Abstract
In this issue of the Biomedical Journal we explore the history of dengue infection in Taiwan and what current trends have to say about the vector responsible for transmitting the disease on the island. We focus on original research reporting the development of a new perfusion bioreactor to engineer bone from human cord blood stem cells. Finally, we look at trends in osteoporosis in Taiwan and how they highlight the success of public health campaigns.
Keywords: Dengue, Aedes aegypti, Mesenchymal stem cells, Osteogenic differentiation
Spotlight on review
Dengue in Taiwan: pointing the finger at Aedes aegypti
About half of the world's population are at risk of the mosquito-viral infection, dengue fever [1]. This number is set to rise as the mosquitos carrying the virus spread to new climates. In this issue of the Biomedical Journal, Chen [2] explores the intimate history of dengue outbreaks in Taiwan.
Dengue fever is transmitted by two of the most medically important insects in the world: A. aegypti and Aedes albopictus, which besides dengue fever, transmit the viruses responsible for yellow fever, chikungungya, Zika and West Nile fever [3]. A. aegypti is the primary vector for dengue and is widespread across most tropical and sub-tropical regions of the world. A. albopictus originated in South East Asia, but has spread to North America and Europe, by hitching a ride within used car tires [4]. The virus is divided into four antigenic subtypes (D1-D4), all of which generally cause flu-like symptoms, which can occasionally develop into potentially lethal dengue hemorrhagic fever. Dengue virus is endemic to 128 countries and is estimated to cause 390 million infections (of which 96 million manifest clinically) worldwide per year [5].
Taiwan was one of the first countries to experience a severe dengue epidemic in recorded history. During the First and Second World War, Taiwan experienced massive dengue outbreaks throughout the island, with approximately 50% and 80% of the population infected, respectively. Yet, following the massive outbreak that occurred in 1942, dengue fell relatively in silent in Taiwan and indeed across much of the world, likely due to worldwide programs for malaria control [6]. Its reemergence in Taiwan in the 1980s has been linked to an explosion in international travel, the fading of herd immunity created by the outbreak during the Second World War, and the termination of spraying programs with the infamous insecticide, dichlorodiphenyltrichloroethane (DDT) [7].
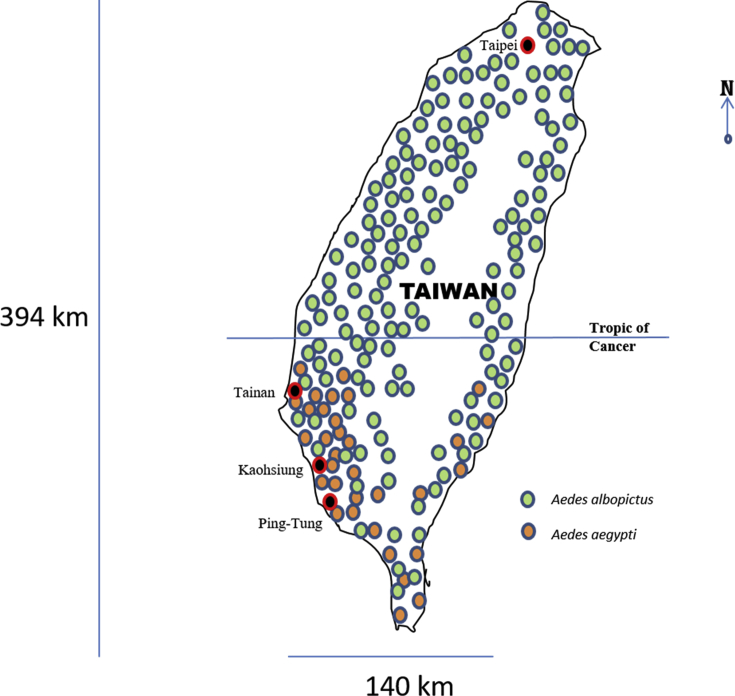
Today indigenous cases of dengue almost exclusively occur in the South of the island in the regions of Tainan, Kaohsiung and Pingtung. These regions lie below the Tropic of Cancer and are known to harbor A. aegypti. In fact, the occurrence of dengue cases in Taiwan in recent years correlates very well with the geographical distribution of A. aegypti, which prefers warm climates [Fig. 1]. A. albopictus on the other hand breeds throughout the island, relatively unfazed by the cold winter weather of the North. To survive the harsh weather A. albopictus enters a state of developmental arrest called diapause, which is typically seen in eggs of this species, but may occur at any developmental stage [8]. Shorter daylight during the fall appears to induce diapause, during which the embryo is confined inside the chorion of the egg, which makes it insensitive to environmental stimuli for hatching.
Fig. 1.
Geographical distribution of Aedes aegypti and Aedes albopictus in Taiwan. A. albopictus can survive the colder weather of the North by entering a state of embryonic arrest called diapause. A. aegypti lacks this property and is limited to the tropics, predominantly in the South West coastal region which have the highest numbers of indigenous dengue cases in Taiwan. Figure kindly provided by Chen [2].
These findings appear to point the finger solely at A. aegypti as the vector responsible for dengue transmission in Taiwan. It is possible that A. albopictus participates in dengue outbreaks in Southern Taiwan but if so, probably only during the later stages of outbreaks initiated by A. aegypti [9]. Thus, A. aegypti appears necessary to initiate dengue outbreaks on the island, and fortunately lacks the cold weather hardiness of its diapause-capable cousin.
Spotlight on original articles
Perfusion bioreactor molds stem cells into bone
Creating artificial tissue for regenerative medicine approaches is no mean feat: source cells, culture systems and scaffolds along with other chemical, biological and physical stimuli must be selected and combined in a “recipe” to produce safe and functional cells for transplantation. In this issue of the Biomedical Journal, Bhaskar et al. [10] test a new dynamic culture system that may help to make bone graft from human cord blood cells.
Tissue engineers have dabbled with multiple stem cell sources in the quest to create tissues for transplantation. Human embryonic stem cells represent the pinnacle of differentiation potential, but their use is ethically fraught. Induced pluripotent stem cells offer the possibility to engineer tissue that is genetically identical to the patient, but the reprogramming process itself is inefficient and there are concerns that it may give rise to cells prone to cancer development [11]. Human umbilical cord blood is a source of mesenchymal stem cells (MSCs), which can differentiate into a variety of tissues including muscle, bone, cartilage and fat. Unlike bone-marrow derived MSCs, cord blood MSCs are obtained non-invasively, which makes them particularly attractive for tissue engineering purposes. Their differentiation is determined by a combination of chemical and physical stimuli, and various bioreactors that apply mechanical stress to cells grown on a scaffold have been developed to promote the osteogenic differentiation of MSCs. In perfusion systems, media is constantly replenished while cells are kept in culture. This not only ensures a steady supply of fresh nutrients and removal of waste, but also generates shear stress which enhances osteogenic differentiation [12]. In a recent work, Birru et al. [13] developed an improved perfusion bioreactor with multiple inlets (instead of the typically used one inlet) deliver nutrients to the central scaffold, which enables an increase in the size of the scaffold. Here, Bhaskar et al. test the suitability of this dynamic culture system to produce osteoblasts from cord blood MSCs.
First, Bhaskar et al. optimized the scaffold on which to grow cord blood MSCs, testing polyactic acid (PLA) and polyethylene glycol (PEG) in varying ratios in the presence of compounds promoting osteogenesis. They found that cell growth, adhesion and differentiation to osteoblasts was best in scaffolds containing PEG, consistent with previous reports [14]. Specifically, scaffolds containing a 90:10 ratio PLA:PEG showed the best results, possibly because the addition of the hydrophilic polymer PEG promoted adhesion while the high PLA content allowed a small pore size. After defining the optimal scaffold, Bhaskar and colleagues turned to the question of whether, and to what extent, perfusion in the bioreactor promoted osteogenesis, by comparing “fluid” (perfusion) conditions with “static” conditions, whereby the media was only changed every 2–3 days. Cells under fluid conditions grew quicker than those under static conditions and showed higher levels of markers of osteoblast differentiation, such as calcium deposition and collagen expression. Thus, consistent with previous reports, shear stress brought about by perfusion appeared to promote osteogenesis.
These findings highlight again just how important mechanical stimuli in the environment are for determining stem cell fate. Moreover, the vehicle for delivering these stimuli, i.e. the perfusion system developed by Bhaskar et al., may be particularly valuable for future tissue engineering applications because its multichannel design can accommodate big scaffolds and hence may be applied to treat larger bone defects. Still, much optimization is required before the engineered bone can be moved to the clinic but the system represents an excellent model to study the effect of perfusion on bone formation and the generation of other tissue types.
Also in this issue
Original articles
Main component of rose oil shows sedative effects
Geraniol is the major constituent of rose and citronella oil and is commonly used in perfumes and flavorings. Besides its industrial value, this monoterpene alcohol has been shown to have host on biological properties, including antioxidant [15] and neuroprotective effects [16]. Here, Medeiros et al. [17] investigate the effects of geraniol on the behavior and brainwave patterns of rats. They find that geraniol made rats more sedentary in various tests of motor behavior and increased sleeping time and the percentage of slow waves, suggesting that geraniol has a depressive effect on the central nervous system.
Factors influencing recovery after surgery for cervical spine infection
Spinal infections involving the cervical (neck) region are rare but particularly dangerous because of the high risk of neurological damage or even death [18]. Multiple factors influence the outcome of surgery to correct cervical spinal infections, but it is not known how these factors influence neurological recovery after surgery. In this retrospective analysis of 27 patients, Luo et al. [19] find that those patients who underwent surgery quickly after being admitted to hospital showed a better neurological outcome. In addition, patients with neck pain before surgery showed poorer neurological improvements, suggesting that clinicians should pay close attention to this symptom when establishing a diagnosis and treatment plan.
Trends in osteoporosis in Taiwan
As the proportion of elderly individuals in the world's population increases, so too should the prevalence of age-related diseases such as osteoporosis. Projections in the US estimate that the number of adults over the age of 50 with osteoporosis will increase by 29% between 2010 and 2020 [20]. But are these trends representative of other world populations? To investigate trends in osteoporosis in Taiwan, Chen et al. [21] examined data from the Taiwan National Health Insurance Research Database, containing approximately 23 million entries for the period under study. They report that the prevalence of osteoporosis increased between 2001 and 2005, but remained steady thereafter. Among individuals below 84 years of age, the prevalence actually decreased between 2001 and 2011. This trends reflects the introduction of public awareness programs for osteoporosis prevention, and likely highlights their success.
Defining “polytrauma”
Patients with severe injuries to multiple body regions are expected to have a worse outcome than those with injury to only one body region. However, the identification of such patients in medical literature is complicated by the fact that there is no universally accepted definition of what constitutes “polytrauma”. Among several definitions, Butcher et al. propose that polytrauma should be defined as an abbreviated injury scale score ≥3 for at least two body regions [22]. Using this definition, Hsieh et al. [23] found no difference in mortality between patients with polytrauma and those with monotrauma who attended a level 1 trauma center over a six year period, suggesting that this definition is not strict enough to distinguish patients with a high risk of death.
Brief communication
Modeling skin lesions in diabetes
Diabetes is associated with several complications including poor wound healing, which is a major cause of hospitalization and poor quality of life. In this brief communication, Leguina-Ruzzi et al. [24] demonstrate that mice treated with streptozotocin, which selectively kills pancreatic beta cells, and fed a high fat diet show the typical skin lesions observed in diabetes, making this model particularly useful for studying this complication.
Organelle sizing: it's all relative
In this brief correspondence, Ma et al. [25] investigate the hypothesis of organelle scaling using the nucleolus of the transparent Caenorhabditis elegans as a model system. Following the knock-down of genes regulating embryo size, they find that the size of the nucleolus scaled with that of the nucleus and cell, i.e. small with small and big with big. These findings support the idea that organelle size is governed by an internal “molecular ruler”, and cannot be completely genetically determined.
Conflicts of interest
The author declares no conflict of interests.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.http://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue [accessed 17 Oct 2018].
- 2.Chen W.J. Dengue outbreaks and the geographic distribution of dengue vectors in Taiwan: a 20-year epidemiological analysis. Biomed J. 2018;41:283–289. doi: 10.1016/j.bj.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leta S., Beyene T.J., De Clercq E.M., Amenu K., Kraemer M.U.G., Revie C.W. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int J Infect Dis. 2018;67:25–35. doi: 10.1016/j.ijid.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawley W.A., Reiter P., Copeland R.S., Pumpuni C.B., Craig G.B., Jr. Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science. 1987;236:1114–1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasheed S.B., Butlin R.K., Boots M. A review of dengue as an emerging disease in Pakistan. Publ Health. 2013;127:11–17. doi: 10.1016/j.puhe.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Kuno G. Emergence of the severe syndrome and mortality associated with dengue and dengue-like illness: historical records (1890 to 1950) and their compatibility with current hypotheses on the shift of disease manifestation. Clin Microbiol Rev. 2009;22:186–201. doi: 10.1128/CMR.00052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson S.M., Craig G.B., Jr. Aedes albopictus (Diptera: culcicidae) eggs : field survivorship during northen Indiana winters. J Med Entomol. 1995;32:599–604. doi: 10.1093/jmedent/32.5.599. [DOI] [PubMed] [Google Scholar]
- 9.Yang C.F., Hou J.N., Chen T.H., Chen Wj. Discriminable roles of Aedes aegypti and Aedes albopictus in establishment of dengue outbreaks in Taiwan. Acta Trop. 2014;130:17–23. doi: 10.1016/j.actatropica.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Bhaskar B., Mekala N.K., Rao Parcha S. Improved osteogenic differentiation of umbilical cord blood MSCs using custom made perfusion bioreactor. Biomed J. 2018;41:290–297. doi: 10.1016/j.bj.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon S., Shailendra S., Renda A., Longaker M., Quarto N. An overview of direct somatic reprogramming: the ins and outs of iPSCs. Int J Mol Sci. 2016;17:141. doi: 10.3390/ijms17010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeatts A.B., Fisher J.P. Bone tissue engineering bioreactors: dynamic culture and the influence of shear stress. Bone. 2011;48:171–181. doi: 10.1016/j.bone.2010.09.138. [DOI] [PubMed] [Google Scholar]
- 13.Bhaskar B., Owen R., Bahmaee H., Rao P.S., Reilly G.C. Design and assessment of a dynamic perfusion bioreactor for large bone tissue engineering scaffolds. Appl Biochem Biotechnol. 2018;5:555–563. doi: 10.1007/s12010-017-2671-5. [DOI] [PubMed] [Google Scholar]
- 14.Ni P., Fu S., Fan M., Guo G., Shi S., Peng J. Preparation of poly(ethylene glycol)/polylactide hybrid fibrous scaffolds for bone tissue engineering. Int J Nanomed. 2011;6:3065–3075. doi: 10.2147/IJN.S25297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carnesecchi S., Schneider Y., Ceraline J., Duranton B., Gosse F., Seiler N. Geraniol , a component of plant essential oils , inhibits growth and polyamine biosynthesis in human colon cancer cells. J Pharmacol Exp Therapeut. 2001;298:197–200. [PubMed] [Google Scholar]
- 16.Khan A.Q., Khan R., Qamar W., Lateef A., Rehman M.U., Tahir M. Geraniol attenuates 12-O-tetradecanoylphorbol-13- acetate (TPA)-induced oxidative stress and inflammation in mouse skin: possible role of p38 MAP Kinase and NF-kB. Exp Mol Pathol. 2013;94:419–429. doi: 10.1016/j.yexmp.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Medeiros K.A.A.L., dos Santos J.R., de S., Melo T.C., de Souza M.F., de G. Depressant effect of geraniol on the central nervous system of rats: behavior and Ecog power spectra. Biomed J. 2018;41:298–305. doi: 10.1016/j.bj.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urrutia J., Zamora T., Campos M. Cervical pyogenic spinal infections: are they more severe diseases than infections in other vertebral locations? Eur Spine J. 2013;22:2815–2820. doi: 10.1007/s00586-013-2995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo C.A., Tsai T.T., Lu M.L., Hsieh M.K., Lai P.L., Fu T.S. Factors related to post surgical neurologic improvement for cervical spine infection. Biomed J. 2018;41:306–313. doi: 10.1016/j.bj.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright N.C., Looker A.C., Saag K.G., Curtis J.R., Delzell E.S., Randall S. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520–2526. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen F.P., Huang T.S., Fu T.S., Sun C.C., Chao A.S., Tsai T.L. Secular trends in incidence of osteoporosis in Taiwan: a nationwide population-based study. Biomed J. 2018;41:314–320. doi: 10.1016/j.bj.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butcher N., Balogh Z.J. AIS>2 in at least two body regions: a potential new anatomical definition of polytrauma. Injury. 2012;43:196–199. doi: 10.1016/j.injury.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh C.H., Chen Y.C., Hsu S.Y., Hsieh H.Y., Chien P.C. Defining polytrauma by abbreviated injury scale ≥ 3 for a least two body regions is insufficient in terms of short-term outcome: a cross-sectional study at a level I trauma center. Biomed J. 2018;41:321–327. doi: 10.1016/j.bj.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leguina-Ruzzi A., Ortiz R., Velarde V. The streptozotocin-high fat diet induced diabetic mouse model exhibits severe skin damage and alterations in local lipid mediators. Biomed J. 2018;41:328–332. doi: 10.1016/j.bj.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma T.H., Chen P.H., Tan B.C.M., Lo S.J. Size scaling of nucleolus in Caenorhabditis elegans embryos. Biomed J. 2018;41:333–336. doi: 10.1016/j.bj.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]