation of Abd-B is orientation dependent just like the bypass activity of this boundary when it is used to replace Fab-7 [30]. When inserted in the reverse orientation, Fab-8 behaves like multimerized dCTCF sites and it fully rescues the Mcp deletion. In contrast, when inserted in the forward orientation, Fab-8 induces the expression of Abd-B in A4 (PS9), and the misspecification of this parasegment. Unlike classical Mcp deletions or the McpPRE replacement described here, expression of the Abd-B gene in PS9 is driven by iab-4, not iab-5. This conclusion is supported by two lines of evidence. First, the mini-y reporter inserted in iab-5 is off in PS9 cells indicating that iab-5 is silenced by PcG factors as it should be in this parasegment. Second, the ectopic expression of Abd-B is eliminated when the iab-4 regulatory domain is inactivated.
Our results, taken together with previous studies [30,59,60], support a model in which the chromatin loops formed by Fab-8 inserted at Mcp in the forward orientation brings the enhancers in the iab-4 regulatory domain in close proximity to the Abd-B promoter, leading to the activation of Abd-B in A4 (PS9). In contrast, when inserted in the opposite orientation, the topology of the chromatin loops formed by the ectopic Fab-8 boundary are not compatible with productive interactions between iab-4 and the Abd-B promoter. Moreover, it would appear that boundary bypass for the regulatory domains that control Abd-B expression is not a passive process in which the boundaries are simply permissive for interactions between the regulatory domains and the Abd-B promoter. Instead, it seems to be an active process in which the boundaries are responsible for bringing the regulatory domains into contact with the Abd-B gene. It also seems likely that bypass activity of Fab-8 (and also Fab-7) may have a predisposed preference, namely it is targeted for interactions with the Abd-B gene. This idea would fit with transgene bypass experiments, which showed that both Fab-7 and Fab-8 interacted with an insulator like element upstream of the Abd-B promoter, AB-I, while the Mcp boundary didn’t [59,60].
Similar conclusions can be drawn from the induction of Abd-B expression in A4 (PS9) when Fab-7 is inserted in place of Mcp. Like Fab-8, this boundary inappropriately targets the iab-4 regulatory domain to Abd-B. Unlike Fab-8, Abd-B is ectopically activated when Fab-7 is inserted in both the forward and reverse orientations. While the effects are milder in the reverse orientation, the lack of pronounced orientation dependence is consistent with experiments in which Fab-7 was inserted at its endogenous location in the reverse orientation. Unlike Fab-8 only very minor iab-6 bypass defects were observed. In addition to the activation of Abd-B in A4 (PS9) the Fab-7 Mcp replacements also alter the pattern of Abd-B regulation in more posterior segments. In the forward orientation, A4 and A5 are transformed towards an A6 identity, while A6 is also misspecified. Similar though somewhat less severe effects are observed in these segments when Fab-7 is inserted in the reverse orientation. At this point the mechanisms responsible for these novel phenotypic effects are uncertain. One possibility is that pairing interactions between the Fab-7 insert and the endogenous Fab-7 boundary disrupt the normal topological organization of the regulatory domains in a manner similar to that seen in boundary competition transgene assays [77]. An alternative possibility is that Fab-7 targets iab-4 to the Abd-B promoter not only in A4 (PS9) but also in cells in A5 (PS10) and A6 (PS11). In this model, Abd-B would be regulated not only by the domain that normally specifies the identity of the parasegment (e.g., iab-5 in PS10), but also by interactions with iab-4. This dual regulation would increase the levels of Abd-B, giving the weak GOF phenotypes. Potentially consistent with this second model, inactivating iab-4 in the McpF8 replacement not only rescues the A4 (PS9) GOF phenotypes but also suppresses the loss of anterior trichomes in the A6 tergite.
Materials and methods
Generation of McpattP by CRISPR/Cas9-induced homologous recombination
The backbone of the recombination plasmid was designed in silico and contains several genetic elements in the following order: [MCS5]-[attP]-[3xP3-EGFP-SV40polyA]-[attP]-[FRT]-[MCS3]. This DNA fragment was synthesized and cloned into pUC57 by Genewiz. The two multiple cloning sites MCS5 and MCS3 were used to clone homology arms into this plasmid. The orientations of the two attP sites are inverted relative to each other and serve as targets for фC31-mediated recombination mediated cassette exchange [62]. The 3xP3-EGFP reporter [78] was introduced as a means to isolate positive recombination events. The Flp-recombinase target FRT [79] was included for the deletion of the selectable mini-yellow marker after recombination mediated cassette exchange.
Homology arms were PCR-amplified from y w genomic DNA using the following primers: CCTGCCGACTGAACGAATGC and ACGCCCTGATCCCGATACACATAC for the proximal arm (iab-4 side; 3967 bp fragment), and GCGTTTGTGTGTAGTAAATGTATC and AAAGGCCAACAAAGAACACATGGACG for the distal arm (iab-5 side; 4323 bp fragment). A successful homologous recombination event will generate a 789 bp deletion within the Mcp region (Genome Release R6.22: 3R:16’868’830–16’869’619; or complete sequence of BX-C: 113821–114610 [4]).
The recombination plasmid was injected into y w vas-Cas9 embryos together with two gRNAs containing the following guides: GCTGGCTTTTACAGCATTTC and GCTTTGTTACCCCTGAAAAT. Concentrations were as described in Gratz et al.[80]. The injected embryos were grown to adulthood and crossed with y w partners. Among the few fertile crosses, one produced many larvae with a clear GFP-signal in the posterior part of their abdomens. This observation suggested that these animals had integrated the recombination plasmid and that the 3xP3-EGFP reporter acts as an enhancer trap for Abd-B specific enhancers. GFP positive larvae were isolated and grown to adulthood. Emerging males showed the expected Mcp phenotype. Also, and as expected for a reporter located in the BX-C, no fluorescence signal could be detected in their eyes, indicating that the 3xP3-EGFP reporter is silenced in eye cells where the 3xP3 promoter is usually active. The planned homologous recombination event could later be verified by PCR and sequencing. We will refer to it as McpattP.
12 EGFP- and Mcp-positive candidate males were individually crossed with y w virgins. Only 2 were fertile. The sterility of others may be caused by presence of off-targets as a frequent non-specific result of CRISPR/Cas9 editing. Starting from the two fertile crosses, 2 independent balanced stocks could be obtained according to established crossing schemes. One of them was used to obtain a y w M{vas-integrase}zh-2A; McpattP/TM3,Sb stock for recombination mediated cassette exchange. Because of poor survival rates in injection experiments, the McpattP chromosome was also temporarily combined with Dp(3;3)P5, Sb (y w M{vas-integrase}zh-2A; McpattP/ Dp(3;3)P5, Sb). By selection we obtained homozygous McpattP line that was subsequently used for fly injections.
Generation of iab-4Δ by CRISPR/Cas9-induced homologous recombination
For generating dsDNA donors for homology-directed repair we used pHD-DsRed vector that was a gift from Kate O'Connor-Giles (Addgene plasmid # 51434). The final plasmid contains genetic elements in the following order: [iab-4 proximal arm]-[attP]- [lox]- [3xP3-dsRed-SV40polyA]-[lox]- [iab-4 distal arm]. Homology arms were PCR-amplified from yw genomic DNA using the following primers: TTTGAATTCTTCCAGACACGCATCGGG and AAACATATGCTTGCTATCGACCCTCCTC for the proximal arm (846 bp fragment), and AATACTAGTCTCGGAAAGGGAAGAAGTTC and TACTCGAGCCGCTAAAGGACGTTCTGC for the distal arm (835 bp fragment). A successful homologous recombination event will generate a 4401 bp deletion within the iab-4 region (Genome Release R6.22: 3R:16,861,368..16,869,768; or complete sequence of BX-C [4]: 120073–115673).
Targets for Cas9 were selected using “CRISPR optimal target finder”–the program from O'Connor-Giles Lab. The recombination plasmid was injected into McpF8 vasa-Cas9 embryos together with two gRNAs containing the following guides: ATAGCAAGTAGGAGTGGAGT and GAACTTCTTCCCTTTCCGAGCGG. Concentrations were as described in Gratz et al. (2014). Injectees were grown to adulthood and crossed with y w; TM6/MKRS partners. Flies with clear dsRed-signal in eyes and the posterior part of their abdomens were selected into a new separate line. The successful integration of the recombination plasmid was verified by PCR.
Cuticle preparations
3 day adult flies were collected in eppendorf tubes and stored in 70% ethanol at least 1 day. Then ethanol was replaced with 10% KOH and flies were heated at 70°C for 1–1.5h. After heating flies were washed with dH2O two times and heated again in dH2O for 45min. Then the digested flies were washed with 70% ethanol and stored in 70% ethanol. The abdomen cuticles were cut from the rest of the digested fly using fine tweezer and a needle of an insulin syringe and put in a droplet of glycerol on a glass slide. Then the abdomens were cut longitudinally on the dorsal side through all of the tergites with the syringe. To spread the cuticles flat on the slides cuts may be done between the tergites. Than the cuticles were flattened with a coverslip. Photographs in the bright or dark field were taken on the Nikon SMZ18 stereomicroscope using Nikon DS-Ri2 digital camera, processed with ImageJ 1.50c4 and Fiji bundle 2.0.0-rc-46.
Embryo immunostaining
Primary antibodies were mouse monoclonal anti-Abd-B at 1:100 dilution (1A2E9, generated by S.Celniker, deposited to the Developmental Studies Hybridoma Bank) and polyclonal rabbit anti-Engrailed at 1:1000 dilution (a kind gift from Judith Kassis). Secondary antibodies were goat anti-mouse Alexa Fluor 647 (Molecular Probes) and anti-rabbit FITC conjugated (Jackson Research) at 1:2000 dilution. Stained embryos were mounted in the following solution: 23% glycerol, 10% Mowiol 4–88, 0.1M Tris-HCl pH 8.3. Images were acquired on Leica TCS SP-2 confocal microscope and processed using GIMP 2.8.16, ImageJ 1.50c4, Fiji bundle 2.0.0-rc-46.
Supporting information
On the top: schematic representation of regulatory region of the abd-A and Abd-B genes (blue and green, respectively). The 789 bp Mcp region that was deleted (coordinates according to complete sequence of BX-C in SEQ89E numbering) and replaced by two attP sites for the integration of the tested constructs. 3xP3-eGFP was used as a marker gene. frt site was used for excision of yellow maker gene. The plasmid that was injected into McpattP line, contains two attB site for integration, iab-5 PRE for restoring functional integrity of the iab-5 domain, the frt site for excision of yellow gene, lox sites for excision of testing element. Testing elements were inserted just in front of iab-5 PRE.
(TIF)
Morphology of the 2nd to 6th abdominal segments in wt, McpF8, McpF8R, McpF7 and McpF7R females. The expression of mini-y (black pigment) is shown on the upper panel. Localization of trichomes on tergites is shown lower.
(TIF)
The scheme of the regulatory region in the distal part of the BX-C. Horizontal arrows represent transcripts for abd-A (blue) and Abd-B (green). The iab-4 region was selected using FlyBase, based on the clustering of multiple binding sites for embryonic gap and pair-rule gene proteins. The screenshot show localization of the 4401 bp of iab-4 deletion with R6 genome release coordinates. The coordinates of iab-4 deletion according to complete sequence of BX-C (in SEQ89E numbering) are 120073–115673 (shown lower). The deletion was made using CRISPR/Cas9 strategy. Targets for Cas9 were selected using “CRISPR optimal target finder”–program from O'Connor-Giles Lab. Vector for generating dsDNA donors for homology-directed repair contains the visible marker 3xP3-DsRed. pHD-DsRed was a gift from Kate O'Connor-Giles (Addgene plasmid # 51434). dsRed gene was using for selection of flies with iab-4 deletion.
(TIF)
Acknowledgments
We thank Farhod Hasanov and Aleksander Parshikov for fly injections. This study was performed using the equipment of the IGB RAS facilities supported by the Ministry of Science and Education of the Russian Federation.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research was supported by the Russian Science Foundation, project no. 14-24-00166 (to PG), by the Kanton Basel-Stadt and Basel-Land (to MA) and by NIH R35GM126975 (to PS). M. Metzler was supported by a Fellowships of Excellence from the Biozentrum of the University of Basel. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978/12/07. 1978;276: 565–570. Available: http://www.ncbi.nlm.nih.gov/pubmed/103000 [DOI] [PubMed] [Google Scholar]
- 2.Mihaly J, Hogga I, Barges S, Galloni M, Mishra RK, Hagstrom K, et al. Chromatin domain boundaries in the Bithorax complex. Cell Mol Life Sci. 1998/03/06. 1998;54: 60–70. Available: http://www.ncbi.nlm.nih.gov/pubmed/9487387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez-Herrero E, Vernos I, Marco R, Morata G. Genetic organization of Drosophila bithorax complex. Nature. 1985/01/10. 1985;313: 108–113. Available: http://www.ncbi.nlm.nih.gov/pubmed/3917555 [DOI] [PubMed] [Google Scholar]
- 4.Martin CH, Mayeda CA, Davis CA, Ericsson CL, Knafels JD, Mathog DR, et al. Complete sequence of the bithorax complex of Drosophila. Proc Natl Acad Sci U S A. 1995;92: 8398–8402. Available: http://www.ncbi.nlm.nih.gov/pubmed/7667301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCall K, O’Connor MB, Bender W. Enhancer traps in the Drosophila bithorax complex mark parasegmental domains. Genetics. 1994;138: 387–399. Available: http://www.ncbi.nlm.nih.gov/pubmed/7828822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celniker SE, Sharma S, Keelan DJ, Lewis EB. The molecular genetics of the bithorax complex of Drosophila: cis-regulation in the Abdominal-B domain. EMBO J. 1990;9: 4277–4286. Available: http://www.ncbi.nlm.nih.gov/pubmed/2265608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender W, Akam M, Karch F, Beachy PA, Peifer M, Spierer P, et al. Molecular Genetics of the Bithorax Complex in Drosophila melanogaster. Science (80-). 1983;221: 23–29. 10.1126/science.221.4605.23 [DOI] [PubMed] [Google Scholar]
- 8.Duncan I. The bithorax complex. Annu Rev Genet. 1987/01/01. 1987;21: 285–319. 10.1146/annurev.ge.21.120187.001441 [DOI] [PubMed] [Google Scholar]
- 9.Karch F, Bender W, Weiffenbach B. abdA expression in Drosophila embryos. Genes Dev. 1990;4: 1573–1587. 10.1101/gad.4.9.1573 [DOI] [PubMed] [Google Scholar]
- 10.François Karch et al. The abdominal region of the bithorax complex. Cell. 1985;43: 81–96. Available: https://www.ncbi.nlm.nih.gov/pubmed/3935319 [DOI] [PubMed] [Google Scholar]
- 11.Boulet AM, Lloyd A, Sakonju S. Molecular definition of the morphogenetic and regulatory functions and the cis-regulatory elements of the Drosophila Abd-B homeotic gene. Development. 1991;111: 393–405. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez-Herrero E. Control of the expression of the bithorax complex genes abdominal-A and abdominal-B by cis-regulatory regions in Drosophila embryos. Development. 1991;111: 437–449. [DOI] [PubMed] [Google Scholar]
- 13.Maeda RK, Karch F. The ABC of the BX-C: the bithorax complex explained. Development. 2006/03/25. 2006;133: 1413–1422. 10.1242/dev.02323 [DOI] [PubMed] [Google Scholar]
- 14.Maeda RK, Karch F. Cis-regulation in the Drosophila Bithorax Complex. Adv Exp Med Biol. 2010/08/28. 2010;689: 17–40. Available: http://www.ncbi.nlm.nih.gov/pubmed/20795320 [DOI] [PubMed] [Google Scholar]
- 15.Maeda RK, Karch F. Gene expression in time and space: additive vs hierarchical organization of cis-regulatory regions. Curr Opin Genet Dev. 2011/02/26. 2011;21: 187–193. 10.1016/j.gde.2011.01.021 [DOI] [PubMed] [Google Scholar]
- 16.Kassis JA, Brown JL. Polycomb group response elements in Drosophila and vertebrates. Adv Genet. 2013/02/20. 2013;81: 83–118. 10.1016/B978-0-12-407677-8.00003-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mihaly J, Hogga I, Gausz J, Gyurkovics H, Karch F. In situ dissection of the Fab-7 region of the bithorax complex into a chromatin domain boundary and a Polycomb-response element. Development. 1997/05/01. 1997;124: 1809–1820. Available: http://www.ncbi.nlm.nih.gov/pubmed/9165128 [DOI] [PubMed] [Google Scholar]
- 18.Mihaly J, Barges S, Sipos L, Maeda R, Cleard F, Hogga I, et al. Dissecting the regulatory landscape of the Abd-B gene of the bithorax complex. Development. 2006/07/05. 2006;133: 2983–2993. 10.1242/dev.02451 [DOI] [PubMed] [Google Scholar]
- 19.Barges S, Mihaly J, Galloni M, Hagstrom K, Muller M, Shanower G, et al. The Fab-8 boundary defines the distal limit of the bithorax complex iab-7 domain and insulates iab-7 from initiation elements and a PRE in the adjacent iab-8 domain. Development. 2000/01/29. 2000;127: 779–790. Available: http://www.ncbi.nlm.nih.gov/pubmed/10648236 [DOI] [PubMed] [Google Scholar]
- 20.Gyurkovics H, Gausz J, Kummer J, Karch F. A new homeotic mutation in the Drosophila bithorax complex removes a boundary separating two domains of regulation. EMBO J. 1990/08/01. 1990;9: 2579–2585. Available: http://www.ncbi.nlm.nih.gov/pubmed/1973385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galloni M, Gyurkovics H, Schedl P, Karch F. The bluetail transposon: evidence for independent cis-regulatory domains and domain boundaries in the bithorax complex. EMBO J. 1993;12: 1087–1097. Available: http://www.ncbi.nlm.nih.gov/pubmed/8384551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karch F, Galloni M, Sipos L, Gausz J, Gyurkovics H, Schedl P. Mcp and Fab-7: molecular analysis of putative boundaries of cis-regulatory domains in the bithorax complex of Drosophila melanogaster. Nucleic Acids Res. 1994/08/11. 1994;22: 3138–3146. Available: http://www.ncbi.nlm.nih.gov/pubmed/7915032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bender W, Lucas M. The border between the ultrabithorax and abdominal-A regulatory domains in the Drosophila bithorax complex. Genetics. 2013;193: 1135–1147. 10.1534/genetics.112.146340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowman SK, Deaton AM, Domingues H, Wang PI, Sadreyev RI, Kingston RE, et al. H3K27 modifications define segmental regulatory domains in the Drosophila bithorax complex. Elife. 2014;3: e02833 10.7554/eLife.02833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iampietro C, Cleard F, Gyurkovics H, Maeda RK, Karch F. Boundary swapping in the Drosophila Bithorax complex. Development. 2008/11/07. 2008;135: 3983–3987. 10.1242/dev.025700 [DOI] [PubMed] [Google Scholar]
- 26.Iampietro C, Gummalla M, Mutero A, Karch F, Maeda RK. Initiator elements function to determine the activity state of BX-C enhancers. PLoS Genet. 2011/01/05. 2010;6: e1001260 10.1371/journal.pgen.1001260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda RK, Karch F. The open for business model of the bithorax complex in Drosophila. Chromosoma. 2015;124: 293–307. 10.1007/s00412-015-0522-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyrchanova O, Mogila V, Wolle D, Magbanua JP, White R, Georgiev P, et al. The boundary paradox in the Bithorax complex. Mech Dev. 2015;138: 122–132. 10.1016/j.mod.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogga I, Mihaly J, Barges S, Karch F. Replacement of Fab-7 by the gypsy or scs insulator disrupts long-distance regulatory interactions in the Abd-B gene of the bithorax complex. Mol Cell. 2001;8: 1145–1151. Available: http://www.ncbi.nlm.nih.gov/pubmed/11741549 [DOI] [PubMed] [Google Scholar]
- 30.Kyrchanova O, Mogila V, Wolle D, Deshpande G, Parshikov A, Cléard F, et al. Functional Dissection of the Blocking and Bypass Activities of the Fab-8 Boundary in the Drosophila Bithorax Complex. PLoS Genet. 2016;12: e1006188 10.1371/journal.pgen.1006188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyrchanova O, Zolotarev N, Mogila V, Maksimenko O, Schedl P, Georgiev P. Architectural protein Pita cooperates with dCTCF in organization of functional boundaries in Bithorax complex. Development. 2017;144: 2663–2672. 10.1242/dev.149815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maksimenko O, Bartkuhn M, Stakhov V, Herold M, Zolotarev N, Jox T, et al. Two new insulator proteins, Pita and ZIPIC, target CP190 to chromatin. Genome Res. 2014/10/25. 2015;25: 89–99. 10.1101/gr.174169.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holohan EE, Kwong C, Adryan B, Bartkuhn M, Herold M, Renkawitz R, et al. CTCF genomic binding sites in Drosophila and the organisation of the bithorax complex. PLoS Genet. 2007/07/10. 2007;3: e112 10.1371/journal.pgen.0030112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magbanua JP, Runneburger E, Russell S, White R. A Variably Occupied CTCF Binding Site in the Ultrabithorax Gene in the Drosophila Bithorax Complex. Mol Cell Biol. 2015;35: 318–330. 10.1128/MCB.01061-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moon H, Filippova G, Loukinov D, Pugacheva E, Chen Q, Smith ST, et al. CTCF is conserved from Drosophila to humans and confers enhancer blocking of the Fab-8 insulator. EMBO Rep. 2005/01/29. 2005;6: 165–170. 10.1038/sj.embor.7400334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gruzdeva N, Kyrchanova O, Parshikov A, Kullyev A, Georgiev P. The Mcp element from the bithorax complex contains an insulator that is capable of pairwise interactions and can facilitate enhancer-promoter communication. Mol Cell Biol. 2005/04/16. 2005;25: 3682–3689. 10.1128/MCB.25.9.3682-3689.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schweinsberg S, Hagstrom K, Gohl D, Schedl P, Kumar RP, Mishra R, et al. The enhancer-blocking activity of the Fab-7 boundary from the Drosophila bithorax complex requires GAGA-factor-binding sites. Genetics. 2004/12/08. 2004;168: 1371–1384. 10.1534/genetics.104.029561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schweinsberg SE, Schedl P. Developmental modulation of Fab-7 boundary function. Development. 2004/08/27. 2004;131: 4743–4749. 10.1242/dev.01343 [DOI] [PubMed] [Google Scholar]
- 39.Perez-Lluch S, Cuartero S, Azorin F, Espinas ML. Characterization of new regulatory elements within the Drosophila bithorax complex. Nucleic Acids Res. 2008;36: 6926–6933. 10.1093/nar/gkn818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou J, Ashe H, Burks C, Levine M. Characterization of the transvection mediating region of the abdominal-B locus in Drosophila. Development. 1999/06/22. 1999;126: 3057–3065. Available: http://www.ncbi.nlm.nih.gov/pubmed/10375498 [DOI] [PubMed] [Google Scholar]
- 41.Zhou J, Barolo S, Szymanski P, Levine M. The Fab-7 element of the bithorax complex attenuates enhancer-promoter interactions in the Drosophila embryo. Genes Dev. 1996/12/15. 1996;10: 3195–3201. Available: http://www.ncbi.nlm.nih.gov/pubmed/8985187 [DOI] [PubMed] [Google Scholar]
- 42.Hagstrom K, Muller M, Schedl P. Fab-7 functions as a chromatin domain boundary to ensure proper segment specification by the Drosophila bithorax complex. Genes Dev. 1996/12/15. 1996;10: 3202–3215. Available: http://www.ncbi.nlm.nih.gov/pubmed/8985188 [DOI] [PubMed] [Google Scholar]
- 43.Zhou J, Levine M. A novel cis-regulatory element, the PTS, mediates an anti-insulator activity in the Drosophila embryo. Cell. 1999/12/28. 1999;99: 567–575. Available: http://www.ncbi.nlm.nih.gov/pubmed/10612393 [DOI] [PubMed] [Google Scholar]
- 44.Chen Q, Lin L, Smith S, Lin Q, Zhou J. Multiple Promoter Targeting Sequences exist in Abdominal-B to regulate long-range gene activation. Dev Biol. 2005;286: 629–636. 10.1016/j.ydbio.2005.08.025 [DOI] [PubMed] [Google Scholar]
- 45.Lin Q.; Wu D.; Zhou J. The promoter targeting sequence facilitates and restricts a distant enhancer to a single promoter in the Drosophila embryo. Development. 2003;130: 519–526. [DOI] [PubMed] [Google Scholar]
- 46.Cai HN, Shen P. Effects of cis arrangement of chromatin insulators on enhancer-blocking activity. Science (80-). 2001/02/13. 2001;291: 493–495. 10.1126/science.291.5503.493 [DOI] [PubMed] [Google Scholar]
- 47.Muravyova E, Golovnin A, Gracheva E, Parshikov A, Belenkaya T, Pirrotta V, et al. Loss of insulator activity by paired Su(Hw) chromatin insulators. Science (80-). 2001/02/13. 2001;291: 495–498. 10.1126/science.291.5503.495 [DOI] [PubMed] [Google Scholar]
- 48.Sigrist CJ, Pirrotta V. Chromatin insulator elements block the silencing of a target gene by the Drosophila polycomb response element (PRE) but allow trans interactions between PREs on different chromosomes. Genetics. 1997/09/01. 1997;147: 209–221. Available: http://www.ncbi.nlm.nih.gov/pubmed/9286681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muller M, Hagstrom K, Gyurkovics H, Pirrotta V, Schedl P. The mcp element from the Drosophila melanogaster bithorax complex mediates long-distance regulatory interactions. Genetics. 1999/11/05. 1999;153: 1333–1356. Available: http://www.ncbi.nlm.nih.gov/pubmed/10545463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li HB, Muller M, Bahechar IA, Kyrchanova O, Ohno K, Georgiev P, et al. Insulators, not Polycomb response elements, are required for long-range interactions between Polycomb targets in Drosophila melanogaster. Mol Cell Biol. 2010/12/08. 2011;31: 616–625. 10.1128/MCB.00849-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujioka M, Mistry H, Schedl P, Jaynes JB. Determinants of Chromosome Architecture: Insulator Pairing in cis and in trans. PLoS Genet. 2016;2: e1005889 10.1371/journal.pgen.1005889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kyrchanova O, Chetverina D, Maksimenko O, Kullyev A, Georgiev P. Orientation-dependent interaction between Drosophila insulators is a property of this class of regulatory elements. Nucleic Acids Res. 2008/11/07. 2008;36: 7019–7028. 10.1093/nar/gkn781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hendrickson JE, Sakonju S. Cis and trans interactions between the iab regulatory regions and abdominal-A and abdominal-B in Drosophila melanogaster. Genetics. 1995;139: 835–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hopmann R, Duncan D, Duncan I. Transvection in the iab-5,6,7 region of the bithorax complex of Drosophila: Homology independent interactions in trans. Genetics. 1995;139: 815–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sipos L, Mihály J, Karch F, Schedl P, Gausz J, Gyurkovics H. Transvection in the Drosophila Abd-B domain: Extensive upstream sequences are involved in anchoring distant cis-regulatory regions to the promoter. Genetics. 1998;149: 1031–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ho MC, Schiller BJ, Akbari OS, Bae E, Drewell RA. Disruption of the Abdominal-B promoter tethering element results in a loss of long-range enhancer-directed Hox gene expression in Drosophila. PLoS One. 2011;6: e16283 10.1371/journal.pone.0016283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akbari OS, Schiller BJ, Goetz SE, Ho MCW, Bae E, Drewell RA. The Abdominal-B promoter tethering element mediates promoter-enhancer specificity at the Drosophila bithorax complex. Fly (Austin). 2007;1: 337–339. 10.4161/fly.5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akbari OS, Bae E, Johnsen H, Villaluz A, Wong D, Drewell RA. A novel promoter-tethering element regulates enhancer-driven gene expression at the bithorax complex in the Drosophila embryo. Development. 2007;135: 123–131. 10.1242/dev.010744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kyrchanova O, Toshchakov S, Podstreshnaya Y, Parshikov A, Georgiev P. Functional Interaction between the Fab-7 and Fab-8 Boundaries and the Upstream Promoter Region in the Drosophila Abd-B Gene. Mol Cell Biol. 2008;28: 4188–4195. 10.1128/MCB.00229-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kyrchanova O, Ivlieva T, Toshchakov S, Parshikov A, Maksimenko O, Georgiev P. Selective interactions of boundaries with upstream region of Abd-B promoter in Drosophila bithorax complex and role of dCTCF in this process. Nucleic Acids Res. 2010/12/15. 2011;39: 3042–3052. 10.1093/nar/gkq1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Busturia a, Lloyd A, Bejarano F, Zavortink M, Xin H, Sakonju S. The MCP silencer of the Drosophila Abd-B gene requires both Pleiohomeotic and GAGA factor for the maintenance of repression. Development. 2001;128: 2163–2173. [DOI] [PubMed] [Google Scholar]
- 62.Bateman JR, Lee AM, Wu CT. Site-specific transformation of Drosophila via??C31 integrase-mediated cassette exchange. Genetics. 2006;173: 769–777. 10.1534/genetics.106.056945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A. 2007/03/16. 2007;104: 3312–3317. 10.1073/pnas.0611511104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gibert JM, Peronnet F, Schlötterer C. Phenotypic plasticity in Drosophila pigmentation caused by temperature sensitivity of a chromatin regulator network. PLoS Genet. 2007;3: e30 10.1371/journal.pgen.0030030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jeong S, Rokas A, Carroll SB. Regulation of Body Pigmentation by the Abdominal-B Hox Protein and Its Gain and Loss in Drosophila Evolution. Cell. 2006;125: 1387–1399. 10.1016/j.cell.2006.04.043 [DOI] [PubMed] [Google Scholar]
- 66.Camino EM, Butts JC, Ordway A, Vellky JE, Rebeiz M, Williams TM. The Evolutionary Origination and Diversification of a Dimorphic Gene Regulatory Network through Parallel Innovations in cis and trans. PLoS Genet. 2015;11: e1005136 10.1371/journal.pgen.1005136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rebeiz M, Williams TM. Using Drosophila pigmentation traits to study the mechanisms of cis-regulatory evolution. Curr Opin Insect Sci. 2017;19: 1–7. 10.1016/j.cois.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bender W, Hudson A. P element homing to the Drosophila bithorax complex. Development. 2000;127: 3981–3992. [DOI] [PubMed] [Google Scholar]
- 69.Bonchuk A, Maksimenko O, Kyrchanova O, Ivlieva T, Mogila V, Deshpande G, et al. Functional role of dimerization and CP190 interacting domains of CTCF protein in Drosophila melanogaster. BMC Biol. 2015; 10.1186/s12915-015-0168-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fedotova AA, Bonchuk AN, Mogila VA, Georgiev PG. C2H2 zinc finger proteins: The largest but poorly explored family of higher eukaryotic transcription factors. Acta Naturae. 2017;9: 47–58. [PMC free article] [PubMed] [Google Scholar]
- 71.Wolle D, Cleard F, Aoki T, Deshpande G, Schedl P, Karch F. Functional Requirements for Fab-7 Boundary Activity in the Bithorax Complex. Mol Cell Biol. 2015;35: 3739–3752. 10.1128/MCB.00456-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cleard F, Wolle D, Taverner AM, Aoki T, Deshpande G, Andolfatto P, et al. Different evolutionary strategies to conserve chromatin boundary function in the bithorax complex. Genetics. 2017;205: 589–603. 10.1534/genetics.116.195586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kyrchanova O., Kurbidaeva A, Sabirov M, Postika N, Wolle D, Aoki T, Maksimenko O, Mogila V SP and GP. The Bithorax comlex iab-7 Polycomb Response Element has a novel role in the functioning of the Fab-7 chromatin boundary. Plos Genet. 2018;14(8):e100 10.1371/journal.pgen.1007442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mishra RK, Mihaly J, Barges S, Spierer A, Karch F, Hagstrom K, et al. The iab-7 polycomb response element maps to a nucleosome-free region of chromatin and requires both GAGA and pleiohomeotic for silencing activity. Mol Cell Biol. 2001/02/07. 2001;21: 1311–1318. 10.1128/MCB.21.4.1311-1318.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chetverina D, Fujioka M, Erokhin M, Georgiev P, Jaynes JB, Schedl P. Boundaries of loop domains (insulators): Determinants of chromosome form and function in multicellular eukaryotes. BioEssays. 2017;39 10.1002/bies.201600233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Starr MO, Ho MCW, Gunther EJM, Tu YK, Shur AS, Goetz SE, et al. Molecular dissection of cis-regulatory modules at the Drosophila bithorax complex reveals critical transcription factor signature motifs. Dev Biol. 2011;359: 290–302. 10.1016/j.ydbio.2011.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gohl D, Aoki T, Blanton J, Shanower G, Kappes G, Schedl P. Mechanism of chromosomal boundary action: roadblock, sink, or loop? Genetics. 2011/01/05. 2011;187: 731–748. 10.1534/genetics.110.123752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horn C, Jaunich B, Wimmer EA. Highly sensitive, fluorescent transformation marker for Drosophila transgenesis. Dev Genes Evol. 2000;210: 623–629. 10.1007/s004270000111 [DOI] [PubMed] [Google Scholar]
- 79.Golic KG, Lindquist S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell. 1989/11/03. 1989;59: 499–509. Available: http://www.ncbi.nlm.nih.gov/pubmed/2509077 [DOI] [PubMed] [Google Scholar]
- 80.Gratz SJ, Ukken FP, Rubinstein CD, Thiede G, Donohue LK, Cummings AM, et al. Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics. 2014;196: 961–971. 10.1534/genetics.113.160713 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
On the top: schematic representation of regulatory region of the abd-A and Abd-B genes (blue and green, respectively). The 789 bp Mcp region that was deleted (coordinates according to complete sequence of BX-C in SEQ89E numbering) and replaced by two attP sites for the integration of the tested constructs. 3xP3-eGFP was used as a marker gene. frt site was used for excision of yellow maker gene. The plasmid that was injected into McpattP line, contains two attB site for integration, iab-5 PRE for restoring functional integrity of the iab-5 domain, the frt site for excision of yellow gene, lox sites for excision of testing element. Testing elements were inserted just in front of iab-5 PRE.
(TIF)
Morphology of the 2nd to 6th abdominal segments in wt, McpF8, McpF8R, McpF7 and McpF7R females. The expression of mini-y (black pigment) is shown on the upper panel. Localization of trichomes on tergites is shown lower.
(TIF)
The scheme of the regulatory region in the distal part of the BX-C. Horizontal arrows represent transcripts for abd-A (blue) and Abd-B (green). The iab-4 region was selected using FlyBase, based on the clustering of multiple binding sites for embryonic gap and pair-rule gene proteins. The screenshot show localization of the 4401 bp of iab-4 deletion with R6 genome release coordinates. The coordinates of iab-4 deletion according to complete sequence of BX-C (in SEQ89E numbering) are 120073–115673 (shown lower). The deletion was made using CRISPR/Cas9 strategy. Targets for Cas9 were selected using “CRISPR optimal target finder”–program from O'Connor-Giles Lab. Vector for generating dsDNA donors for homology-directed repair contains the visible marker 3xP3-DsRed. pHD-DsRed was a gift from Kate O'Connor-Giles (Addgene plasmid # 51434). dsRed gene was using for selection of flies with iab-4 deletion.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.