Abstract
Background
We recently reported that myeloid sirtuin 6 (Sirt6) is a critical determinant of phenotypic switching and the migratory responses of macrophages. Given the prominent role of macrophages in the pathogenesis of rheumatoid arthritis (RA), we tested whether myeloid Sirt6 deficiency affects the development and exacerbation of RA.
Methods
Arthritis was induced in wild type and myeloid Sirt6 knockout (mS6KO) mice using collagen-induced and K/BxN serum transfer models. Sirt6 expression (or activity) and inflammatory activities were compared in peripheral blood mononuclear cells (PBMCs) and monocytes/macrophages obtained from patients with RA or osteoarthritis.
Findings
Based on clinical score, ankle thickness, pathology, and radiology, arthritis was more severe in mS6KO mice relative to wild type, with a greater accumulation of macrophages in the synovium. Consistent with these findings, myeloid Sirt6 deficiency increased the migration potential of macrophages toward synoviocyte-derived chemoattractants. Mechanistically, Sirt6 deficiency in macrophages caused an inflammation with increases in acetylation and protein stability of forkhead box protein O1. Conversely, ectopic overexpression of Sirt6 in knockout cells reduced the inflammatory responses. Lastly, PBMCs and monocytes/macrophages from RA patients exhibited lower expression of Sirt6 than those from patients with osteoarthritis, and their Sirt6 activity was inversely correlated with disease severity.
Interpretation
Our data identify a role of myeloid Sirt6 in clinical and experimental RA and suggest that myeloid Sirt6 may be an intriguing therapeutic target.
Fund
Medical Research Center Program and Basic Science Research Program through the National Research Foundation of Korea.
Keywords: Sirt6, RA, Macrophage, Inflammation, FoxO1
Graphical abstract
Highlights
-
•
Myeloid Sirt6 deficiency aggravates the joint destruction by increasing recruitment of macrophages into arthritic joints.
-
•
Myeloid Sirt6 deacetylates FoxO1 to promote proteasomal degradation.
-
•
Overexpression of Sirt6 greatly attenuates inflammatory activity of human macrophages.
-
•
Sirt6 expression and activity decrease in blood monocytes and joint macrophages from RA patients.
Research in context.
Evidence before this study
Monocyte infiltration and macrophage proliferation in joint synovium play an important role in rheumatoid arthritis by secreting various cytokines and chemical mediators which amplify and perpetuate synovial inflammation.The numbers of accumulated macrophages in the synovium correlate well with clinical symptoms and degree of joint damage in rheumatoid arthritis. Thus, monocytes/macrophages have become a promising target in the treatment of rheumatoid arthritis. We previously have shown that sirtuin 6 is a critical determinant of macrophage migration and infiltration into local tissues.
Added value of this study
This study demonstrated that myeloid sirtuin 6 is essential in inhibiting synovial macrophage infiltration and thus suppressing the development and progression of rheumatoid arthritis in mice. To this end, we analyzed functional phenotypes of blood monocytes and joint macrophages from patients with rheumatoid arthritis and utilized experimental model of rheumatoid arthritis in myeloid-specific sirtuin 6 knockout mice.
Implications of all the available evidence
Our study suggests that sirtuin 6 activation by virtue of pharmacological or genetic modulation might be an effective therapeutic approach to prevent disease progression in rheumatoid arthritis. The implication of this finding for the control of rheumatoid arthritis is profound, as searching small molecule activators of sirtuin 6 are gaining attention in the field.
Alt-text: Unlabelled Box
1. Introduction
Histopathological findings of rheumatoid arthritis (RA) include synovial hyperplasia, bone and cartilage destruction, and inflammatory cell infiltration [1]. Among inflammatory cells, macrophages/monocytes are major source of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 [2]. These cytokines are involved in the development and progression of RA. Indeed, the degree of macrophage infiltration in the joint tissues and serum levels of monocyte-derived cytokines are positively correlated with disease severity [[3], [4], [5]]. Furthermore, anti-rheumatic drugs reduce synovial macrophage numbers in close association with clinical benefit [6,7]. Together, these reports suggest that macrophages and macrophage-derived cytokines might be an effective therapeutic targets to prevent disease onset and progression in RA.
Recently, we demonstrated that sirtuin 6 (Sirt6) in myeloid cells has a pivotal role in the phenotypic switch and migration response of macrophages [8]. As a histone deacetylase, Sirt6 deacetylates histone H3 lysine 9 (H3K9) and H3K56 and represses the transcriptional activity of nuclear factor κB (NF-κB) [9]. Accordingly, overexpression of Sirt6 downregulates local and systemic levels of proinflammatory cytokines and attenuates bone destruction in mice with collagen-induced arthritis [10,11]. As a non-histone protein deacetylase, Sirt6 deacetylates GCN5 [12], pyruvate kinase M2 [13], forkhead box protein O1 (FoxO1) [14], and GATA binding protein 3 [15]. Based on the prominent role of Sirt6 in inflammatory and migration responses of macrophages, we investigated the functional responses of wild type (WT) and myeloid Sirt6 knockout (mS6KO) mice in collagen-induced arthritis (CIA) and K/BxN serum transfer arthritis models, which are known to be highly dependent on chronic adaptive immunity and acute passive immunity, respectively. To explore the clinical implication of myeloid Sirt6 in RA, we analyzed Sirt6 expression and/or activity in circulating peripheral blood mononuclear cells (PBMCs) and macrophages isolated from synovial fluid of RA patients and determined its relationship with disease severity and inflammatory activity.
2. Materials and methods
2.1. Animals
Sirt6flox/flox mice (B6; 129-Sirt6tm1Ygu/J) were crossed to LysM-Cre (B6.129P2-lyz2tm1(cre)Ifo/J) mice to generate mS6KO mice [8]. KRN TCR-transgenic mice were gifted by D. Mathis and C. Benosit (Harvard Medical School, Boston, MA, USA). NOD mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). K/BxN mice were generated by crossing KRN TCR-transgenic and NOD mice. All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (NIH Publication No. 85-23, revised 2011) and approved by the Institutional Animal Care and Use Committee of Chonbuk National University (Permit No: CBNU 2016-46).
2.2. CIA induction
The modified protocol was used to induce CIA using C57BL/6 strain of mS6KO mice [16]. WT or mS6KO mice (14–18 weeks old) were immunized with 150 μg of chicken type II collagen (CII; Chondrex, Redmond, WA, USA) emulsified in 4 mg/ml of complete Freund's adjuvant (CFA; Chondrex) in equal volumes. The day of initial immunization was designated Day 0. Immunization was boosted by an equal volume of emulsion of CII and incomplete Freund's adjuvant (IFA; Chondrex) on Day 21 and by intraperitoneal injection of lipopolysaccharide (LPS, 5 μg/100 ml) on Day 28.
2.3. K/BxN serum transfer arthritis induction and arthritis scoring
K/BxN serum transfer arthritis models were induced as described previously [17]. WT or mS6KO mice (8–10 weeks old) were intraperitoneally injected with K/BxN serum (50 μl) pooled from arthritic K/BxN mice on Days 0 and 2.
2.4. Preparation of recombinant adenovirus
Adenovirus expressing Sirt6 (AdSirt6) or β-galactosidase (AdLacZ) was prepared as described previously [11].
2.5. Micro–computed tomography (micro-CT) imaging
A SkyScan 1076 micro-CT apparatus was used to evaluate structural changes in the ankle joints. Ankle joints obtained from CIA mice were scanned and reconstructed into a 3-dimensional structure with a voxel size of 18 μm. The projection images were reconstructed into 3-dimensional images using NRecon software (version 1.6.1.5) and CT Analyzer (version 1.10.0.1) (both from SkyScan, Kontich, Belgium).
2.6. Histology
Paraffin sections of murine paws were stained with H&E, Safranin-O, and tartrate-resistant acid phosphatase (TRAP) for evaluation of inflammation and joint destruction. After deparaffinization, tissue sections were immunostained with antibodies against CD68 (Santa Cruz Biotechnology, Dallas, TX, USA), Ly6G (Abcam, Cambridge, UK), Sirt6 (Cell Signaling Technology, Beverly, MA, USA), FoxO1 (Cell Signaling Technology) or acetyl-FoxO1 (Santa Cruz Biotechnology).
2.7. Antibodies
Antibodies against the following proteins were used: HSP90 (Enzo Life Sciences, Plymouth Meeting, PA, USA), FoxO1, Sirt6, Ac-Lys (Cell Signaling Technology), Ac-FoxO1, ubiquitin (Santa Cruz Biotechnology), CCR3, FoxO3a, FoxO4 (Abcam), lamin B (Bioworld Technology, St Louis Park, MN, USA) and β-actin (Sigma-Aldrich, St. Louis, MO, USA).
2.8. RNA isolation and real-time RT-PCR
Total RNA was extracted from frozen joint tissues or bone marrow macrophages (BMMs) using Trizol reagent (Invitrogen, Carlsbad, CA, USA). First-strand cDNA was generated with oligo dT-adaptor primers by reverse transcriptase (TaKaRa, Tokyo, Japan). Specific primers were designed using qPrimerDepot (http://mouseprimerdepot.nci.nih.gov, Table S1).
2.9. Biochemical analysis
Specific enzyme-linked immunosorbent assay (ELISA) kits were used to measure the concentrations of IL-6, TNF-α (eBioscience, San Diego, CA, US), MCP-1, MIP-1α (CUSABIO, Houston, TX, USA), and RANTES/CCL5 (R&D systems, Minneapolis, MN, USA). Quantitative measurement of Sirt6 activity from PBMCs was performed using a fluorometric Sirt6 activity assay kit (Abcam).
2.10. Isolation of PBMCs and monocytes/macrophages
PBMCs were freshly isolated from whole blood by Ficoll (GE Healthcare Life Science, Marlborough, MA, USA) density gradient centrifugation. Human PBMC-derived and synovial fluid-derived monocytes were freshly isolated using anti-CD14 magnetic beads (Miltenyi Biotec, Auburn, CA, USA). For preparation of mouse bone marrow-derived macrophages (BMMs), bone marrow cells were isolated from the femurs and tibias of mice. Cells were cultured for 6 days in complete α-MEM medium supplemented with 30% L929-conditioned medium (CM) to provide M-CSF. The adherent cells were used as BMMs.
2.11. Cell proliferation and viability assay
The cell proliferation rate was measured using 5-bromo-2-deoxyuridine (BrdU)-labeling cell proliferation assay (BioVision, Milpitas, CA, USA). Cell viability was determined by MTT assay (Sigma-Aldrich).
2.12. Cell migration and invasion assay
Cell migration and invasion assays were performed using transwell migration assay chambers (BD Life Sciences, Franklin Lakes, NJ, USA) with an 8-μm pore size. Bone marrow macrophages isolated from WT or mS6KO mice were layered onto a transwell insert, and migration was assessed with fibroblast-like synoviocyte (FLS) conditioned medium (CM), MCP-1, or RANTES present in the lower well.
2.13. Patients
The demographic and clinical characteristics of the RA and OA patients included in this study are shown in Table S2. Patients with RA were classified according to the 2010 rheumatoid arthritis classification criteria [18], and all RA patients were disease modifying anti-rheumatic drug (DAMARD) naïve state. The disease activity of RA was determined by the disease activity score (DAS) 28-C-reactive protein (CRP) using clinical and laboratory data. Patients with DAS28-CRP > 5.1 were considered to have severe RA [19]. All patients gave informed consent. The biospecimens and data used for this study were provided by Gyeongsang National University Hospital, a member of the Korea Biobank Network. This study was approved by the Institutional Review Board of the Hospital of Gyeongsang National University (Permit No: GNUH 2014-02-013).
2.14. Statistical analysis
Data are expressed as mean ± SEM. Statistical comparisons were made using two-way analysis of variance followed by Fisher's post hoc analysis. The significance of differences between groups was determined using Student's unpaired t-test. A p value <0.05 was considered significant.
3. Results
3.1. Myeloid Sirt6 deficiency increases macrophage infiltration and aggravates joint destruction in experimental arthritic mice
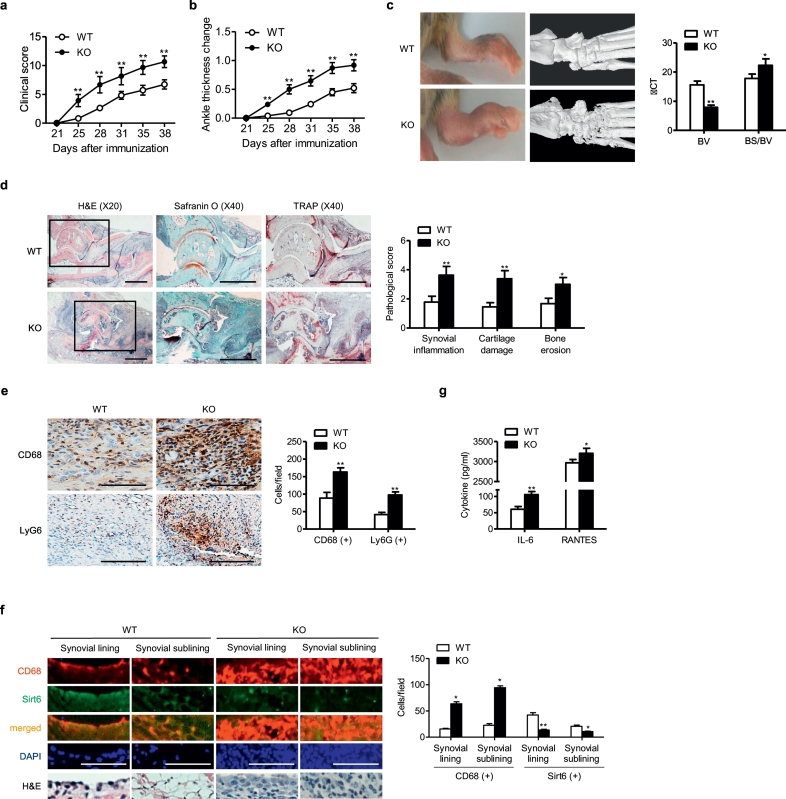
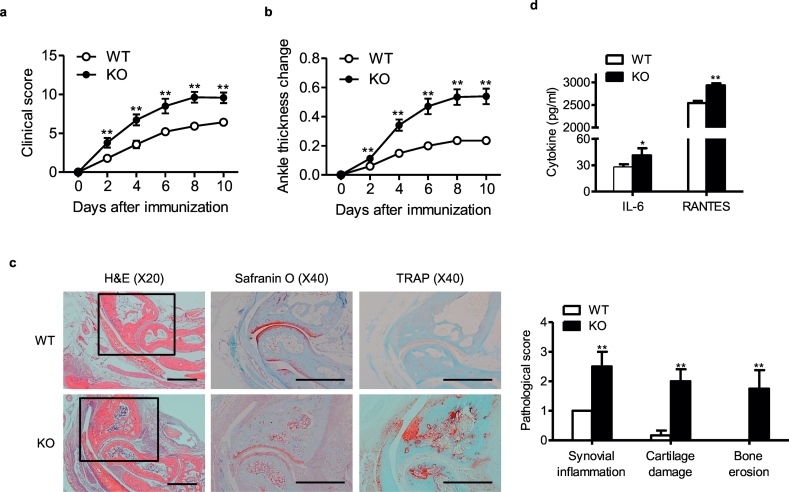
To gain insights into the influence of myeloid Sirt6 suppression on rheumatoid arthritis development, we generated mS6KO mice. Western blotting confirmed successful deletion of Sirt6 in the BMMs of mS6KO mice (Fig. S1). Arthritis was induced in mS6KO mice and their WT littermates using CIA (Fig. 1) and K/BxN serum transfer models (Fig. 2). The arthritis severity determined by clinical score (Figs. 1a and 2a), ankle thickness change (Figs. 1b and 2b), bone destruction in micro-CT examination (Fig. 1c), and pathologic abnormalities such as synovial inflammation, cartilage damage, and bone erosion (Figs. 1d and 2c) were greater in mS6KO mice than in WT mice. We focused on the roles of macrophages because these cells were the predominant cell type infiltrating joint tissues of CIA mice (Fig. 1e). To investigate the impact of myeloid Sirt6 deficiency on the infiltration of macrophages in arthritic joints of CIA, we performed co-staining of CD68, as a marker for macrophages, and Sirt6. Sirt6 expression was decreased, whereas CD68 immunoreactivity was remarkably increased in the lining and sublining layers of hyperplastic synovium in the mS6KO mice compared with WT mice (Fig. 1f). Real-time RT-PCR confirmed the increased accumulation of proinflammatory macrophages in the ankles of the mS6KO mice compared with WT mice; mRNA levels of M1 macrophage-related genes (Figs. S2a and S2e), chemokines and their receptors (Figs. S2b and S2f), and protease (Figs. S2c and S2 g) were increased, while M2 macrophage marker genes were decreased (Figs. S2d and S2 h) in mS6KO mice with CIA and K/BxN serum transfer arthritis. Accordingly, serum levels of IL-6 and RANTES were increased in mS6KO mice (Figs. 1g and 2d). Collectively, these results suggest that myeloid Sirt6 deficiency aggravates the inflammatory response and joint destruction by increasing recruitment of proinflammatory macrophages into arthritic joints.
Fig. 1.
Worsening of arthritis in mS6KO mice with CIA. (a) Mean clinical score (n = 10–12), (b) ankle thickness change (n = 10–12), and (c) gross and micro-CT images and score for bone volume (BV) and ratio of bone surface to bone volume (BS/BV) (n = 7) of CIA mice. (d) Pathological staining with H&E, Safranin-O, and TRAP (bar = 1 mm), and scores for synovial inflammation, cartilage damage, and bone erosion (n = 9). (e) Staining with CD68 (for macrophages) or Ly6G (for neutrophils) and mean number of CD68+ macrophages or Ly6G+ neutrophils in the ankle joints (bar = 50 μm) (n = 5). (f) Staining with CD68 or Sirt6 and mean number of CD68- or Sirt6-positive cells in the synovial lining and sub-lining of ankle joints (bar = 100 μm) (n = 3). (g) Serum concentrations of IL-6 and RANTES (n = 10–12). Values are mean ± SEM. ⁎, p < 0.05 and ⁎⁎, p < 0.01 vs. WT.
Fig. 2.
Worsening of arthritis in mS6KO mice with K/BxN serum transfer arthritis. (a) Mean clinical score (n = 14), (b) ankle thickness change (n = 14), and (c) pathological staining with H&E, Safranin-O, and TRAP (bar = 1 mm) and scores for synovial inflammation, cartilage damage, and bone erosion (n = 4–6). (d) Serum concentrations of IL-6 and RANTES (n = 5–6). Values are mean ± SEM. ⁎, p < 0.05 and ⁎⁎, p < 0.01 vs. WT.
3.2. Myeloid Sirt6 deficiency augments the migration potential of macrophages toward synoviocyte-derived chemoattractants
To investigate the effects of Sirt6 deficiency on the migration and invasion of macrophages, we isolated BMMs from WT or mS6KO mice and performed transwell migration assays. It has previously been reported that MCP-1 and RANTES are major chemokines produced by RA-FLS and contributes to the chemotaxis of monocytes [20,21]. We observed significant excretion of MCP-1 and RANTES in the culture supernatants after stimulation of FLS with LPS or TNF-α (Fig. 3a). When MCP-1 and RANTES were used as chemoattractants, BMMs from mS6KO mice showed increased migratory activity and invasion rate compared with those from WT mice (Fig. 3b and Fig. S3a). Similarly, when LPS– or TNF-α–stimulated FLS-CM was used as the chemoattractant, BMMs from mS6KO mice exhibited enhanced migration and invasion compared to WT cells (Fig. 3c and Fig. S3b). Additionally, CCR3 (a receptor for chemokines including RANTES) was highly expressed in LPS-treated BMMs from mS6KO mice compared with WT (Fig. 3d–f), suggesting that Sirt6-deficient macrophages have an increased potential for migration toward joint synovium in part via CCR3 upregulation through a yet unidentified mechanism.
Fig. 3.
Regulation of macrophage migration by Sirt6. (a) FLS were cultured with 10 ng/ml LPS or 20 ng/ml TNF-α for 24 h, and chemokine concentrations in the culture supernatants were analyzed by ELISA (n = 4–5). (b, c) BMMs from WT or mS6KO mice were allowed to migrate through porous membranes for 3 h for cell migration toward 10 ng/ml MCP-1, 20 ng/ml RANTES or fibroblast-like synoviocyte-conditioned medium (FLS-CM). Representative microphotographs (original magnification ×40). Mean number of migratory cells in trans-well chamber were counted (n = 3). (d–f) BMMs from WT or mS6KO mice were treated with 10 ng/ml LPS for 3 h and subjected to analysis of CCR3 expression (n = 4–5) and confocal microscopic analysis for subcellular localization of CCR3. Values are mean ± SEM. ⁎, p < 0.05 and ⁎⁎p < 0.01 vs. WT; #, p < 0.05 and ##, p < 0.01 vs. VEH. VEH, Vehicle.
3.3. Myeloid Sirt6 deficiency increases the proliferation and viability of BMMs
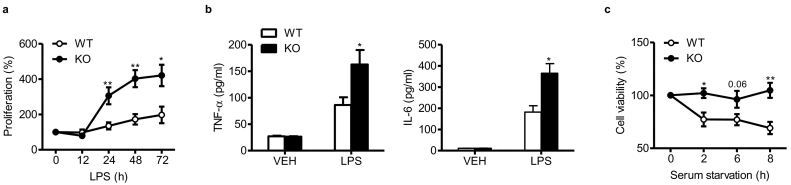
The proliferative response was significantly increased in LPS-treated BMMs isolated from mS6KO mice compared with those from WT mice with concomitant excretion of TNF-α and IL-6 (Fig. 4a and b), indicating an anti-inflammatory function of Sirt6. Moreover, viability under serum-free conditions was significantly higher in BMMs from mS6KO mice than those from WT mice (Fig. 4c). Together with the increased migratory activity of Sirt6-deficient macrophages, these results confirm the in vivo finding of increased numbers of macrophages in the synovium of mS6KO arthritic mice.
Fig. 4.
Regulation of macrophage survival and cytokine excretion by Sirt6. (a, b) BMMs (2 × 105) from WT or mS6KO mice were treated with 10 ng/ml LPS as indicated (a) or for 24 h (b), followed by washing twice with PBS. Proliferation rate and excretion of TNF-α and IL-6 into culture medium were determined by BrdU incorporation assay and ELISA analysis, respectively. (c) BMMs (2 × 105) were cultured in serum-starved conditions as indicated, and cell viability was determined by MTT assay. Values are mean ± SEM (n = 5–7). ⁎, p < 0.05 and ⁎⁎, p < 0.01 vs. WT.
3.4. Sirt6 deacetylates FoxO1 to trigger its nuclear export and proteasomal degradation
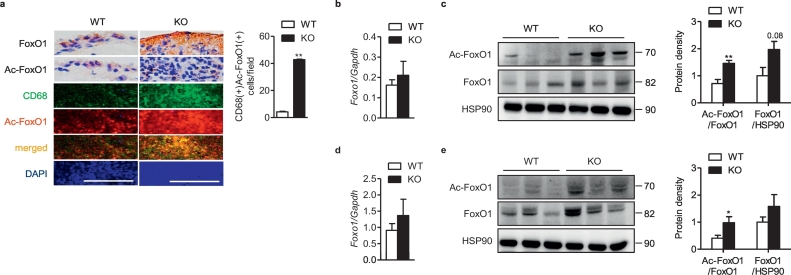
Knowing that acetylated FoxO1 regulates diverse proinflammatory functions of monocytes/macrophages including chemotaxis [[22], [23], [24]], and that FoxO1 is deacetylated by Sirt6 [14], we hypothesized that myeloid Sirt6 would regulate the inflammatory response by deacetylating FoxO1 in arthritic joints. To address this, we examined FoxO1 acetylation in joint tissues of CIA mice. Our results showed that expression levels of FoxO1 and Ac-FoxO1 in joint tissues and specific localization of Ac-FoxO1 in CD68-positive macrophages were noticeably increased in the synovium of mS6KO mice with CIA (Fig. 5a–c) or K/BxN serum transfer arthritis (Fig. 5d and e), indicating the role of Sirt6 in the control of FoxO1 acetylation in macrophages infiltrating arthritic joint tissues. The expression of two other members of this family, FoxO3a and FoxO4, were also highly enhanced in RA synovium (Fig. S4a-S4c) compared with OA synovium. Interestingly, the protein level of FoxO4, but not of FoxO3a, was significantly increased in KO joint tissues with CIA compared to WT mice (Figs. S4d and S4e).
Fig. 5.
Total- and acetylated-FoxO1 in mice with CIA (a-c) or K/BxN serum transfer arthritis (d, e). All experimental procedures were same as described in Fig. 1, Fig. 2 legends. (a) Staining of FoxO1, Ac-FoxO1, and CD68 in ankle joints (bar = 100 μm), and mean number of CD68 and Ac-FoxO1 double-positive cells (n = 3). (b, c) mRNA and protein levels of total- and Ac-FoxO1 in the ankle joints of CIA mice (n = 9–11). (d, e) mRNA (n = 5–6) and protein levels (n = 3–5) of total- and Ac-FoxO1 in the ankle joints of K/BxN serum transfer arthritis. Values are mean ± SEM. ⁎, p < 0.05 and ⁎⁎, p < 0.01 vs. WT.
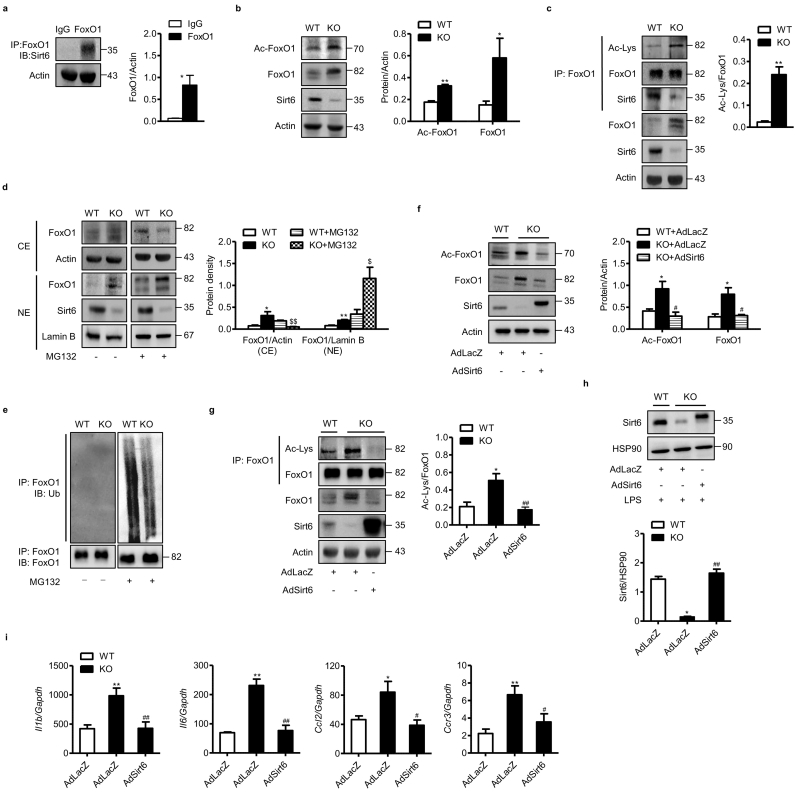
To further validate these in vivo results, we tested FoxO1 deacetylation by Sirt6 in BMMs isolated from WT and mS6KO mice. Co-immunoprecipitation assays showed a direct physical interaction between FoxO1 and Sirt6 in BMMs (Fig. 6a). Additionally, total- and acetylated-FoxO1 protein levels were elevated significantly in the KO BMMs compared with WT BMMs (Fig. 6b and c), suggesting that Sirt6 plays a critical role in the regulation of FoxO1 protein levels. To evaluate if proteasome-ubiquitin pathway is primarily responsible for the increase of FoxO1 in KO BMMs, we treated BMMs with the proteasome inhibitor MG132. In myeloid Sirt6 KO BMMs, MG132 treatment significantly increased FoxO1 protein level in the nuclear fraction (Fig. 6d) and suppressed its ubiquitination in the cytosol (Fig. 6e). Lastly, adenoviral-mediated Sirt6 overexpression in KO BMMs decreased the protein levels of total- and acetylated-FoxO1 (Fig. 6f and g) as well as the expression of FoxO1 downstream target genes (Fig. 6h and i). Taken together, Sirt6 deacetylates FoxO1 in the nucleus, and facilitates its proteasome-mediated degradation in the cytosol.
Fig. 6.
Regulation of acetylation and degradation of FoxO1 by Sirt6. (a) BMMs were prepared from WT mice, and the interaction between Sirt6 and FoxO1 was analyzed. (b, c) Protein levels of FoxO1 and acetylated FoxO1 in BMMs from WT and mS6KO mice. (d) BMMs from WT or mS6KO mice were treated with 10 μM MG132 for 3 h, and protein levels of FoxO1 in cytosolic extract (CE) and nuclear extract (NE) were determined. (e) Total lysates of BMMs were immunoprecipitated with anti–FoxO1-antibodies and immunoblotted with anti-ubiquitin antibodies. (f, g) Protein levels of total and acetylated FoxO1 in BMMs whole lysates following transduction with AdLacZ or AdSirt6. (h, i) Following transduction of BMMs with AdLacZ or AdSirt6, cells were treated with 10 ng/ml LPS for 3 h and protein levels of Sirt6 and the expression of cytokines were determined. Values are mean ± SEM (n = 6). ⁎, p < 0.05 and ⁎⁎, p < 0.01 vs. WT; #, p < 0.05 and ##, p < 0.01 vs. AdLacZ.
3.5. Expression and activity of Sirt6 are suppressed in PBMCs and macrophages of RA patients
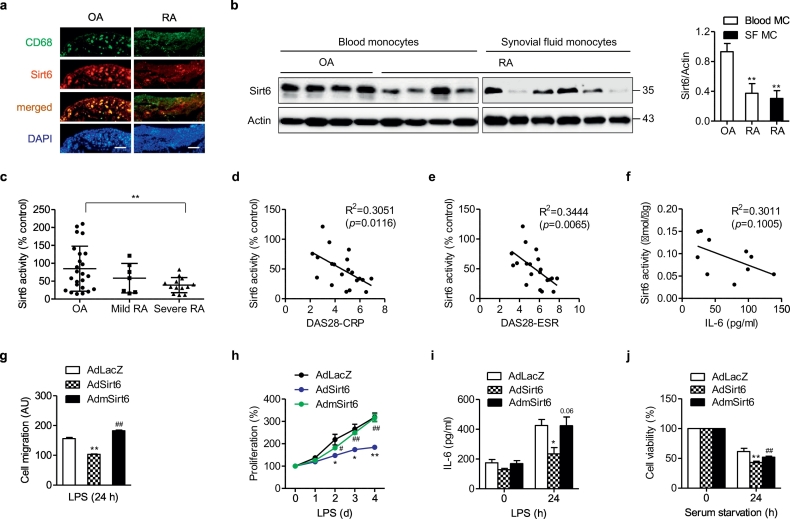
To provide clinical relevance, we compared Sirt6 expression and/or activity in patients with RA and osteoarthritis (OA). The protein levels of Sirt6 were markedly decreased in synovial tissues, blood-derived monocytes, and synovial fluid monocytes of RA patients compared with OA patients (Fig. 7a and b). The Sirt6 activity was also decreased in PBMCs of patients with severe RA compared with OA patients (Fig. 7c). Furthermore, Sirt6 activity in PBMCs negatively correlated with disease activity and IL-6 concentration in RA patients (Figs. 7d–f). These findings suggest that reduced expression and enzymatic activity of Sirt6 were closely related to the inflammatory activity of RA patients.
Fig. 7.
Expression and activity of Sirt6 in RA patients and relationship with disease severity. (a) Staining with CD68 and Sirt6 in the synovial tissue from osteoarthritis (OA) and RA patients (bar = 100 μm). (b) Protein levels of Sirt6 in blood monocytes and synovial fluid monocytes from OA patients and RA patients (n = 4–6). (c) Sirt6 enzyme activity in PBMCs from patients with OA (n = 23), mild RA (DAS 28 ≤ 5.0, n = 7), and severe RA (DAS28 > 5.1, n = 13). (d) Correlation analysis of Sirt6 enzyme activity in PBMCs with RA severity (DAS28-CRP, n = 20), (e) DAS28-ESR (n = 20), and (f) IL-6 concentration (n = 10) in serum. (g–j) Synovial fluid monocytes/macrophages were transduced with AdSirt6 or AdmSirt6 and then incubated with 10 ng/ml LPS for 24 h (G) or as indicated (H, I). Cell migration (n = 4), cell proliferation (n = 3), IL-6 excretion (n = 4), and cell viability under serum-starved condition (n = 4) were determined. Values are mean ± SEM. ⁎, p < 0.05 and ⁎⁎, p < 0.01 vs. OA or AdLacZ. #, p < 0.05 and ##, p < 0.01 vs. AdSirt6. Blood MC, blood monocytes; SF MC, synovial fluid monocytes; DAS, disease activity score.
Finally, we overexpressed Sirt6 in the synovial fluid macrophages of RA patients to validate the requirement for Sirt6 in the suppression of inflammation. Compared with macrophages transduced with AdLacZ, those transduced with AdSirt6 exhibited decreased LPS-stimulated migration, proliferation, IL-6 excretion, and survival (Figs. 7g–j). However, transduction with mutant Sirt6 adenovirus had no effects on RA macrophages, indicating that deacetylase activity is required for Sirt6-dependent immunosuppression.
4. Discussion
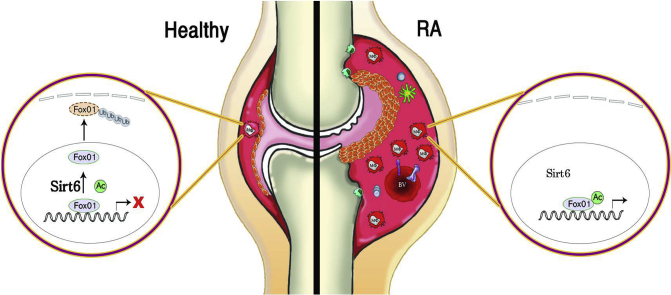
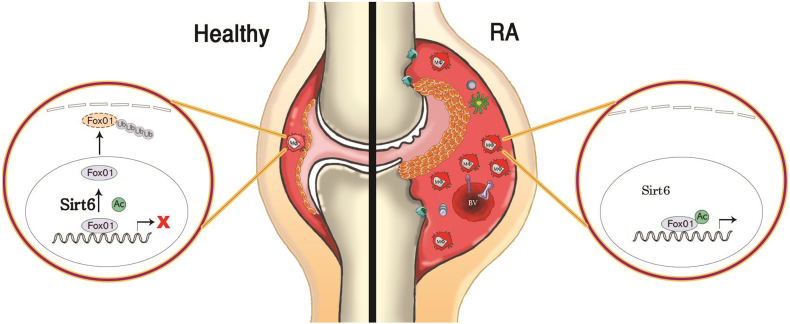
As an extension of our previous finding that myeloid Sirt6 regulates macrophage phenotypes and migration [8], the present study demonstrates a novel role of myeloid Sirt6 in the pathogenesis of RA. In CIA and K/BxN serum transfer arthritis models, mS6KO mice display augmented joint destruction compared with WT mice. Two probable mechanisms underlie the arthritic effects of myeloid Sirt6 deficiency: regulatory effects on macrophage polarization and enhanced macrophage infiltration in the joints (Fig. 8).
Fig. 8.
Proposed scheme. A healthy joint (left side) contains 1–2 layers of FLS with a few macrophages (Mφ) in the synovium. FoxO1 is deacetylated by Sirt6, exported from the nucleus to the cytosol, and targeted for degradation through the ubiquitination-proteasome pathway. In arthritic joint tissues of mS6KO mice (right side), the synovial lining is thickened, the vascularity is increased, and the hyperplastic synovium is infiltrated by inflammatory cells such as macrophages, dendritic cells, and T cells. Sirt6-mediated FoxO1 deacetylation in macrophages is impaired, which recruits more inflammatory cells into synovium. This inflamed condition damages cartilage, bone, and surrounding tissue, causing joint deformity.
Monocytes are critical in the pathogenesis of autoimmune RA through their roles in the production of proinflammatory cytokines [2,3]. In particular, infiltration of circulating monocytes into the joint tissues and subsequent polarization into proinflammatory M1 macrophages are critical for the development and exacerbation of RA [25,26]. Here, we demonstrated that the number of CD68-positive macrophages was increased in the RA synovial compartment in the CIA model, and that this upregulation was associated with higher levels of the inflammatory mediators IL-6 and RANTES (CCL5) in systemic circulation. Consistent with these findings, gene expression analysis showed that mRNA levels of numerous M1 marker genes and genes related to macrophage chemotaxis were highly elevated in the joint tissues of mS6KO mice. These in vivo data are consistent with the results of in vitro transwell migrations showing that macrophages from mS6KO mice migrated faster than WT macrophages in response to FLS CM. Together with our recent report that liver and adipose tissue from mS6KO mice after high-fat diet feeding are highly infiltrated with proinflammatory M1 macrophages [8], these results suggest that myeloid Sirt6 deficiency contributes to RA pathogenesis through accelerating macrophage migration from circulation to joint tissue and phenotypic switching to M1 type.
To evaluate the clinical implications of our study, we further compared Sirt6 activity and expression levels in PBMCs from RA patients and OA patients as controls. We included only DMARD-naïve RA patients to minimize the effect on Sirt6 of anti-rheumatic drugs. In accordance with findings in other inflammatory disease models [8,27], we observed decreased expression and activity of Sirt6 in monocytes/macrophages and PBMCs from RA patients compared with those from OA patients. Additionally, Sirt6 activity was inversely correlated with disease activity score of RA and levels of IL-6, indicating that suppression of Sirt6 activity in PBMCs might be a precondition for increased susceptibility to the development of inflammation or an initial change in the pathogenesis of RA. Additionally, we observed the resolution of inflammatory activity in RA macrophages by overexpression of Sirt6, but not mutant Sirt6, confirming the deacetylase-dependent anti-inflammatory activity of Sirt6 [11]. Consistent with the foregoing discussion, this finding suggests that macrophages with lower Sirt6 activity are an important causative factor in RA pathogenesis.
Given the central role of FoxO1 in the transcriptional regulation of genes involved in the inflammatory pathway [22,24], FoxO1 upregulation in KO macrophages most likely reflects its functional activation. At a mechanistic level, consistent with our previous report [14], Sirt6-mediated deacetylation of FoxO1 provoked nuclear extrusion of FoxO1, which is critical for FoxO1 protein stability. This concept was supported by the findings that pretreatment with MG132 suppressed proteasomal degradation of FoxO1 and ectopic expression of Sirt6 significantly downregulated the expression of FoxO1 target genes. These results are also consistent with recent studies performed by Zhang et al. [28] and Kuang et al. [29] in which Sirt6 deacetylated FoxO1 and suppressed the expression of gluconeogenic and lipolytic genes, respectively. Previous studies have shown that FoxO3a and FoxO4 contribute to the pathogenesis of RA by stimulating cysteine-rich protein 61 (CYR-61)-mediated CCL20 production in RA FLSs [30] and by possibly regulating macrophage function in RA synovium [31], respectively. Consistent with these reports, we observed an increase of FoxO3a and FoxO4 in joint tissues of RA patients with difference of FoxO4 between WT and mS6KO mice, suggesting that FoxO4 may also play an important role in the RA development of mS6KO mice.
Since inflammatory bone resorption is a major cause of morbidity of RA, strategy that restrains bone loss in inflammatory setting is attractive. Several studies support a central role of Sirt6 in inflammation. Sirt6 suppresses NF-κB mediated inflammatory responses by its interaction with the RelA subunit of NF-κB; the recruitment of the Sirt6 to the chromatin at the promoters of RelA targets represses NF-κB target gene expression by deacetylating histone H3K9 [9]. Using well-established joint inflammation models, we and others provide evidence that Sirt6-mediated suppression of NF-κB is effective to prevent joint destruction. In our previous study, local overexpression of Sirt6 was shown to reduce the joint inflammation and tissue destruction in mice with CIA [11]. We also showed that overexpression of Sirt6 in FLS suppressed the production of inflammatory mediators by TNF-α treatment via deacetylation of H3K9 at the NF-κB target gene promoter. Similar to our results, overexpression of Sirt6 in chondrocytes decreased matrix metalloproteinases (MMPs) and intra-articluar injection of Sirt6 lentivirus preserved the cartilage structure in mice [32]. Similarly, Engler et al. [33] also observed a protective effect of Sirt6 in FLS stimulated with cigarette smoke extract or TNF-α, identifying Sirt6 as a regulator of cigarette smoke-induced development and progression of RA. In addition to joint inflammation, a number of studies have demonstrated that Sirt6 exhibits pronounced anti-inflammatory properties in various inflammatory disease models, such as allergic airway inflammation, steatohepatitis, and proteinuric kidney disease [34].
Using animal models and human samples, we demonstrated that myeloid Sirt6 deficiency augments inflammatory arthritis. This conclusion is concordant with our previous study showing the dramatic attenuation of RA severity by Sirt6 overexpression [11]. Together, these observations suggest Sirt6 as a clinical therapeutic target in RA.
Footnotes
This work was presented at the Annual European Congress of Rheumatology took place on 13–16 June 2018 in Amsterdam, Netherlands.
Declarations of interest
None.
Author contributions
SJW, HSN, YHC, SMY, and HMJ performed the experiments and analyzed the data. EJB interpreted the data and wrote the manuscript. SIL and BHP designed the experiments, interpreted the data, and wrote the manuscript. All authors reviewed the manuscript.
Acknowledgements
This research was supported by grants from the Medical Research Center Program (2015R1A5A2008833 and 2017R1A5A2015061) and Basic Science Research Program (2018R1D1A1B07051017) through the National Research Foundation of Korea (NRF), funded by the Korean government. The funders had no role in study design, data collection, data analysis, interpretation, or writing of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.11.005.
Contributor Information
Sang-Il Lee, Email: goldgu@gnu.ac.kr.
Byung-Hyun Park, Email: bhpark@jbnu.ac.kr.
Appendix A. Supplementary data
Supplementary material
References
- 1.Muller-Ladner U., Pap T., Gay R.E., Neidhart M., Gay S. Mechanisms of disease: the molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat Clin Pract Rheumatol. 2005;1:102–110. doi: 10.1038/ncprheum0047. [DOI] [PubMed] [Google Scholar]
- 2.Gierut A., Perlman H., Pope R.M. Innate immunity and rheumatoid arthritis. Rheum Dis Clin North Am. 2010;36:271–296. doi: 10.1016/j.rdc.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinne R.W., Stuhlmuller B., Burmester G.R. Cells of the synovium in rheumatoid arthritis. Macrophages Arthritis Res Ther. 2007;9:224. doi: 10.1186/ar2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulherin D., Fitzgerald O., Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39:115–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- 5.Yanni G., Whelan A., Feighery C., Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994;53:39–44. doi: 10.1136/ard.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haringman J.J., Gerlag D.M., Zwinderman A.H., Smeets T.J., Kraan M.C., Baeten D. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64:834–838. doi: 10.1136/ard.2004.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bresnihan B., Pontifex E., Thurlings R.M., Vinkenoog M., El-Gabalawy H., Fearon U. Synovial tissue sublining CD68 expression is a biomarker of therapeutic response in rheumatoid arthritis clinical trials: consistency across centers. J Rheumatol. 2009;36:1800–1802. doi: 10.3899/jrheum.090348. [DOI] [PubMed] [Google Scholar]
- 8.Lee Y., Ka S.O., Cha H.N., Chae Y.N., Kim M.K., Park S.Y. Myeloid sirtuin 6 deficiency causes insulin resistance in high-fat diet-fed mice by eliciting macrophage polarization toward an M1 phenotype. Diabetes. 2017;66:2659–2668. doi: 10.2337/db16-1446. [DOI] [PubMed] [Google Scholar]
- 9.Kawahara T.L., Michishita E., Adler A.S., Damian M., Berber E., Lin M. SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou K.L., Lin S.K., Chao L.H., Hsiang-Hua Lai E., Chang C.C., Shun C.T. Sirtuin 6 suppresses hypoxia-induced inflammatory response in human osteoblasts via inhibition of reactive oxygen species production and glycolysis-a therapeutic implication in inflammatory bone resorption. Biofactors. 2017;43:170–180. doi: 10.1002/biof.1320. [DOI] [PubMed] [Google Scholar]
- 11.Lee H.S., Ka S.O., Lee S.M., Lee S.I., Park J.W., Park B.H. Overexpression of sirtuin 6 suppresses inflammatory responses and bone destruction in mice with collagen-induced arthritis. Arthritis Rheum. 2013;65:1776–1785. doi: 10.1002/art.37963. [DOI] [PubMed] [Google Scholar]
- 12.Dominy J.E., Jr., Lee Y., Jedrychowski M.P., Chim H., Jurczak M.J., Camporez J.P. The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis. Mol Cell. 2012;48:900–913. doi: 10.1016/j.molcel.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhardwaj A., Das S. SIRT6 deacetylates PKM2 to suppress its nuclear localization and oncogenic functions. Proc Natl Acad Sci U S A. 2016;113 doi: 10.1073/pnas.1520045113. [E538-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song M.Y., Wang J., Ka S.O., Bae E.J., Park B.H. Insulin secretion impairment in Sirt6 knockout pancreatic β cells is mediated by suppression of the FoxO1-Pdx1-Glut2 pathway. Sci Rep. 2016;6:3032. doi: 10.1038/srep30321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang H.Y., Gu S., Lee S.M., Park B.H. Overexpression of sirtuin 6 suppresses allergic airway inflammation through deacetylation of GATA3. J Allergy Clin Immunol. 2016;138:1452–1455. doi: 10.1016/j.jaci.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Woo S.J., Lee S.M., Lim H.S., Hah Y.S., Jung I.D., Park Y.M. Myeloid deletion of SIRT1 suppresses collagen-induced arthritis in mice by modulating dendritic cell maturation. Exp Mol Med. 2016;48 doi: 10.1038/emm.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hah Y.S., Cheon Y.H., Lim H.S., Cho H.Y., Park B.H., Ka S.O. Myeloid deletion of SIRT1 aggravates serum transfer arthritis in mice via nuclear factor-κB activation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aletaha D., Neogi T., Silman A.J., Funovits J., Felson D.T., Bingham C.O., 3rd 2010 Rheumatoid arthritis classification criteria: an American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 19.Fransen J., van Riel P.L. The disease activity score and the EULAR response criteria. Rheum Dis Clin North Am. 2009;35:745–757. doi: 10.1016/j.rdc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Rathanaswami P., Hachicha M., Sadick M., Schall T.J., McColl S.R. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J Biol Chem. 1993;268:5834–5839. [PubMed] [Google Scholar]
- 21.Volin M.V., Shah M.R., Tokuhira M., Haines G.K., Woods J.M., Koch A.E. RANTES expression and contribution to monocyte chemotaxis in arthritis. Clin Immunol Immunopathol. 1998;89:44–53. doi: 10.1006/clin.1998.4590. [DOI] [PubMed] [Google Scholar]
- 22.Su D., Coudriet G.M., Hyun Kim D., Lu Y., Perdomo G., Qu S. FoxO1 links insulin resistance to proinflammatory cytokine IL-1β production in macrophages. Diabetes. 2009;58:2624–2633. doi: 10.2337/db09-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miao H., Zhang Y., Lu Z., Yu L., Gan L. FOXO1 increases CCL20 to promote NF-κB-dependent lymphocyte chemotaxis. Mol Endocrinol. 2012;26:423–437. doi: 10.1210/me.2011-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan W., Morinaga H., Kim J.J., Bae E., Spann N.J., Heinz S. FoxO1 regulates Tlr4 inflammatory pathway signalling in macrophages. EMBO J. 2010;29:4223–4236. doi: 10.1038/emboj.2010.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misharin A.V., Cuda C.M., Saber R., Turner J.D., Gierut A.K., Haines G.K., 3rd Nonclassical Ly6C(−) monocytes drive the development of inflammatory arthritis in mice. Cell Rep. 2014;9:591–604. doi: 10.1016/j.celrep.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Udalova I.A., Mantovani A., Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol. 2016;12:472–485. doi: 10.1038/nrrheum.2016.91. [DOI] [PubMed] [Google Scholar]
- 27.Ka S.O., Bang I.H., Bae E.J., Park B.H. Hepatocyte-specific sirtuin 6 deletion predisposes to nonalcoholic steatohepatitis by up-regulation of Bach1, an Nrf2 repressor. FASEB J. 2017;31:3999–4010. doi: 10.1096/fj.201700098RR. [DOI] [PubMed] [Google Scholar]
- 28.Zhang P., Tu B., Wang H., Cao Z., Tang M., Zhang C. Tumor suppressor p53 cooperates with SIRT6 to regulate gluconeogenesis by promoting FoxO1 nuclear exclusion. Proc Natl Acad Sci U S A. 2014;111:10684–10689. doi: 10.1073/pnas.1411026111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuang J., Zhang Y., Liu Q., Shen J., Pu S., Cheng S. Fat-specific Sirt6 ablation sensitizes mice to high-fat diet-induced obesity and insulin resistance by inhibiting lipolysis. Diabetes. 2017;66:1159–1171. doi: 10.2337/db16-1225. [DOI] [PubMed] [Google Scholar]
- 30.Kok S.H., Lin L.D., Hou K.L., Hong C.Y., Chang C.C., Hsiao M. Simvastatin inhibits cysteine-rich protein 61 expression in rheumatoid arthritis synovial fibroblasts through the regulation of sirtuin-1/FoxO3a signaling. Arthritis Rheum. 2013;65:639–649. doi: 10.1002/art.37807. [DOI] [PubMed] [Google Scholar]
- 31.Ludikhuize J., de Launay D., Groot D., Smeets T.J., Vinkenoog M., Sanders M.E. Inhibition of forkhead box class O family member transcription factors in rheumatoid synovial tissue. Arthritis Rheum. 2007;56:2180–2191. doi: 10.1002/art.22653. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y., Chen L., Wang Y., Li W., Lin Y., Yu D. Overexpression of Sirtuin 6 suppresses cellular senescence and NF-κB mediated inflammatory responses in osteoarthritis development. Sci Rep. 2015;5:17602. doi: 10.1038/srep17602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engler A., Niederer F., Klein K., Gay R.E., Kyburz D., Camici G.G. SIRT6 regulates the cigarette smoke-induced signalling in rheumatoid arthritis synovial fibroblasts. J Mol Med (Berl) 2014;92:757–767. doi: 10.1007/s00109-014-1139-0. [DOI] [PubMed] [Google Scholar]
- 34.Liu M., Liang K., Zhen J., Zhou M., Wang X., Wang Z. Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat Commun. 2017;8:413. doi: 10.1038/s41467-017-00498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material