Abstract
Background
DNA methylation at the GFI1-locus has been repeatedly associated with exposure to smoking from the foetal period onwards. We explored whether DNA methylation may be a mechanism that links exposure to maternal prenatal smoking with offspring's adult cardio-metabolic health.
Methods
We meta-analysed the association between DNA methylation at GFI1-locus with maternal prenatal smoking, adult own smoking, and cardio-metabolic phenotypes in 22 population-based studies from Europe, Australia, and USA (n = 18,212). DNA methylation at the GFI1-locus was measured in whole-blood. Multivariable regression models were fitted to examine its association with exposure to prenatal and own adult smoking. DNA methylation levels were analysed in relation to body mass index (BMI), waist circumference (WC), fasting glucose (FG), high-density lipoprotein cholesterol (HDL—C), triglycerides (TG), diastolic, and systolic blood pressure (BP).
Findings
Lower DNA methylation at three out of eight GFI1-CpGs was associated with exposure to maternal prenatal smoking, whereas, all eight CpGs were associated with adult own smoking. Lower DNA methylation at cg14179389, the strongest maternal prenatal smoking locus, was associated with increased WC and BP when adjusted for sex, age, and adult smoking with Bonferroni-corrected P < 0·012. In contrast, lower DNA methylation at cg09935388, the strongest adult own smoking locus, was associated with decreased BMI, WC, and BP (adjusted 1 × 10−7 < P < 0.01). Similarly, lower DNA methylation at cg12876356, cg18316974, cg09662411, and cg18146737 was associated with decreased BMI and WC (5 × 10−8 < P < 0.001). Lower DNA methylation at all the CpGs was consistently associated with higher TG levels.
Interpretation
Epigenetic changes at the GFI1 were linked to smoking exposure in-utero/in-adulthood and robustly associated with cardio-metabolic risk factors.
Fund
European Union's Horizon 2020 research and innovation programme under grant agreement no. 633595 DynaHEALTH.
Research in context.
Evidence before this study
Maternal prenatal smoking is associated with unfavourable birth outcomes and has implications on offspring cardio-metabolic health in later life. Despite the widely known risk, a recent global systematic review reported that 52.9% of women, who smoke daily, continue smoking during pregnancy, with the highest prevalence in the European region and most stable over the years. As of yet, the mechanism underlying smoking associated adverse cardio-metabolic health outcomes remains poorly understood and is suggestive of the associated DNA methylation changes. We searched PubMed for articles on maternal smoking associated DNA methylation changes in offspring using the search terms ‘maternal smoking’, ‘pregnancy’, ‘DNA methylation’, ‘epigenetic markers’, and ‘offspring’ for work published until December 2017. We noted that previous studies have identified many epigenetic markers, especially DNA methylation changes in the offspring exposed to maternal prenatal smoking, but no study has investigated the potential underlying role of these epigenetic markers on long-term health. One study identified the mediating role of maternal smoking related DNA methylation changes at the GFI1 in the association between maternal prenatal smoking and low birth weight, and thus our research aimed to go beyond the direct effect of maternal smoking on birth weight.
Added value of this study
To the best of our knowledge, our study is one of the largest meta-analysis conducted and includes 22 studies from Europe, US and Australia to substantiate the debate of the epigenetic pathways of life-long health. Our research is key to understanding the causal, molecular pathways associated with such consistently observed DNA methylation patterns, and how they may mediate the association between smoking and clinical risks attributed to smoking. In the present research, we bring evidence for ascertaining the clinical relevance of these findings in the emerging field of epigenomics. Epigenomics helps to understand why the risk for diseases, in our case chronic cardio-metabolic diseases, may exist even in the absence of a direct exposure. We uniquely identified lower DNA methylation at cg14179389, a strong maternal smoking locus as a risk factor for adult adiposity, higher triglycerides levels, and blood pressure. The study delivers strong evidence to support the concept for the early life epigenetic influence on adult health.
Implications of all the available evidence
We report novel findings on the maternal smoking-related epigenetic factors at the GFI1-locus linking it to cardio-metabolic health in the adult. The findings matched known cardio-metabolic diseases risk attributed to maternal smoking exposure or adult smoking, supporting an underlying epigenetic component that can help bio-marking exposure to past risk. These findings provide a strong foundation for further work to unravel emerging smoking epigenetic markers with downstream detrimental health outcomes and further draws attention to increase awareness on smoking cessation and better prevention strategies.
Alt-text: Unlabelled Box
1. Introduction
Cigarette smoking, including second-hand exposure, is estimated to account for nearly 6 million deaths annually [1]. First and second-hand exposures are widely recognized as independent risk factors for cardiovascular diseases (CVD), largely determined by dose and duration [1,2]. Proposed direct mechanisms linking cigarette smoking and CVD include increased heart rate and myocardial contractility, inflammation, insulin resistance, and oxidative stress [3,4]. Moreover, the risk may remain even after successful long-term smoking cessation [5]. Similarly, maternal prenatal smoking has implications for birth outcomes, including low birth weight and risk of preterm birth [6], as well as increased risk to the offspring's later cardio-metabolic health [7,8]. A recent global review reported the highest estimated prevalence of maternal prenatal smoking in Europe, despite the widely known risks [9].
Emerging research suggests that part of the downstream impact of smoking likely persists through altered epigenetic patterns, many of which have been associated with alterations in the gene expression [10]. Of particular importance, altered DNA methylation at AHHR, GFI1, and MYO1G genes is consistently observed among both adult smokers and new-borns exposed to maternal prenatal smoking [[10], [11], [12], [13]]. Evidence on the stability of smoking-related-loci DNA methylation over the lifetime is inconsistent. Many CpGs in former smokers show a reversal of disrupted DNA methylation equivalent to non-smokers within five years of cessation, whereas others show no reversibility even 20–30 years after cessation [10,14]. Similarly, Richmond et al. suggested there were both reversible and permanent changes at smoking-related DNA methylation loci in offspring exposed to maternal smoking during pregnancy [15].
Furthermore, eight GFI1-linked-CpGs with aberrant DNA methylation were reported to partially mediate the association of maternal prenatal smoking with birthweight.16 Considering the consistent association observed between low birth weight and adverse adult cardio-metabolic health [8], we aimed to pursue a life-course approach to evaluate the possibility that exposure to maternal smoking in pregnancy influences the health of offspring via epigenetic mechanisms. We hypothesized that DNA methylation changes at GFI1-CpGs, a potential smoking biomarker, persist throughout the life-course and associate with cardio-metabolic phenotypes in adults. We tested this hypothesis in a large meta-analysis involving 22 population-based studies.
2. Material and methods
2.1. Participating studies
We included 22 studies consisting of 18,212 participants, including five pregnancy-birth cohorts, 17 other population-based datasets and their sub-studies: the Avon Longitudinal Study of Parents and Children (ALSPAC) (specifically subset with DNA methylation profiles in the Accessible Resource for Integrated Epigenomic Studies), two studies from the Bogalusa Heart Study (BHS – the European-American and African-American cohorts), the BIOS consortium, the Estonian Genome Centre University of Tartu (EGCUT), the European Prospective Investigation into Cancer and Nutrition (EPIC), the Italian Cardiovascular section (EPICOR), two independent subsets of the ESTHER study, the Cooperative Health Research in the Augsburg Region F4 (KORAF4), the Lifelines Deep (LLD), the London Life Science Population study (LOLIPOP), two follow-up datasets from the Northern Finland Birth cohort 1966 (NFBC1966 - 31 years and NFBC1966 - 46 years) and Northern Finland Birth cohort 1986 (NFBC1986), the Western Australian Pregnancy Cohort (RAINE) study, two independent studies from the Rotterdam Study (RS) –RSIII-1 and RSII-3_III-2, the Study of Health In Pomerania – Trend (SHIP-Trend), and the Young Finns Study 2011 (YFS). The BIOS consortium represents four studies with coordinated DNA methylation measurements: the Cohort On Diabetes And Atherosclerosis Maastricht (CODAM), the Leiden Longevity Study (LLS), the Netherlands Twin Register Study (NTR) and the prospective Amyotrophic Lateral Sclerosis (ALS) study, the Netherlands (PAN). Among these, five studies (ALSPAC, NFBC1966–31 yr, NFBC1966–46 yr, NFBC1986, and RAINE) participated in the meta-analysis of associations between the eight GFI1-CpGs and maternal prenatal smoking (n = 4230). Detailed data collection and ethical approval of each study are described in supplementary methods in the Appendix A. Subjects with missing information on DNA methylation and multiple births were excluded.
2.2. Smoking
Maternal prenatal smoking and offspring's own adult smoking were self-reported. Questions were harmonized to derive a dichotomous variable for maternal smoking as ‘no maternal smoking’ and ‘any maternal smoking’ during pregnancy. Adult own smoking was categorized as current non-smokers and smokers (adult own smoking ≥ one cigarette/day).
2.3. DNA methylation measurement and quality control
We used eight GFI1-linked-CpGs: cg04535902, cg09662411, cg09935388, cg10399789, cg12876356, cg18146737, cg14179389, and cg18316974. Each study conducted DNA methylation measurements and quality control. DNA methylation was measured in peripheral whole blood by standard procedures for Illumina HumanMethylation450 or EPIC array. DNA Methylation is described as β-value ranging between 0 (no cytosine methylation) and 1 (complete cytosine methylation). Each study excluded failed samples based on detection P-values, CpG-specific percentage, low DNA concentration, bisulphite conversion efficiency, and other study-specific control metrics (Appendix A) [17].
2.4. Covariates
Covariates were age, sex, and technical covariates for CpGs (batch effects, control probe adjustments, and cell type proportions). Adjustments for technical variation and cell type proportion in each study are described in the Appendix A.
2.5. Cardio-metabolic phenotypes
We used seven cardio-metabolic phenotypes derived from clinical examinations: body mass index (BMI, weight(kg)/height(m)2), waist circumference (WC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), fasting glucose (FG), diastolic blood pressure (DBP), and systolic blood pressure (SBP). All cardio-metabolic phenotypes were used as continuous variables and standardized (mean = 0, standard deviation = 1). Correction constants were applied to HDL-C, TG, and BP values, if participant reported lipid or blood pressure medication use (Appendix A). According to availability in the participating studies, BMI was available in 18,212, WC in 14,665, HDL-C in 18,212, TG in 18,212, FG in 16,529, DBP in 16,529, and SBP in 16,529 individuals of the total.
2.6. Study-specific statistical analyses
Each study conducted statistical analyses according to the analysis plan. Frequencies and means were computed for descriptive purposes. We used multivariate regression to evaluate three sets of associations: GFI1-CpGs with (i) maternal prenatal smoking (n = 4230), (ii) adult own smoking (n = 13,551), and (iii) cardio-metabolic phenotypes (n = 18,212) (Appendix B Fig. S1). Firstly, analyses in five pregnancy-birth cohort studies were performed using: baseline model, which used any maternal smoking during pregnancy as an exposure plus technical covariates regressed on DNA methylation as an outcome (beta-values), and adjusted model with sex, age (where applicable) and adult smoking as covariates. To assess the impact of adult own smoking on DNA methylation level, we included: baseline model, which used adult own smoking as an exposure plus technical covariates, and DNA methylation as an outcome, and adjusted model including sex and age as covariates. These two analyses were assessed in 20 participating studies. Both maternal and adult smoking showed lower DNA methylation at GFI1-CpGs, and thus we assessed cardio-metabolic phenotypes with respect to risk for lower DNA methylation. In the final analyses, covariate-adjusted models were performed in all participating studies with: baseline model, using DNA methylation as an exposure plus technical covariates, and each cardio-metabolic phenotype as an outcome, and adjusted model, including sex, age and adult smoking as additional covariates.
2.7. Meta-analysis
We used METAL software to conduct inverse variance-weighted fixed effects meta-analysis. We assessed heterogeneity using the I2 statistic (low-heterogeneity = I2 < 50%). Statistical significance was defined by Bonferroni correction for multiple testing as 0·05/4 (P ≤ 0·012), accounting for four clusters of cardio-metabolic phenotypes.
2.8. Supplementary analyses
In the NFBC1966 and 1986, we also examined the correlation between eight GFI1-CpGs. In a conditional analysis, we assessed association between adult own smoking and GFI1-CpGs additionally adjusted for all other GFI1-CpGs. Furthermore, as the full sample is multi-ethnic, the sensitivity analysis was performed to investigate the association between lower DNA methylation at eight GFI1-CpGs and cardio-metabolic phenotypes in a subset of European ancestry. Additionally, we also assessed the association of the eight GFI1-CpGs with former and current adult own smoking in NFBC1966 (Appendix B Tables S2, S5, S7 and S8).
3. Results
3.1. Participant characteristics
Participants were aged 16–81 years at the time of cardio-metabolic phenotype measurements, with the majority between 40 and 60 years. Among these, 17% were current smokers (Table 1). 18% of the participants were exposed to maternal prenatal smoking in the five studies (Appendix B Table S1). All eight GFI1-CpGs had lower mean DNA methylation levels in the group exposed to maternal prenatal smoking compared with unexposed group.
Table 1.
Characteristics of the participants of studies in the meta-analysis.
| Study Acronyma | Sample sizeb | Males, N (%) | Age, mean (SD), years | Current smokersc, N (%) | BMI, mean (SD), kg/m2 | WC, mean (SD), cm | TG, mean (SD), mmol/l | HDL-C, mean (SD), mmol/l | FG, mean (SD), mmol/l | DBP, mean (SD), mmHg | SBP, mean (SD), mmHg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALSPAC | 1530 | 554 (36) | 49·1 (5·8) | 172 (11) | 26·8 (4·8) | 89·2 (13·2) | 1·2 (0·7) | 1·4 (0·4) | 5·4 (1·1) | 74 (10) | 123 (14) |
| BHS_EA | 680 | 308 (45) | 43·2 (4·5) | 168 (25) | 30·0 (6·9) | 98·6 (16·4) | 1·7 (1·2) | 1·2 (0·3) | 4·7 (1·2) | 81 (9) | 117 (14) |
| BHS_AA | 288 | 113 (39) | 43·2 (4·5) | 96 (33) | 32·5 (8·6) | 100·9 (17·7) | 1·3 (1·0) | 1·3 (0·4) | 5·0 (2·0) | 89 (14) | 131 (22) |
| CODAM | 160 | 86 (54) | 65·5 (6·8) | 25 (16) | 28·9 (4·3) | NA | 1·5 (0·7) | 1·4 (0·3) | NA | NA | NA |
| EGCUT | 312 | 156 (50) | 50·2 (17) | 56 (18) | 27·4 (5·6) | 91·6 (14·9) | 1·3 (0·8) | 1·6 (0·5) | 5·1 (0·7) | 80 (10) | 128 (18) |
| EPICOR | 584 | 376 (64) | 53 | 194 (33) | 26·6 (3·8) | 90·6 (11·7) | 1·5 (0·9) | 1·7 (0·5) | 6·1 (1·6) | 87 (11) | 140 (21) |
| ESTHERa | 1000 | 500 (50) | 62·1 (6·5) | 186 (19) | 27·8 (4·3) | NA | 1·3 (0·9) | 1·3 (0·4) | 5·6 (1·2) | 87 (12) | 146 (22) |
| ESTHERb | 864 | 390 (45) | 62·1 (6·5) | 174 (21) | 27·7 (4·8) | NA | 1·5 (0·9) | 1·3 (0·4) | 5·7 (2·0) | 89 (12) | 148 (22) |
| KORAF4 | 1701 | 831 (49) | 60·9 (8·9) | 243 (14) | 28·1 (4·6) | 95·4 (13·9) | 1·6 (1·1) | 1·5 (0·4) | 5·0 (0·9) | 79 (11) | 130 (21) |
| LLD | 1057 | 446 (42) | 45·2 (13·5) | 439 (42) | 25·3 (4·1) | 88·2 (12·5) | 1·4 (0·2) | 1·6 (0·4) | 5·2 (0·1) | 70 (9) | 119 (13) |
| LLS | 631 | 300 (48) | 58·9 (6·6) | 85 (13) | 25·4 (3·5) | NA | 1·9 (1·2) | 1·4 (0·4) | NA | NA | NA |
| LOLIPOP | 3842 | 2386(62) | 52 (10·3) | 304 (8) | 27·5 (4·4) | 96·9 (11·2) | 1·7 (1·1) | 1·3 (0·3) | 5·4 (1·1) | 81 (11) | 131 (19) |
| NFBC1966–31 | 740 | 325 (44) | 31 | 194 (26) | 24·5 (4·0) | 82·7 (11·4) | 1·1 (0·7) | 1·6 (0·4) | 5·0 (0·8) | 76 (11) | 124 (13) |
| NFBC1966–46 | 716 | 315 (44) | 46 | 113 (16) | 26·8 (4·8) | 91·4 (13·2) | 1·3 (0·9) | 1·6 (0·4) | 6·1 (0·7) | 86 (11) | 129 (17) |
| NFBC1986 | 512 | 232 (45) | 16 | 101 (20) | 21·4 (3·5) | 74·4 (9·2) | 0·9 (0·4) | 1·4 (0·3) | 5·2 (0·5) | 68 (7) | 115 (12) |
| NTR | 729 | 256 (35) | 40·3 (15·1) | 137 (19) | 24·6 (4·1) | NA | 1·3 (0·7) | 1·5 (0·4) | NA | NA | NA |
| PAN | 163 | 100 (61) | 62·6 (9·5) | 45 (28) | 26·1 (3·7) | NA | 1·9 (1·1) | 1·4 (0·3) | NA | NA | NA |
| RAINE | 819 | 418 (51) | 17 | NA | 23·2 (4·5) | 79·7 (11·6) | 1·1 (0·5) | 1·3 (0·3) | 4·7 (0·6) | 58 (6) | 113 (11) |
| RSIII-1 | 731 | 336 (46) | 59·9 (8·2) | 197 (27) | 27·5 (4·8) | 93·5 (12·8) | 1·5 (0·8) | 1·4 (0·4) | 5·6 (1·2) | 81 (11) | 132 (20) |
| RSII-3_III-2 | 719 | 305 (42) | 67·6 (5·9) | 77 (11) | 27·7 (4·1) | 94·4 (12·0) | 1·5 (0·9) | 1·5 (0·4) | 5·7 (1·2) | 84 (11) | 144 (21) |
| SHIP | 248 | 118 (48) | 51·6(13·8) | 53 (21) | 27·3 (4·0) | 89·0 (12·5) | 1·5 (0·8) | 1·4 (0·4) | 5·4 (0·6) | 76 (9) | 124 (17) |
| YFS | 186 | 72 (39) | 44·2 | 21 (11) | 26·2 (4·7) | 88·2 (13·7) | 1·2 (0·8) | 1·4 (0·3) | 5·4 (1·1) | 73 (9) | 119 (13) |
Data shown as N (%) or mean (SD). According to availability in the participating studies, BMI was available in 18212, WC in 14665, HDL-C in 18212, TG in 18212, FG in 16529, DBP in 16529, SBP in 16529 individuals of the total.
Abbreviations: BMI – Body Mass Index; WC – Waist Circumference; TG – Triglycerides; HDL-C – High Density Lipoprotein Cholesterol; FG – Fasting Glucose; DBP – Diastolic Blood Pressure; SBP – Systolic Blood Pressure, NA - not available.
Study names: The Avon Longitudinal Study of Parents and Children (ALSPAC) (specifically subset with DNA methylation profiles in the Accessible Resource for Integrated Epigenomic Studies, ARIES), the two studies from Bogalusa Heart Study (BHS – European American (EA) and African American (AA)), the Cohort On Diabetes And Atherosclerosis Maastricht (CODAM),the Estonian Genome Centre, University of Tartu (EGCUT), the Italian cardiovascular section of EPIC (EPICOR), the Cooperative Gesundheitsforschung in der Region Augsburg (Cooperative Health Research in the Augsburg Region) F4 (KORAF4), the two independent subsets of the Epidemiologische Studie zu Chancen der Verhütung, Früherkennung und optimierten Therapie chronischer Erkrankungen in der älteren Bevölkerung (ESTHERa and ESTHERb), the Lifelines Deep (LLD), the Leiden Longevity Study (LLS), the London Life Science Population study (LOLIPOP), the two follow-up datasets from Northern Finland Birth cohort 1966 (NFBC1966–31 years and NFBC1966–46 years), Northern Finland Birth cohort 1986 (NFBC1986), the Netherlands Twin Register study (NTR), the Prospective Amyotrophic Lateral Sclerosis study Netherlands (PAN), The Western Australian Pregnancy Cohort study (RAINE), the two independent studies from Rotterdam Study (RS) –RSIII-1 and RSII-3_III-2, the Study of Health in Pomerania – Trend (SHIP-Trend), and the Young Finns Study 2011 (YFS). The CODAM, LLS, NTR, and PAN belong to the BIOS consortium with coordinated DNA methylation measurements.
Sample size of the studies with DNA methylation data.
Current smoking was defined as smoking ≥1 cigarette per day.
3.2. Correlation structure of the GFI1-CpGs
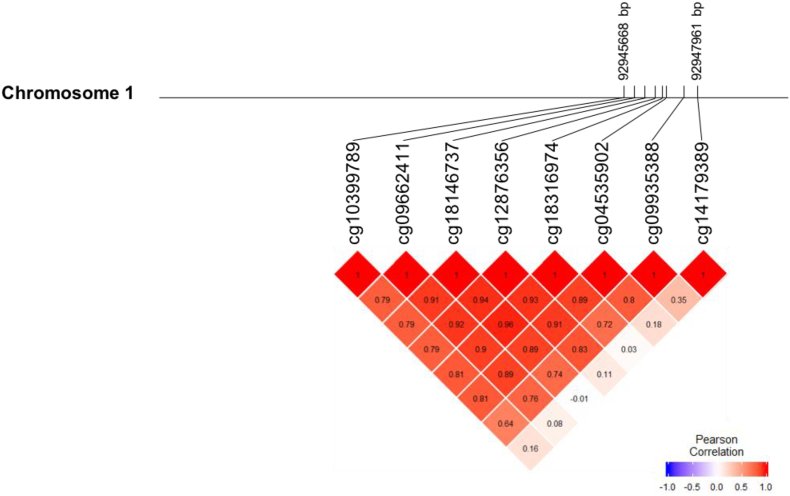
Fig. 1 displays the correlation matrix between the eight studied GFI1-CpGs in relation to their genomic location. The analysis performed in the NFBC1986 and NFBC1966 described a strong correlation between seven CpGs (cg04535902, cg09662411, cg09935388, cg10399789, cg12876356, cg18146737, and cg18316974). In contrast cg14179389 was weakly correlated with cg09935388 (0·35; P < 0·0001) only (Fig. 1 and Appendix B Table S2).
Fig. 1.
Map and correlation clustering of DNA methylation at eight GFI1 CpGs on human chromosome 1 (HapMap build 37).
3.3. GFI1-CpGs DNA methylation and prenatal maternal smoking and offspring's own smoking exposures
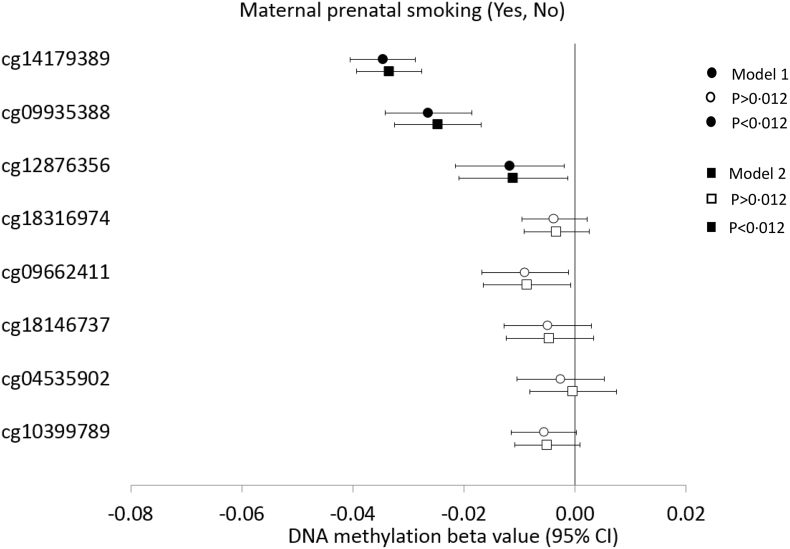
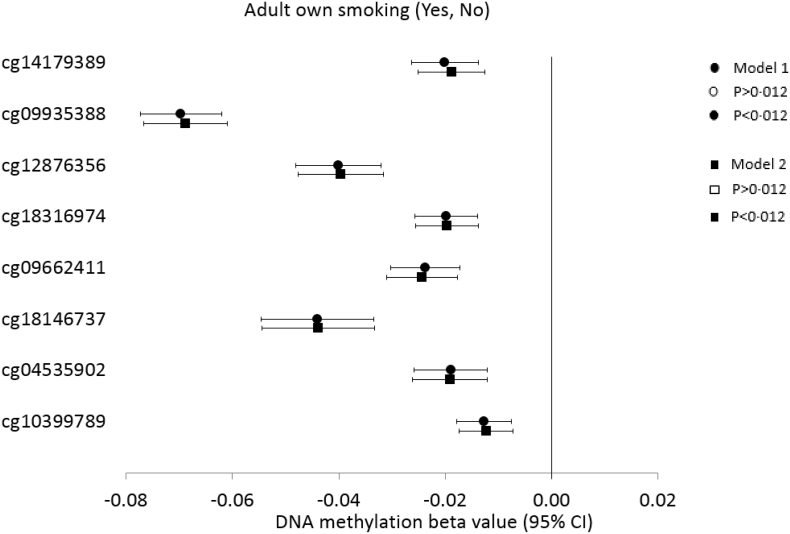
Following meta-analysis from five studies, the prenatal maternal smoking exposure status was associated with lower DNA methylation at cg14179389 (P = 6 × 10−30), cg09935388 (P = 9 × 10−11), and cg12876356 (P = 0·008) (Fig. 2, Appendix B Table S3). Cg14179389 was found to be the strongest maternal smoking locus and the association was not attenuated when adjusted for age, sex, and adult own smoking (β = −0·03, P = 2·0 × 10−27, I2 = 19·3). Similarly, adult own smoking status was associated with lower DNA methylation at all the studied CpGs. However, cg09935388 was found to be the strongest adult smoking locus (β = −0·07, P = 4·4 × 10−67); the association being independent of other CpGs in the conditional analysis (Fig. 3, Appendix B Table S4 and S5). In contrast, Cg14179389, the strongest above-mentioned prenatal maternal smoking signal did not show association with adult smoking status when conditioned by the DNA methylation at the other seven GFI1-CpGs. In fact, of the eight CpGs studied, only three of them remained associated with adult smoking following conditional analysis including cg09935388, cg18316974, and cg18146737 (P < 0·001) (Appendix B Table S5).
Fig. 2.
Forest plot showing meta-analysis effect sizes of DNA methylation at eight GFI1-CpGs by maternal prenatal smoking across five studies (n = 4230).
Model 1: CpG = maternal prenatal smoking + technical covariates; Model 2: CpG = maternal prenatal smoking + technical covariates + sex + age + adult own smoking.
95% CI, 95% Confidence Interval. Bonferroni corrected P[HYPHEN]value threshold of P<0·012. Open and closed symbol indicate p>0·012 and p<0·012, respectively.
Maternal prenatal smoking was defined as any maternal smoking during pregnancy (0 No, 1 Yes).
Standardized values with mean = 0 and standard deviation = 1 were used for CpG methylation across all the studies.
DNA methylation beta values can be interpreted as SD change in methylation for maternal prenatal smoking status from 0 to 1.
Fig. 3.
Forest plot showing meta-analysis effect sizes of DNA methylation at eight GFI1-CpGs by adult smoking across 20 participating studies (n = 13,551).
Model 1: CpG = adult own smoking + technical covariates; Model 2: CpG = adult own smoking + technical covariates + sex + age.
CI: Confidence Interval. Bonferroni corrected P[HYPHEN]value threshold of P<0.012. Open and closed symbol indicate p>0·012 and p<0·012, respectively.
Adult own smoking was defined as 1 or more cigarette per day (0 No, 1 Yes).
Standardized values with mean = 0 and standard deviation = 1 were used for CpG methylation across all the studies.
Since smoking exposures were consistently negatively associated with DNA methylation at the GFI1-locus, we assessed the associations of cardio-metabolic phenotypes against lower DNA methylation, to be consistent with the environmental risk itself i.e. increase in smoking.
3.4. Meta-analysis: eight GFI1-CpGs with lower DNA methylation and cardio-metabolic phenotypes
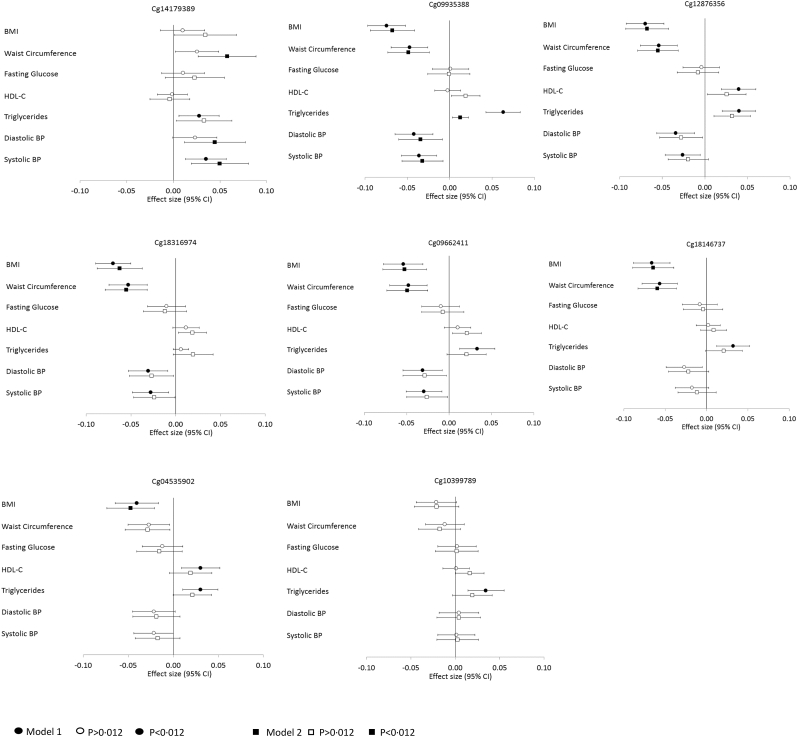
The associations between GFI1-CpGs and cardio-metabolic phenotypes from the meta-analysis are presented in Fig. 4 and Appendix B Table S6. Lower DNA methylation at cg14179389 was associated with increased WC, TG, and BP after a Bonferroni-correction set at P ≤ 0.012, with associations being enhanced with WC and BP when adjusted for sex, age, and adult own smoking (WC β = 0·04; BP β = 0·04; 0.0002 ≤ P ≤ 0.001). Cg14179389 consistently showed the lowest heterogeneity of the eight CpGs (I2 ≤ 25·4). In contrast, lower DNA methylation at cg09935388 was associated with decreased BMI, WC, and BP, although similarly to cg14179389 showed association with increased TG. After adjustments, the associations remained showing moderate attenuation with TG (BMI β = −0·06, WC β = −0·05; BP β = −0·03, TG β = 0·01; 1 × 10−7 ≤ P ≤ 0.01). Lower DNA methylation at cg12876356, cg18316974, and cg09662411 was associated with decreased BMI, WC, BP and increased TG and after adjustments, associations with decreased BMI and WC survived Bonferroni-correction (5 × 10−8 ≤ P < 9 × 10−5). Similarly, lower DNA methylation at cg18146737 was associated with decreased BMI and WC and at cg04535902, with decreased BMI when adjusted (1 × 10−7 ≤ P ≤ 0.001). Lower DNA methylation at cg10399789 showed no associations following adjustments (P ≥ 0.04). Lower heterogeneity was observed in only European ancestry individuals, rather than the full sample, for the association between GFI1-CpGs with BMI and WC (0 ≤ I2 < 40) (Appendix B Table S7). The independent results of all the associations from each of the 22 studies are present in the Appendix B Tables S9, S10, S11 and S12.
Fig. 4.
Forest plot showing meta-analysis effect sizes of cardio-metabolic phenotypes in SD change by one SD lower DNA methylation at eight GFI1-CpGs across all the participating studies (n = 18,212).
Model 1: Cardio-metabolic Phenotype = CpG + technical covariates; Model 2: Cardio-metabolic Phenotype = CpG + technical covariates + sex + adult smoking + age.
Bonferroni corrected P-value threshold of P < 0·012 has been used for this analysis. Open and closed symbol indicate p>0·012 and p<0·012, respectively.
Standardized values with mean = 0 and standard deviation = 1 were used for cardio-metabolic phenotypes and CpG methylation across all the studies.
β can be interpreted as SD change in cardio-metabolic phenotype per 1-SD decrease in methylation.
According to availability in the participating studies, we had BMI for 18212, WC for 14665, HDL-C for 18212, TG for 18212, FG for 16529, DBP for 16529, and SBP for 16529 of the total 18212 individuals.
Abbreviations: BMI – Body Mass Index, BP – Blood Pressure, CI - Confidence Interval, HDL-C – High Density Lipoprotein Cholesterol. Measurement units for each cardio-metabolic phenotype are given in Table 1.
4. Discussion
The present meta-analysis has corroborated the association of lower DNA methylation at the eight GFI1-CpGs with maternal prenatal and adult own smoking exposure, as well as uniquely identifying lower DNA methylation at cg14179389, a prenatal maternal smoking-related locus, as a risk factor for adult adiposity and blood pressure levels. Importantly, lower DNA methylation at all the CpGs indicates risk for higher triglyceride levels.
Recently, studies have shown GFI1-CpGs to mediate low birth weight due to prenatal maternal smoking exposure [16], and to associate with sudden infant death syndrome (SIDS) [18]. One striking finding from our study was the long lasting association between exposure to maternal prenatal smoking and lower DNA methylation at cg14179389 until adulthood. Moreover, lower DNA methylation at cg14179389 was also associated with increased adult WC, SBP, and DBP, suggesting a risk for adiposity and hypertension. The meta-analysis revealed a consistent effect size and direction of association in all studies, highlighting the reproducibility of findings. Furthermore, the associations persisted and were reinforced after adjusting for adult own smoking, supporting robustness and postnatal stability of maternal smoking-related DNA methylation locus. Previous studies have observed cg14179389 as the most consistent and strongest signal associated with maternal prenatal smoking among GFI1-CpGs [13,16]. These findings also include an appreciable overlap with previously identified evidence for influence of maternal smoking on the offspring's risk for obesity, hypertension, hyperlipidaemia and cardiovascular disease [19,20]. As hypothesized, similarity in influences of maternal smoking and cg14179389 on cardio-metabolic health identifies consequences for childhood development, and suggests there may be an underlying regulatory role for epigenetic changes in relation to detrimental cardio-metabolic health outcomes. We speculate that functionally important DNA methylation changes at cg14179389 in adults are present from birth due to smoking exposure in-utero.
In contrast, lower DNA methylation of the other six GFI1-CpGs (cg09935388, cg12876356, cg18316974, cg09662411, cg18146737, cg04535902) was associated with decreased BMI, WC, and BP. Of these all BMI and WC and the most of BP associations survived Bonferroni correction. The associations were of similar magnitude, although directionally opposite to cg14179389. Furthermore, adult own smoking showed a confounding effect in attenuating the associations. These findings are in agreement with the observational studies that show highly complex and non-linear associations between smoking and cardio-metabolic health [2,5,21]. Sneve et al. observed a U-shaped relationship between the number of cigarettes/day and BMI, with lowest BMI in those smoking 6–10 cigarettes/day; smoking cessation was associated with an initial increase in weight compared to those who continued smoking [22]. Increased risk of obesity among smokers is observed in a dose dependent manner, where former heavy smokers are more likely to be obese than former light smokers and have greater risk for CVD events [5,21]. Higher BMI in heavy smokers likely reflects clustering of risky behaviours that is conducive to weight gain. Paradoxically, whilst smoking acutely increases BP, smokers are observed to have slightly lower BP levels than non-smokers, especially in young adulthood, in larger epidemiological studies [23]. The comparable observations between six GFI1-CpGs and smoking with cardio-metabolic phenotypes raises the intriguing possibility that cigarette smoking induces epigenetic modifications at these CpGs, which, at least in part, may reflect the detrimental impact of smoking on cardio-metabolic health. Significant associations in our study between adult own smoking and lower DNA methylation at GFI1-CpGs across the participating studies support this hypothesis (Fig. 3, Appendix B Table S4). The observed epigenetic alterations may also partly indicate potential pathways for complex associations between smoking and BP.
Interestingly, lower DNA methylation at all eight CpGs showed association with higher TG in technically corrected models, but adjustment for adult smoking attenuated the associations, indicating a strong confounding or mediation effect. Consistency in direction of effect across all CpGs implies a concordant influence of both maternal and adult smoking induced epigenetic alterations at the GFI1-CpGs on TG. Previous evidence shows that maternal prenatal smoking exposure is associated with hyperlipidaemia in offspring [19]. Similarly, adult smokers have hyperlipidaemia and the influence of smoking cessation on lipid profiles seems to be quite modest and higher triglyceride levels pose significant risk to CVDs [24,25].
Lower heterogeneity was observed only in the European ancestry individuals, rather than the full sample, for the association between GFI1-CpGs with BMI and WC, indicating population-specific influence pertaining to adiposity (Appendix B Table S7). The studies excluded here were of the African American and South Asian ancestry. There is strong evidence that at any given BMI, the health risks are markedly higher in some ethnic groups than others. Asians have higher weight-related disease risks at a lower BMI and South Asians, in particular, have especially high levels of body fat and are more prone to developing abdominal obesity than Caucasians [26].
We observed contrasting associations of the different GFI1-CpGs (cg14179389 vs six other CpGs) with cardio-metabolic phenotypes. In the NFBC1966 and NFBC1986, we observed that cg14179389 differed and did not correlate with the other CpGs, while the other seven CpGs were highly correlated with each other (Fig. 1, Appendix B Table S2). This is supported by a recent population-level study that identified differential DNA methylation quantitative trait loci (meQTL) at these eight GFI1-CpGs, where all but one of the CpG sites (cg14179389) were highly correlated with the others, and formed contiguous clusters under the control of one meQTL [27]. Furthermore, cg14179389 association with adult smoking disappeared when adjusted for other CpGs whilst cg09935388, cg18316974, and cg18146737 showed independent associations (Appendix B Table S5). This explains the differences in their independent biological functions. Although some CpGs were associated with both maternal and adult smoking, perhaps due to the reversible nature of DNA methylation, their association with cardio-metabolic phenotypes was similar to functional consequence of own smoking in later life. Longitudinal analysis has provided evidence of rapid reversibility of DNA methylation in general during early development, particularly during the immediate postnatal years, with stabilization beyond age 7, suggesting a ‘catch up’ mechanism in early life [15,28]. In contrast, the unperturbed effect of cg14179389 due to maternal smoking in our study indicates persistent disruption of DNA methylation due to in-utero smoking exposure at this particular site.
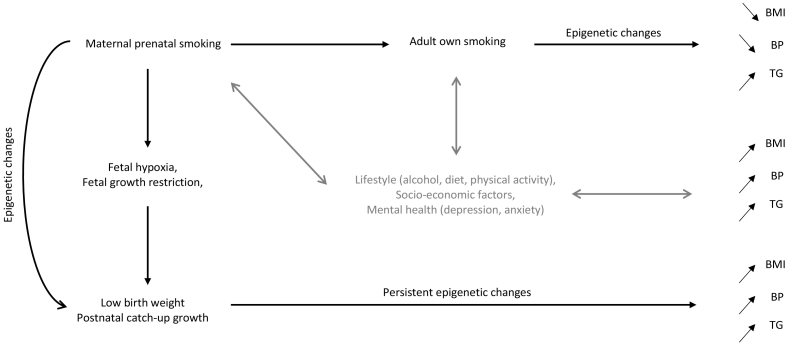
Collectively, these observations suggest evidence of two concepts (Fig. 5). First, maternal prenatal smoking induces a foetal response modulated through persistent epigenetic disruption. Second, adult own smoking, that may be potentially influenced by maternal smoking, induces similar epigenetic changes, which may play a role in the underlying pathways towards adverse consequences of smoking on cardiovascular risk. It is important to consider that many other environmental factors contribute to the cardiovascular risk (e.g. physical activity, stress, sedentary lifestyle, diet, alcohol consumption), and associated DNA methylation disruption following exposures to the maternal prenatal and own smoking could only explain a partial mediating role.
Fig. 5.
Model representing the potential mechanistic pathways in the study.
This study represents a major effort to perform a large-scale meta-analysis of maternal prenatal smoking, DNA methylation at GFI1-locus and cardio-metabolic phenotypes. A wide range of phenotypic data was available, facilitating assessment of the functional consequences of DNA methylation changes over a varied age range. The study replicates and confirms previously reported associations of lower DNA methylation at the GFI1-CpGs with exposure to own and maternal prenatal smoking.
We acknowledge that large collaborations utilizing summary level data, although useful in enhancing power to detect associations, may limit the ability to undertake multiple sensitivity analyses. We were unable to fully analyse DNA methylation changes over the life-course and disentangle the interaction of age with DNA methylation, to support emerging evidence that shows reversibility of DNA methylation patterns [29]. Another limitation was the use of leucocytes, which were the source of DNA used. They are composed of several cell types each with cell-type specific DNA methylation patterns and thus differences in these cell types could potentially confound the observed associations. Adjustment for derived cell type proportions was included in the analysis to overcome this eventuality. Our study included eight GFI1-sites associated with maternal prenatal smoking. We recognize that further work exploring the associations between DNA methylation of other adult and maternal prenatal smoking related loci and cardio-metabolic phenotypes could yield additional insights into the role of epigenetic markers that may jointly affect cardio-metabolic health. In addition, lack of gene expression data across studies limited insight into the molecular mechanisms. Additional evidence is needed to support GFI1 as the causal gene responsible for the observed findings. However, in a recent animal study, GFI1 did affect the systemic inflammation through the NE-dependent-C/EBPa-GFI1 pathway that predisposes to metabolic dysfunction and obesity [30]. Translating these findings to human data would be clinically relevant in light of our findings.
5. Conclusion
Our findings support evidence that epigenetic factors at the GFI1-locus, that are associated with exposures to smoking in-utero or adulthood are also linked to cardio-metabolic risk factors, specifically suggesting a role in hypertriglyceridemia. The findings support an underlying epigenetic component of the epidemiologically observed cardio-metabolic risk by maternal prenatal and adult smoking. The fact that these epigenetic factors associate with cardio-metabolic risk in later life even among non-smokers exposed to in-utero smoking may have important clinical implications. Such epigenetic loci might serve as objective biomarkers of past environmental exposures that could be used for preventive health measures. Our findings provide a strong foundation for further work to unravel emerging epigenetic markers with downstream detrimental health outcomes, and deliver strong evidence to support the early origin of adult health. It draws attention to increase awareness on smoking cessation and better prevention strategies.
Funding statements
The UK Medical Research Council and Wellcome (grant number 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and Matthew Suderman will serve as guarantor for the ALSPAC-related contents of this paper. Analysis of the ALSPAC data was funded by UK Economic & Social Research Council grant (grant number ES/N000498/1). ARIES was funded by the BBSRC (BBI025751/1 and BB/I025263/ 1). Supplementary funding to generate DNA methylation data which are (or will be) included in ARIES has been obtained from the MRC, ESRC, NIH and other sources. ARIES is maintained under the auspices of the MRC Integrative Epidemiology Unit at the University of Bristol (grant numbers MC_UU_12013/2, MC_UU_12013/8 and MC_UU_12013/9). Matthew Suderman was supported by the BBSRC and ESRC (grant number ES/N000498/1). BHS The study is supported by grant 2R01AG016592 from the National Institute on Aging. Shengxu Li was partly supportly by grant 13SDG14650068 from the American Heart Association. NTR, LLS, CODAM and PAN were supported from the Netherlands Cardiovascular Research Initiative (the Dutch Heart Foundation, Dutch Federation of University Medical Centres, the Netherlands Organization for Health Research and Development, and the Royal Netherlands Academy of Sciences) for the GENIUS project “Generating the best evidence-based pharmaceutical targets for atherosclerosis” (CVON2011–19). This work was performed within the framework of the Biobank-Based Integrative Omics Studies (BIOS) Consortium funded by BBMRI-NL, a research infrastructure financed by the Dutch government (NWO 184.021.007). EGCUT was supported by the Estonian Research Council Grants PRG184, PUT1665, IUT20-60, EU H2020 grant ePerMed (Grant No. 692145), and European Union through the European Regional Development Fund (Project No. 2014–2020.4.01.15–0012). Lili Milani was supported by Uppsala University Strategic Research Grant as part of the Science for Life Laboratory fellowship program. EPICOR was supported by the Compagnia di San Paolo for the EPIC-Italy and EPICOR projects, the Italian Institute for Genomic Medicine (IIGM, formerly Human Genetics Foundation-Torino, HuGeF, Turin, Italy) and the MIUR ex60% grant. EPIC-Italy is further supported by a grant from the “Associazione Italiana per la Ricerca sul Cancro” (AIRC, Milan). The EPICOR study was also supported by a MIUR grant to the Department of Medical Sciences, project “Dipartimenti di Eccellenza 2018 – 2022”. The ESTHER study was supported by the Baden-Württemberg State Ministry of Science, Research and Arts (Stuttgart, Germany), the Federal Ministry of Education and Research (Berlin, Germany), and the Federal Ministry of Family Affairs, Senior Citizens, Women and Youth (Berlin, Germany). The KORA study was initiated and financed by the Helmholtz Zentrum München – German Research Centre for Environmental Health, which is funded by the German Federal Ministry of Education and Research (BMBF) and by the State of Bavaria. Furthermore, KORA research has been supported within the Munich Centre of Health Sciences (MC-Health), Ludwig-Maximilians-Universität, as part of LMUinnovativ. The research leading to these results has received funding from the European Union Seventh Framework Program under grant agreement [n°602736] (Multi-dimensional omics approach to stratification of patients with low back pain - PAIN-OMICS; http://www.painomics.eu/) and under grant agreement [n°603288] (Systems Biology to Identify Molecular Targets for Vascular Disease Treatment – SysVasc; http://www.sysvasc.eu/). This work was further supported by a grant (WA 4081/1–1) from the German Research Foundation. N. Verweij was supported by NWO VENI (016.186.125). L. Franke is supported by ZonMW-VIDI 917.14.374 and ERC Starting Grant, grant agreement 637640 (ImmRisk). The LOLIPOP study is supported by the National Institute for Health Research (NIHR) Comprehensive Biomedical Research Centre Imperial College Healthcare NHS Trust, the British Heart Foundation (SP/04/002), the Medical Research Council (G0601966, G0700931), the Wellcome Trust (084723/Z/08/Z, 090532 & 098381) the NIHR (RP-PG-0407-10371), the NIHR Official Development Assistance (ODA, award 16/136/68), the European Union FP7 (EpiMigrant, 279143) and H2020 programs (iHealth-T2D, 643774). John Chambers is supported by Singapore Ministry of Health's National Medical Research Council under its Singapore Translational Research Investigator (STaR) Award (NMRC/STaR/0028/2017). NFBC1966 received financial support from University of Oulu Grant no. 65354, Oulu University Hospital Grant no. 2/97, 8/97, Ministry of Health and Social Affairs Grant no. 23/251/97, 160/97, 190/97, National Institute for Health and Welfare, Helsinki Grant no. 54121, Regional Institute of Occupational Health, Oulu, Finland Grant no. 50621, 54231. NFBC1986 received financial support from EU QLG1-CT-2000-01643 (EUROBLCS) Grant no. E51560, NorFA Grant no. 731, 20056, 30167, USA / NIHH 2000 G DF682 Grant no. 50945. MRC no: MR/M013138/1, ERDF European Regional Development Fund Grant no. 539/2010 A31592. Matthias Wielscher was supported by the European Union's Horizon 2020 research and innovation program under grant agreement No 633212. Priyanka Parmar, Sylvain Sebert and Marjo-Riitta Jarvelin received support by H2020–633595 DynaHEALTH, H2020 733206 LifeCycle, the academy of Finland EGEA-project (285547) and the Biocenter Oulu. The authors acknowledge the contributions to core funding of the Raine Study by the University of Western Australia, the Telethon Kids Institute, the Raine Medical Research Foundation, the Faculty of Medicine, Dentistry and Health Science (UWA), the Women and Infants Research Foundation, Curtin University, and Edith Cowan University. The authors also acknowledge the long-term support of the National Health and Medical Research Council of Australia (NHMRC). The epigenetic data collection is supported by NHMRC grant #1059711. Rae-Chi Huang and Trevor A Mori are supported by NHMRC Research Fellowships #1053384 and #1042255, respectively. The Rotterdam Study is funded by Erasmus Medical Centre and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. The EWAS data was funded by the Genetic Laboratory of the Department of Internal Medicine, Erasmus Medical Centre, and by the Netherlands Organization for Scientific Research (NWO; project no. 184021007) and made available as a Rainbow Project (RP3; BIOS) of the Bio banking and Biomolecular Research Infrastructure Netherlands (BBMRI-NL). SHIP is part of the Community Medicine Research net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (grants no. 01ZZ9603, 01ZZ0103, and 01ZZ0403), the Ministry of Cultural Affairs as well as the Social Ministry of the Federal State of Mecklenburg-West Pomerania, and the network ‘Greifswald Approach to Individualized Medicine (GANI_MED)’ funded by the Federal Ministry of Education and Research (grant 03IS2061A). DNA methylation data have been supported by the DZHK (grant 81 × 34000104). The University of Greifswald is a member of the Caché Campus program of the InterSystems GmbH. The YFS has been financially supported by the Academy of Finland: grants 286284, 134309 (Eye), 126925, 121584, 124282, 129378 (Salve), 117787 (Gendi), and 41071 (Skidi); the Social Insurance Institution of Finland; Competitive State Research Financing of the Expert Responsibility area of Kuopio, Tampere and Turku University Hospitals (grant X51001); Juho Vainio Foundation; Paavo Nurmi Foundation; Finnish Foundation for Cardiovascular Research; Finnish Cultural Foundation; The Sigrid Juselius Foundation; Tampere Tuberculosis Foundation; Emil Aaltonen Foundation; Yrjö Jahnsson Foundation; Signe and Ane Gyllenberg Foundation; Diabetes Research Foundation of Finnish Diabetes Association; and EU Horizon 2020 (grant 755320 for TAXINOMISIS); and European Research Council (grant 742927 for MULTIEPIGEN project); Tampere University Hospital Supporting Foundation.
The funding bodies had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. PP, MRJ and SS had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflicts of interest
Niek Verweij is employee at Genomic Plc. Other authors have nothing to disclose.
Contributors
SS, MRJ, and PP conceptualised and designed the study. PP wrote the first draft and analysed the data. PP, MRJ, and SS had full access to the data. All authors acquired and interpreted the data, critically reviewed the manuscript, provided technical or material support and approved the final version to be published. SS and MRJ supervised the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.10.066.
Contributor Information
Marjo-Riitta Järvelin, Email: m.jarvelin@imperial.ac.uk.
Sylvain Sebert, Email: sylvain.sebert@oulu.fi.
Appendix. Supplementary data
Appendix A and B
Supplementary material 1
Supplementary material 2
References
- 1.WHO WHO Report on the Global Tobacco Epidemic, 2017: Monitoring Tobacco Use and Prevention Policies. 2017. http://www.who.int/tobacco/global_report/en/
- 2.U.S. Department of Health and Human Services The Health Consequences of Smoking—50 Years of Progress: A Report of Surgeon General. 2014. http://www.ncbi.nlm.nih.gov/pubmed/24455788
- 3.Ambrose J.A., Barua R.S. The pathophysiology of cigarette smoking and cardiovascular disease. J Am Coll Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (US), National Center for Chronic Disease Prevention and Health Promotion (US), Office on Smoking and Health (US), US Centers for Disease Control and Prevention How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. 2010. http://www.ncbi.nlm.nih.gov/pubmed/21452462 [PubMed]
- 5.Clair C., Rigotti N.A., Porneala B. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA. 2013;309:1014. doi: 10.1001/jama.2013.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer M.S. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 7.Gluckman P.D., Hanson M.A. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 2004;15:183–187. doi: 10.1016/j.tem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Horikoshi M., Beaumont R.N., Day F.R. Genome-wide associations for birth weight and correlations with adult disease. Nature. 2016;4:1–20. doi: 10.1038/nature19806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange S., Probst C., Rehm J., Popova S. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob Heal. 2018;6:e769–e776. doi: 10.1016/S2214-109X(18)30223-7. [DOI] [PubMed] [Google Scholar]
- 10.Joehanes R., Just A.C., Marioni R.E. Epigenetic signatures of cigarette smoking. Circ Cardiovasc Genet. 2016;9:436–447. doi: 10.1161/CIRCGENETICS.116.001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shenker N.S., Polidoro S., van Veldhoven K. Epigenome-wide association study in the European prospective Investigation into Cancer and Nutrition (EPIC-Turin) identifies novel genetic loci associated with smoking. Hum Mol Genet. 2013;22:843–851. doi: 10.1093/hmg/dds488. [DOI] [PubMed] [Google Scholar]
- 12.Zeilinger S., Kühnel B., Klopp N. Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joubert B.R., Felix J.F., Yousefi P. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am J Hum Genet. 2016;98:680–696. doi: 10.1016/j.ajhg.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y., Yang R., Burwinkel B., Breitling L.P., Brenner H. F2RL3 methylation as a biomarker of current and lifetime smoking exposures. Environ Health Perspect. 2014;122:131–137. doi: 10.1289/ehp.1306937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richmond R.C., Simpkin A.J., Woodward G. Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: Findings from the Avon Longitudinal Study of parents and Children (ALSPAC) Hum Mol Genet. 2015;24:2201–2217. doi: 10.1093/hmg/ddu739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Küpers L.K., Xu X., Jankipersadsing S.A. DNA methylation mediates the effect of maternal smoking during pregnancy on birthweight of the offspring. Int J Epidemiol. 2015;44:1224–1237. doi: 10.1093/ije/dyv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehne B., Drong A.W., Loh M. A coherent approach for analysis of the Illumina HumanMethylation450 BeadChip improves data quality and performance in epigenome-wide association studies. Genome Biol. 2015;16:37. doi: 10.1186/s13059-015-0600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwender K., Holtkötter H., Johann K.S. Sudden infant death syndrome: exposure to cigarette smoke leads to hypomethylation upstream of the growth factor independent 1 (GFI1) gene promoter. Forensic Sci Med Pathol. 2016;12:399–406. doi: 10.1007/s12024-016-9812-y. [DOI] [PubMed] [Google Scholar]
- 19.Power C., Atherton K., Thomas C. Maternal smoking in pregnancy, adult adiposity and other risk factors for cardiovascular disease. Atherosclerosis. 2010;211:643–648. doi: 10.1016/j.atherosclerosis.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Brion M.-J.A., Leary S.D., Lawlor D.A., Smith G.D., Ness A.R. Modifiable maternal exposures and offspring blood pressure: a review of epidemiological studies of maternal age, diet, and smoking. Pediatr Res. 2008;63:593–598. doi: 10.1203/PDR.0b013e31816fdbd3. [DOI] [PubMed] [Google Scholar]
- 21.Tolstrup J.S., Hvidtfeldt U.A., Flachs E.M. Smoking and risk of coronary heart disease in younger, middle-aged, and older adults. Am J Public Health. 2014;104:96–102. doi: 10.2105/AJPH.2012.301091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sneve M., Jorde R. Cross-sectional study on the relationship between body mass index and smoking, and longitudinal changes in body mass index in relation to change in smoking status: the Tromso Study. Scand J Public Health. 2008;36:397–407. doi: 10.1177/1403494807088453. [DOI] [PubMed] [Google Scholar]
- 23.Keto J., Ventola H., Jokelainen J. Cardiovascular disease risk factors in relation to smoking behaviour and history: a population-based cohort study. Open Hear. 2016;3 doi: 10.1136/openhrt-2015-000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nordestgaard B.G., Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 25.Maeda K., Noguchi Y., Fukui T. The effects of cessation from cigarette smoking on the lipid and lipoprotein profiles: a meta-analysis. Prev Med (Baltim) 2003;37:283–290. doi: 10.1016/s0091-7435(03)00110-5. [DOI] [PubMed] [Google Scholar]
- 26.Expert Consultation W.H.O. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y., Li X., Aryee M.J. GeMes, clusters of DNA methylation under genetic control, can inform genetic and epigenetic analysis of disease. Am J Hum Genet. 2014;94:485–495. doi: 10.1016/j.ajhg.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee K.W.K., Richmond R., Hu P. Prenatal exposure to maternal cigarette smoking and DNA methylation: Epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age. Environ Health Perspect. 2015;123:193–199. doi: 10.1289/ehp.1408614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones M.J., Goodman S.J., Kobor M.S. DNA methylation and healthy human aging. Aging Cell. 2015;14:924–932. doi: 10.1111/acel.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J.-Y., Zhou Q.L., Huang C.-H. Neutrophil elastase regulates emergency myelopoiesis preceding systemic inflammation in diet-induced obesity. J Biol Chem. 2017;292:4770–4776. doi: 10.1074/jbc.C116.758748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A and B
Supplementary material 1
Supplementary material 2