Abstract
Background
Soluble suppression of tumorigenicity-2 (sST2) is a novel biomarker shown to be useful for prognostic assessment in heart failure (HF). However, very limited data exists about its prognostic utility in patients with HF in India.
Methods
We studied 150 patients [mean age 67.7 ± 13.3, 93 (62%) males], hospitalized with clinical HF, irrespective of their left ventricular ejection fraction (LVEF). HF was confirmed by N-terminal probrain natriuretic peptide (NT-proBNP) value above 125 ng/L. Primary end point was death or cardiac transplant at 1-year follow-up, with additional telephonic follow-up performed at 2 years. The clinical outcomes were correlated with the sST2 values obtained at the time of initial hospitalization.
Results
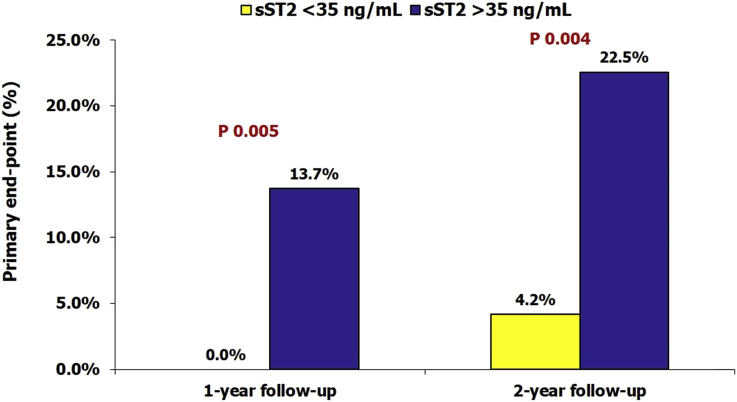
HF was ischemic in origin in 82.0% patients. The primary outcome occurred in 9.3% patients at the end of 1-year follow-up and in 16.7% patients at the end of 2 years. The patients who had events had significantly higher NT-proBNP and sST2 values, but there was no difference in the clinical characteristics, cause of HF, baseline LVEF, or serum creatinine. The patients with elevated sST2 levels (>35 ng/mL) had substantially higher event rates than those with normal sST2 levels (13.7% vs 0.0% at 1-year, P = 0.005; 22.5% vs 4.2% at 2-years, P = 0.004). On multivariate analysis, sST2 was the strongest predictor of adverse outcomes at both 1-year and 2-year follow-ups.
Conclusion
In patients hospitalized for HF, elevated sST2 >35 ng/mL at the time of initial hospitalization was associated with significantly high mortality over a 2-year period. The prognostic value of sST2 was incremental to that of NT-proBNP. These findings suggest that a single elevated sST2 value at the time of hospitalization should alert the physicians about the high risk of adverse outcomes and should help facilitate timely intensification of HF treatment.
Keywords: ST2, Heart failure, Biomarkers, BNP, NT-ProBNP, Natriuretic peptides
1. Introduction
As in the rest of the world, heart failure (HF) is also a growing problem in India. This is due to improved survival after acute coronary syndromes and increased longevity. At the same time, novel treatment options are also growing rapidly, making it important to identify patients who are more likely to have adverse outcomes and in whom more aggressive guideline-mandated therapies should be directed.
Currently, natriuretic peptides, such as brain natriuretic peptide (BNP) and N-terminal probrain natriuretic peptide (NT-proBNP), are the most commonly used biomarkers for diagnosis and prognostication in HF. Soluble suppression of tumorigenicity-2 (sST2), also known as interleukin-1 receptor-like 1 (IL1RL1), is a member of the interleukin-1 receptor family and is a relatively novel biomarker developed for this purpose.1, 2 The ST2 protein has two isoforms directly implicated in the progression of cardiac disease: the circulation, soluble isoform (sST2) and a cell membrane-bound isoform, ST2L. When sST2 levels are low, sST2's ligand, interleukin-33 (IL-33), is available to bind to ST2L and has a cardioprotective effect resulting in preserved cardiac function. However, when the levels of sST2 are elevated, sST2 competitively binds to IL-33, making IL-33 less likely to bind to ST2L and ultimately making IL-33 unavailable for cardioprotective signaling. IL-33/ST2L signaling is a mechanically activated, cardioprotective system, which is therapeutically beneficial in regulating the myocardial response to injury. sST2 acts as a decoy receptor and, by sequestering IL-33, antagonizes the cardioprotective effects of IL-33/ST2L interaction. The heart is subjected to greater stress in the presence of high levels of sST2, leading to cellular death and tissue fibrosis, with reduced cardiac function, and an increase in the rate of disease progression.3, 4 Although natriuretic peptides, such as BNP and NT-proBNP, are well established in the diagnosis and prognosis in HF, several studies have demonstrated that sST2 is a stronger predictor of cardiac outcomes in both acute and chronic HF.5, 6, 7, 8, 9, 10, 11, 12, 13 However, while there is now extensive data available about the prognostic utility of sST2, only limited such data are available for patients with HF in India.14
2. Material and methods
This was a single-center study at a tertiary care hospital from 2014 to 2016 of all patients hospitalized under our care with clinical evidence of chronic HF, and an NT-proBNP value of >125 ng/L, considered diagnostic of HF as per the 2015 European Society of Cardiology guidelines.5 The patients were either admitted for the first time or were repeat hospitalization for chronic HF. The patients were excluded if they had presented with HF due to acute coronary syndromes or accelerated hypertension or were in cardiogenic shock or suffered from any serious illness other than HF at the time of presentation. A total of 150 patients with the aforementioned criteria were included in the study.
All the subjects were directly under the care of the principal author. Clinical examination, laboratory evaluation, and echocardiography were performed as part of their routine cardiac care. Laboratory evaluation included measurement of NT-proBNP and sST2, along with other routine investigations as per clinical judgment. For NT-proBNP and sST2 measurement, blood samples were drawn in fasting or nonfasting state within 12 h of hospitalization. Nonfasting state samples were deemed acceptable, as serum/plasma sST2 concentrations are independent of the fasting status. Determination of serum NT-proBNP was done by electrochemiluminescence immunoassay on a cobas® e 411-immunoassay analyzer (Roche Diagnostics, IN, USA). The kit assay contains two monoclonal antibodies, which recognize epitopes located in the N-terminal region of proBNP.
Three different enzyme-linked immunosorbent assays (ELISA) are currently being used to determine circulating sST2 concentrations—the Presage® ST2 assay (Critical Diagnostics, San Diego, CA, USA), the MBL ST2 assay (Medical & Biological Laboratories), and the R&D ST2 assay (R&D Systems). The US Food and Drug Administration (FDA) has approved Presage® assay for clinical use, and in the present study, sST2 was quantitatively measured using the Presage® ST2 assay.15, 16 The kit is an in vitro diagnostic device that quantitatively measures sST2 in serum by ELISA method in a microtiter plate format. The assay utilizes two monoclonal antibodies against sST2 and can measure sST2 in serum, ethylenediaminetetraacetic acid plasma, or heparinized plasma.
All the patients received guideline-directed medical therapy, optimized to maximally tolerable doses. After the initial hospitalizations, the patients were followed up in the outpatient department for a minimum study period of 12 months from the day of initial hospitalization. The primary end point of the study was either cardiac transplantation or death because of cardiac cause at the end of 1-year follow-up. Subsequently, telephonic follow-up was performed to record occurrence of these events during the second year of follow-up. Repeat hospitalizations were not taken into account, as patients often got admitted at different health-care facilities because of a variety of reasons, not always related to HF.
The study protocol was approved by the institutional ethics committee.
2.1. Statistical analysis
The data were managed on Microsoft Excel spreadsheet and analyzed using SPSS for Windows (release 20.0; SPSS Inc, Chicago, IL, USA). All the quantitative values were expressed as mean ± standard deviation, but NT-proBNP and sST2 were presented as median with interquartile ranges (IQR) as they were not normally distributed. Categorical variables were presented as actual number with percentages. Standard descriptive analysis was performed to analyze the baseline characteristics of the study population. The comparisons among different groups were performed using chi-square test for categorical variables and Student's independent samples t-test and Mann–Whitney U test for continuous variables. A multivariate analysis was performed to determine independent predictors of primary outcome event. Because the US FDA has accepted sST2 values >35 ng/mL as a predictor of worse prognosis, the patients were also categorized into two groups based on this cutoff value, and the two groups were compared for baseline characteristics and clinical outcomes. The Presage® ST2 assay has an upper limit of measurement of 200 ng/mL with values higher than this reported as >200 ng/mL. For the purpose of the present analysis, all the values >200 ng/mL were treated as 200 ng/mL.
3. Results
A total of 150 subjects [mean age 67.7 ± 13.3, 93 (62%) males] meeting the eligibility criteria were included in the study.
3.1. Baseline characteristics
The baseline characteristics of the study subjects are presented in Table 1. Ischemic heart disease was the dominant cause of HF, seen in 82.0% cases. Mean left ventricular ejection fraction (LVEF) was 38.4 ± 14.5% with 56% having LVEF ≤40% and the remaining 44% having relatively preserved LVEF (>40%). The median NT-proBNP level was 3303 ng/mL (IQR 1227–9544), and the median sST2 was 58.5 ng/mL (IQR 29–117.5).
Table 1.
Baseline characteristics of the study subjects.
| Parameter | Values |
|---|---|
| Age, years | 67.7 ± 13.3 |
| Male gender | 93 (62%) |
| Hypertension | 109 (72.7%) |
| Diabetes mellitus | 86 (57.3%) |
| Cause of heart failure | |
| Ischemic | 123 (82.0%) |
| Nonischemic | 27 (18.0%) |
| Serum creatinine, mg/dL | 1.5 ± 1.2 |
| LVEF | |
| Mean value, % | 38.4 ± 14.5 |
| LVEF > 40% | 66 (44.0%) |
| LVEF ≤ 40% | 84 (56.0%) |
| NT-proBNP, ng/L | 3303 (1227–9544) |
| sST2, ng/mL | 58.5 (29–117.5) |
Quantitative variables are expressed as actual numbers with percentages in parentheses; quantitative variables are expressed as mean ± standard deviation, except NT-proBNP and sST2 which are presented as median with interquartile ranges.
LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal probrain natriuretic peptide; sST2, soluble suppression of tumorigenicity-2.
3.2. Primary end point during follow-up
The follow-up information about the occurrence of the primary end point could be obtained for all the patients included in the study.
There were a total of 13 deaths and one cardiac transplant (overall primary event rate, 9.3%) at the end of 1-year follow-up. There was no difference in the clinical characteristics, cause of HF, and baseline LVEF or serum creatinine levels between those who did and those who did not have primary end point (Table 2). However, the patients who had a primary event had significantly higher NT-proBNP levels [12710 ng/L (IQR 3830–28943) vs 2943 ng/L (IQR 1158–7989), P = 0.001] and ST2 values [157.5 ng/mL (IQR 80.8–200.0) vs 53.5 ng/mL (27.3–106.0)] as compared to those who did not have a primary end point (Table 2).
Table 2.
Baseline characteristics of the study subjects categorized according to the occurrence of primary outcome event (death or cardiac transplant) at 2 years.
| Parameter | 1-year follow-up |
2-year follow-up |
||||
|---|---|---|---|---|---|---|
| Without event (n = 136) | With event (n = 14) | P-value | Without event (n = 125) | With event (n = 25) | P-value | |
| Age, years | 67.9 ± 12.7 | 65.53 ± 18.4 | 0.52 | 67.4 ± 12.8 | 69.3 ± 15.8 | 0.51 |
| Male gender | 84 (61.8%) | 9 (64.3%) | 1.0 | 78 (62.4%) | 15 (60%) | 0.83 |
| Hypertension | 100 (73.5%) | 9 (64.3%) | 0.53 | 92 (73.6%) | 17 (68.0%) | 0.63 |
| Diabetes | 79 (58.1%) | 7 (50.0%) | 0.58 | 73 (58.4%) | 13 (52.0%) | 0.66 |
| Cause of heart failure | ||||||
| Ischemic | 113 (83.1%) | 10 (71.4%) | 0.28 | 102 (81.6%) | 21 (84%) | 1.0 |
| Nonischemic | 23 (16.9%) | 4 (28.6%) | 23 (18.4%) | 4 (16%) | ||
| Serum creatinine, mg/dL | 1.4 ± 1.2 | 1.9 ± 1.3 | 0.16 | 1.4 ± 1.2 | 1.8 ± 1.2 | 0.21 |
| LVEF | ||||||
| Mean value, % | 39.0 ± 14.2 | 32.3 ± 16.8 | 0.11 | 39.0 ± 13.9 | 35.2 ± 16.8 | 0.23 |
| LVEF > 40% | 60 (44.1%) | 6 (42.9%) | 1.0 | 55 (44%) | 11 (44%) | 1.0 |
| LVEF ≤ 40% | 76 (55.9%) | 8 (57.1%) | 70 (56%) | 14 (56%) | ||
| NT-proBNP, ng/L | 2943 (1158–7989) | 12710 (3830–28943) | 0.001 | 2807 (1103–6223) | 11253 (2995–24660) | <0.001 |
| sST2, ng/mL | 53.5 (27.3–106.0) | 157.5 (80.8–200.0) | <0.001 | 53.0 (27.0–97.5) | 141.0 (57.0–200.0) | <0.001 |
Quantitative variables are expressed as actual numbers with percentages in parentheses; quantitative variables are expressed as mean ± standard deviation, except NT-proBNP and sST2 which are presented as median with interquartile ranges.
LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal probrain natriuretic peptide; sST2, soluble suppression of tumorigenicity-2.
Another 11 patients died during the second year of follow-up with no further cardiac transplant, resulting in an overall primary event rate of 16.7%. The patient who had undergone transplant during the first year was alive at the end of 2-year follow-up. Comparison of the patients based on 2-year events yielded same results as observed for 1-year outcomes (Table 2).
3.3. sST2 subgroups and primary end point
A total of 48 (32.0%) subjects had normal sST2 values (<35 ng/mL) at baseline. The patients who had elevated sST2 were more likely to be male (67.6% vs 50.0%, P = 0.048) and had higher serum creatinine values (1.7 ± 1.4 vs 1.1 ± 0.6 mg/dL, P = 0.012) but did not differ in terms of other clinical characteristics or LVEF (Table 3).
Table 3.
Baseline characteristics of the study subjects categorized according to the sST2 levels.
| Parameter | Normal sST2 (n = 48) | Elevated sST2 (n = 102) | P-value |
|---|---|---|---|
| Age, years | 65.2 ± 13.5 | 68.9 ± 13.1 | 0.11 |
| Male gender | 24 (50.0%) | 69 (67.6%) | 0.048 |
| Hypertension | 34 (70.8%) | 75 (73.5%) | 0.85 |
| Diabetes | 28 (58.3%) | 58 (56.9%) | 1.0 |
| Cause of heart failure | |||
| Ischemic | 38 (79.2%) | 85 (83.3%) | 0.65 |
| Nonischemic | 10 (20.8%) | 17 (16.7%) | |
| Serum creatinine, mg/dL | 1.1 ± 0.6 | 1.7 ± 1.4 | 0.012 |
| LVEF | |||
| Mean value, % | 40.6 ± 13.7 | 37.4 ± 14.8 | 0.211 |
| LVEF > 40% | 24 (50.0%) | 42 (41.2%) | 0.38 |
| LVEF ≤ 40% | 24 (50.0%) | 60 (58.8%) | |
| NT-proBNP, ng/L | 1233 (393–3446) | 4380 (2000–11829) | <0.001 |
| sST2, ng/mL | 22.5 (14.3–28.8) | 86 (56–172.3) | <0.001 |
| Primary end point | |||
| At 1-year follow-up | 0 (0%) | 14 (13.7%) | 0.005 |
| At 2-year follow-up | 2 (4.2%) | 23 (22.5%) | 0.004 |
Quantitative variables are expressed as actual numbers with percentages in parentheses; quantitative variables are expressed as mean ± standard deviation, except NT-proBNP and sST2 which are presented as median with interquartile ranges.
LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal probrain natriuretic peptide; sST2, soluble suppression of tumorigenicity-2.
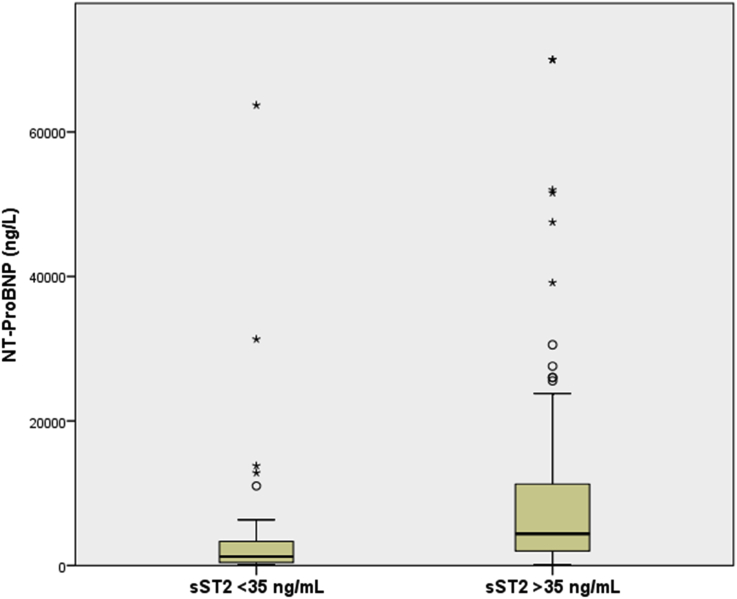
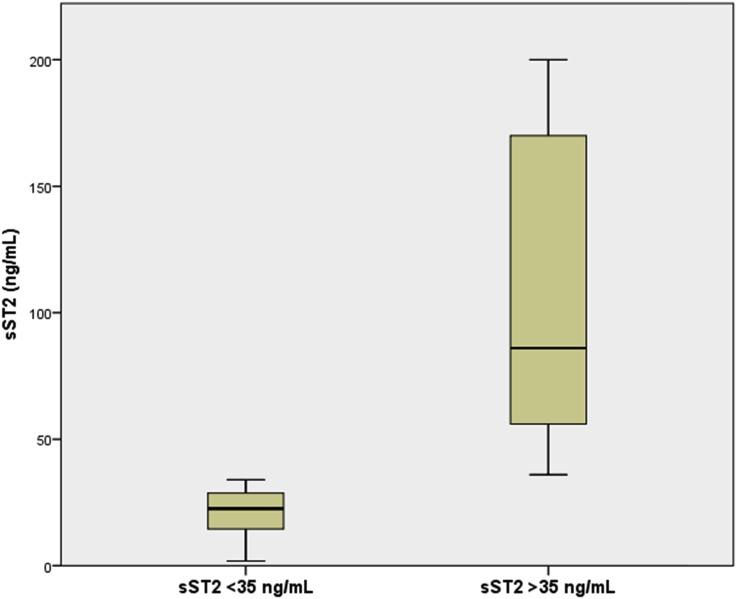
Distribution of NT-proBNP and sST2 values in patients with and without elevated sST2 (>35 ng/mL) are depicted in Fig. 1, Fig. 2. As expected, the patients who had higher sST2 levels also had significantly elevated NT-proBNP values [4380 (IQR 2000–11829) vs 1233 (IQR 393–3446), P < 0.001]. The patients with elevated sST2 levels had significantly higher incidence of primary outcomes at 1- and 2-year follow-ups with only two events (4.2%) occurring in the normal sST2 groups and 23 events (22.5%, P = 0.004) in the elevated sST2 group at the end of 2 years (Fig. 3).
Fig. 1.
Distribution of NT-proBNP values in patients with and without elevated sST2 levels at baseline. NT-proBNP, N-terminal probrain natriuretic peptide; sST2, soluble suppression of tumorigenicity-2.
Fig. 2.
Distribution of sST2 values in patients with and without elevated sST2 levels at baseline. sST2, soluble suppression of tumorigenicity-2.
Fig. 3.
Incidence of primary end point in patients with and without elevated sST2 levels at baseline. sST2, soluble suppression of tumorigenicity-2.
3.4. Independent prognostic value of sST2
Because only NT-proBNP and sST2 were associated with the primary outcome, a multivariate analysis was performed using only these parameters as independent variables and the primary outcome as the dependent variable. At 1-year follow-up, only sST2 was found to be the independent predictor of primary outcome (P = 0.002), whereas NT-proBNP showed only a trend (P = 0.051) (Table 4). At 2-year follow-up, both sST2 and NT-proBNP were predictive of outcome (P values 0.001 and 0.013, respectively), with sST2 having greater prognostic power (reflected in higher Wald value) (Table 4).
Table 4.
Multivariate logistic regression analysis for prediction of primary outcome.
| Parameter | Unstandardized coefficient B | Standard error | Wald | P-value | Exp(B) |
|---|---|---|---|---|---|
| One-year follow-up | |||||
| NT-proBNP | <0.001 | <0.001 | 3.808 | 0.051 | 1.000 |
| SST2 | 0.014 | 0.004 | 9.336 | 0.002 | 1.014 |
| Constant | −4.115 | 0.691 | 35.427 | <0.001 | 0.016 |
| Two-year follow-up | |||||
| NT-proBNP | <0.001 | <0.001 | 6.196 | 0.013 | 1.000 |
| SST2 | 0.011 | 0.004 | 10.685 | 0.001 | 1.012 |
| Constant | −3.107 | 0.485 | 41.002 | <0.001 | 0.045 |
NT-proBNP, N-terminal probrain natriuretic peptide; sST2, soluble suppression of tumorigenicity-2.
4. Discussion
HF is one of the commonest causes of hospitalization in cardiology practice and is a major cause of morbidity and mortality. Worldwide, the incidence of HF continues to increase because of rising prevalence of risk factors such as hypertension and obesity; improved survival after acute coronary syndromes; and overall increased longevity. At the same time, novel treatment options for HF are becoming available, making it important to identify patients who are more likely to have adverse outcomes and in whom more aggressive guideline-mandated therapies need to be instituted.
Although the clinical status of the patient may itself provide a clue to the prognosis, accurate assessment of prognosis on the basis of clinical assessment alone remains challenging. Natriuretic peptides such as BNP and NT-proBNP provide useful prognostic information in these patients, but their inherent variability compromises their prognostic utility at individual patient level. sST2 is a novel biomarker found to have excellent prognostic value in patients with HF. Unlike the natriuretic peptides, sST2 is less influenced by age, gender, body mass index, etiology of HF, and comorbidities such as renal dysfunction, atrial fibrillation, and so forth.17
sST2 has been shown to have excellent prognostic value in both acute and chronic HF,5, 6, 7, 8, 9, 10, 11, 12, 13 and this prognostic value is incremental to most other biomarkers studied, including natriuretic peptides. Aimo et al5 recently performed a meta-analysis of 10 studies collectively recruiting 4835 patients with acute HF. Admission sST2 was predictive of both all-cause and cardiovascular death, whereas discharge sST2 was predictive of all-cause death, cardiovascular death, and HF hospitalization. Lassus et al,6 through an international collaborative network, collected individual patient data on 5306 patients hospitalized for acute decompensated HF. A large number of biomarkers were analyzed. Their analysis showed that demonstrated that sST2 and midregional pro-adrenomedullin had powerful independent predictive value over clinical risk factors for 30-day and 1-year outcomes in these patients. Similarly, numerous studies have also demonstrated strong, independent prognostic value of sST2 in chronic HF.9, 11, 12, 18 Importantly, sST2 has been shown to predict adverse outcomes in both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF).7 Moreover, sST2 predicts not only prognosis in patients with HF but also future development of HF among the “at-risk” population who have not yet developed HF.19
It has also been demonstrated that serial monitoring of sST2 provides additional prognostic information, as compared with single measurement alone.20, 21 The patients who have an increase in sST2 levels during follow-up have much worse prognosis as compared to those who show a decline in sST2 and those who maintain low sST2 levels throughout their clinical course. In addition, various HF therapies such as beta-blockers, angiotensin-receptor inhibitor/neprilysin inhibitor have been shown to favorably influence sST2 levels, and this effect translates into better clinical outcomes.22, 23, 24, 25 These findings suggest that serial sST2 monitoring may be helpful in assessing the patient's response to treatment and determining the need for further therapeutic adjustments.
Based on the available impressive data regarding prognostic value of sST2, the 2013 American College of Cardiology and American Heart Association guideline recommended measurement of sST2 for additive risk stratification in patients with chronic HF.26 A subsequent international sST2 consensus panel has provided stronger recommendation for its use in acute HF, with on-admission measurement recommended for initial risk assessment and triage and at discharge measurement for informing post-treatment decision-making.27
Consistent with the available evidences, we too found that admission sST2 value was highly predictive of the risk of death or cardiac transplant at a follow-up of 2-years. An sST2 value <35 ng/mL virtually excluded the possibility of death over the next 2-year period. The prognostic value of sST2 was similar in patients with or without reduced LVEF, which too is consistent with previous studies. There are currently very limited data available from India about prognostic utility of ST2 in HF. Only one study has been published very recently, which included 141 patients with HFrEF who were followed up for 1 year.14 The study showed that sST2 concentration at baseline was significantly higher among patients with adverse events in comparison to those without adverse events (P < 0.001). Receiver-operating characteristic curve for baseline sST2 concentration identified 49 ng/mL as the cutoff value to predict cardiac death and rehospitalization, with a sensitivity and specificity of 72% and 75%, respectively. The patients who had significant decline at sST2 level by discharge had lower risk of adverse events. The study concluded that in patients with HFrEF, sST2 concentration at baseline as well as on serial testing was significantly correlated with cardiac death and rehospitalization for worsening of HF. Our study further supports these observations by demonstrating strong prognostic value of single elevated ST2 at the time of hospitalization. However, compared to the study by Bahuleyan et al, we extended these observations to 2-year follow-up and to both HFrEF and HFpEF.
4.1. Limitations
Our study had some important limitations which merit attention. First, we measured sST2 only once and therefore were not able to assess the prognostic value of serial testing. Second, we could also not evaluate the impact of therapeutic changes on sST2 levels. Finally, although we showed that the prognostic value of sST2 was independent of LVEF, a more detailed analysis of the impact of type and etiology of HF as well as the presence of comorbidities on the prognostic value of sST2 could not be performed. However, we wish to reiterate the primary objective of our study was to find out the prognostic value of a single measurement of sST2 at the time of hospitalization in our patients with HF. The issue was relevant given the high cost of sST2 assay. Further studies are now needed to determine the optimum sST2 monitoring protocol and the role of serial sST2 monitoring in therapeutic decision-making in these patients.
5. Conclusion
Despite important progress in recent decades, mortality remains high for patients with chronic HF. Novel biomarkers to guide therapy and help in prognosis are becoming standard of care in patients with HF. BNP and NT-proBNP are currently the most commonly used biomarkers for this purpose. However, the present study clearly demonstrates that sST2 is a better marker for prognostication and helps to further risk stratify patients with elevated NT-proBNP values. The strong prognostic value of admission sST2 observed in our study suggests that a single elevated sST2 value at the time of hospitalization should alert the physicians about the high risk of adverse outcomes and should help facilitate timely intensification of HF treatment.
Conflict of interest
All authors have none to declare.
Contributor Information
Jamshed J. Dalal, Email: jjdalal@hotmail.com.
Aarti Digrajkar, Email: aarti1610@yahoo.com.
Barnali Das, Email: barnali.das@relianceada.com.
Avinash Toomu, Email: avitoomu@gmail.com.
Alan S. Maisel, Email: asmaisel@gmail.com.
References
- 1.de Boer R.A., Daniels L.B., Maisel A.S., Januzzi J.L., Jr. State of the art: newer biomarkers in heart failure. Eur J Heart Fail. 2015;17:559–569. doi: 10.1002/ejhf.273. [DOI] [PubMed] [Google Scholar]
- 2.Wettersten N., Maisel A.S. Biomarkers for heart failure: an update for practitioners of internal medicine. Am J Med. 2016;129:560–567. doi: 10.1016/j.amjmed.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Kakkar R., Lee R.T. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seki K., Sanada S., Kudinova A.Y. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail. 2009;2:684–691. doi: 10.1161/CIRCHEARTFAILURE.109.873240. [DOI] [PubMed] [Google Scholar]
- 5.Aimo A., Vergaro G., Ripoli A. Meta-analysis of soluble suppression of tumorigenicity-2 and prognosis in acute heart failure. JACC Heart Fail. 2017;5:287–296. doi: 10.1016/j.jchf.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Lassus J., Gayat E., Mueller C. Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: the Multinational Observational Cohort on Acute Heart Failure (MOCA) study. Int J Cardiol. 2013;168:2186–2194. doi: 10.1016/j.ijcard.2013.01.228. [DOI] [PubMed] [Google Scholar]
- 7.Manzano-Fernandez S., Mueller T., Pascual-Figal D., Truong Q.A., Januzzi J.L. Usefulness of soluble concentrations of interleukin family member ST2 as predictor of mortality in patients with acutely decompensated heart failure relative to left ventricular ejection fraction. Am J Cardiol. 2011;107:259–267. doi: 10.1016/j.amjcard.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Januzzi J.L., Jr., Peacock W.F., Maisel A.S. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol. 2007;50:607–613. doi: 10.1016/j.jacc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Aimo A., Vergaro G., Passino C. Prognostic value of soluble suppression of tumorigenicity-2 in chronic heart failure: a meta-analysis. JACC Heart Fail. 2017;5:280–286. doi: 10.1016/j.jchf.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Bayes-Genis A., de Antonio M., Galan A. Combined use of high-sensitivity ST2 and NTproBNP to improve the prediction of death in heart failure. Eur J Heart Fail. 2012;14:32–38. doi: 10.1093/eurjhf/hfr156. [DOI] [PubMed] [Google Scholar]
- 11.Bayes-Genis A., de Antonio M., Vila J. Head-to-head comparison of 2 myocardial fibrosis biomarkers for long-term heart failure risk stratification: ST2 versus galectin-3. J Am Coll Cardiol. 2014;63:158–166. doi: 10.1016/j.jacc.2013.07.087. [DOI] [PubMed] [Google Scholar]
- 12.Gaggin H.K., Szymonifka J., Bhardwaj A. Head-to-head comparison of serial soluble ST2, growth differentiation factor-15, and highly-sensitive troponin T measurements in patients with chronic heart failure. JACC Heart Fail. 2014;2:65–72. doi: 10.1016/j.jchf.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Mebazaa A., Di Somma S., Maisel A.S., Bayes-Genis A. ST2 and multimarker testing in acute decompensated heart failure. Am J Cardiol. 2015;115:38b–43b. doi: 10.1016/j.amjcard.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 14.Bahuleyan C.G., Alummoottil G.K., Abdullakutty J. Prognostic value of soluble ST2 biomarker in heart failure patients with reduced ejection fraction – a multicenter study. Indian Heart J. 2018;70(Suppl1):S79–S84. doi: 10.1016/j.ihj.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller T., Zimmermann M., Dieplinger B., Ankersmit H.J., Haltmayer M. Comparison of plasma concentrations of soluble ST2 measured by three different commercially available assays: the MBL ST2 assay, the Presage ST2 assay, and the R&D ST2 assay. Clin Chim Acta. 2012;413:1493–1494. doi: 10.1016/j.cca.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 16.Mueller T., Dieplinger B. The Presage((R)) ST2 Assay: analytical considerations and clinical applications for a high-sensitivity assay for measurement of soluble ST2. Expert Rev Mol Diagn. 2013;13:13–30. doi: 10.1586/erm.12.128. [DOI] [PubMed] [Google Scholar]
- 17.Maisel A.S., Di Somma S. Do we need another heart failure biomarker: focus on soluble suppression of tumorigenicity 2 (sST2) Eur Heart J. 2017;38:2325–2333. doi: 10.1093/eurheartj/ehw462. [DOI] [PubMed] [Google Scholar]
- 18.Ky B., French B., McCloskey K. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4:180–187. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang T.J., Wollert K.C., Larson M.G. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boisot S., Beede J., Isakson S. Serial sampling of ST2 predicts 90-day mortality following destabilized heart failure. J Card Fail. 2008;14:732–738. doi: 10.1016/j.cardfail.2008.06.415. [DOI] [PubMed] [Google Scholar]
- 21.Manzano-Fernandez S., Januzzi J.L., Pastor-Perez F.J. Serial monitoring of soluble interleukin family member ST2 in patients with acutely decompensated heart failure. Cardiology. 2012;122:158–166. doi: 10.1159/000338800. [DOI] [PubMed] [Google Scholar]
- 22.Gaggin H.K., Motiwala S., Bhardwaj A., Parks K.A., Januzzi J.L., Jr. Soluble concentrations of the interleukin receptor family member ST2 and beta-blocker therapy in chronic heart failure. Circ Heart Fail. 2013;6:1206–1213. doi: 10.1161/CIRCHEARTFAILURE.113.000457. [DOI] [PubMed] [Google Scholar]
- 23.O'Meara E., Prescott M.F., Claggett B. Independent prognostic value of serum soluble ST2 measurements in patients with heart failure and a reduced ejection fraction in the PARADIGM-HF trial (Prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure) Circ Heart Fail. 2018;11:e004446. doi: 10.1161/CIRCHEARTFAILURE.117.004446. [DOI] [PubMed] [Google Scholar]
- 24.Anand I.S., Rector T.S., Kuskowski M., Snider J., Cohn J.N. Prognostic value of soluble ST2 in the Valsartan Heart Failure Trial. Circ Heart Fail. 2014;7:418–426. doi: 10.1161/CIRCHEARTFAILURE.113.001036. [DOI] [PubMed] [Google Scholar]
- 25.Maisel A., Xue Y., van Veldhuisen D.J. Effect of spironolactone on 30-day death and heart failure rehospitalization (from the COACH Study) Am J Cardiol. 2014;114:737–742. doi: 10.1016/j.amjcard.2014.05.062. [DOI] [PubMed] [Google Scholar]
- 26.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Januzzi J.L., Mebazaa A., Di Somma S. ST2 and prognosis in acutely decompensated heart failure: the international ST2 consensus panel. Am J Cardiol. 2015;115:26b–31b. doi: 10.1016/j.amjcard.2015.01.037. [DOI] [PubMed] [Google Scholar]