Abstract
Geraniol is a monoterpene alcohol that is derived from the essential oils of aromatic plants, with anti-inflammatory, antimicrobial, antioxidant and neuroprotective properties. This study characterized the effect of geraniol on behavior and brainwave patterns in rats. Male rats were submitted to administration of geraniol (25, 50 and 100 mg/kg). The hole board (HB) and open field (OF) tests were performed to evaluate anxiety and motor behavior, respectively. In addition, barbiturate-induced sleeping time (BIST) was used to analyze sedative effect. Finally, electrocorticogram (ECoG) recordings were used to characterize brain-wave patterns. The results showed that geraniol treatment in rats decreased the distance traveled, rearing numbers and lead to increase in immobility time in HB and OF tests. In BIST test, geraniol treatment increased sleep duration but not sleep latency in the animals. Furthermore, geraniol-treated animals demonstrated an increase in the percentage of delta waves in the total spectrum power. Taken together, our results suggested that geraniol exerted a depressant effect on the central nervous system of rats.
Keywords: Sedation, Monoterpene, Brain waves, Hypnose, Natural product
At a glance commentary
Scientific background on the subject
Geraniol is an important constituent of essential oils. Some studies have reported geraniol effects on the central nervous system in invertebrates and vertebrates, like neuroprotective, anti-inflammatory, neuroprotective and antidepressant effects. Its depressant effect has not been reported before.
What this study adds to the field
Many monoterpenes have depressant, sedative–hypnotic and anxiolytic effects. Given to this, we hypothesized that geraniol has also a depressant effect on the central nervous system. Therefore, this study aimed to investigate the central effects of geraniol on rat behavior associated with electrocorticogram (ECoG) power spectra.
Geraniol is an important constituent of essential oils of ginger, lemon, lime, lavender, nutmeg, orange, rose, etc. It is the main acyclic monoterpenoid of several aromatic herb oils [1] and isolated from Rosa damascene [2], Monarda fistulosa [3], Dracocephalum moldavica [4] and Cymbopogon winterianus [5]. This substance is widely used in perfumes and toilet solutions owing to its rose-like aroma [6].
Some studies have reported geraniol effects on the central nervous system (CNS) in invertebrates and vertebrates. Geraniol showed neuroprotective effect against acrylamide-induced oxidative stress, mitochondrial dysfunction and neurotoxicity in a model using the fruit fly [7]. Geraniol was also neuroprotective in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced mice model of Parkinsonism, i.e., geraniol was capable of traversing the cellular membrane and suppressed MPP+ (a toxic metabolite from MPTP)-induced lipid peroxidation and mRNA expressions [8]. In another study, geraniol pretreatment improved neuromuscular impairment, tyrosine hydroxylase expression and decreased α-synuclein expression in MPTP-intoxicated Parkinsonian mice, in a dose dependent manner [9]. In both studies there was improvement of motor deficits in the animals pre-treated with geraniol.
Relevant to the present study, geraniol showed antidepressant effect in mice exposed to a chronic unpredictable mild stress model. Animals treated with geraniol for three weeks showed attenuation of the depression-related behaviors [10]. Futhermore, it was demonstrated that geraniol is a probable anticonvulsant. In this study was seen that geraniol increased the latency for the first pentylenetetrazole-induced convulsion and protected the animals against seizures. Pentylenetetrazole is a competitive GABAA receptor antagonist [11].
However, its depressant effect has not been reported before. Many monoterpenes have depressant, sedative–hypnotic and anxiolytic effect. The aqueous extracts of peanut stems and leaves had linalool (a monoterpene) as one of the main constituents. This extract had a sedative−hypnotic effect by affecting neuro-transmitter levels in mice in the cerebrum, brain stem and cerebellum [12]. Isopulegol, neoisopulegol, (±)-isopinocampheol, (−)-myrtenol, (+)-cis-myrtanol, (+)-p-menth-1-en-9-ol and (±)-neomenthol also showed a depressant effect in the pentobarbital-induced sleep test, indicating a sedative property [13].
Given this, we hypothesized that geraniol has also a depressant effect on the central nervous system. Therefore, this study aimed to investigate the central effects of geraniol on rat behavior associated with electrocorticogram (ECoG) power spectra.
Material and methods
Animals
Wistar rats (3–4 months) were randomly housed in appropriate cages with free access to food and water, under controlled conditions of temperature (22 ± 1 °C) and a 12 h light/dark cycle (lights on 06:00 a.m.). The animals' age (90–120 days) and route of administration choices were based on previous studies that performed behavioral tests and ECoG in Wistar rats, with or without pretreatment [14], [15], [16], [17], [18], [19], [20], [21]. Animals were handled according to the Brazilian law for the use of animals in scientific research (Law number 11.794). Experimental protocols and all procedures described were approved by local ethical committee (CEPA/UFS Nº 26/2013). All efforts were made to minimize animal pain, suffering, or discomfort during treatment.
Drugs
Diazepam (DZP, Teuto, Anápolis, Brazil), thiopental (Cristália, Itapira, Brazil), polyoxyethylene-sorbitan monolated (Tween 80, Synth, Diadema, Brazil) and geraniol (98%, Sigma Chemical Co. USA) were diluted in saline solution and given intraperitoneally (i.p.) at 1 ml/kg.
Hole-board test
The hole-board set-up apparatus is a wooden box (66 × 56 × 47 cm) with sixteen equidistant holes (3 cm diameter) on the floor. Animals were randomly assigned to one of the following groups: a control group that received vehicle (saline, n = 7); three experimental groups that received 25, 50 or 100 mg/kg (n = 15–16) of geraniol; and a DZP group (0.5 mg/kg, n = 7). After 30 minutes, each rat was placed in the inferior right corner of the hole-board and was recorded for 5 minutes. Below the surface of the holes, a sensor counted the head-dippings duration (number of head dives). The evaluated parameters were distance traveled, immobility time, rearings numbers and head-dippings duration. After each trial, the floor of the apparatus was carefully cleaned to remove traces of previous paths.
Open field test
The open field test consists of a circular apparatus (81 cm diameter and 65 cm tall), made of wood and painted black. Rats were randomly assigned to one of five groups: SLN (saline 0.9%, n = 11), DZP 1.5 mg/kg (anxiolytic positive control, n = 7), DZP 5.0 mg/kg (sedative positive control, n = 8), geraniol 25, 50 or 100 mg/kg (n = 6–8). After 30 minutes, each rat was placed in the center of the open field by free exploration (5 min). Distance traveled in the whole arena (m), immobility time (s) and rearing frequency were evaluated.
Barbiturate-induced sleeping time (BIST)
Animals were assigned to one of three groups: saline (n = 12), geraniol 100 mg/kg (n = 12) and DZP 5.0 mg/kg (n = 14). After 30 minutes, each rat was administered with thiopental (60 mg/kg)and placed in a box. For each animal was evaluated the time to loss (latency) and recovery (sleeping time) of the righting reflex.
Surgical procedure
Prior to surgery, rats were anesthetized intraperitoneal with ketamine (100 mg/kg) plus xylazine (10 mg/kg). After verification of the anesthetic state, the animal was placed in stereotaxic frame (Insight, Ribeirão Preto, Brazil). Local anesthetic (0.1 mL of lidocaine 2%) was injected percutaneously to the exposed tissue of the head. Three holes were created to implant the electrode sets using the following stereotaxic coordinates from bregma: frontal (AP: + 2 mm; ML: +1.5 mm), parietal (AP: − 4.3 mm; ML: − 2 mm) and occipital (AP: − 10.1 mm; ML: +1.5 mm). Three screws (stainless steel annealed) were implanted in three cortical areas epidurally and served as electrodes. They were connected to pin with a small insert, where the data collection wires were inserted. The electrodes were anchored to the skull with dental acrylic. Animals were given 5–7 days of post-operative recovery prior to the start of the experimental proceedings.
Electrocorticogram data acquisition and processing
Electrocorticogram (ECoG) recordings were obtained with the animal awake after the administration of geraniol (100 mg/kg) during 1 hour. Signals were amplified. Digitalization (sampling rate 1000 Hz) and data recording were done using a Windaq (Dataq Instruments) acquisition system. ECoG recordings were inspected visually and artifact-containing EEGs were discarded before and during analysis.
Analysis ECoG data
After recording, raw ECoG was divided into eleven intervals of 5 minutes each. Power spectrum analysis of ECoG was performed using MATLAB code (MathWorks Inc.), which computes power by pwelch function. The digital values of power spectra of four major bandwidths of ECoG – delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–13 Hz) and beta (13–30 Hz) – were obtained. The power of ECoG waves was expressed in percentage of total spectrum power.
Statistical analysis
Data normality and homogeneity of variances were tested by Shapiro–Wilk and Levene tests. For the behavioral data, one-way ANOVA was used followed by Fisher's LSD test for multiple comparisons. Two-way ANOVA followed by Bonferroni's multiple comparisons test was performed on the ECoG data. All statistical analyses were conducted with Statistica 8.0 and Graphpad Prism 5.0 software. Values of p < 0.05 were accepted as significant.
Results
Effects of geraniol on behavior
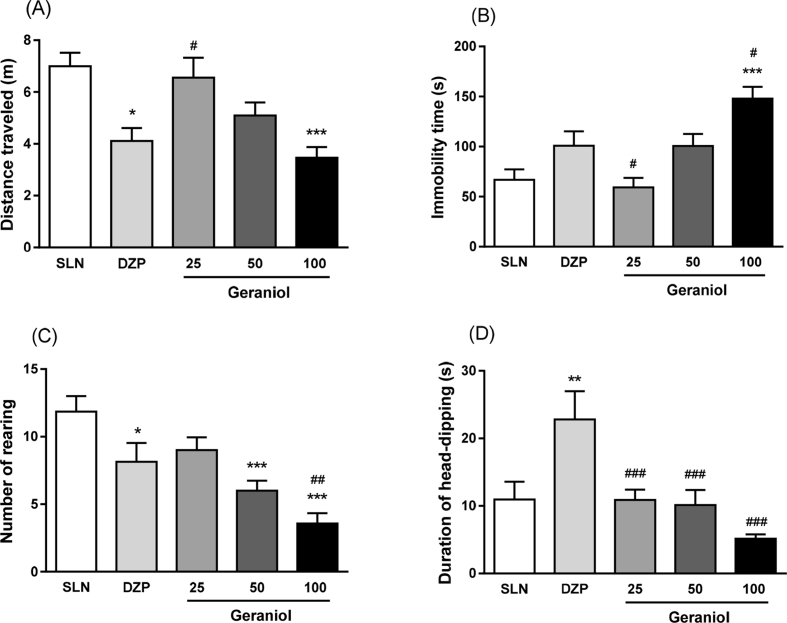
For hole-board test analysis, one-way analysis of variance (ANOVA) revealed significant effect of treatment for all parameters: distance traveled [F(4,56) = 5.94, p < 0.001], immobility time [F(4,56) = 9.99, p < 0.001), rearing number [F(4,56) = 10.02, p < 0.001] and head-dipping duration [F(4.56) = 7.92, p < 0.001]. Fisher's LSD post-hoc test showed that the geraniol (100 mg/kg) and DZP 0.5 groups decreased the distance traveled when compared to saline group (p = 0.014; p < 0.001, respectively) [Fig. 1A]. Furthermore, immobility time was higher in geranioltreated animals (100 mg/kg) when compared with saline group (p < 0.001) [Fig. 1B]. In addition, geraniol (25 and 50 mg/kg) treatment decreased immobility time compared with DZP-treated animals (p = 0.033; p = 0.017, respectively). In relation to rearing number, geraniol-treated animals (50 and 100 mg/kg) showed a decrease in the frequency when compared with saline(p < 0.001; p < 0.001, respectively) and DZP 0.5 (p = 0.004) groups [Fig. 1C]. For head-dipping duration, animals receiving geraniol (100 mg/kg) showed a significant reduction when compared with DZP-treated rats (p < 0.001). However, DZP group (0.5 mg/kg) had a increase when compared with saline (p = 0.002) and geraniol, at 25 mg/kg (p < 0.001), 50 mg/kg (p < 0.001) and 100 mg/kg (p < 0.001), groups.
Fig. 1.
Effect of geraniol (25, 50 and 100 mg/kg) in rats submitted to hole-board test. (A) Distance traveled, (B) immobility time, (C) number of rearing and (D) duration of head-dipping. One way ANOVA followed by Fisher's LSD test for multiple comparisons (*p < 0.05, **p < 0.01 and ***p < 0.001 compared with control group; #p < 0.05, ##p < 0.01 and ###p < 0.001 compared with DZP (0.5 mg/kg) group. Data expressed as mean ± SEM. Abbreviations: SLN: saline; DZP: diazepam. Experimental groups: SLN (n = 7), DZP 0.5 mg/kg (n = 7), geraniol 25 (n = 16), 50 (n = 15) and 100 mg/kg (n = 16).
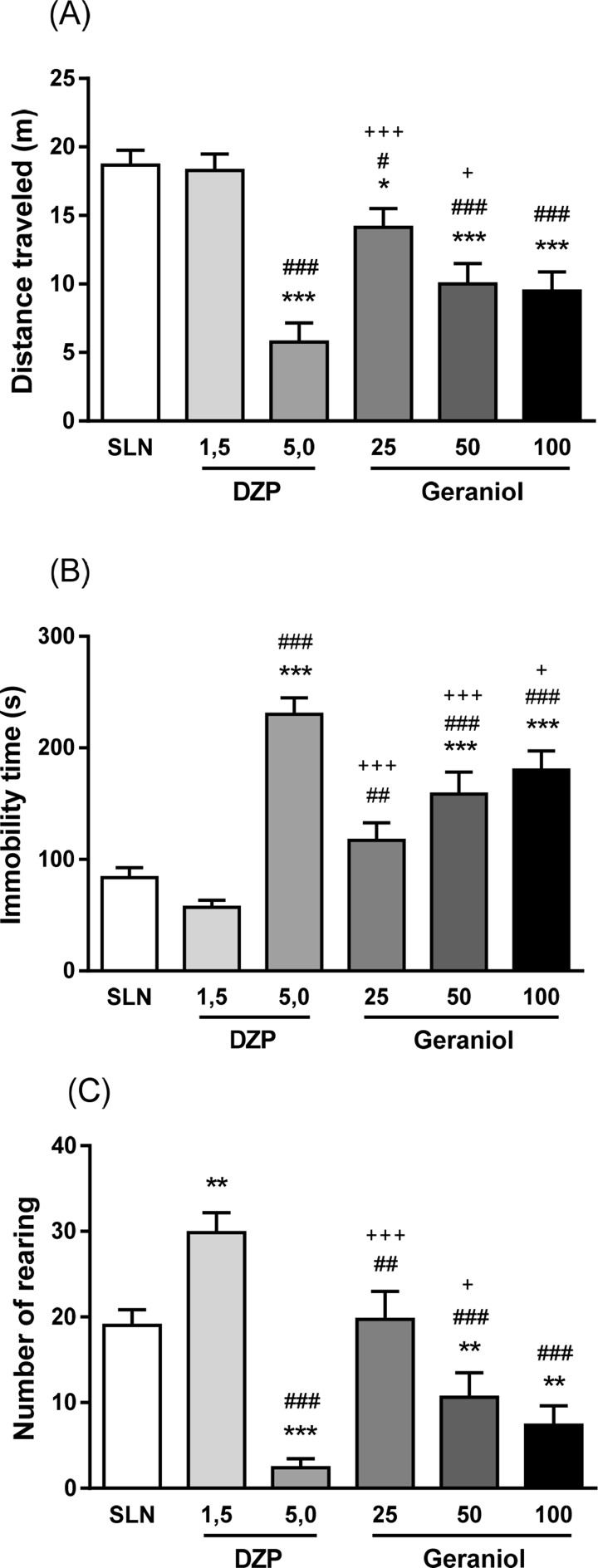
In the open field test, one way ANOVA showed differences in all parameters: distance traveled [F(5,41) = 16.39; p < 0.001], immobility time [F(5,41) = 20.54; p < 0.001] and rearing number [F(5,41) = 17.40; p < 0.001). Post hoc analysis revealed a decrease in the distance traveled on geraniol (25, 50 and 100 mg/kg) and DZP (5.0 mg/kg) when compared with saline (p = 0.015; p < 0.001; p < 0.001; p < 0.001, respectively) and diazepam 1.5 mg/kg (DZP 1.5) groups (p < 0.001; p = 0.042; p < 0.001; p < 0.001, respectively). Geraniol-treated animals (25 and 50 mg/kg) also showed reduction in comparison to DZP 5.0 group (p < 0.001; p = 0.027, respectively). However, DZP 5.0 and geraniol (50 and 100 mg/kg) groups showed an increase in immobility time when compared with saline group (p < 0.001; p < 0.001; p < 0.001, respectively) [Fig. 2B]. All geraniol-treated animals increased the immobility time compared with DZP 1.5 (p = 0.007; p < 0.001; p < 0.001, respectively) and DZP 5.0 (p < 0.001; p < 0.001; p < 0.025, respectively).
Fig. 2.
Effect of geraniol (25, 50 and 100 mg/kg) in rats submitted to open field test. (A) Distance traveled, (B) immobility time and (C) number of rearing. One way ANOVA followed by Fisher's LSD test for multiple comparisons (*p < 0.05, **p < 0.01 and ***p < 0.001 compared with control group; #p < 0.05, ##p < 0.01 and ###p < 0.001 compared with DZP (1.5 mg/kg) group; +p < 0.05, ++p < 0.01 and +++p < 0.001 compared with DZP (5.0 mg/kg) group. Data expressed as mean ± SEM. Abbreviations: SLN: saline; DZP: diazepam. Experimental groups: SLN (n = 11), DZP (n = 7) e 5.0 mg/kg (n = 8), geraniol 25 (n = 7), 50 (n = 8) e 100 mg/kg (n = 6).
For the rearing number, the Fisher's LSD test revealed decrease for DZP 5.0 and geraniol (50 and 100 mg/kg) groups (p < 0.001; p = 0.008; p = 0.001, respectively), however an increase for DZP 1.5 group (p = 0.001), when compared with saline group. Geraniol treatment (25, 50 and 100 mg/kg) and DZP (5.0 mg/kg) decreased the rearing number compared with DZP-treated animals (1.5 mg/kg) (p = 0.005; p < 0.001; p < 0.001; p < 0.001, respectively). Geraniol-treated rats (25 and 50 mg/kg) showed higher rearing frequency when compared with DZP 5.0 group (p < 0.001; p = 0.015, respectively).
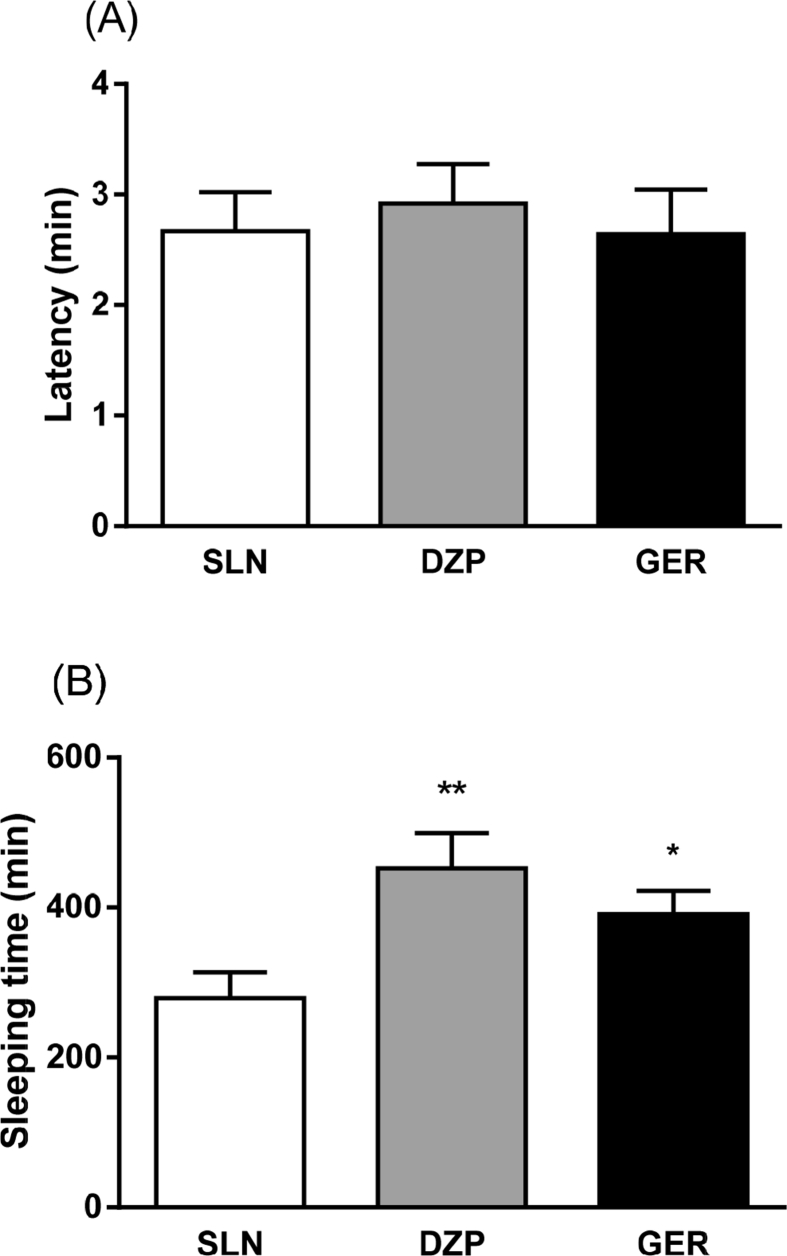
For barbiturate-induced sleeping time [Fig. 3], no differences were found in sleep latency (p = 0.873). However, one way ANOVA showed alterations in sleep time [F(2350 = 5.16; p = 0.011]. Fisher's LSD test revealed increase in the duration of sleeping time induced by thiopental in animals pre-treated with DZP 5.0 (p = 0.003) and geraniol (100 mg/kg) (p = 0.040).
Fig. 3.
Effect of geraniol (100 mg/kg) and diazepam (5 mg/kg) in rats in relation to (A) latency and (B) barbiturate-induced sleeping time (60 mg/kg). One way ANOVA followed by Fisher's LSD test for multiple comparisons (*p < 0.05 and **p < 0.01 compared with control group). Data expressed as mean ± SEM. Abbreviations: SLN: saline; DZP: diazepam; GER: geraniol. Experimental groups: SLN (n = 12), DZP 5.0 mg/kg (n = 14), geraniol 100 mg/kg (n = 12).
Effect of geraniol in ECoG power spectra
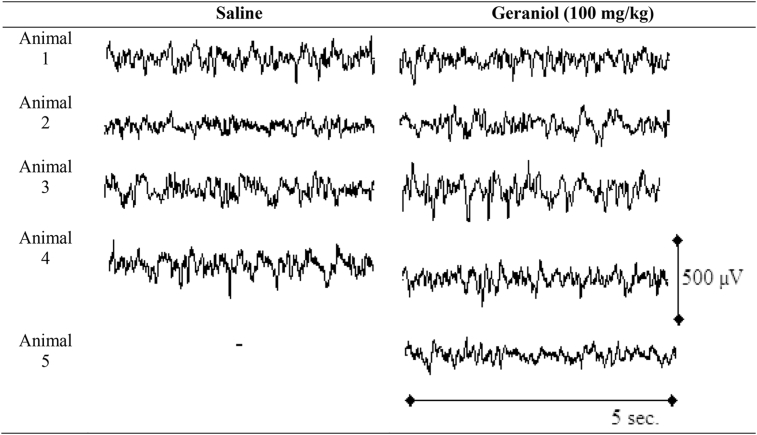
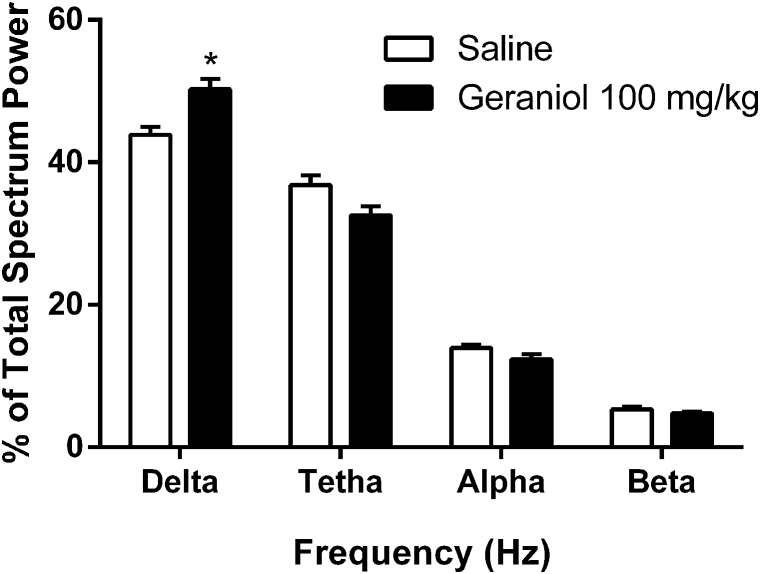
Systemic administration of geraniol (100 mg/kg) changed the ECoG power spectra of rats. Raw ECoG samples for each animal are shown in Table 1. Indeed, ANOVA showed that there are differences among the four bandwidths [F(3.28) = 758.2; p < 0.001] and interaction effects [F(3.28) = 10.50; p < 0.001]. Bonferroni's test showed that delta waves was bigger (p < 0.001), and theta was smaller (p = 0.021), in the animals treated with geraniol [Fig. 4]. No alterations were observed in other bandwidths (alpha and beta).
Table 1.
Raw ECoG for each animal treated with saline or geraniol (100 mg/kg). Five seconds cut of raw ECoG after 30 minutes of recording.
Fig. 4.
ECoG relative spectrum power in four bandwidths, delta (0.5–4 Hz), tetha (4–8 Hz), alpha (8–13 Hz) and beta (13–30 Hz) recorded from the cortex of rats treated with saline (n = 4) or geraniol 100 mg/kg (n = 5). Data evaluated by two way ANOVA followed by Bonferroni's test (*p < 0.05 compared with control group). Data expressed as mean ± SEM.
Discussion and conclusion
In this study, we investigated the central effects of geraniol treatment on rat behavior. To the best of our knowledge, this is the first study report the depressant effect of geraniol on the central nervous system of rats. We have chosen the open field test to evaluated if geraniol affected the exploratory activity of the animals, the hole-board because it is an efficient test to measure anxiety state in rodents and we have wanted to know if geraniol is an anxiolytic drug in smaller doses [22], [23], [24], [25]. After this, we did the barbiturate-induced sleeping time test because geraniol showed some signs of depression on CNS and this is a specific test to evaluate depressant drugs [26], [27]. Finally, we evaluated if geraniol affected the patterns of brain waves, since this measure express the state of sleep/wake of the individual [28]. In order to come to such a conclusion, rats under acute effect of geraniol were submitted to hole board, open field, and barbiturate-induced sleeping time tests, and patterns of brain waves were analyzed.
Regarding the hole-board test, as indicated by our data [Fig. 1], it was observed that geraniol (100 mg/kg) decreased distance traveled and number of rearing and increased immobility time, in comparison with control groups (saline and DZP 0.5 mg/kg). No differences were observed in relation to head-dipping time, but the geraniol-treated animals (25, 50 and 100 mg/kg) showed lower duration of head-dippings compared to the DZP-treated animals at anxiolytic dose (0.5 mg/kg) for this test, because the animals showed low locomotor activity. In other words, geraniol-treated animals were lethargic and as a consequence there was decrease motor activity (walk, rearing or head-dipping).
Similarly, the results obtained in open field test corroborated this hypothesis, because geraniol-treated animals (50 and 100 mg/kg) also showed reduction of the distance traveled and rearing frequency, moreover there was an increase in the immobility time in the animals compared with saline and DZP 1.5 groups.
Notwithstanding, geraniol is not the first monoterpene that caused depressant effect in rodents. This effect on the CNS was also reported in citronellal-treated mice. The citronellal chemical structure is similar to that of geraniol. There is only one difference between these two molecules: in the geraniol there is a double bond between the sixth and seventh carbon, whereas in the citronellal the double bond occurs between the eighth carbon and oxygen. Citronellal-treated mice demonstrate decreased motor activity in the remaining time on the rota-rod apparatus and increased latency for onset of convulsions induced by pentylenetetrazole [29].
Our research group in previous study demonstrated that the treatment with geraniol (200 mg/kg) and the inclusion complex geraniol/β-cyclodextrin (100 and 200 mg/kg) in rats significantly increased the latency for the first pentylenetetrazole-induced convulsion and protected 50% of the animals against seizures [11]. Pentylenetetrazole is a competitive GABAA receptor antagonist that is well established as a model for the study of seizures in rodents and scanning potential anticonvulsant and sedative drugs [30]. GABAA receptors have historically been the main targets for commercially-useful hypnotics. Benzodiazepines, the most widely-used class of hypnotics, are high-affinity allosteric modulators of GABAA receptors and act indirectly by potentiating GABA activation of these receptors [31].
Taking into account the above information, we suggest that the mechanism of action of geraniol may be related to modulation of the GABA receptor. Under physiological conditions, GABA receptor activation produces neuronal inhibition that is vital to the normal function of the CNS [32].
Other monoterpenes (Linalool, Myrtenol, Verbenol and Menthol) showed sedative effect mediated by GABA receptor. Linalool, a major component of lavender oil harbors, is a positive allosteric potential at GABAA receptors. Myrtenol and verbenol also act on GABAA receptors, by decrease phasic and tonic GABAergic inhibition [12], [33]. Menthol is another monoterpene and its chemical structure is also similar to that of geraniol, both have a hydroxyl in a similar position and two methyls attached to the tertiary carbon. Menthol has been described as a potent and stereoselective positive allosteric modulator of GABAA receptors expressed in Xenopus laevis oocytes, such effect is a consequence of its functional groups (OH) [34].
In order to clarify the possible depressant effect of geraniol, we performed the barbiturate-induced sleeping time test (BIST). This test is a classic pharmacological method for the screening of sedative-hypnotic drugs in mice [35]. BIST is the main test used to assess the potentiating effect of substances that prolonged sleep induced by hypnotics such as pentobarbital [27]. In our study, geraniol (100 mg/kg) increased the animals' sleeping time but did not modify the sleep latency [Fig. 3]. Based on this, we can affirm that there was a synergistic effect in the association of geraniol with thiopental.
This effect was observed in other studies, for example, Rosa damascena essential oil, rich in monoterpenes (geraniol included), caused depressant effects when administered in mice. The results showed that the ethanolic and aqueous extracts (500 and 1000 mg/kg) increased pentobarbital-induced sleeping time compared with diazepam [2]. Futhermore, the essential oil of D. moldavica (oil consisted of 55,63% of geraniol) prolonged the pentobarbital-induced sleeping time, induced sedation in the hole-board test, decreased spontaneous activity and produced motor coordination impairment in mice [4].
Finally, we evaluated the pattern of brain waves in geraniol-treated rats, because the brain waves generally express the state of sleep/wake of the individual. The predominance of slow waves in sleep and sedation states is well established in the literature. Under hyperpolarizing influences such as anesthetics, cortical neurons oscillate close to the frequency of 1 Hz, which is classified as slow-wave in the electroencephalogram [36].
According to our results, we observed that geraniol caused increase of the frequency of slow waves (delta waves) [Fig. 4]. Based on this information, we may suppose that geraniol (100 mg/kg) interferes with the excitatory postsynaptic potentials in the cortex, because ECoG relative spectrum power of delta waves was decreased. Similar results were reported by previous studies which showed that bergamot oil (rich in monoterpenes) in low dose induced a long period of immobility associated with increase in the amplitude of delta waves in the hippocampus and cortex of rats [37].
The genesis of slow wave occurs in the cortical regions through repetitive postsynaptic inhibitory potential [38], which is mostly generated by GABA receptors. The slow wave is a fundamental cortical rhythm that emerges in conditions of high inhibition or low arousal, lethargy, depression, low blood pressure, drowsiness, sleep and even coma.
We also observed that geraniol caused a decrease of theta waves [Fig. 4]. This data differs from that it is found in the literature. Theta waves used to show an increase associated with the immobility behavior and a decreased associated with the increase motor activity [14], [37]. Maybe, this variance is related with methodological differences. More studies are necessary to clarify this point.
Given the aforementioned and taken together with our results, we conclude that geraniol has a depressant effect on the central nervous system, reducing the animal's overall exploratory activity, increasing barbiturate-induced sleeping time and increasing the percentage of slow waves (ultra-slow and delta) in rats.
Conflicts of interest
The authors report no conflicts of interest.
Acknowledgements
This research was supported by fellowships from National Council for Scientific and Technological Development, São Paulo Research Foundation (FAPESP, grant #2015/20785-8), Coordination for the Improvement of Higher Education Personnel, Research Supporting Foundation of State of Sergipe (FAPITEC-SE) and Pro-rectory of Research of Federal University of Sergipe (POSGRAP/UFS).
Footnotes
Peer review under responsibility of Chang Gung University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bj.2018.08.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Carnesecchi S., Schneider Y., Ceraline J., Duranton B., Gosse F., Seiler N. Geraniol, a component of plant essential oils, inhibits growth and polyamine biosynthesis in human colon cancer cells. J Pharmacol Exp Therapeut. 2001;298:197–200. [PubMed] [Google Scholar]
- 2.Rakhshandah H., Hosseini M. Potentiation of pentobarbital hypnosis by Rosa damascena in mice. Indian J Exp Biol. 2006;44:910–912. [PubMed] [Google Scholar]
- 3.Simon D.Z., Beliveau J. Extraction by hydrodiffusion of the essential oil of MonardufistuZosu grown in the province of Quebec : assay of geraniol in the hydrodiffused oil. Int J Crude Drug Res. 1986;24:120–122. [Google Scholar]
- 4.Martínez-Vázquez M., Estrada-Reyes R., Martínez-Laurrabaquio a, López-Rubalcava C., Heinze G. Neuropharmacological study of Dracocephalum moldavica L. (Lamiaceae) in mice: sedative effect and chemical analysis of an aqueous extract. J Ethnopharmacol. 2012;141:908–917. doi: 10.1016/j.jep.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 5.Rajeswara Rao B.R., Bhattacharya A.K., Mallavarapu G.R., Ramesh S. Yellowing and crinkling disease and its impact on the yield and composition of the essential oil of citronella(Cymbopogon winterianus Jowitt.) Flavour Fragrance J. 2004;19:344–350. [Google Scholar]
- 6.Tiwari M., Kakkar P. Plant derived antioxidants – geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol In Vitro. 2009;23:295–301. doi: 10.1016/j.tiv.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Prasad S.N., Muralidhara Neuroprotective effect of geraniol and curcumin in an acrylamide model of neurotoxicity in Drosophila melanogaster: relevance to neuropathy. J Insect Physiol. 2014;60:7–16. doi: 10.1016/j.jinsphys.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Rekha K.R., Selvakumar G.P., Sethupathy S., Santha K., Sivakamasundari R.I. Geraniol ameliorates the motor behavior and neurotrophic factors inadequacy in MPTP-induced mice model of Parkinson's disease. J Mol Neurosci. 2013;51:851–862. doi: 10.1007/s12031-013-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rekha K.R., Selvakumar G.P., Santha K., Inmozhi Sivakamasundari R. Geraniol attenuates α-synuclein expression and neuromuscular impairment through increase dopamine content in MPTP intoxicated mice by dose dependent manner. Biochem Biophys Res Commun. 2013;440:664–670. doi: 10.1016/j.bbrc.2013.09.122. [DOI] [PubMed] [Google Scholar]
- 10.Deng X.-Y., Xue J.-S., Li H.-Y., Ma Z.-Q., Fu Q., Qu R. Geraniol produces antidepressant-like effects in a chronic unpredictable mild stress mice model. Physiol Behav. 2015;152:264–271. doi: 10.1016/j.physbeh.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Lins R.F., Cristina L., Santos M.A., Melo De S., Saraújo A., Nunes D.S. The anticonvulsant effect of geraniol and inclusion complex geraniol : β -cyclodextrin. Boletín Latinoam y Del Caribe Plantas Med y Aromáticas. 2014;13:557–565. [Google Scholar]
- 12.Deng L., Shi A.-M., Wang Q. Sedative-hypnotic and anxiolytic effects and the mechanism of action of aqueous extracts of peanut stems and leaves in mice. J Sci Food Agric. 2018 doi: 10.1002/jsfa.9020. [DOI] [PubMed] [Google Scholar]
- 13.Pergentino De Sousa D.O., Raphael E., Brocksom U., Brocksom T.J. Sedative effect of monoterpene alcohols in mice: a preliminary screening. Z Naturforsch. 2007;62:563–566. doi: 10.1515/znc-2007-7-816. [DOI] [PubMed] [Google Scholar]
- 14.Bellesi M., Vyazovskiy V.V., Tononi G., Cirelli C., Conti F. Reduction of EEG theta power and changes in motor activity in rats treated with ceftriaxone. PLoS One. 2012;7:e34139. doi: 10.1371/journal.pone.0034139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang X., Yang L., Sanford L.D. Sleep and EEG spectra in rats recorded via telemetry during surgical recovery. Sleep. 2007;30:1057–1061. doi: 10.1093/sleep/30.8.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Souza de T.K.M., Silva-Gondim M.B., Rodrigues M.C.A., Guedes R.C.A. The facilitating effect of unfavorable lactation on the potentiation of electrocorticogram after spreading depression in awake and anesthetized adult rats. Nutr Neurosci. 2016;0:1–9. doi: 10.1080/1028415X.2016.1210878. [DOI] [PubMed] [Google Scholar]
- 17.Souza de T.K.M., Silva-Gondim M.B., Rodrigues M.C.A., Guedes R.C.A. Anesthetic agents modulate ECoG potentiation after spreading depression, and insulin-induced hypoglycemia does not modify this effect. Neurosci Lett. 2015;592:6–11. doi: 10.1016/j.neulet.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Karimzadeh F., Hosseini M., Mangeng D., Alavi H., Hassanzadeh G.R., Bayat M. Anticonvulsant and neuroprotective effects of Pimpinella anisum in rat brain. BMC Complement Altern Med. 2012;12:2–9. doi: 10.1186/1472-6882-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leite M.P., Fassin J., Jr., Baziloni E.M.F., Almeida R.N., Mattei R., Leite J.R. Behavioral effects of essential oil of Citrus aurantium L. inhalation in rats. Braz J Pharmacogn. 2008;18:661–666. [Google Scholar]
- 20.Saiyudthong S., Pongmayteegul S., Marsden C.A., Phansuwan-Pujito P. Anxiety-like behaviour and c-fos expression in rats that inhaled vetiver essential oil. Nat Prod Res. 2015;29:2141–2144. doi: 10.1080/14786419.2014.992342. [DOI] [PubMed] [Google Scholar]
- 21.Majnooni M.B., Mohammadi-Farani A., Gholivand M.B., Nikbakht M.R., Bahrami G.R. Chemical composition and anxiolytic evaluation of Achillea Wilhelmsii C. Koch essential oil in rat. Res Pharm Sci. 2013;8:269–275. [PMC free article] [PubMed] [Google Scholar]
- 22.Onaolapo J.O., Onaolapo Y.A., Akanmu A.M., Olayiwola G. Caffeine and sleep-deprivation mediated changes in open-field behaviours, stress response and antioxidant status in mice. Sleep Sci. 2016;9:236–243. doi: 10.1016/j.slsci.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreira M.R.C., da Salvadori M.G.S.S., de Almeida A.A.C., de Sousa D.P., Jordan J., Satyal P. Anxiolytic-like effects and mechanism of (-)-myrtenol: a monoterpene alcohol. Neurosci Lett. 2014;579:119–124. doi: 10.1016/j.neulet.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Assad T., Khan R.A. Effect of methanol extract of Trigonella foenum-graecum L. seeds on anxiety, sedation and motor coordination. Metab Brain Dis. 2016:1–7. doi: 10.1007/s11011-016-9914-y. [DOI] [PubMed] [Google Scholar]
- 25.Casarrubea M., Sorbera F., Santangelo A., Crescimanno G. Microstructure of rat behavioral response to anxiety in hole-board. Neurosci Lett. 2010;481:82–87. doi: 10.1016/j.neulet.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C., Zhao X., Mao X., Liu A., Liu Z., Li X. Pharmacological evaluation of sedative and hypnotic effects of schizandrin through the modification of pentobarbital-induced sleep behaviors in mice. Eur J Pharmacol. 2014;744:157–163. doi: 10.1016/j.ejphar.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Birhanie M.W., Walle B., Rebba K. Hypnotic effect of the essential oil from the leaves of Myrtus communis on mice. Nat Sci Sleep. 2016;8:267–275. doi: 10.2147/NSS.S101493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knyazev G.G. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci Biobehav Rev. 2012;36:677–695. doi: 10.1016/j.neubiorev.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Melo M.S., Santana De M.T., Guimarães A.G., Siqueira R.S., Sousa De D.P., Santos R.V. Bioassay-guided evaluation of central nervous system effects of citronellal in rodents. Rev Bras Farmacogn. 2011;21:697–703. [Google Scholar]
- 30.Fisher R.S. Animal models of the epilepsies. Brain Res Brain Res Rev. 1989;14:245–278. doi: 10.1016/0165-0173(89)90003-9. [DOI] [PubMed] [Google Scholar]
- 31.Harrison N.L. Mechanisms of sleep induction by GABA(A) receptor agonists. J Clin Psychiatr. 2007;68:6–12. [PubMed] [Google Scholar]
- 32.Granger R.E., Campbell E.L., Johnston G. a R. (+)- and (-)-borneol: efficacious positive modulators of GABA action at human recombinant alpha1beta2gamma2L GABA(A) receptors. Biochem Pharmacol. 2005;69:1101–1111. doi: 10.1016/j.bcp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 33.van Brederode J., Atak S., Kessler A., Pischetsrieder M., Villmann C., Alzheimer C. The terpenoids Myrtenol and Verbenol act on δ subunit-containing GABAAreceptors and enhance tonic inhibition in dentate gyrus granule cells. Neurosci Lett. 2016;628:91–97. doi: 10.1016/j.neulet.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 34.Hall A.C., Turcotte C.M., Betts B.A., Yeung W.-Y., Agyeman A.S., Burk L.A. Modulation of human GABAA and glycine receptor currents by menthol and related monoterpenoids. Eur J Pharmacol. 2004;506:9–16. doi: 10.1016/j.ejphar.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 35.Estrada-Reyes R., Martínez-Vázquez M., Gallegos-Solís A., Heinze G., Moreno J. Depressant effects of Clinopodium mexicanum Benth. Govaerts (Lamiaceae) on the central nervous system. J Ethnopharmacol. 2010;130:1–8. doi: 10.1016/j.jep.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Ní Mhuircheartaigh R., Warnaby C., Rogers R., Jbabdi S., Tracey I. Slow-wave activity saturation and thalamocortical isolation during propofol anesthesia in humans. Sci Transl Med. 2013;5:208ra148. doi: 10.1126/scitranslmed.3006007. [DOI] [PubMed] [Google Scholar]
- 37.Rombolá L., Corasaniti M.T., Rotiroti D., Tassorelli C., Sakurada S., Bagetta G. Effects of systemic administration of the essential oil of bergamot (BEO) on gross behaviour and EEG power spetra redorded from the rat hippocampus. Funct Neurol. 2009;24:107–112. [PubMed] [Google Scholar]
- 38.Steriade M., Nuiiez A., Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: despolarizing and hyperpolarizing components. J Neurosci. 1993;73:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.