Abstract
Background
There is evidence of structural brain alterations in major depressive disorder (MDD), but little is known about how these alterations might be affected by age at onset or genetic vulnerability. This study examines whether lifetime episodes of MDD are associated with specific alterations in grey-matter volume, and whether those alterations vary according to sex or serotonin transporter-linked promoter region (5-HTTLPR) genotype (LL, SL or SS).
Methods
We used structural MRI to acquire anatomic scans from 610 community-dwelling participants. We derived quantitative regional estimates of grey-matter volume in 16 subregions using FreeSurfer software. We diagnosed MDD according to DSM-IV criteria. We adjusted analyses for age, sex, total brain volume, education level, head injury and comorbidities.
Results
Lifetime MDD was associated with a smaller insula, thalamus, ventral diencephalon, pallidum and nucleus accumbens and with a larger pericalcarine region in both men and women. These associations remained after adjustment for false discovery rate. Lifetime MDD was also associated with a smaller caudate nucleus and amygdala in men and with a larger rostral anterior cingulate cortex in women. Late-onset first episodes of MDD (after age 50 years) were associated with a larger rostral anterior cingulate cortex and lingual and pericalcarine regions; early-onset MDD was associated with a smaller ventral diencephalon and nucleus accumbens. Some associations differed according to 5-HTTLPR genotype: the thalamus was smaller in participants with MDD and the LL genotype; pericalcarine and lingual volumes were higher in those with the SL genotype.
Limitations
This study was limited by its cross-sectional design.
Conclusion
Major depressive disorder was associated with persistent volume reductions in the deep nuclei and insula and with enlargements in visual cortex subregions; alterations varied according to age of onset and genotype.
Introduction
The identification of sociodemographic, environmental and physiopathological factors associated with onset, recovery and relapse in major depressive disorder (MDD) has become a major public health priority, given the association of MDD with high rates of mortality and comorbidity. While considerable research has been done into the clinical characterization of MDD and its associated risk factors, the neuroanatomical substrates involved in the disorder are still unclear; meta-analyses of structural and functional imaging studies report inconsistent findings.1 These inconsistencies are likely due to heterogeneity in study design (case–control v. cohort), setting (general population, inpatient or outpatient), population (age, sex), depression characteristics (diagnosis or symptoms, comorbidity, age of onset, recurrent episodes, antidepressant treatment)1 and methodological issues (not controlling for total brain volume, potential confounding or modifying factors, and different neuroimaging techniques).
The most consistent findings suggest that MDD is associated with dysregulation in neural networks that are implicated in affective and cognitive processing, or in autonomic system activity, resulting in a heterogeneous array of emotive, cognitive and behavioural abnormalities.2 Grey-matter volume changes constitute network nodes in MDD, of which the hippocampus, amygdala and prefrontal cortex have been extensively examined.1,3–6 Conversely, structural alterations in deep nuclei (notably the pallidum, thalamus and hypothalamus), as well as in the insula and occipital regions, have rarely been studied despite accumulating evidence for their role in emotion and the neuropathology of stress-related affective disorder.7–10
The nature and course of volumetric changes may also vary across the lifespan.11 Although grey-matter abnormalities in frontal–subcortical and limbic networks are thought to play a key role in the pathophysiology of depression, recent meta-analyses in late-life depression have shown that the most consistent evidence for brain-volume reductions is for the hippocampus, and not for other brain areas.1,5,12 These meta-analyses made no distinction between current and past (remitted) depression and rarely considered age of onset. Most studies have been limited to clinical cohorts, which may not be representative of the case heterogeneity in the general population. In addition, studies have generally been limited in the brain regions they examine and in sample size, so they lack the power to explore modifying factors. More particularly, the influence of sex has rarely been evaluated, despite evidence for sexually dimorphic structural and functional brain differences across the lifespan, and the potential implications of those differences in sex- biased psychiatric conditions.13 This may be especially important for MDD, because prevalence, age of onset, symptomatology and etiology differ between the sexes, and steroid hormones influence brain development and onset of MDD throughout life.14 Genetic risk factors may also influence brain volumes and depression,15,16 but are seldom considered. Serotonergic genes — notably genetic variants in the serotonin transporter- linked promoter region (5-HTTLPR) — have been reported to influence the structure and function of certain brain regions in depressed patients.17 Whether grey-matter volume alterations are influenced by age-related characteristics (physical and psychiatric comorbidities) also remains to be addressed.
To address the limitations of previous studies, we investigated the association between lifetime MDD and various fronto– subcortical and limbic subregions in a large, community-dwelling, elderly population. We tested the hypothesis that regional brain structure abnormalities would be more extensive in participants with a lifetime MDD diagnosis and may persist after recovery. We further hypothesized that these abnormalities would differ according to sex and genetic vulnerability to 5-HTTLPR. We also considered age of onset and the effect of physical and psychiatric comorbidities. Because prospective lifetime birth cohort data were unavailable, we conducted the study retrospectively in elderly people, for whom both lifetime MDD episodes and genotype had been recorded.
Methods
Participants
We derived data from a longitudinal study of neuropsychiatric disorders in community-dwelling French elderly adults, called “Enquête de Santé Psychologique — Risques, Incidence et Traitemement” (ESPRIT).18 Eligible participants who were at least 65 years of age and not institutionalized were recruited by random selection from the electoral rolls between 1999 and 2001. Ethics approval for the study was provided by the national ethics committee, and written informed consent was obtained from all participants. Of the 1863 participants initially recruited to the study, only those aged 80 years or younger were invited for an MRI; 760 participants were randomly selected to take part in the imaging study, of whom 668 had complete volumetric data for analysis. Participants with dementia (n = 14), those who were left-handed (n = 16) or were missing data about lifetime MDD or other main covariates (n = 28) were excluded, leaving 610 participants. Compared with those who were excluded, included participants were younger, more frequently men, more likely to live alone and less likely to have cognitive impairment (p < 0.001 for all characteristics). They were less likely to have never smoked and to have cardiovascular ischemic pathologies (p = 0.02 for both). They did not differ with respect to other characteristics, including the prevalence of lifetime MDD (p = 0.78).
MRI protocol and image analysis
We acquired all neuroimaging scans using the same scanner at the examination centre (Gui de Chauliac Neurology Hospital, Montpellier, France). We used a 1.5 T GE Signa Imaging system to acquire a contiguous T1-weighted sequence for volumetric estimates (axial inversion recovery prepared, spoiled gradient recalled) that was aligned on the anterior–posterior commisure (repetition time 12 ms, echo time 2.8 ms, inversion time 600 ms, matrix size 256 × 256, pixel spacing 0.9375 × 0.9375 mm, number of excitations = 1, slice thickness 1.0 mm). We performed regional reconstruction and segmentation using the FreeSurfer (5.3) image analysis suite (http://surfer.nmr.mgh.harvard.edu/) as described previously.19 We inspected the FreeSurfer outputs of each scan for errors or misclassifications (from 2D and 3D perspectives), and we excluded 28 scans with clear errors. We defined 16 regions of interest (ROIs) using the Desikan atlas.20 The regions of primary interest were the hippocampus, amygdala, orbitofrontal cortex and anterior cingulate cortex (ACC), as well as several subcortical structures (thalamus, caudate nucleus, putamen, pallidum and accumbens nuclei) that might show neuroimaging abnormalities in depressed patients.1,3,5,6,21,22 We also examined ROIs that had rarely been evaluated despite accumulating evidence for abnormalities in MDD and their potential role in emotional processing and symptom characteristics, including the insula,7,8,23 ventral diencephalon (a region primarily comprising the hypothalamus in FreeSurfer)9,24,25 and occipital visual cortex.10,23,26,27 We calculated the total brain volume (grey plus white matter) for each participant using the segment m-file in SPM5 (Wellcome Department of Cognitive Neurology), which showed greater accuracy28 and consistency29 and less systematic bias evaluation30 than FreeSurfer for this measure.
Diagnosis of lifetime psychiatric disorder
Current and previous MDD and anxiety disorders (phobias, generalized anxiety disorder, panic disorder, obsessive compulsive disorder and posttraumatic stress disorder) were diagnosed by psychologists and psychiatric nurses according to DSM-IV criteria and using the Mini-International Neuropsychiatric Interview (MINI, French version 5.00),31 a standardized psychiatric examination that has been validated in the general population. Positive cases were reviewed by a panel of independent psychiatrists.32
Sociodemographic and clinical variables
We used a standardized interview to obtain information on sociodemographic characteristics, physical health and medical history. We used detailed medical questionnaires (with additional information from general practitioners) to obtain participants’ history of cardiovascular ischemic pathologies (angina pectoris, myocardial infarction, stroke, cardiovascular surgery and arteritis). We recorded all drugs used by participants in the preceding month (including antidepressants) from medical prescriptions and drug packaging. We evaluated global cognitive function using the Mini-Mental State Examination;33 a score below 26 indicated cognitive impairment. Dementia was diagnosed by a neurologist as part of a standardized examination and validated by a panel of independent neurologists.34
5-HTTLPR genotyping
We collected blood samples after the baseline clinical interview, enabling DNA extraction and 5-HTTLPR genotyping as described previously.35 We performed replicate independent genotyping using buccal DNA extracts.32
Statistical analysis
Brain-volume measurements were normally distributed. We evaluated associations between brain regions and lifetime MDD using analysis of covariance adjusted for age, sex and total brain volume (model M0). Where we observed significant associations, we used exploratory analyses to assess the specificity of these findings. We tested the sex × diagnosis interaction by adding a lifetime MDD × sex term to the model. Where we observed an interaction effect (p < 0.10), we stratified analyses by sex. We made further adjustments for other covariates that have been reported to modify the association between MDD and brain volume: education level, head injury, cardiovascular ischemic pathologies and antidepressant use (model M1), as well as lifetime anxiety disorder (model M2). For significant bivariate associations, we evaluated the effect of 5-HTTLPR by stratifying it into 3 genotypes (LL, SL and SS) because of a lack of consensus about the choice of a genetic model and frequent reports of heterosis for 5-HTTLPR.36,37 To account for the fact that we examined multiple brain regions, we adjusted significance levels using the false discovery rate (FDR) method.38 All tests were 2-sided, and we used SAS (version 9.4, SAS Institute, Inc.) for the statistical analyses.
Results
Participant characteristics
The baseline characteristics of the 610 participants are summarized in Table 1. The median age of participants was 70.7 years. Of the total population, 47.5% were male, and 26.6% had lifetime MDD, of whom 38.3% had recurrent episodes. Only 2.1% had current MDD, and 5.1% were taking an antidepressant. Men and women differed with respect to most characteristics: women were younger, more frequently living alone and more likely to report a lifetime psychiatric disorder, but they had fewer head injuries and fewer cardiovascular risk factors.
Table 1.
Participant characteristics (n = 610*)
| Group, median (IQR) or % | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | Whole sample (n = 610) | Men (n = 290) | Women (n = 320) | p value† |
| Age, yr | 70.7 (67.8–74.0) | 71.0 (68.4–74.4) | 70.1 (67.6–73.5) | 0.03 |
| Body mass index, kg/m2 (n = 604) | 24.8 (22.8–27.1) | 25.8 (24.0–27.7) | 24.0 (21.6–26.4) | < 0.001 |
| Cortex volume, cm3 | 358 (336–382) | 376 (353–402) | 344 (324–365) | < 0.001 |
| Total brain volume, cm3 | 882 (816–958) | 925 (858–998) | 850 (788–908) | < 0.001 |
| Education ≤ 5 yr | 25.7 | 23.1 | 28.1 | 0.16 |
| Living alone (n = 609) | 19.7 | 6.2 | 32.0 | < 0.001 |
| Smoking (n = 609) | ||||
| Never | 53.4 | 26.9 | 77.5 | < 0.001 |
| Former | 38.7 | 62.1 | 17.5 | |
| Current | 7.9 | 11.0 | 5.0 | |
| Head injury | 10.2 | 13.5 | 7.2 | 0.01 |
| Lifetime number of major depressive episodes‡ | ||||
| 1 | 16.4 | 11.7 | 20.6 | < 0.001 |
| ≥ 2 | 10.2 | 3.8 | 16.0 | |
| Current major depressive disorder‡ | 2.1 | 0.7 | 3.4 | 0.02 |
| Age at first episode (n = 609) | ||||
| None | 73.6 | 84.5 | 63.6 | < 0.001 |
| < 50 yr | 14.9 | 7.9 | 21.3 | |
| ≥ 50 yr | 11.5 | 7.6 | 15.1 | |
| Antidepressant use | 5.1 | 2.4 | 7.5 | 0.004 |
| Lifetime anxiety disorder‡ (n = 576) | 26.4 | 16.4 | 35.6 | < 0.001 |
| Hypertension§ | 68.5 | 74.4 | 63.1 | 0.003 |
| Cardiovascular ischemic pathologies¶ | 12.8 | 17.2 | 8.8 | 0.002 |
| Diabetes** (n = 606) | 8.8 | 12.9 | 5.0 | < 0.001 |
| Cognitive impairment†† | 13.4 | 10.7 | 15.9 | 0.06 |
IQR = interquartile range.
Unless otherwise specified.
Kruskal–Wallis tests for continuous variables and χ2 tests for categorical variables.
Diagnosis of past and current major depression or anxiety disorder (phobias, generalized anxiety disorder, posttraumatic stress disorder, panic disorder or obsessive–compulsive disorder) according to DSM-IV criteria and using the Mini-International Neuropsychiatric Interview.31
Hypertension ≥ 140/90 mm Hg or treated.
History of cardiovascular ischemic pathologies (angina pectoris, myocardial infarction, stroke, cardiovascular surgery, arteritis).
Fasting blood glucose ≥ 7.0 mmol/L or treatment.
Mini-Mental State Examination score < 26.
Subsegmental brain regions according to lifetime MDD
After adjustment for age, sex and total brain volume, lifetime MDD was associated with smaller grey-matter volumes in the insula, diencephalic structures and deep nuclei, and with larger volumes in the rostral ACC and 2 visual cortex subregions (Table 2). Alterations in the insula, thalamus, ventral diencephalon, nucleus accumbens, pallidum and pericalcarine region survived FDR correction. We observed similar alterations in the multivariate model that was further adjusted for education level, head injury, cardiovascular ischemic pathologies and antidepressant use (Appendix 1, Table S1, available at jpn.ca/180026-a1). These covariates accounted for a relatively small proportion of the variance in each volume: the standardized regression coefficients ranged between −0.338 and 0.257 (Appendix 1, Table S2). In our sample, 576 participants were also evaluated for lifetime anxiety disorder, and we observed a similar pattern after controlling for anxiety disorder (data not shown). We conducted a sensitivity analysis excluding 41 participants who were currently depressed or taking antidepressants, and observed similar patterns, except for a slightly weaker association for the nucleus accumbens but a stronger association for the lingual region (Appendix 1, Table S3).
Table 2.
Association of subsegmental brain region volumes with lifetime major depressive disorder (n = 610)
| Brain region | No lifetime MDD (n = 448), mean ± SD* | Lifetime MDD (n = 162), mean ± SD* | p value† | pFDR value‡ |
|---|---|---|---|---|
| Medial orbitofrontal cortex | 8 962.94 ± 43.20 | 8 939.55 ± 73.61 | 0.79 | 0.79 |
| Lateral orbitofrontal cortex | 11 652.40 ± 46.37 | 11 718.13 ± 79.01 | 0.48 | 0.55 |
| Rostral anterior cingulate cortex | 3 347.51 ± 28.37 | 3 458.73 ± 48.34 | 0.05 | 0.09 |
| Caudal anterior cingulate cortex | 2 993.71 ± 25.67 | 3 065.27 ± 43.73 | 0.16 | 0.22 |
| Hippocampus | 7 061.54 ± 33.88 | 7 033.17 ± 57.73 | 0.68 | 0.72 |
| Amygdala | 2 634.05 ± 14.88 | 2 580.65 ± 25.34 | 0.07 | 0.12 |
| Insula | 11 954.83 ± 48.80 | 11 722.33 ± 83.14 | 0.017 | 0.045 |
| Thalamus | 11 806.32 ± 43.44 | 11 575.64 ± 74.01 | 0.008 | 0.038 |
| Ventral diencephalon | 6 794.71 ± 28.62 | 6 624.44 ± 48.76 | 0.003 | 0.038 |
| Caudate nucleus | 6 852.97 ± 52.58 | 6 669.85 ± 89.58 | 0.08 | 0.12 |
| Putamen | 9 453.97 ± 52.75 | 9 215.18 ± 89.87 | 0.024 | 0.06 |
| Nucleus accumbens | 997.39 ± 7.14 | 960.92 ± 12.17 | 0.011 | 0.038 |
| Pallidum | 2 983.73 ± 16.17 | 2 893.75 ± 27.55 | 0.005 | 0.038 |
| Cuneus | 4 765.02 ± 30.27 | 4 839.00 ± 51.58 | 0.22 | 0.27 |
| Pericalcarine region | 3 404.66 ± 28.46 | 3 547.02 ± 48.48 | 0.012 | 0.038 |
| Lingual region | 10 714.10 ± 67.51 | 10 984.78 ± 115.01 | 0.045 | 0.09 |
FDR = false discovery rate; MDD = major depressive disorder; SD = standard deviation.
Values expressed in mm3 and adjusted for age, sex and total brain volume.
Raw p values not adjusted for multiple comparisons.
False-discovery rate–corrected.
Subsegmental brain regions according to age at first major depressive episode
Of the participants with lifetime MDD, 56.5% reported first onset before 50 years of age. Compared with participants who did not have lifetime MDD, grey-matter volumes in the rostral ACC and lingual and pericalcarine regions were larger for those with late onset (after age 50 years). Smaller volumes in the ventral diencephalon and nucleus accumbens appeared to be more related to earlier onset (Appendix 1, Table S4).
Exploratory analyses according to sex
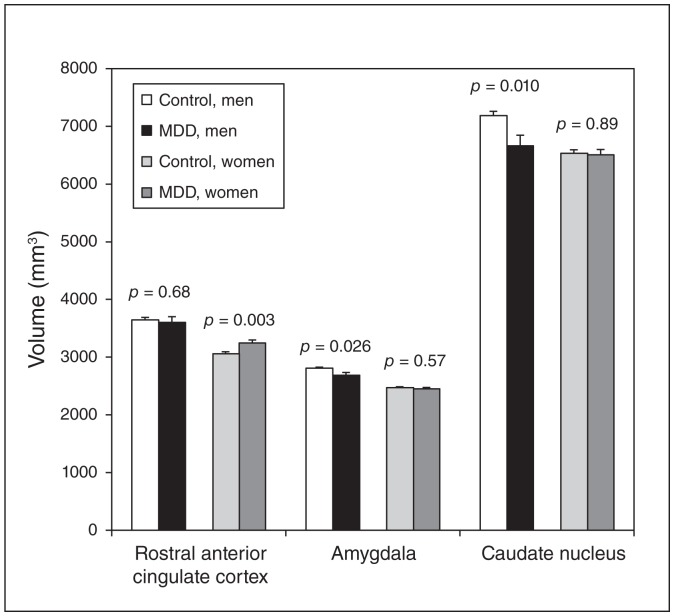
We observed an interacting effect of sex on the amygdala (p = 0.09), caudate nucleus (p = 0.018) and rostral ACC (p = 0.06). Lifetime MDD was associated with smaller grey-matter volumes in the amygdala and caudate nucleus in men (−4.3%, p = 0.026, and −7.2%, p = 0.010, respectively) but not in women (−0.8%, p = 0.57, and −0.2%, p = 0.89, respectively). Conversely, only women with lifetime MDD had larger volumes in the rostral ACC (+6.3%, p = 0.003 v. −1.2% in men, p = 0.68; Fig. 1).
Fig. 1.
Association between lifetime major depressive disorder (MDD) and volumes of rostral anterior cingulate cortex, amygdala and caudate nucleus in elderly men and women. Mean ± standard deviation (SD); values expressed in cubic millimetres and adjusted for age and total brain volume. Members of the control group did not have lifetime MDD; members of the MDD group had lifetime MDD.
Exploratory analyses as a function of 5-HTTLPR genotype
Of all participants, 30% were homozygous carriers of the L allele, and 23.2% were SS homozygotes. The frequency of the 5-HTTLPR genotypes did not deviate significantly from Hardy–Weinberg equilibrium (p = 0.17). Lifetime MDD was associated with a smaller thalamus in LL participants only (−4.9%, p = 0.002, v. −1.2%, p = 0.27 in SL participants and +0.3%, p = 0.86, in SS participants; Table 3). We found significant differences in insula, ventral diencephalon, lingual and (most significantly) pericalcarine volume for SL participants with lifetime MDD (+8.2%, p = 0.003 v. +2.5%, p = 0.41 for LL participants and +2%, p = 0.55 for SS participants). We observed the same pattern after excluding participants who had current MDD or were taking antidepressants, although association with the ventral diencephalon was weakened (p = 0.060) and that with the lingual region was strengthened (p = 0.005 in M0 and p = 0.001 in M2; data not shown).
Table 3.
Association of subsegmental brain region volumes with lifetime MDD according to 5-HTTLPR genotype
| LL (n = 160) | SL (n = 250) | SS (n = 124) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| 5-HTTLPR | No lifetime MDD (n = 112), mean ± SD* | Lifetime MDD (n = 48), mean ± SD* | p value† | No lifetime MDD (n = 184), mean ± SD* | Lifetime MDD (n = 66), mean ± SD* | p value† | No lifetime MDD (n = 93), mean ± SD* | Lifetime MDD (n = 31), mean ± SD* | p value† |
| Rostral anterior cingulate cortex | 3 394.88 ± 60.80 | 3 537.88 ± 94.35 | 0.22 | 3 345.48 ± 43.42 | 3 400.87 ± 74.31 | 0.52 | 3 346.62 ± 63.48 | 3 498.30 ± 112.93 | 0.24 |
| Insula | 11 847.14 ± 99.97 | 11 963.30 ± 155.14 | 0.54 | 11 996.04 ± 78.88 | 11 628.15 ± 134.99 | 0.020 | 11 833.83 ± 103.71 | 11 520.94 ± 184.48 | 0.14 |
| Thalamus | 11 966.39 ± 98.90 | 11 376.42 ± 153.47 | 0.002 | 11 843.10 ± 65.47 | 11 700.58 ± 112.05 | 0.27 | 11 685.44 ± 89.60 | 11 717.36 ± 159.39 | 0.86 |
| Ventral diencephalon | 6 726.12 ± 57.73 | 6 524.76 ± 89.59 | 0.07 | 6 869.44 ± 46.83 | 6 659.23 ± 80.15 | 0.025 | 6 765.45 ± 59.92 | 6 731.95 ± 106.59 | 0.78 |
| Putamen | 9 530.94 ± 131.13 | 9 093.75 ± 203.49 | 0.08 | 9 591.21 ± 75.65 | 9 323.70 ± 129.47 | 0.08 | 9 257.61 ± 108.03 | 8 955.26 ± 192.18 | 0.17 |
| Nucleus accumbens | 996.60 ± 14.19 | 966.80 ± 22.02 | 0.27 | 1 003.50 ± 11.03 | 984.31 ± 18.88 | 0.38 | 980.00 ± 16.80 | 921.09 ± 29.89 | 0.09 |
| Pallidum | 2 957.48 ± 32.98 | 2 852.01 ± 51.18 | 0.10 | 3 033.56 ± 24.32 | 2 952.72 ± 41.62 | 0.10 | 2 994.12 ± 37.38 | 2 869.91 ± 66.50 | 0.11 |
| Pericalcarine region | 3 422.87 ± 54.66 | 3 509.63 ± 84.83 | 0.41 | 3 362.83 ± 47.23 | 3 639.30 ± 80.83 | 0.003 | 3 444.39 ± 56.29 | 3 512.98 ± 100.13 | 0.55 |
| Lingual region | 10 870.88 ± 135.40 | 10 875.23 ± 210.12 | 0.99 | 10 620.67 ± 110.25 | 11 142.98 ± 188.68 | 0.018 | 10 943.15 ± 133.37 | 10 991.36 ± 237.26 | 0.86 |
MDD = major depressive disorder; SD = standard deviation.
Values expressed in mm3 and adjusted for age, sex and total brain volume.
Raw p values.
Discussion
Lifetime MDD was associated with many grey-matter volume differences. The most robust finding was smaller volumes in the deep nuclei and insula and larger volumes in the occipital visual cortex. These findings were independent of age, sex, education level, head injury, cardiovascular ischemic pathologies, antidepressant use and lifetime anxiety disorder. They were also seen in individuals who had been free of MDD for many years (median [interquartile range] = 15 [5–24] years). Lifetime MDD was also associated with smaller caudate nuclei and amygdalae in men, and a larger rostral ACC in women. Some associations varied by 5-HTTLPR genotype: most importantly, the thalamus was smaller in LL participants with lifetime MDD, whereas the pericalcarine and lingual regions were larger in SL participants only.
Previous reports of morphological brain changes relative to MDD have mostly been based on small case–control studies that focused on the hippocampus, amygdala, orbitofrontal cortex and ACC.1,3,5,6 Inconsistent findings have been reported, probably attributable to heterogeneity in study design, size, setting, population and depression characteristics.1 Meta-analyses reported smaller volumes in adults, but the effects were small and/or nonsignificant; effects depended on patients’ age and whether they were experiencing a first episode, and were less likely in the community-dwelling population or in patients with remitted MDD compared with those who had current or recurrent episodes.1,3,5,6,21,39,40 In the community-dwelling elderly population in our study, most participants reported only 1 previous depressive episode, and the median (interquartile range) age of first onset was 47 (35–57) years, which may explain the lack of significant associations. However, we did find a highly significant difference for the rostral ACC, which was larger in women with lifetime MDD. Voxel-based morphometry studies have reported larger cingulate gyri in geriatric patients with remitted depression,41 and a larger ACC in medication-washout young adults with MDD.42 In healthy adults, a negative association has been shown between grey-matter volume and stress-related brain activity in the perigenual ACC.43
Basal nuclei
In our study, lifetime MDD was associated with decreased grey-matter volumes in several basal nuclei. Smaller caudate nuclei have been reported in adult patients3,40 and, in late-life depression, effect sizes increased with age and with a smaller percentage of women.6 Consistent with these results, we found that lifetime MDD was associated with a smaller caudate nucleus in men, and the association was strengthened after adjusting for several confounders, including antidepressant use and the presence of an anxiety disorder (−8.6%, p = 0.003, in M2).
Some meta-analyses on the putamen have reported significant grey-matter volume reduction with lifetime MDD,3,6 and others in early- but not late-onset40 or current MDD.5 Other studies have found that associations were limited to severe or persistent subtypes.39 We found a marginal association between lifetime MDD and a smaller putamen (pFDR = 0.055) and a greater grey-matter volume reduction in those with recurrent episodes (global p value = 0.042).
We also found an association between lifetime MDD and smaller volumes in the nucleus accumbens and pallidum. These regions have been examined rarely, and meta-analyses of studies including mainly adult patients with acute or lifetime MDD have failed to report significant associations.5,21 In an analysis of a high-dimensional set of more than 11 000 traits, the pallidum volume was reported to be a main endophenotype associated with recurrent depression.9 We also observed a greater grey-matter volume reduction in participants who reported multiple MDD episodes compared with those who reported 1 or no previous episodes (global p = 0.013).
Lifetime MDD was also associated with a smaller thalamus and ventral diencephalon — a region primarily comprising the hypothalamus. Small meta-analyses of the thalamus have reported moderate6,21 or no significant3,5,40 volume reduction, but none has included the hypothalamus, despite its crucial role in emotional behaviour and stress response as part of the hypothalamic–pituitary–adrenal axis. However, smaller ventral diencephalon volume has been reported to be the top-ranked neuroimaging endophenotype associated with recurrent MDD in adults9 and to correlate with the number of depressive episodes in late-life depression.25
Insula
The insula plays a role in emotional, sensorimotor and interoceptive processing, but it has rarely been examined, despite some evidence for abnormalities in MDD and related phenotypes (sadness, irritability, sleep disorders).7,8 A small case–control study reported reduced anterior insular cortex in current and remitted young adult patients compared with healthy controls.44 A meta-analysis of voxel-based morphometry studies also showed left insula volume reductions in young adults with first-episode depression.8 Our study is, to our knowledge, the first ROI study to show a significant smaller insula volume in an elderly general population with lifetime and remitted MDD.
Visual cortex
Another original finding concerns the association of MDD with larger grey-matter volume in the pericalcarine and lingual ROIs, a finding that was especially marked for late-onset MDD. The visual cortex plays a central role in the fear-conditioning paradigm in humans.45 The pericalcarine region is the initial region of visual processing, and the lingual gyrus is associated with high-level visual processing and visual memory. The associations were highly significant after excluding participants who were currently depressed or treated for depression (p = 0.006 and 0.004, respectively, in M2). The link between the visual cortex and MDD has been described only in voxel-based morphometry studies; a meta-analysis reported a significant grey-matter volume increase in the right lingual gyrus in late-life depression.10 A larger lingual gyrus volume was also found to predict early antidepressant response in adults and was linked to better performance on visual neuropsychological tests.46 Greater sensory reactivity in the visual cortex could predict resilience against depressive relapse.47 In healthy young adults, facilitation of processing of aversive stimuli was associated with the breaking of subcortical limbic connections (insula, putamen, amygdala, hippocampus), together with a compensatory emerging centrality of the visual cortex.45 Whether the pericalcarine and lingual regions could participate in a neuronal compensatory process to facilitate the processing of aversive stimuli and fear or emotional learning in response to abnormal input from other structures, or whether they reflect resilience against relapse, remains to be examined.
5-HTTLPR genotype
Most previous studies have examined the effect of 5-HTTLPR on grey-matter volumes in either depressed or healthy participant groups, but rarely as a modifying factor between MDD risk and grey-matter alterations.17 These studies were generally size-limited and focused on the hippocampus and amygdala, rarely on the striatum or thalamus, where 5-HTT is highly expressed. The vast majority of the structural imaging genetic studies did not consider the SL genotype individually, despite a lack of consensus regarding the genetic model and frequent report of heterosis for 5-HTTLPR.37 Heterogeneity in age is another potential source of concern:48 in younger populations, the S allele is a risk factor for mental and physical distress, but the LL genotype appears to be a risk factor in elderly people, who are highly exposed to chronic disorders and severe stressors.49
We found no significant volumetric differences according to 5-HTTLPR within groups (with or without lifetime MDD; data not shown) but we did find significant between-group differences. In particular, the thalamus was smaller in LL homozygotes with lifetime MDD, but only SL heterozygotes with lifetime MDD had larger pericalcarine and lingual volumes compared with their non-MDD counterparts. The thalamus is rich in serotonergic neurons, and reduced 5-HTT availability has been described in the thalamus of depressed patients,50 but data on the effect of 5-HTTLPR genotypes are lacking, and no studies have examined the pericalcarine or lingual regions, despite some indication for serotonergic occipital dysfunction in depression.51 Our findings were unchanged after excluding patients who were currently depressed or treated for depression, suggesting that they were related to serotonergic vulnerability to the disorder.
In a subsample of the ESPRIT study, we reported that past MDD and stressful events were risk factors for current depression in LL homozygotes specifically, whereas SL heterozygotes were more resilient to these factors.36 We also found that some adverse events during childhood (for example, sexual or physical abuse, or having a mother with mental illness) were associated with higher risk of late-life depression35 but lower risk of cognitive decline, notably in visual memory, 52 suggesting a possible cognitive adaptation or resilience effect. Although these findings are speculative, they may suggest that the pericalcarine and lingual regions could participate in a persistent neuronal compensatory process in SL heterozygotes with a history of MDD.
Sex differences
We found some evidence for sexually dimorphic alterations: smaller caudate nuclei and amygdalae in men with lifetime MDD, and larger rostral ACC in women. Specific sex differences in the depression symptomatology of older adults have been described, with women showing more mood-related symptoms and appetite disturbance and men showing more motivation-related symptoms and psychomotor changes;14 these differences may involve distinct biological correlates. However, only a few neuroimaging studies have investigated sex effects, many including predominantly women and, likely as a consequence, meta-analyses have seldom reported sex differences.5,10
In a healthy sample consisting mainly of older women, a larger ACC was associated with higher levels of anhedonia.53 Valence-dependent sex differences in emotional reactivity have been reported, with divergent activation patterns, notably in the ACC and amygdala, suggesting a difference in recognizing, expressing or responding to emotions.54 The rostral ACC and caudate nucleus are involved in impulse inhibition in young adults in a sex-specific manner, suggesting different processing strategies (e.g., inhibiting inappropriate response in males v. eliciting appropriate response in females).55 In adults with attention-deficit/hyperactivity disorder, a smaller caudate nucleus was associated with impulsive/hyperactive symptoms in men, but not in women.56 In addition, increased resting rostral ACC activity has been linked to adaptive cognitive aspects of rumination and could predict better antidepressant response and recovery.57 Whether sex influences the nature of changes in some structures or is associated with specific symptoms, processing strategies or characteristics of depression (e.g., rumination, irritability, impulsivity) remains to be examined.
Context of the findings
We found that retrospectively determined incidence of lifetime MDD was associated with a smaller striatum, pallidum, thalamus, hypothalamus and insula, but with larger pericalcarine and lingual regions, even many years after recovery. These findings are partly consistent with a neurobiological model of current depression that posits dysfunction of the cortico–striatal–pallidal–thalamic network involved in emotion, cognition and motor control; reward and stress systems; and sensorimotor and interoceptive processing.23,58,59 There is also some evidence for sex differences with respect to emotion production and regulation. We did not observe the reduced volumes in the hippocampus or frontal subregions previously reported in currently depressed adults. In a meta-analysis of functional MRI studies, Graham and colleagues23 suggested that frontal areas could be state markers of MDD, but that striatal regions were trait vulnerability markers that might be less affected by treatment. They also stressed the potential key roles of regions that are not included in prevailing models of MDD, such as the insula and occipital subregions, the latter showing overactivity in MDD.23 Sustained remission from MDD has been associated with normalized reactivity of certain prefrontal and limbic regions and greater sensory reactivity in visual cortices,47 as well as hyperactivity and/or reduced deactivation in the rostral ACC.57 Our data further suggest a key role of the visual corticostriatal loop in elderly patients with remitted MDD, with volume enlargement in visual occipital regions and reduction in subcortical structures.60 They also suggest a link between certain sensory/visual functions (thalamus for sensory relay, and pericalcarine and lingual regions for visual processing and visual memory) and 5-HTTLPR vulnerability (to stress-induced relapse) or resilience to MDD. However, it remains to be determined whether these abnormalities represent biological long-term vulnerability (endophenotype as intermediate expression of genetic vulnerability factors).
Limitations
Limitations of our study include the cross-sectional design; we could not determine whether volume alterations preceded or followed MDD. Data related to lifetime MDD were retrospective, which may have introduced recall bias and led to an underestimation of associations, even if we had excluded participants diagnosed with probable/possible dementia to minimize inaccuracies. The volume variations associated with lifetime MDD were 2% to 5% for deep nuclei and insula and 5% to 8% for visual cortex ROIs, suggesting a relatively small effect size. We did not examine state-like characteristics because of the low prevalence of current MDD in this relatively healthy community sample, and the lack of associations with some ROIs could have been related to normalization after sustained remission/treatment. Finally, we performed multiple analyses, potentially increasing the risk of type 1 errors, but most findings remained significant even after correction for multiple comparisons.
This study constitutes the largest structural MRI investigation targeting lifetime MDD, in terms of the number of participants and ROIs examined. We measured brain volumes using FreeSurfer automated segmentation, enabling accurate evaluation of volumetric changes of smaller deep brain structures. Lifetime MDD was assessed by trained staff using a standardized psychiatric evaluation, according to DSM-IV criteria. Further clinical validation of the cases minimized false positives. Extensive information available on participants’ clinical status and medications helped minimize exposure misclassification. In contrast to previous studies, we controlled for numerous potential confounding factors, particularly education level, head injury, and physical and mental comorbidities.
Conclusion
We observed grey-matter volume differences between those who retrospectively reported lifetime MDD and those who did not. These structural correlates may constitute useful imaging phenotypes of depression: treatment responsiveness or resilience. It remains to be determined whether grey-matter volume increases are linked to a neuronal adaptive compensatory process in response to dysfunction in other structures, or whether they represents trait-like, developmental differences that underlie a neurobiological vulnerability associated with etiological pathways. Whether sex influences the nature of changes in some brain structures (which could help account for the sex differences observed in epidemiological and clinical studies of depression) or is associated with sex-specific traits or dimensions of depression also remains to be examined. Further work is required to understand the significance of these volumetric differences, including prospective multimodal and complementary imaging studies. This will help distinguish between causal roles and neural correlates of MDD, the result of shared underlying causes (for example, genetic predisposition) or bidirectional/mutual reinforcement, opening up the potential for effective therapeutic strategies.
Acknowledgements
The ESPRIT project is financed by the regional government of Languedoc-Roussillon, the Agence Nationale de la Recherche Project 07 LVIE 004 and an unconditional grant from Novartis. This work was also supported by France Alzheimer. The funders had no role in the design and conduct of the study, or in data collection, management, analysis or interpretation, and they were not involved with the writing, preparation, review or approval of the manuscript.
Footnotes
Competing interests: None declared.
Contributors: M-L. Ancelin designed the study. M-L. Ancelin and K. Ritchie led the ESPRIT study and the collection of data. J. Maller and C. Meslin processed all neuroimaging data. I. Carrière performed all statistical analyses. M-L. Ancelin, S. Artero, J. Ryan and I. Chaudieu were involved in the interpretation of the data. M-L. Ancelin drafted the manuscript, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Geerlings MI, Gerritsen L. Late-life depression, hippocampal volumes, and hypothalamic-pituitary-adrenal axis regulation: a systematic review and meta-analysis. Biol Psychiatry. 2017;82:339–50. doi: 10.1016/j.biopsych.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 2.Jaworska N, Yang XR, Knott V, et al. A review of fMRI studies during visual emotive processing in major depressive disorder. World J Biol Psychiatry. 2015;16:448–71. doi: 10.3109/15622975.2014.885659. [DOI] [PubMed] [Google Scholar]
- 3.Arnone D, McIntosh AM, Ebmeier KP, et al. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol. 2012;22:1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, et al. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–35. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmaal L, Veltman DJ, van Erp TG, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA major depressive disorder working group. Mol Psychiatry. 2016;21:806–12. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sexton CE, Mackay CE, Ebmeier KP. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am J Geriatr Psychiatry. 2013;21:184–95. doi: 10.1016/j.jagp.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Sliz D, Hayley S. Major depressive disorder and alterations in insular cortical activity: a review of current functional magnetic imaging research. Front Hum Neurosci. 2012;6:323. doi: 10.3389/fnhum.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Li L, Wu M, et al. Brain gray matter alterations in first episodes of depression: a meta-analysis of whole-brain studies. Neurosci Biobehav Rev. 2016;60:43–50. doi: 10.1016/j.neubiorev.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Glahn DC, Curran JE, Winkler AM, et al. High dimensional endophenotype ranking in the search for major depression risk genes. Biol Psychiatry. 2012;71:6–14. doi: 10.1016/j.biopsych.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du M, Liu J, Chen Z, et al. Brain grey matter volume alterations in late-life depression. J Psychiatry Neurosci. 2014;39:397–406. doi: 10.1503/jpn.130275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol. 2009;5:363–89. doi: 10.1146/annurev.clinpsy.032408.153621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sexton CE, Le Masurier M, Allan CL, et al. Magnetic resonance imaging in late-life depression: vascular and glucocorticoid cascade hypotheses. Br J Psychiatry. 2012;201:46–51. doi: 10.1192/bjp.bp.111.105361. [DOI] [PubMed] [Google Scholar]
- 13.Ruigrok AN, Salimi-Khorshidi G, Lai MC, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kockler M, Heun R. Gender differences of depressive symptoms in depressed and nondepressed elderly persons. Int J Geriatr Psychiatry. 2002;17:65–72. doi: 10.1002/gps.521. [DOI] [PubMed] [Google Scholar]
- 15.Hibar DP, Stein JL, Renteria ME, et al. Common genetic variants influence human subcortical brain structures. Nature. 2015;520:224–9. doi: 10.1038/nature14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brouwer RM, Panizzon MS, Glahn DC, et al. Genetic influences on individual differences in longitudinal changes in global and subcortical brain volumes: results of the ENIGMA plasticity working group. Hum Brain Mapp. 2017;38:4444–58. doi: 10.1002/hbm.23672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Won E, Ham BJ. Imaging genetics studies on monoaminergic genes in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:311–9. doi: 10.1016/j.pnpbp.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Ritchie K, Artero S, Beluche I, et al. Prevalence of DSM-IV psychiatric disorder in the French elderly population. Br J Psychiatry. 2004;184:147–52. doi: 10.1192/bjp.184.2.147. [DOI] [PubMed] [Google Scholar]
- 19.Calati R, Maller JJ, Meslin C, et al. Repatriation is associated with isthmus cingulate cortex reduction in community-dwelling elderly. World J Biol Psychiatry. 2018;19:421–30. doi: 10.1080/15622975.2016.1258490. [DOI] [PubMed] [Google Scholar]
- 20.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Kempton MJ, Salvador Z, Munafo MR, et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–90. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 22.Bora E, Fornito A, Pantelis C, et al. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 23.Graham J, Salimi-Khorshidi G, Hagan C, et al. Meta-analytic evidence for neuroimaging models of depression: state or trait? J Affect Disord. 2013;151:423–31. doi: 10.1016/j.jad.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Sacchet MD, Livermore EE, Iglesias JE, et al. Subcortical volumes differentiate major depressive disorder, bipolar disorder, and remitted major depressive disorder. J Psychiatr Res. 2015;68:91–8. doi: 10.1016/j.jpsychires.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebedeva AK, Westman E, Borza T, et al. MRI-based classification models in prediction of mild cognitive impairment and dementia in late-life depression. Front Aging Neurosci. 2017;9:13. doi: 10.3389/fnagi.2017.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheuerecker J, Meisenzahl EM, Koutsouleris N, et al. Orbitofrontal volume reductions during emotion recognition in patients with major depression. J Psychiatry Neurosci. 2010;35:311–20. doi: 10.1503/jpn.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monkul ES, Hatch JP, Nicoletti MA, et al. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry. 2007;12:360–6. doi: 10.1038/sj.mp.4001919. [DOI] [PubMed] [Google Scholar]
- 28.Malone IB, Leung KK, Clegg S, et al. Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage. 2015;104:366–72. doi: 10.1016/j.neuroimage.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sargolzaei S, Sargolzaei A, Cabrerizo M, et al. A practical guideline for intracranial volume estimation in patients with Alzheimer’s disease. BMC Bioinformatics. 2015;16(Suppl 7):S8. doi: 10.1186/1471-2105-16-S7-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sargolzaei S, Sargolzaei A, Cabrerizo M, et al. Estimating intracranial volume in brain research: an evaluation of methods. Neuroinformatics. 2015;13:427–41. doi: 10.1007/s12021-015-9266-5. [DOI] [PubMed] [Google Scholar]
- 31.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 32.Ancelin ML, Carriere I, Scali J, et al. Angiotensin-converting enzyme gene variants are associated with both cortisol secretion and late-life depression. Transl Psychiatry. 2013;3:e322. doi: 10.1038/tp.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Ancelin ML, Ripoche E, Dupuy AM, et al. Sex differences in the associations between lipid levels and incident dementia. J Alzheimers Dis. 2013;34:519–28. doi: 10.3233/JAD-121228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritchie K, Jaussent I, Stewart R, et al. Association of adverse childhood environment and 5-HTTLPR genotype with late-life depression. J Clin Psychiatry. 2009;70:1281–8. doi: 10.4088/JCP.08m04510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ancelin ML, Scali J, Norton J, et al. Heterogeneity in HPA axis dysregulation and serotonergic vulnerability to depression. Psychoneuroendocrinology. 2017;77:90–4. doi: 10.1016/j.psyneuen.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Ancelin ML, Ryan J. 5-HTTLPR × stress hypothesis: Is the debate over? Mol Psychiatry. 2017 Sep 17; doi: 10.1038/mp.2017.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 39.Lorenzetti V, Allen NB, Fornito A, et al. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 40.Bora E, Harrison BJ, Davey CG, et al. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol Med. 2012;42:671–81. doi: 10.1017/S0033291711001668. [DOI] [PubMed] [Google Scholar]
- 41.Yuan Y, Zhu W, Zhang Z, et al. Regional gray matter changes are associated with cognitive deficits in remitted geriatric depression: an optimized voxel-based morphometry study. Biol Psychiatry. 2008;64:541–4. doi: 10.1016/j.biopsych.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 42.Zhao YJ, Du MY, Huang XQ, et al. Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychol Med. 2014;44:2927–37. doi: 10.1017/S0033291714000518. [DOI] [PubMed] [Google Scholar]
- 43.Boehringer A, Tost H, Haddad L, et al. Neural correlates of the cortisol awakening response in humans. Neuropsychopharmacology. 2015;40:2278–85. doi: 10.1038/npp.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi T, Yucel M, Lorenzetti V, et al. Volumetric MRI study of the insular cortex in individuals with current and past major depression. J Affect Disord. 2010;121:231–8. doi: 10.1016/j.jad.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Lithari C, Moratti S, Weisz N. Limbic areas are functionally decoupled and visual cortex takes a more central role during fear conditioning in humans. Sci Rep. 2016;6:29220. doi: 10.1038/srep29220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung JY, Kang J, Won E, et al. Impact of lingual gyrus volume on antidepressant response and neurocognitive functions in major depressive disorder: a voxel-based morphometry study. J Affect Disord. 2014;169:179–87. doi: 10.1016/j.jad.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 47.Farb NA, Anderson AK, Bloch RT, et al. Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biol Psychiatry. 2011;70:366–72. doi: 10.1016/j.biopsych.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–46. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- 49.Grabe HJ, Schwahn C, Appel K, et al. Update on the 2005 paper: moderation of mental and physical distress by polymorphisms in the 5-HT transporter gene by interacting with social stressors and chronic disease burden. Mol Psychiatry. 2011;16:354–6. doi: 10.1038/mp.2010.45. [DOI] [PubMed] [Google Scholar]
- 50.Reimold M, Batra A, Knobel A, et al. Anxiety is associated with reduced central serotonin transporter availability in unmedicated patients with unipolar major depression: a [11C]DASB PET study. Mol Psychiatry. 2008;13:606–13. 557. doi: 10.1038/sj.mp.4002149. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez A, Rodriguez-Palancas A, Lopez-Ibor M, et al. Increased occipital delta dipole density in major depressive disorder determined by magnetoencephalography. J Psychiatry Neurosci. 2005;30:17–23. [PMC free article] [PubMed] [Google Scholar]
- 52.Ritchie K, Jaussent I, Stewart R, et al. Adverse childhood environment and late-life cognitive functioning. Int J Geriatr Psychiatry. 2011;26:503–10. doi: 10.1002/gps.2553. [DOI] [PubMed] [Google Scholar]
- 53.McLaren ME, Szymkowicz SM, O’Shea A, et al. Dimensions of depressive symptoms and cingulate volumes in older adults. Transl Psychiatry. 2016;6:e788. doi: 10.1038/tp.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Filkowski MM, Olsen RM, Duda B, et al. Sex differences in emotional perception: meta analysis of divergent activation. Neuroimage. 2017;147:925–33. doi: 10.1016/j.neuroimage.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 55.Liu J, Zubieta JK, Heitzeg M. Sex differences in anterior cingulate cortex activation during impulse inhibition and behavioral correlates. Psychiatry Res. 2012;201:54–62. doi: 10.1016/j.pscychresns.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Onnink AM, Zwiers MP, Hoogman M, et al. Brain alterations in adult ADHD: effects of gender, treatment and comorbid depression. Eur Neuropsychopharmacol. 2014;24:397–409. doi: 10.1016/j.euroneuro.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 57.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 59.Tadayonnejad R, Ajilore O. Brain network dysfunction in late-life depression: a literature review. J Geriatr Psychiatry Neurol. 2014;27:5–12. doi: 10.1177/0891988713516539. [DOI] [PubMed] [Google Scholar]
- 60.Seger CA. The visual corticostriatal loop through the tail of the caudate: circuitry and function. Front Syst Neurosci. 2013;7:104. doi: 10.3389/fnsys.2013.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]