Abstract
Background
The customary puerperal practice of Natron consumption has been identified as one of the predisposing factors in the etiology of peripartum cardiomyopathy (PPCM). This study was designed to investigate the effect of Natron in postpartum Wistar albino rats.
Methods
A total of 30 postpartum Wistar rats were exposed to different doses (50 mg/kg, 100 mg/kg, 200 mg/kg and 300 mg/kg) of Natron for 28 days. After the treatment, we carried out biochemical analyses and histological evaluations of kidney, liver and heart.
Results
The study revealed that the exposure of postpartum rats to 100 mg/kg of Natron and above significantly (p < 0.05) increase the cardiac markers; myoglobin, creatine kinase-MB, troponin I and T as compared with control. The result of liver function indicated no significant difference in alanine aminotransferase, aspartate aminotransferase, gamma-glutamyltransferase, albumin and total protein of the Natron treated groups as compared with control. However, at higher doses, the levels of total protein, globulin and alkaline phosphatase activity were significantly increased in comparison to the control. There was no significant difference in the kidney function markers of the treatment groups as compared with control. Histological examinations revealed no changes in the kidney of the treated groups. Mild portal triaditis was observed in the liver of the treated rats. The heart of the rats administered ≥100 mg/kg of Natron showed myocyte hypertrophy.
Conclusion
The study demonstrated that the administration of Natron for 28 days caused changes in the heart of postpartum rats and thus may contribute to the pathogenesis of PPCM.
Keywords: Peripartum cardiomyopathy, Natron, Myocyte hypertrophy, Postpartum, Wistar rat
1. Introduction
Peripartum cardiomyopathy (PPCM) is an infrequent form of dilated cardiomyopathy that causes heart failure occurring typically between the last month of pregnancy and up to five months after giving birth.1 The disease is characterized by systolic dysfunction of the heart with a left ventricular ejection fraction of less than 0.452 which could increase the risk of atrial and ventricular arrhythmias, blockage of blood vessels by clot as well as cardiac death. As a result, the heart muscle cannot contract vehemently enough to supply adequate amounts of blood needed by the vital organs of the body.1, 3, 4, 5 The exact incidence of PPCM is still unknown worldwide, but the accepted incidence is 1 per 3000–4000 births which are more common in West Africa6 and Haiti.7 The disease is common among the Hausa tribe in Nigeria, which accounts for about 13% of all female admissions.8 Studies have shown that the incidence of PPCM varies between 1 in 100 to 1 in 15,000 live births, and this could be ascribed to divergent definitions of PPCM and referral pattern in different nations.9, 10 Unique customary puerperal practices such as postpartum consumption of excess Natron and daily hot water bath for 40 days8, 11 by the Hausa and Fulani women in northern Nigeria have been hypothesized as contributing factors to the incidence of PPCM in Nigeria. Isezuo and Abubakar11 reported that these practices are still common in Sokoto and have been suggested to cause volume overload and heart failure. These puerperal practices could cause the initial accumulation of fluid due to the increase of the peripheral resistance. As a result, the volume expansion overburdens the dilated heart leading to myocardial damage.12
Natron (known as kanwa) is a hydrated sodium carbonate with water of crystallization, which contains traces of sodium chloride and silicon dioxide.13 Different varieties exist which differ in composition with deference to their locations.14 It is also widely used in Nigeria as a cooking ingredient in soups. Natron has been reported for the treatment of various diseases such as skin diseases, reproductive disorders, and stomach pain.15 Therefore, the study was designed to investigate the hypothesis that consumption of Natron as a puerperal practice is a predisposing factor in the pathogenesis of PPCM using Wistar albino rats.
2. Materials and methods
2.1. Chemicals and reagents
Analytical graded chemicals were used for the study. Assay kits were obtained from Agappe Diagnostics Ltd, India and WKEA Med Supplies, China.
2.2. Natron
The Natron was sourced from Sokoto Central Market, Nigeria, pulverized into a fine powder using pestle and mortar, sieved and stored in a clean airtight container until required. It was identified and authenticated at the Department of Chemistry, Usmanu Danfodiyo University, Sokoto, Nigeria. Appropriate measurement of different concentrations per kg body weight was prepared daily for the administration.
2.3. Experimental animals
This study was conducted in accordance with the standard set for the Care and Use of Laboratory Animals. The protocol was approved by the Ethics Committee on Animal Use of the Usmanu Danfodiyo University, Sokoto, Nigeria. The rats were purchased from the Nigerian Institute for Trypanosomiasis Research, Kaduna, Nigeria. The animals were kept in cages under supervision in the animal house with environment temperature and controlled light (21 ± 2 °C, 12 h cycles of light/dark). They were allowed free access to feed and tap water ad libitum before and during the experimental period. The rats were grown into maturity and co-habited with male rats which were separated after the female rats got pregnant. The rats were randomly grouped after delivery.
2.4. Experimental protocol
Thirty postpartum rats (weighing between 160 and 180 g) were used for the study. The animals were randomly divided into 5 groups of 6 rats each and were fed pelletized growers’ feed (Vital Feeds, Jos, Nigeria). Group I served as control and was given distilled water, group II, III, IV and V were administered 50 mg/kg, 100 mg/kg, 200 mg/kg and 300 mg/kg, respectively, of Natron solution by oral gavage using cannula for 4 weeks. At the end of the experimental period, the animals were anesthetized and blood sample was collected for biochemical assays. The rats were then euthanized; the heart, kidney and liver were collected for histological assessment. The blood samples were allowed to clot and centrifuged at 4000 rpm for 5 min and serum was then collected using a Pasteur pipette for biochemical assays.
2.5. Cardiac function biomarkers
The activities of lactate dehydrogenase (LDH), creatine kinase-MB (CK-MB), creatine kinase-NAC (CK-NAC), myoglobin, troponin T and I levels were measured using commercial kits according to the manufacturer’s instructions. CK-MB, LDH and CK-NAC kits were purchased from Agappe Diagnostics Ltd, India. Myoglobin, troponin T and I ELISA kits were products of WKEA Med Supplies, China. Microplate reader (RT6000, Germany) was used for the assay.
2.6. Liver function biomarkers
Serum bilirubin16, alanine transaminases (ALT) and aspartate transaminases (AST)17, alkaline phosphatase (ALP)18, total Protein19 and albumin20 were estimated as described previously. The method of Szasz21 was used for the estimation of gamma-glutamyltransferase (GGT). The parameters were assayed using Semi-auto Chemistry Analyser (RT9200, China)
2.7. Kidney function biomarkers
Serum Urea was estimated according to the method of Young22 while the method of Bartels and Bohmer23 was used for the estimation of serum creatinine by Semi-auto Chemistry Analyser (RT9200, China). Flame photometer (Jenway PFP7, UK) was used for the estimation of sodium and potassium.24 The method relied on the principle of Atomic Emission Spectrophotometry, which the intensity of atomization is measured with a photocell in a selected wavelength range corresponding to the given element. Chloride ion and bicarbonate were determined as described previously.25, 26
2.8. Tissue processing for histopathologic study
The organs (heart, kidney, and liver) of the rats were harvested and preserved in 10% formalin for histologic analysis. The organs were fixed in 10% buffered formalin for 72 h. The tissues were then dehydrated in alcohol of graded concentrations and embedded in paraffin. Embedded tissues were cut into sections of 5 μm thick and stained with hematoxylin and eosin for microscopic assessment. Photomicrographs of the samples were then taken.
2.9. Data analysis
The results are expressed as the mean ± standard error of the mean (SEM) with six rats per group. Data were analyzed using SPSS version 20. The differences between the five groups were compared using one way ANOVA, followed by Turkey multiple comparison tests. p < 0.05 was used as statistically significant.
3. Results
The result of the effect of Natron on cardiac function markers of postpartum rats is presented in Table 1. The result indicated that Natron significantly (p < 0.05) increase the activities of CK-MB and myoglobin, troponin I and troponin T levels at higher doses of the Natron as compared with control. There was no significant difference in the activities of CK-MB and troponin I and T levels at 50 mg/kg of Natron but the myoglobin level was significantly higher in comparison to the control. The activities of lactate dehydrogenase in the Natron exposed groups at a dose of 100 mg/kg and above were higher than the control.
Table 1.
Effect of Natron on Cardiac Function Markers of Postpartum Rats.
| Biomarkers | Groups |
||||
|---|---|---|---|---|---|
| I Control | II 50 mg/kg | III 100 mg/kg | IV 200 mg/kg | V 300 mg/kg | |
| LDH (U/L) | 10.88 ± 0.89a | 10.77 ± 0.90a | 11.27 ± 1.44a | 12.07 ± 0.70a | 14.27 ± 1.37a |
| Myoglobin (ng/mL) | 0.92 ± 0.10a | 1.32 ± 0.04b | 1.77 ± 0.08c | 1.96 ± 0.11c | 3.14 ± 0.14d |
| CK-MB (ng/mL) | 6.60 ± 0.54a | 8.39 ± 1.47a | 13.61 ± 0.44b | 12.49 ± 0.69b | 11.74 ± 0.29b |
| CK-NAC (ng/mL) | 32.41 ± 2.25a | 33.80 ± 4.91a | 45.06 ± 2.84b | 43.43 ± 3.35b | 47.55 ± 1.86b |
| TnI (ng/mL) | 1.42 ± 0.03a | 1.45 ± 0.06a | 1.50 ± 0.09b | 1.70 ± 0.07b | 1.77 ± 0.07c |
| TnT (μg/L) | 25.75 ± 0.82a | 25.55 ± 0.90a | 28.68 ± 0.14b | 30.87 ± 0.36c | 34.99 ± 0.75d |
Values are expressed as mean ± Standard Error of Mean (n = 6). Mean value having different superscript letters in rows are significantly different (p < 0.05).
LDH = Lactate Dehydrogenase; CK-MB = Creatinine Kinase (Isoform); CK-NAC = Creatinine Kinase (N- Acetyl Cysteine); TnI = Troponin I; TnT = Troponin T.
The result of the effect of Natron on kidney function parameters of postpartum rats is presented in Table 2. The result showed no significant difference in urea, creatinine, and electrolytes of the exposed groups as compared with control.
Table 2.
Effect of Natron on Kidney Function Parameters of Postpartum Rats.
| Parameters | Groups |
||||
|---|---|---|---|---|---|
| I Control | II 50 mg/kg | III 100 mg/kg | IV 200 mg/kg | V 300 mg/kg | |
| Urea (mg/dL) | 5.57 ± 0.36 | 4.75 ± 0.42 | 5.23 ± 0.49 | 4.35 ± 0.25 | 5.31 ± 0.54 |
| Creatinine (mg/dL) | 1.03 ± 0.43 | 0.98 ± 0.23 | 1.08 ± 0.08 | 1.09 ± 0.05 | 1.21 ± 0.04 |
| Na+ (mMol/L) | 133.50 ± 0.56 | 133.83 ± 0.94 | 132.67 ± 1.65 | 133.83 ± 1.74 | 133.73 ± 1.19 |
| K+ (mMol/L) | 4.02 ± 0.13 | 4.12 ± 0.19 | 3.92 ± 0.09 | 4.12 ± 0.02 | 4.12 ± 0.18 |
| Cl− (mMol/L) HCO3− (mMol/L) |
94.67 ± 1.09 25.33 ± 1.33 |
94.17 ± 1.01 23.50 ± 0.85 |
94.83 ± 1.19 24.50 ± 1.20 |
96.00 ± 1.71 23.17 ± 0.70 |
96.00 ± 0.68 23.67 ± 0.76 |
Values are expressed as mean ± Standard Error of Mean (n = 6). Mean values of all the parameters are not statistically different as compared with control at p < 0.05. Na+-sodium ion, K+-potassium ion, Cl− − chloride ion, HCO3− − bicarbonate.
The result of the effect of Natron on liver function biomarkers of postpartum rats is presented in Table 3. The result indicated no significant difference was observed in AST, ALT, Albumin, GGT and total bilirubin of treatment groups as compared with control. The treatments with ≥100 mg/kg significantly (p < 0.05) increase the activity of ALP. The levels of total protein and globulin were also increased at high doses of Natron.
Table 3.
Effect of Natron on Liver Function Biomarkers of Postpartum Rats.
| Biomarkers | Groups |
||||
|---|---|---|---|---|---|
| I Control | II 50 mg/kg | III 100 mg/kg | IV 200 mg/kg | V 300 mg/kg | |
| AST (U/L) | 191.11 ± 1.15a | 190.81 ± 0.79a | 193.03 ± 0.78a | 192.16 ± 1.00a | 193.27 ± 1.54a |
| ALT (U/L) | 170.65 ± 1.68a | 170.17 ± 1.16a | 170.19 ± 2.21a | 171.07 ± 2.41a | 169.32 ± 1.57a |
| ALP (U/L) | 535.65 ± 1.46a | 535.66 ± 3.88a | 568.79 ± 2.09b | 564.66 ± 1.74b | 569.16 ± 3.36b |
| TP (g/dL) | 6.16 ± 0.43a | 6.36 ± 0.26a | 7.84 ± 0.23b | 7.05 ± 0.28a | 7.56 ± 0.23b |
| ALB (g/dL) | 2.64 ± 0.06a | 2.62 ± 0.07a | 2.71 ± 0.04a | 2.57 ± 0.06a | 2.67 ± 0.05a |
| GLO (g/dL) | 3.53 ± 0.41a | 3.74 ± 0.22a | 5.13 ± 0.24b | 4.48 ± 0.31a | 4.90 ± 0.27b |
| TBIL (mg/dL) | 0.35 ± 0.04a | 0.32 ± 0.08a | 0.31 ± 0.06a | 0.23 ± 0.06a | 0.22 ± 0.03a |
| GGT (U/L) | 0.23 ± 0.12a | 0.03 ± 0.01a | 0.30 ± 0.20a | 0.17 ± 0.11a | 0.34 ± 0.16a |
Values are expressed as mean ± Standard Error of Mean (n = 6). Mean Value Having Different Superscript Letters in rows are significantly different (p < 0.05).
ALT = Alanine Amino Transferase; AST = Aspartate Amino Transferase; ALP = Alkaline Phosphatase; TP = Total Protein; ALB = Albumin; GLO = Globulin; TBIL = Total Bilirubin; GGT = ɣ-Glutamyl Transferase.
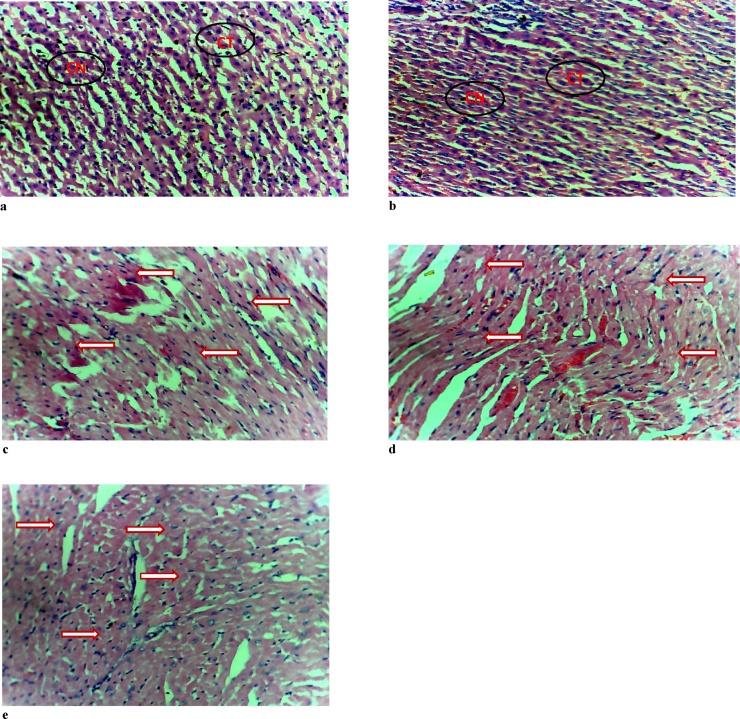
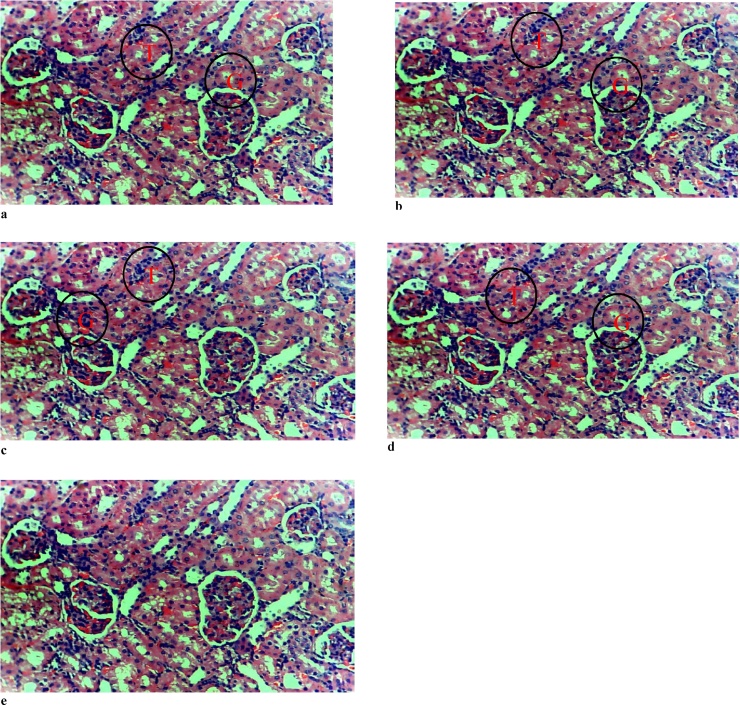
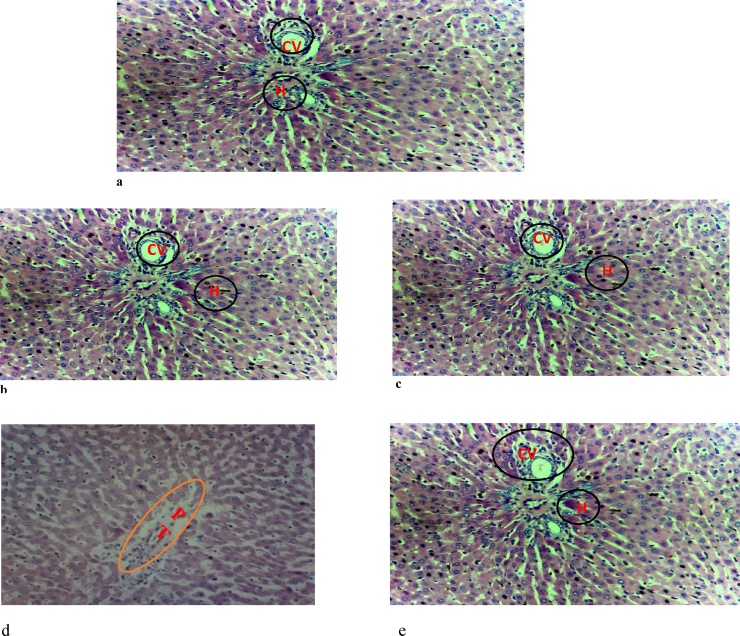
The results of the histology of the heart, liver, and kidney are presented in Fig. 1, Fig. 2, Fig. 3, respectively. The heart of the rats exposed to ≥100 mg/kg body weight of Natron showed myocyte hypertrophy. There was no effect of the administered substance on the kidney of the postpartum rats. However, mild to moderate portal triaditis was observed in the liver, which was more pronounced in the group administered 200 mg/kg of Natron.
Fig. 1.
Photomicrograph of the Heart.1
1(a) and (b) control and group administered 50 mg/kg of Natron showing normal histologic structures of heart cells and connective tissues. (c), (d) and (e) are groups administered 100, 200 and 300 mg/kg of Natron, respectively with arrows showing myocyte hypertrophy. Hematoxylin & Eosin ×20. CT- connective tissue, CN-cell nucleus.
Fig. 2.
Photomicrograph of the Kidney.2
2(a) control (b) group administered 50 mg/kg of Natron (c) group administered 100 mg/kg of Natron (d) group administered 200 mg/kg of Natron (e) group administered 300 mg/kg showing normal glomeruli and renal tubule. Hematoxylin & Eosin ×20. G-glomerulus, T-renal tubule.
Fig. 3.
Photomicrograph of the Liver.3
3(a) control (b) group administered 50 mg/kg of Natron (c) group administered 100 mg/kg of Natron showing the central vein and normal hepatocytes (d) group administered 200 mg/kg showing mild portal triaditis (e) group administered 300 mg/kg of Natron showing the central vein and normal hepatocytes. Hematoxylin & Eosin ×20. CV-central vein, H-hepatocyte, PT- portal triaditis.
4. Discussion
Peripartum cardiomyopathy is uncommon, potentially life-threatening disease, which presents with symptoms of heart failure mainly due to left ventricular systolic dysfunction.27 This occurs in women of childbearing age with no preexisting heart disease during the last month of pregnancy or in the first five months postpartum.5 In this study, the effect of Natron at different doses was investigated in postpartum rats after 28 days treatment. Exposure of postpartum rats to the Natron increased myoglobin, troponin I and T levels and creatine kinase-MB and creatine kinase-NAC activities. These markers, especially cardiac troponins are intricate intracellular contractile-regulating proteins of the heart muscles that are important for calcium-mediated regulation of heart muscle contraction.28 Elevated levels of troponin I and T could reflect a direct effect of Natron that subsequently leads to myocyte hypertrophy observed in our study as shown by the results of heart histology. The increase activities of CK release from the myocardium may be associated with membrane damage or left ventricular hypertrophy, which could be secondary to inflammatory responses due to Natron assault. The progressive loss of myocyte through apoptotic or necrotic cell death has been recognized as a key mechanistic pathophysiology in the progression of heart dysfunction in dilated cardiomyopathy and heart failure.29 The finding of this study is consistent with our earlier study of which administration of dried lake salt (kanwa) to postpartum rats caused cardiac chamber dilation, hypertrophy and focal atrophy.30 High circulating cardiac troponin I (cTnI) have been shown to be associated with larger left ventricular, left atrial and right ventricular diameters which correlated with higher mortality rates in idiopathic dilated cardiomyopathy, demonstrating a link between cTnI levels and cardiac remodeling.28 PPCM shared similar clinical characteristics as dilated cardiomyopathy, such as ventricle dilation and systolic dysfunction, but the former is thought to be dilated cardiomyopathy activated by pregnancy.31 During pregnancy, physiological changes due to the hemodynamic load caused by the rise in the blood volume bring about physiological hypertrophy. Consequently, the chamber dimensions would increase blood volume with a compensatory increase in wall thickness to handle the increased hemodynamic load and to ease cardiac output.32 These physiological changes in PPCM differ because an increase in chamber dimension does not increase the wall thickness proportionately in response to increased hemodynamic load.33 Therefore, it is evident from this study that Natron could cause volume overload without a compensatory increase in wall thickness and couples with oxidative stress is probably the mechanism underlying the changes observed in the Natron treated rats. Increased oxidative stress levels have been reported in both animal models and human PPCM patients.34
The measurement of activities of various enzymes in the tissues and body fluids plays a significant role in disease investigation and diagnosis.35 ALT, AST, and ALP are biomarkers used to predict hepatic injury. Although ALT is a more specific marker of liver damage. The serum levels of ALT, AST, and ALP are usually elevated in conditions associated with injuries or diseases affecting the liver, which leads to the release of these hepatocellular enzymes into the bloodstream.36 The significant increase in total protein, globulin and ALP activities due to Natron might be due to mild portal triaditis observed in the liver as revealed by the result of histology. Furthermore, increased in ALP activities could also be from the circulating placental ALP isoform or the tissue non-specific isoform. The histological analysis of the kidneys revealed no changes due to Natron treatment as glomeruli and renal tubule were normal just as the control. Treatment of postpartum rats with Natron caused significant alterations in the heart as evidenced by the results of biochemical and histological analyses.
5. Conclusion
The result of this study revealed that the exposure of postpartum rats to Natron for 28 days caused myocyte hypertrophy and changes in the heart biomarkers. It can be concluded that consumption of Natron at a higher dose may be a predisposing factor in the pathogenesis of PPCM.
Conflict of interest
The authors have not declared any conflict of interest.
Acknowledgement
The authors thank TETFUND and Federal University, Birnin Kebbi, for providing the grant used for this work.
References
- 1.Sliwa K., Fett J., Elkayam U. Peripartum cardiomyopathy. Lancet. 2006;368:687–693. doi: 10.1016/S0140-6736(06)69253-2. [DOI] [PubMed] [Google Scholar]
- 2.Hibbard J.U., Lindheimer M., Lang R.M. A modified definition for peripartum cardiomyopathy and prognosisbased on echocardiography. Obstet Gynecol. 1999;94:311–316. doi: 10.1016/s0029-7844(99)00293-8. [DOI] [PubMed] [Google Scholar]
- 3.Elkayam U., Akhter M.W., Singh H.S. Pregnancy-associated cardiomyopathy:clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111:2050–2055. doi: 10.1161/01.CIR.0000162478.36652.7E. [DOI] [PubMed] [Google Scholar]
- 4.Murali S., Baldisseri M.R. Peripartum cardiomyopathy. Crit Care Med. 2005;33:340–346. doi: 10.1097/01.ccm.0000183500.47273.8e. [DOI] [PubMed] [Google Scholar]
- 5.Pearson G.D., Veille J.C., Rahimtoola S. Peripartum cardiomyopathy: national heart, lung, and blood institute and office of rare diseases (National Institutes of Health) workshop recommendations and review. J Am Med Assoc. 2000;28:1183–1188. doi: 10.1001/jama.283.9.1183. [DOI] [PubMed] [Google Scholar]
- 6.Ferriere M., Sacrez A., Bouhour J.B. Cardiomyopathy in the peripartum period: current aspects. A multicenter study. 11 cases. Arch. Mal Coeur Vaiss. 1990;83:1563–1569. [PubMed] [Google Scholar]
- 7.Cenac A., Djibo A. Postpartum cardiac failure in Sudanese-Sahelian Africa: clinical prevalence in western Niger. Am J Trop Med Hyg. 1998;58:319–323. doi: 10.4269/ajtmh.1998.58.319. [DOI] [PubMed] [Google Scholar]
- 8.Davidson N.M., Parry E.H.O. Peripartum cardiac failure. Q J Med. 1978;47:431–461. [PubMed] [Google Scholar]
- 9.Lampert M.B., Lang R.M. Peripartum cardiomyopathy. Am Heart J. 1995;130:860–870. doi: 10.1016/0002-8703(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 10.Demakis J.G., Rahimtoola S.H., Sutton G.C. Natural course of peripartum cardiomyopathy. Circulation. 1971;44:1053–1061. doi: 10.1161/01.cir.44.6.1053. [DOI] [PubMed] [Google Scholar]
- 11.Isezuo A.S., Abubakar A.S. Epidemiology of peripartum cardiomyopathy. Ethn Dis. 2007;17:228–233. [PubMed] [Google Scholar]
- 12.Sanderson J.E., Adesanya C.O., Anjorin F.I., Parry E.H.O. Post-partum cardiac failure −heart failure due to cardiac overload. Am Heart J. 1979;97:613–621. doi: 10.1016/0002-8703(79)90189-3. [DOI] [PubMed] [Google Scholar]
- 13.Ekosse G.E. X-ray diffraction study of kanwa used as active ingredient in achu soup in Cameroon. Afr J Biotechnol. 2010;9:7928–7929. [Google Scholar]
- 14.Ekanem E.J., Harrison G.F.S. Assessment of Lake salt for sourcing dietary minerals. Niger J Chem Res. 1997;2:33–38. [Google Scholar]
- 15.Alawa C.B.I., Adamu A.M., Ehoche O.W., Lamidi O.S., Oni O.O. Performance of Bunaji Bulls fed maize stover supplemented with urea and local mineral lick (Kanwa) J Agric Environ. 2000;1:35–42. [Google Scholar]
- 16.Jendrassik L., Grof P. Simplified photometric methods for the determination of bilirubin. Biochem Zchr. 1938;297:81–89. [Google Scholar]
- 17.Reitman S., Frankel S. A calorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 18.Sood R. Jaypee Brothers Medical Publishers Ltd; New Delhi: 1999. Medical Laboratory Technology. Method and Interpretation; p. 488. [Google Scholar]
- 19.Lowry O.H., Rosenbrough N.T., Farr A.L., Randall R.J. Protein measurements with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Doumas B.T., Watson W., Briggs H.G. Albumin standards and the measurement of serum albumin with bromocresol green. Clin Chim Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 21.Szasz G.U.M.Z. New substrates for measuring gamma-glutamyl transpeptidase activity. Z Klin Chem Biochem. 1974;12:228. [PubMed] [Google Scholar]
- 22.Young D.S. Effects of drugs on clinical laboratory test. Ann Clin Biochem. 1997;34:579–581. doi: 10.1177/000456329703400601. [DOI] [PubMed] [Google Scholar]
- 23.Bartels H., Bohmer M. Micro determination of creatinine. Clin Chim Acta. 1971;32:81–85. doi: 10.1016/0009-8981(71)90467-0. [DOI] [PubMed] [Google Scholar]
- 24.Willebrands A.F. The determination of sodium and potassium in blood serum and urine by means of the flame photometer. Rec Trav Chim Pays Bas. 1950;69:799–821. [Google Scholar]
- 25.Gerlach J.L., Frazier R.G. Spectrophotometric determination of chloride in sweat and serum with diphenylcarbazone. Anal Chem. 1958;30:1142–1146. [Google Scholar]
- 26.Scribner B.H., Caillouette J.C. Improved method for the bedside determination of bicarbonate in serum. JAMA. 1954;155:644–648. doi: 10.1001/jama.1954.03690250024008. [DOI] [PubMed] [Google Scholar]
- 27.Pyatt J.R., Dubey G. Peripartum cardiomyopathy: current understanding, comprehensive management review and new developments. Postgrad Med J. 2011;87:34–39. doi: 10.1136/pgmj.2009.096594. [DOI] [PubMed] [Google Scholar]
- 28.Li X., Luo R., Jiang R. The prognostic use of serum concentrations of cardiac troponin-I, CK-MB and myoglobin in patients with idiopathic dilated cardiomyopathy. Heart Lung: J Acute Crit Care. 2014;43:219–224. doi: 10.1016/j.hrtlng.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Logeart D., Beyne P., Cusson C. Evidence of cardiac myolysis in severe nonischemic heart failure and the potential role of increased wall strain. Am Heart J. 2001;141:247–253. doi: 10.1067/mhj.2001.111767. [DOI] [PubMed] [Google Scholar]
- 30.Muhammad A.S., Saidu Y., Bilbis L.S., Onu A., Isezuo S.A., Sahabi S. Effect of dried lake salt (Kanwa) on lipid profile and heart histology of female albino rats. Nig J Basic Appl Sci. 2014;22:73–78. [Google Scholar]
- 31.Bollen I.A., Van Deel E.D., Kuster D.W., Van Der Velden J. Peripartum cardiomyopathy and dilated cardiomyopathy: different at heart. Front Physiol. 2015;5:531. doi: 10.3389/fphys.2014.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umar S., Nadadur R., Iorga A., Amjedi M., Matori H., Eghbali M. Cardiac structural and hemodynamic changes associated with physiological heart hypertrophy of pregnancy are reversed postpartum. J Appl Physiol. 2012;113:1253–1259. doi: 10.1152/japplphysiol.00549.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaasch W.H., Zile M.R. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol. 2011;58:1733–1740. doi: 10.1016/j.jacc.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 34.Hilfiker-Kleiner D., Kaminski K., Podewski E. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 35.Malomo S.O. Toxicological implication of ceftriaxone administration in rats. Niger J Biochem Mol Biol. 2000;15:33–38. [Google Scholar]
- 36.Pagana K.D., Pagana T.J. St Louis, Mosby Elsevier; USA: 2006. Mosby’s Manual of Diagnostic and Laboratory Tests; p. 40. [Google Scholar]