Abstract
Glenoid chondral injuries constitute challenging injuries to treat because of the limited access and the limited options and evidence available for their resolution. The purpose of this Technical Note is to describe the procedure, pearls, and pitfalls of implantation of a cryopreserved osteochondral allograft (Cartiform) for the treatment of full-thickness cartilage defects of the shoulder. Cartiform is a cryopreserved osteochondral allograft composed of chondrocytes, chondrogenic growth factors, and extracellular matrix proteins that can be implanted through a single-stage procedure.
Chondral and osteochondral lesions in the shoulder can cause significant pain and can be challenging to treat. Lesions of the glenoid are the most difficult to manage and typically occur in young patients as a result of an acute traumatic shoulder dislocation or chronic shoulder instability that results in delamination of the articular cartilage.1 There is no established treatment in the literature; however, a trial of conservative treatment with physical therapy, anti-inflammatory medications, and corticosteroid injections is often performed.2, 3 If conservative treatment fails, there are a number of available surgical techniques to address the pathology. However, limited clinical evidence exists to support the superiority of 1 technique. The treatments are often extrapolated from clinical evidence in similar defects present in the knee.
Available surgical techniques for glenoid cartilage defects include debridement, microfracture, autologous chondrocyte implantation, osteochondral allograft, interpositional arthroplasty with dermal allograft, or arthroplasty.4 Osteochondral allografts contain viable chondrocytes, chondrogenic growth factors, and extracellular matrix proteins to promote cartilage repair.5 They are available in fresh, fresh-frozen, or cryopreserved forms. Fresh osteochondral allografts have the highest percentage of viable chondrocytes but suffer from a shelf life of approximately 30 days and require precise preoperative sizing, whereas fresh-frozen allografts have minimal viable chondrocytes.6 Cryopreserved osteochondral grafts were reported to have up to a 2-year shelf life and may maintain up to 70.5% of viable chondrocytes.6 The advantages and disadvantages of cryopreserved osteochondral allografts are outlined in Table 1. The purpose of this Technical Note is to describe the surgical procedure, pearls, and pitfalls of implantation of a cryopreserved osteochondral allograft for the treatment of full-thickness cartilage defects of the shoulder.
Table 1.
Advantages and Disadvantages of Cryopreserved Osteochondral Allograft Implantation
| Advantages | Disadvantages |
|---|---|
| No donor-site morbidity | Treatable defect limited to 20-mm × 25-mm defect |
| Single operation | Allograft disease transmission is theoretically possible |
| Long shelf life | Technically demanding |
| Implant flexibility, easily contoured | |
| Minimal bone and low risk of reaction |
Surgical Technique
Indications
Indications include large focal osteochondral lesions or lesions not amenable to microfracture at the glenoid surface.
Preoperative Preparation and Positioning
The patient is positioned in the lateral decubitus position under general anesthesia. Prophylactic antibiotics are given intravenously, and the operative arm is prepared in the standard sterile fashion and draped free (Video 1).
Portal and Cannula Placement
The inflow cannula is inserted into the standard posterior portal, followed by the creation of a high anterior portal, anterior to the supraspinatus, using an outside-in technique. The portal incision should be large enough to allow placement of an 8-mm Gemini cannula (Arthrex, Naples, FL), which has inner and outer sheaths. The outer sheath has a dam to prevent fluid escape, but once the inner sheath is removed, the outer sheath can remain in place to allow for unresisted graft passage. A diagnostic arthroscopy is then performed. A second, percutaneous, 5.4-mm metal cannula is inserted through a trans-subscapularis (5-o’clock) portal.
Graft Site Preparation
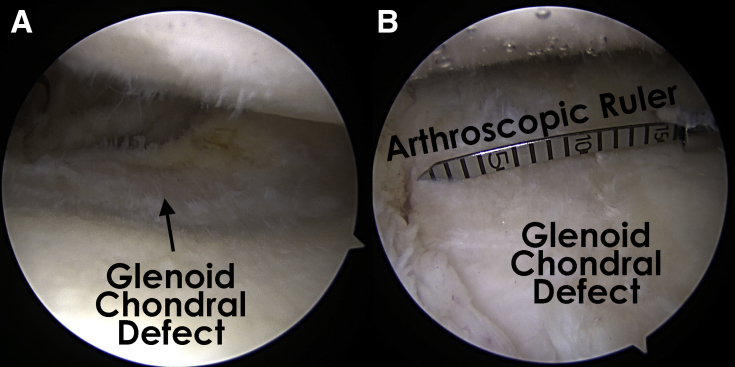
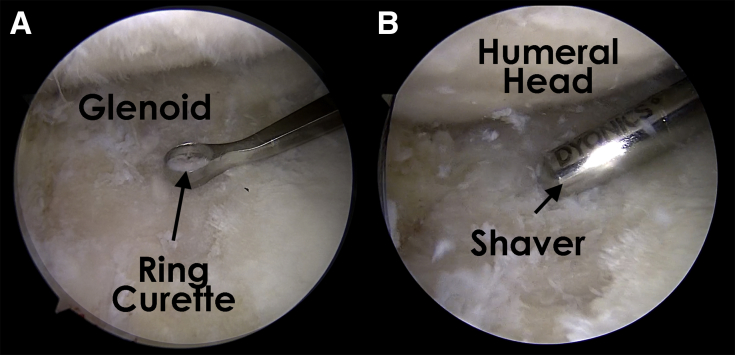
A combination of shaver and curettes is used to debride and remove the pathologic cartilage and the calcified cartilage layer throughout the base of the defect, leaving healthy subchondral bone exposed. Next, curettes are used to develop sharp borders of normal cartilage around the defect. Creation of a stable border with normal cartilage and a healthy layer of subchondral bone is the key to successful integration. A calibrated probe or arthroscopic ruler is used to measure the defect to accurately size the graft (Figs 1 and 2). Microfracture of the defect may be performed per surgeon discretion.
Fig 1.
(A) Glenoid chondral defect as viewed from the posterior portal in a right shoulder with the patient in the lateral decubitus position. (B) An arthroscopic ruler can be used to measure the chondral defect.
Fig 2.
Glenoid chondral defect as viewed from the anterosuperior portal in a right shoulder with the patient in the lateral decubitus position. The ring curette (A) and shaver (B) are used to debride the lesion to create sharp edges.
Graft Preparation
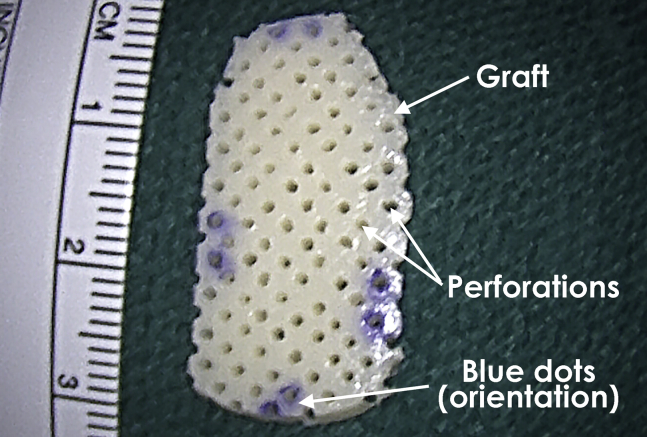
The osteochondral allograft consists of full-thickness articular cartilage and a thin layer of subchondral bone. In the case of Cartiform (Osiris, Columbia, MD), the graft comes with perforations in the articular cartilage, which allow for flexible conformity and improved integration to the underlying subchondral bone. The graft comes in a sterile jar, which should be placed into a sterile basin. Saline solution is added until the liquid level rests just below the lid. The jar is left in place for approximately 10 minutes or until there are no visible ice crystals. Once thawed, the graft may be removed from the jar with forceps and placed into a basin of sterile saline solution at room temperature. The graft should remain in the saline solution for at least 1 minute but may remain for up to 2 hours (Fig 3).
Fig 3.
Cryopreserved osteochondral allograft (Cartiform) showing the perforations that allow graft flexibility. The blue dots orient the graft poles, which are used to match the positions marked on the glenoid defect.
Graft Implantation
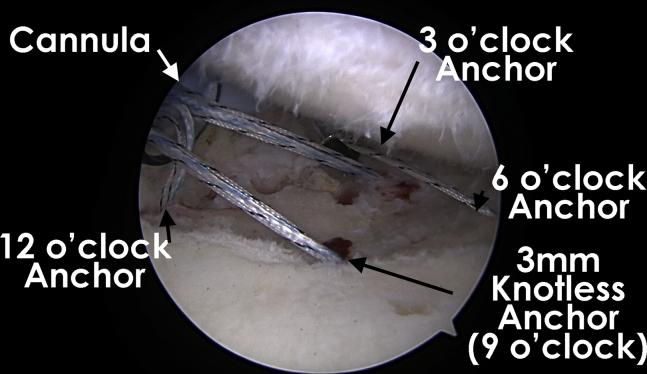
The allograft is sized and cut using a scalpel or scissors to match the desired shape and size. A metal drill sleeve is inserted through the anterosuperior (Gemini) cannula, and a 2.4-mm drill is used to drill a hole for the anchor at the 12-o’clock position of the lesion. A 3-mm BioComposite Knotless SutureTak (Arthrex) is then inserted. Another anchor is placed at the 3-o’clock position through the trans-subscapularis metal cannula. A third anchor is placed at the 6-o’clock position of the lesion through the same cannula. The fourth and final anchor is placed at the 9-o’clock position of the lesion from the anterosuperior cannula. Each anchor has 3 suture strands that are then meticulously retrieved, brought out of the anterosuperior (Gemini) cannula, and kept separated (Fig 4).
Fig 4.
Glenoid chondral defect as viewed from a posterior portal in a right shoulder with the patient in the lateral decubitus position showing the position of the 4 knotless suture anchors and the orientation to the anterosuperior (Gemini) cannula (left) and trans-subscapularis portal (center).
A measuring tool is used to confirm the distance between the anchors to ensure the graft has been sized correctly. A needle is then used to pass the FiberWire suture (Arthrex) end (white) from the 12-o’clock anchor through the perforations of the graft with the corresponding 12-o’clock position. The free end of the FiberWire suture is placed through the loop of a 2-mm FiberLink (Arthrex) to shuttle the FiberWire into the locking mechanism of the anchor. We strongly recommend passing the sutures through the second perforation of the graft to prevent suture cutting through the graft. The same step is repeated with the other 3 anchors with their corresponding positions to the graft. The inflow is then turned off, and the graft is covered with a thin layer of fibrin glue using a dry arthroscopic technique (Fig 5). The surgical pearls and pitfalls are summarized in Table 2.
Fig 5.
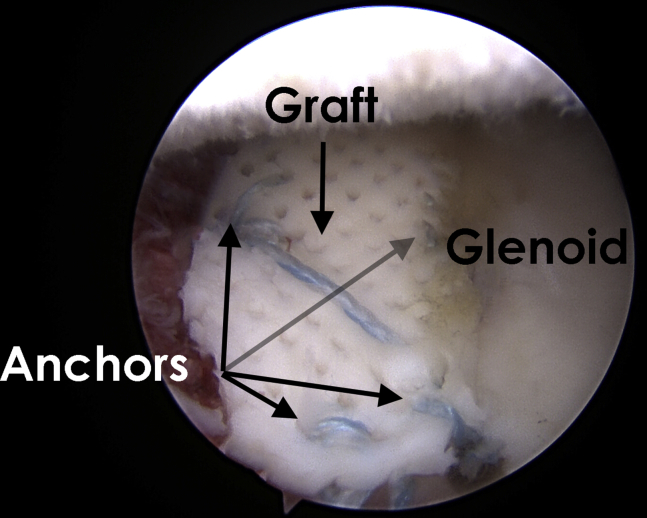
Glenoid as viewed from an anterosuperior portal in a right shoulder with the patient in the lateral decubitus position showing a cryopreserved osteochondral allograft secured in place by 4 knotless suture anchors.
Table 2.
Surgical Pearls and Pitfalls
| Pearls | Pitfalls |
|---|---|
| The surgeon should perform diagnostic arthroscopy before opening the graft to ensure the patient is a candidate. | The graft should be oriented to prevent placing the wrong side down. |
| The inner (clear) sheath from the anterosuperior (Gemini) cannula is removed, leaving the blue outer sheath of the cannula in the joint. The outer sheath does not have a dam within it, thus allowing easy passage of the graft and sutures without resistance. Once the graft is inserted into the joint, the suture limb that tightens the knotless anchors is sequentially pulled from each anchor until all the slack is removed from each anchor. | Placement of microfracture holes too closely should be avoided. |
| If a suture cuts through the graft, a suture-shuttling device can be used to pass the suture limbs through the graft and reanchor it with a PushLock anchor (Arthrex). | Insufficient debridement and preparation of the cartilage borders and bony bed should be avoided. |
| The surgeon should use a probe to remove suture slack to avoid cutout from the graft. | |
| Microfracture of the defect may be performed based on surgeon discretion. |
Postoperative Management
The patient is placed in a padded shoulder sling for comfort for 2 to 3 weeks. The patient is encouraged to perform immediate pendulum and passive range-of-motion exercises. No active range of motion or lifting is allowed for the first 6 weeks. After 6 weeks, active range of motion is initiated along with a strengthening program. Throwing is not permitted until 6 months after surgery if symptoms have resolved, with a return to sport at 10 to 12 months.
Discussion
Chondral and osteochondral defects of the glenohumeral joint can cause significant pain and disability. A variety of surgical techniques are available without the demonstrated superiority of 1 technique. Small lesions may respond well to microfracture and medium-sized lesions may respond well to autologous chondrocyte implantation, whereas large defects with an osseous component are often best treated with osteochondral allografts to prevent significant donor-site morbidity and multiple procedures. Cryopreserved osteochondral allografts (Cartiform) maintain a high percentage of viable chondrocytes and contain chondrogenic factors and extracellular matrices, which provide a sound scaffolding for cartilage repair without sacrificing shelf life. There are limited studies on osteochondral allograft use in shoulders, and these are composed mainly of case reports and case series.
A recent study by Riff et al.7 evaluated 20 patients (average age, 24.8 years) who had undergone osteochondral allograft transplantation for glenohumeral defects. At a mean of 67 months, 90% of grafts had incorporated, 20% of patients had undergone subsequent arthroplasty, and 55% of patients were satisfied. Similar results were seen in 13 patients with humeral head defects treated with an osteochondral allograft in a case series by Diklic et al.8 At a mean of 54 months, 69% of patients were pain free with no activity restrictions. These studies indicate that osteochondral allografts may be beneficial for glenohumeral joint treatment. At this time, there are no studies using cryopreserved osteochondral allograft in the shoulder; however, good short-term clinical results have been seen in the knee and ankle in case series.9, 10
This article describes a surgical technique for implantation of a cryopreserved osteochondral allograft (Cartiform) for the treatment of full-thickness cartilage defects of the shoulder. The risks and limitations of this technique are minimal. There is a potential risk of disease transmission from an allograft. The graft is soft and malleable; thus, sutures can potentially pull through the graft if pulled under tension. We recommend placing the sutures in the second hole from the periphery of the graft to minimize suture cutting through. The graft is obtained from the knee of a donor; therefore, the cartilage will be thicker than the native glenoid articular cartilage. This did not appear to be clinically significant. Further clinical research is needed to elucidate the outcomes and optimal fixation of cryopreserved osteochondral allografts in the shoulder.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: R.M. has received honoraria from Arthrex and research funding from Arthrex and Joint Restoration Foundation; receives royalties from Lippincott and Thieme; and owns stocks in Alignmed. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Treatment of a glenoid osteochondral defect with a cryopreserved osteochondral allograft (Cartiform) performed in a right shoulder. The patient is positioned in the lateral decubitus position with induction of general anesthesia. The inflow cannula is inserted into the standard posterior portal, followed by the creation of a high anterior portal, anterior to the supraspinatus, using an outside-in technique. The defect is located, and a percutaneous metal cannula is placed through the subscapularis at the 5-o’clock position. A combination of a shaver and curettes is used to debride and remove the pathologic cartilage and the calcified cartilage layer throughout the base of the defect, leaving healthy subchondral bone exposed. A calibrated probe or arthroscopic ruler is used to measure the defect to accurately size the graft. The allograft is sized and cut using a scalpel or scissors to match the desired shape and size. A metal drill sleeve is inserted through the anterosuperior (Gemini) cannula, and a 2.4-mm drill is used to drill a hole for the anchor at the 12-o’clock position of the lesion. A 3-mm Knotless SutureTak (Arthrex) is then inserted. Another anchor is placed at the trans-subscapularis metal cannula. A third anchor is placed at the 6-o’clock position of the lesion through the same cannula. The fourth and final anchor is placed at the 9-o’clock position of the lesion from the anterosuperior cannula. Each anchor has 3 suture strands that are then meticulously retrieved, brought out of the anterosuperior (Gemini) cannula, and kept separated. A needle is used to pass the FiberWire suture end (white) from the 12-o’clock anchor through the perforations of the graft with the corresponding 12-o’clock position. The free end of the FiberWire suture is then placed through the loop of a 2-mm FiberLink to shuttle the FiberWire into the locking mechanism of the anchor. The same step is repeated with the other 3 anchors with their corresponding positions to the graft. The tails of the FiberLinks are then tensioned, thus delivering the graft through the outer sheath of the Gemini cannula. Finally, the inflow is turned off, and the graft is covered with a thin layer of fibrin glue using a dry arthroscopic technique.
References
- 1.Edmonds E.W., Heyworth B.E. Osteochondritis dissecans of the shoulder and hip. Clin Sports Med. 2014;33:285–294. doi: 10.1016/j.csm.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 2.McCarty L.P., III, Cole B.J. Nonarthroplasty treatment of glenohumeral cartilage lesions. Arthroscopy. 2005;21:1131–1142. doi: 10.1016/j.arthro.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Seidl A.J., Kraeutler M.J. Management of articular cartilage defects in the glenohumeral joint. J Am Acad Orthop Surg. 2018;26:e230–e237. doi: 10.5435/JAAOS-D-17-00057. [DOI] [PubMed] [Google Scholar]
- 4.Saltzman B.M., Leroux T., Cole B.J. Management and surgical options for articular defects in the shoulder. Clin Sports Med. 2017;36:549–572. doi: 10.1016/j.csm.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Becerra J., Andrades J.A., Guerado E., Zamora-Navas P., Lopez-Puertas J.M., Reddi A.H. Articular cartilage: Structure and regeneration. Tissue Eng Part B Rev. 2010;16:617–627. doi: 10.1089/ten.TEB.2010.0191. [DOI] [PubMed] [Google Scholar]
- 6.Geraghty S., Kuang J.Q., Yoo D., LeRoux-Williams M., Vangsness C.T., Jr., Danilkovitch A. A novel, cryopreserved, viable osteochondral allograft designed to augment marrow stimulation for articular cartilage repair. J Orthop Surg Res. 2015;10:66. doi: 10.1186/s13018-015-0209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riff A.J., Yanke A.B., Shin J.J., Romeo A.A., Cole B.J. Midterm results of osteochondral allograft transplantation to the humeral head. J Shoulder Elbow Surg. 2017;26:e207–e215. doi: 10.1016/j.jse.2016.11.053. [DOI] [PubMed] [Google Scholar]
- 8.Diklic I.D., Ganic Z.D., Blagojevic Z.D., Nho S.J., Romeo A.A. Treatment of locked chronic posterior dislocation of the shoulder by reconstruction of the defect in the humeral head with an allograft. J Bone Joint Surg Br. 2010;92:71–76. doi: 10.1302/0301-620X.92B1.22142. [DOI] [PubMed] [Google Scholar]
- 9.Vangsness C.T., Jr., Higgs G., Hoffman J.K. Implantation of a novel cryopreserved viable osteochondral allograft for articular cartilage repair in the knee. J Knee Surg. 2018;31:528–535. doi: 10.1055/s-0037-1604138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez E.G., Hall J.P., Smith R.L., Rachoy J.P., Szmyd T. Treatment of osteochondral lesions of the talus with cryopreserved talar allograft and ankle distraction with external fixation. Surg Technol Int. 2006;15:282–288. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Treatment of a glenoid osteochondral defect with a cryopreserved osteochondral allograft (Cartiform) performed in a right shoulder. The patient is positioned in the lateral decubitus position with induction of general anesthesia. The inflow cannula is inserted into the standard posterior portal, followed by the creation of a high anterior portal, anterior to the supraspinatus, using an outside-in technique. The defect is located, and a percutaneous metal cannula is placed through the subscapularis at the 5-o’clock position. A combination of a shaver and curettes is used to debride and remove the pathologic cartilage and the calcified cartilage layer throughout the base of the defect, leaving healthy subchondral bone exposed. A calibrated probe or arthroscopic ruler is used to measure the defect to accurately size the graft. The allograft is sized and cut using a scalpel or scissors to match the desired shape and size. A metal drill sleeve is inserted through the anterosuperior (Gemini) cannula, and a 2.4-mm drill is used to drill a hole for the anchor at the 12-o’clock position of the lesion. A 3-mm Knotless SutureTak (Arthrex) is then inserted. Another anchor is placed at the trans-subscapularis metal cannula. A third anchor is placed at the 6-o’clock position of the lesion through the same cannula. The fourth and final anchor is placed at the 9-o’clock position of the lesion from the anterosuperior cannula. Each anchor has 3 suture strands that are then meticulously retrieved, brought out of the anterosuperior (Gemini) cannula, and kept separated. A needle is used to pass the FiberWire suture end (white) from the 12-o’clock anchor through the perforations of the graft with the corresponding 12-o’clock position. The free end of the FiberWire suture is then placed through the loop of a 2-mm FiberLink to shuttle the FiberWire into the locking mechanism of the anchor. The same step is repeated with the other 3 anchors with their corresponding positions to the graft. The tails of the FiberLinks are then tensioned, thus delivering the graft through the outer sheath of the Gemini cannula. Finally, the inflow is turned off, and the graft is covered with a thin layer of fibrin glue using a dry arthroscopic technique.