Abstract
Patients with type 2 diabetes mellitus (T2DM) exhibit an increased risk for cardiovascular (CV) events. Hyperglycemia itself contributes to the pathogenesis of atherosclerosis and heart failure (HF) in these patients, but glucose-lowering strategies studied to date have had little or no impact on reducing CV risk, especially in patients with a long duration of T2DM and prevalent CV disease (CVD). Sodium-glucose cotransporter-2 (SGLT2) inhibitors are the new class of glucose-lowering medications that increase urinary glucose excretion, thus improving glycemic control, independent of insulin. The recently published CV outcome trial, Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients—Removing Excess Glucose (EMPA-REG OUTCOME), demonstrated that the SGLT2 inhibitor empagliflozin significantly reduced the combined CV end point of CV death, nonfatal myocardial infarction, and nonfatal stroke vs. placebo in a population of patients with T2DM and prevalent atherosclerotic CVD. In addition, and quite unexpectedly, empagliflozin significantly and robustly reduced the individual end points of CV death, overall mortality, and hospitalization for HF in this high-risk population. Several beneficial factors beyond glucose control, such as weight loss, lowering blood pressure, sodium depletion, renal hemodynamic effects, effects on myocardial energetics, and/or neurohormonal effects, have been seen with SGLT2 inhibition.
Keywords: SGLT2, Glycosuria, Osmotic diuresis, Empagliflozin, Major adverse cardiovascular events
1. Introduction
Type 2 diabetes mellitus (T2DM) is associated with a substantially increased cardiovascular (CV) risk,1, 2 and several international guideline statements addressing the management of T2DM3, 4 underscore the need to prevent and reduce CV complications. Although it is true that glycemic control plays an important role in this process, as suggested by epidemiological studies, there remains great controversy concerning the impact of glucose lowering on CV outcomes from intensive glycemic control trials.5 Thus, and in the light of the multiple CV risk factors beyond hyperglycemia that exist in most patients with T2DM, a multipronged approach to address CV risk is of immense importance. This includes, in addition to glucose lowering, the control of blood pressure (BP) and lipids, weight management, smoking cessation, and, when indicated, antiplatelet therapy.3, 4 Despite these recommendations, it is difficult for most patients in clinical practice to reach their therapeutic goals. In light of the multifaceted pathogenesis of CV disease (CVD) in diabetes, it is imperative to have a specific intervention that could attenuate atherosclerosis risk in a multidimensional fashion and beyond glycemic control.
The CV safety of antidiabetic medications has become an acute area of concern. Rosiglitazone, sulfonylureas, and insulin are three traditional antidiabetic medications that have been associated with increased risk of CV events in patients with T2DM.6 In 2008, the U.S. Food and Drug Administration mandated all new antidiabetic medications to provide evidence that they do not increase the risk of CVDs.7, 8
However, to date, the potential effects of specific glucose-lowering agents i.e., sulfonylureas, glinides, metformin, thiazolidinediones, and insulin, on CV events in patients with T2DM remain uncertain.9 A neutral effect for the composite CV death, myocardial infarction (MI), or stroke have been observed from the first two placebo-controlled trials involving the dipeptidyl peptidase 4 inhibitors saxagliptin [i.e. Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)—Thrombolysis in Myocardial Infarction (TIMI) 53 (SAVOR-TIMI 53)]10 and alogliptin [i.e. Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE)].11 This class of drugs has been associated with beneficial effects on several factors and biological processes linked to atherogenesis in few mechanistic and preclinical studies.12 It should be noted that both SAVOR-TIMI 53 and EXAMINE were relatively short in duration (median follow-up 2.2 and 1.5 years, respectively) and included patients predominantly, or exclusively, with overt CVD. A sufficient duration of treatment might be important because macrovascular (and microvascular) disease may be a relatively late complication of a complex and progressive pathogenic process that spans years.13 Subsequently, the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) with sitagliptin showed no CVD risks or heart failure (HF) hospitalization14 in high-risk diabetic patients. Regarding glucagon-like peptide-1 receptor analogs, the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial15 showed reduction in the rate of the first occurrence of death from CV causes, nonfatal MI, or nonfatal stroke among patients with T2DM with liraglutide than with placebo. In addition, in T2DM patients with established CV complications, who are often targeted by these studies, it may be more difficult to further reduce the residual CV risk beyond that which standard of care can offer.16 Recent trials with sodium-glucose cotransporter-2 (SGLT2) inhibitors in T2DM showed promise related to not only CV safety but also CV risk reduction.
2. Role of the kidney in glucose metabolism
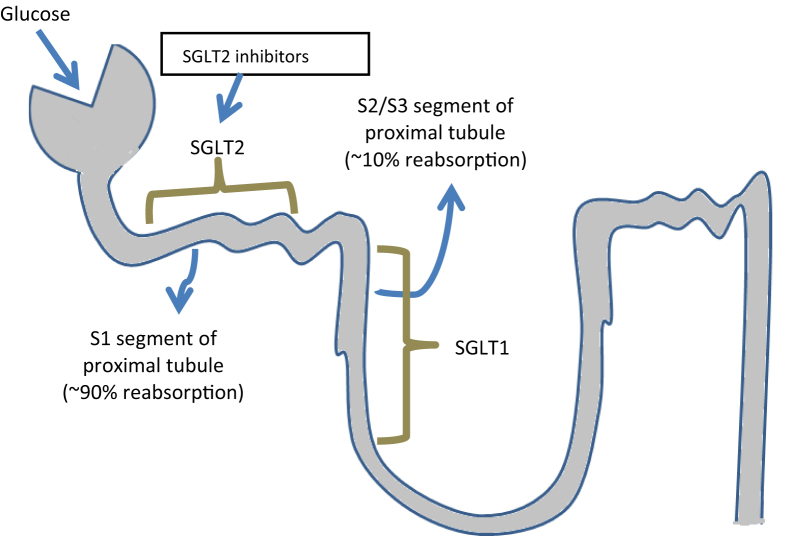
Through its contribution to gluconeogenesis and its capacity to reabsorb glucose from the urine, the kidney plays an important role in glucose homeostasis. In individuals without DM, approximately 160–180 g of glucose is filtered by the kidneys per day,17 and virtually all this filtered glucose is reabsorbed in the proximal tubule. As long as the filtered glucose does not exceed the maximum renal glucose reabsorption capacity, filtered glucose is reabsorbed, thus allowing energy conservation.18 About 90% of glucose filtered in the glomeruli is reabsorbed in the first segment of the proximal tubule by SGLT2 receptors, which is a low-affinity, high-capacity transporter.19 The remaining 10% is reabsorbed in the more distal part of the tubule by SGLT1 receptor, a high-affinity, low-capacity transporter.20, 21 Sodium-glucose cotransporter proteins are proteins localized in the apical membrane which are capable of actively transporting glucose along with sodium against a concentration gradient into the cell. This process is driven by the active transport of sodium out of the cell by the adenosine triphosphate–dependent sodium-potassium pump. Once in the intracellular space, glucose then passively diffuses from the basolateral membrane of the tubule cell into the blood via glucose transporter 2 (GLUT2), a member of the GLUT family of proteins.22 If the blood glucose load exceeds the renal tubular glucose excretion threshold of 180 mg/dL, glucosuria occurs. In patients with DM and chronic hyperglycemia, the threshold paradoxically increases to 220 mg/dL because of an enhanced maximum renal glucose reabsorption capacity mediated by upregulation of SGLT2 in the proximal tubule. This maladaptive mechanism seems to be explained by increased SGLT2 transcription and translation resulting in increased SGLT2 density on the apical membrane in the proximal tubule.19, 21
2.1. SGLT2 inhibitors: mode of action and glucose-lowering properties
SGLT2 inhibitors are a novel class of glucose-lowering drugs that act in the kidney by inhibiting SGLT2-mediated glucose reabsorption in the proximal tubule. The resulting increase in urinary glucose excretion leads to a reduction in plasma glucose levels (Fig. 1).22, 23 The concept of SGLT2 inhibition is different from other glucose-lowering strategies because glucose is removed from the ‘system’, thus reducing total body and cellular glucose toxicity, a mechanism that is completely independent of insulin. Overall, 24-h urinary glucose excretion in patients treated with SGLT2 inhibitors lies between 60 and 100 g/day, corresponding to a loss of 240–400 kcal/day on chronic administration.24, 25 In addition to their glucosuric effects, SGLT2 inhibitors lead to an increase in sodium excretion26 and a reduction in plasma volume due to glucose osmotic diuretic effects and natriuresis.27 Currently, three SGLT2 inhibitors are approved in Europe and the USA: dapagliflozin, canagliflozin, and empagliflozin.28 Recently, ertugliflozin has also been approved in the USA. Meta-analyses for these SGLT2 inhibitors suggest that they lower glycosylated hemoglobin (HbA1c) levels between 0.7 and 0.8% relative to placebo.29 SGLT2 inhibitor effects are glucose dependent, thus leading to a very low risk of hypoglycemia. In addition, SGLT2 inhibitors can be combined with any other antihyperglycemic medication because the mechanism of action is different from all other agents presently available and is completely independent of insulin.30 Interestingly, various studies suggest that SGLT2 inhibitors may improve insulin sensitivity potentially through an increase in insulin-mediated glucose tissue disposal31, 32 and overtime, because of the net caloric loss and enhanced insulin sensitivity mediated by weight loss. Recent data have shown that SGLT2 inhibitors increase glucagon secretion from alpha-cells in the pancreatic islet, a mechanism that may contribute to enhanced endogenous glucose production in treated patients.33 In addition to these effects on glucose homeostasis, SGLT2 inhibitors exhibit potential beneficial effects on CV risk factors.34, 35
Fig. 1.
Glucose-lowering mechanism of SGLT2 inhibitors. Source: Fioretto et al. Cardiovasc Diabetol (2015) 14:142.
3. Effects of SGLT2 inhibitors beyond glucose control
There are pleiotropic effects of SGLT2 inhibitors beyond its glucose control effect through different mechanisms (Table 1).
Table 1.
Pleiotropic effects of SGLT2 inhibitors.
|
SGLT2, sodium-glucose cotransporter-2; SBP, systolic blood pressure; SNS, sympathetic nervous system.
3.1. Anthropometrics
The glucosuric effect of SGLT2 inhibitors causing a negative energy balance results in an average weight reduction of 2–3 kg which occurs gradually over the first few months on treatment, a consistent observation across the class of medications in studies over 1–2 years.36 Interestingly, the weight loss appears to reach a nadir and thereafter stabilizes after 3–6 months, most likely through a compensatory increased energy intake.37 These medications seem to have no effect on energy expenditure.38, 39, 40
3.2. BP and diuresis
A reduction in BP in patients treated with SGLT2 inhibitors is another effect beyond glucose control consistently observed across the class of medications. Several trials have shown that SGLT2 inhibitors lead to a reduction in systolic BP in a range of 3–5 mmHg in systolic and 2–3 mmHg in diastolic BP. In addition, SGLT2 inhibitors reduce pulse pressure, mean arterial pressure, and arterial stiffness.41 Interestingly, these BP effects occurred without a compensatory increase in the heart rate, suggesting a lack of compensatory sympathetic activation. In addition, clamp studies in patients with uncomplicated type 1 diabetes suggest that empagliflozin reduces carotid-radial pulse wave velocity also without inducing a reflex sympathomimetic activity.42
3.3. Renal hemodynamic effects
SGLT2 inhibitors have also been suggested to directly affect the tubuloglomerular feedback mechanism in the kidney. The increased delivery of solute (sodium and chloride) to the macula densa in the setting of SGLT2 inhibition may reduce hyperglycemia-induced glomerular hyperfiltration via tubuloglomerular feedback invoking adenosine-dependent pathways, with direct effects on afferent glomerular arteriolar tone that may diminish hyperfiltration acutely and consistently during treatment.43
3.4. Effects on mediators and markers of CV risk
With respect to lipids, SGLT2 inhibitors mildly increase both low-density lipoprotein-cholesterol (LDL-C) and high-density lipoprotein-cholesterol (HDL-C) through an as of yet unexplained mechanism.44, 45 These observations may reflect direct effects on lipoprotein particle metabolism but could also simply reflect hemoconcentration resulting from the diuretic effects of SGLT2 inhibition, thereby having no net effect on circulating lipid particle numbers.
3.5. Cardiac effects
Experimental data in obese and diabetic mice demonstrated that the SGLT2 inhibitor empagliflozin significantly ameliorates cardiac fibrosis, coronary arterial thickening, and cardiac macrophage infiltration, suggesting a direct cardiac effect along with an attenuation of oxidative stress on the myocardium.46 Other potential direct or indirect cardiac effects might include alterations of myocardial energetics and potential antiarrhythmic effects. From a myocardial energetics perspective, SGLT1 but not SGLT2 is expressed in cardiac myocytes.47 In the kidney, SGLT2 inhibition results in increased SGLT1-mediated glucose reabsorption,48 and if this is a humoral-mediated response, the possibility remains for upregulation of cardiac SGLT1. This could directly affect myocardial substrate metabolism and energetics with enhanced glucose and decreased fatty acid (FA) metabolism that could favorably affect myocardial function. In addition, shifting from beta-oxidation of free FA to glycolysis in the myocardium might reduce the potential proarrhythmia effects of free FA metabolites.49
3.6. Reduction in the levels of uric acid
Increased uric acid levels have, however, also been associated with chronic kidney disease,50 CV complications,51 and congestive HF,52 although a cause-and-effect relationship of uric acid and CV outcomes has yet to be proved.53 Reduction in the levels of uric acid has consistently been seen with SGLT2 inhibitors,20, 54, 55 presumably mediated by the actions of solute carrier family 2, facilitated glucose transporter member 9, also called GLUT9, a urate transporter that secretes urate back into the urine in exchange for glucose.
3.7. Effect on lipid parameters
SGLT2 inhibitors are associated with a small increase in HDL-C and an increase in LDL-C with concomitant reductions in triglyceride levels.45, 56, 57 Whether these small lipid changes are clinically relevant and whether they could potentially offset any potential CV benefit with SGLT2 inhibitors will need further clarification.
3.8. Effects on other CV risk pathways
In animal and mechanistic models, SGLT2 inhibitors have been shown to reduce leukocytosis induced by hyperglycemia58 and to reduce inflammation and oxidative stress,59, 60, 61 which are processes involved in the pathophysiology of atherosclerosis. Because inhibition of SGLT2 leads to glucosuria with an accompanying diuresis and weight and BP reductions, all of which are theoretically beneficial in patients with HF, it is also conceivable that impaired ventricular function and remodeling could be improved with such an intervention. Although clinical data are yet to be reported, an animal study suggested that SGLT2 inhibition could attenuate the increase in the left ventricle mass and left ventricle end-diastolic diameter in a rat model of progressive HF.62
4. Clinical landmark trials of SGLT2 inhibitors and CV risk
4.1. Meta-analysis of 21 trials with dapagliflozin
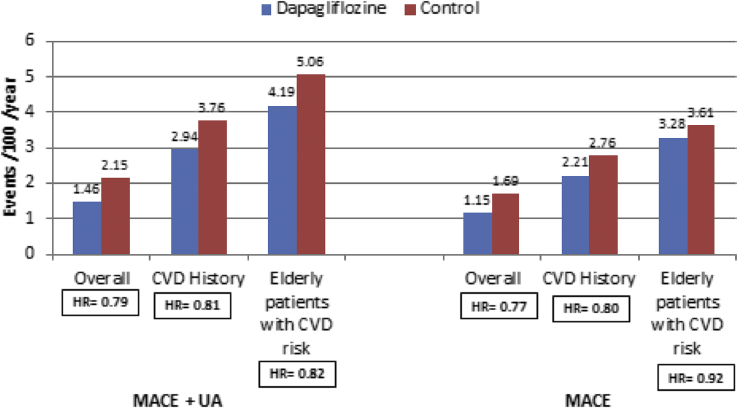
It has been performed in T2DM patients with CV risk. Analysis was based on time to first event comparing dapagliflozin versus control. Results showed that dapagliflozin is not associated with increased CV risk; more over, there was a potential benefit in the overall population for major adverse cardiac event (MACE) and in those with a history of CVD. These findings were consistent in patients with varying degrees of CV risk, including age, number, and type of CVD events in medical history and the number of CV risk factors present (Fig. 2).63
Fig. 2.
Meta-analysis of 21 trials with dapagliflozin. CVD, cardiovascular disease; MACE, major adverse cardiac event; UA, unstable angina. Source: Sonesson et al. Cardiovasc Diabetol (2016) 15:37.
5. EMPA-REG OUTCOME trial with empagliflozin
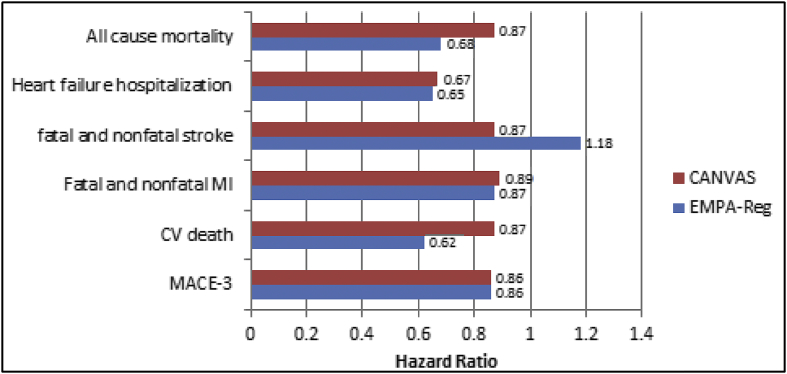
The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients—Removing Excess Glucose (EMPA-REG OUTCOME) study64 is the first study to provide evidence that an antidiabetic agent decreases CV events. In 7020 T2DM patients with established CVD, empagliflozin significantly reduced the primary MACE outcome (CV death, nonfatal MI, nonfatal stroke). The outcome was surprising; CV death decreased significantly by 38% (p = 0.001) unlike other interventions that reduce CV risk, e.g., lowering LDL-C65 and BP.66 The separation of the Kaplan–Meier curves for CV mortality and hospitalization for HF was observed within the first 3 months of the trial. This indicates that the mechanisms of empagliflozin therapy had an early and profound effect on the risk of death or HF. The study population included patients older than 18 yrs with T2DM, body mass index (BMI) ≤45 kg/m2, effective glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2, prior CVD (defined by ≥ 1 of the following: MI > 2 months prior, multivessel coronary artery disease, single-vessel coronary artery disease with positive stress test or unstable angina hospitalization in prior year, stroke >2 months prior and occlusive peripheral artery disease) (Fig. 3).64
Fig. 3.
Key outcomes in EMPA-REG and CANVAS trials. MI, myocardial infarction; EMPA-REG, Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients—Removing Excess Glucose; CANVAS, Canagliflozin Cardiovascular Assessment Study; CV, cardiovascular; MACE, major adverse cardiac event. Source: Ahmed R et al. BLDE Univ J Health Sci 2017; 2:75–79.
5.1. Potential mechanisms explaining the results in EMPA-REG OUTCOME
The surprising and unexpected results of EMPA-REG OUTCOME on mortality and HF cannot be explained by glucose control per se nor by a reduction of atherosclerotic events because the effect of empagliflozin on nonfatal MI and hospitalization for unstable angina was not statistically significant. More likely, the favorable outcomes are related to the effects of empagliflozin on HF and CV mortality, direct or indirect, that are independent of effects on hyperglycemia or on atherosclerotic CVD. In the trial, 10% of the patients enrolled had a history of HF, but no data on the left ventricular function or surrogate parameters such as N terminal prohormone brain natriuretic peptide (NT-proBNP) were collected. Empagliflozin led to a highly significant reduction in hospitalization for HF with an effect that became evident very early in the trial in the overall study population. Subgroup analyses stratified by presence or absence of HF at baseline suggest a consistent benefit observed in patients with and without baseline HF with no evident heterogeneity of efficacy by the HF status.67 Various factors may contribute to the observed reduction in CV death and HF hospitalization. As with the atherosclerotic event end points discussed previously, blood glucose reduction itself is very unlikely to account for the results observed, given the minor difference in HbA1c levels between groups and the fact that previous studies showed that intensified glucose control does not influence the incidence of these events in similar populations. The significant reductions in BP and body weight seen in EMPA-REG OUTCOME have been proposed as potential contributors to the beneficial results. However, BP lowering only translates into CV risk reduction after 6–12 months or even longer, making the early beneficial effects seen unlikely to be attributable to BP reduction per se. Still, the decrease in markers of arterial stiffness found in SGLT2 inhibitor–treated patients, all closely linked to BP reduction, may have a direct, potentially beneficial effect on myocardial oxygen consumption via afterload reduction. Weight loss seems to play a minor role in this context, given the data from the Look Action for Health in Diabetes study, showing that intensive lifestyle intervention, focused on weight loss, did not improve CV risk in patients with T2DM.68
The diuretic effect of empagliflozin has been discussed as a potential contributor to the observed reduction of hospitalization for HF through an at least temporary reduction in plasma volume as shown for SGLT2 inhibitors.27
5.1.1. Nondiuretic hypothesis
Similar or greater decreases in intravascular volume and net sodium balance are affected by most commonly used diuretic medications but in contrast to empagliflozin, treatment with loop diuretics or thiazides has not been demonstrated to reduce CV death in previous studies, and their effects on hospitalization for HF risk, when demonstrated, have been much more modest in magnitude compared with the large reduction observed with empagliflozin, probably some nondiuretic mechanisms are responsible for its CV effects. SGLT2 inhibitors are different from the loop and thiazide diuretics in several important ways. First, they do not lead to a reflex activation of the sympathetic nervous system. In addition, thiazides work in the distal tubule, while SGLT2 inhibitors act proximal of the macula densa, thus leading to an increased urinary sodium and chloride delivery to the juxtaglomerular apparatus. This has been suggested to restore the tubuloglomerular feedback mechanism in diabetes with afferent arteriolar vasoconstriction and subsequent reduction of hyperfiltration and normalization of transglomerular perfusion pressures. Similarly, the increased sodium and chloride delivery to the macula densa on SGLT2 inhibitor treatment may affect other neurohormonal factors such as local renin angiotensin aldosterone system (RAAS) inhibition,69, 70, 71 which may have contributed to preglomerular vasoconstriction in diabetes. The depletion of sodium and potential reduction in body sodium content by SGLT2 inhibition may play a crucial role in HF in patients with diabetes. It has been hypothesized that patients with diabetes exhibit an excess of total body sodium, mainly because of increased sodium retention in the kidney as a consequence of hyperglycemia and hyperinsulinemia.72, 73, 74 Sodium-loading studies in animals and humans suggest that excess sodium is not only distributed in the extracellular space but possibly also intracellularly and in osmotically inactive compartments, e.g. matrix components of skin and muscle. Increased intracellular sodium in the myocardium may increase the risk of arrhythmias and impair myocardial function,75, 76 most likely through an impairment of mitochondrial function. Interestingly, experimental data in an animal model of HF showed an increase in myocardial intracellular sodium and in this model, blockade of the mitochondrial Na+/Ca2+ exchange prolonged the survival of these animals and significantly decreased arrhythmias.77 SGLT2 inhibition leads to an early and transient increase in urinary sodium excretion that normalizes after 2 weeks.78 Other mechanisms that may explain the profound effects of empagliflozin on CV events include antioxidative, anti-inflammatory, or antiapoptotic properties of SGLT2 inhibitors as shown in experimental models, as well as counterbalancing effects of these drugs on cellular senescence.59, 79 There is also a major role of myocardial energy kinetics and use of ketone bodies as efficient energy sources as one of the major mechanisms postulated.80
Increase in glucagon secretion induced by SGLT2 inhibition33, 81, 82 may be interesting in light of the EMPA-REG OUTCOME results. Glucagon exhibits inotropic effects with an increased myocardial contractility and cardiac output by generating cyclic adenosin mono phosphate (cAMP). In addition, glucagon has been shown to exhibit antiarrhythmic effects. Clinically, glucagon has previously been used as an adjunctive therapy in shock situations and HF but given that catecholamines are much more effective in these conditions, glucagon is no longer used for this indication. Still, a continuous increase in glucagon levels in SGLT2 inhibitor–treated patients could contribute to a cardioprotective effect.
Based on the effect of empagliflozin in HF in EMPA-REG OUTCOME it has been included in ESC 2016 Guidelines for Diagnosis and Treatment of Acute and Chronic Heart Failure as class 2A, level b recommendation.
5.2. CANVAS trial with canagliflozin
Canagliflozin Cardiovascular Assessment Study (CANVAS) trial was performed with canagliflozin, another SGLT2 inhibitor;83 the mean age of the participants was 63.3 years; 35.8% were women; the mean duration of diabetes was 13.5 years; and 65.6% had a history of CVD. As compared with placebo, the rate of primary outcome was lower with canagliflozin (occurring in 26.9 vs. 31.5 participants per 1000 patient-years). Canagliflozin reduces the MACE by 14%, all-cause and CV deaths by 13%, and HF by 33%, cuts renal decline by 40% but doubles the risk of lower limb amputations (Fig. 3).
In a recent meta-analysis of 35 eligible studies (nine with canagliflozin, eight with empagliflozin, 18 with dapagliflozin), consisting of 34,987 patients with T2DM, pooled results show that SGLT2 inhibitors, when compared with placebo, significantly reduce all-cause mortality, MACE, nonfatal MI, and HF/hospitalization for HF. No significant difference was noted in the occurrence of stroke, atrial fibrillation, or unstable angina. In addition, there was no heterogeneity among different drugs in the SGLT2 inhibitor class for all the clinical outcomes studied.84
5.3. Ongoing trial—DECLARE-TIMI 5885
The Dapagliflozin Effect on Cardiovascular Events-TIMI 58 (DECLARE-TIMI 58) is designed to test that in patients with T2DM, long-term treatment with dapagliflozin will reduce one or both of the coprimary end points: (1) the incidence CV death, MI, or ischemic stroke or (2) the incidence of CV death or hospitalization for HF. It has randomized approximately 17,150 patients with T2DM having secondary prevention cohort or primary prevention cohort. Mean age of the participants was 63.8 ± 6.8 years, 62.6% were male, and their mean diabetes duration was 11.8 ± 7.8 years; HbA1c, 8.3% ± 1.2%; and BMI, 32.1 ± 6.0 kg/m2. About 40.6% patients had atherosclerotic CVD and 59.4% patients had multiple risk factors (MRFs) for CVD (defined as men age ≥ 55 years or women ≥60 years, with at least one of dyslipidemia, hypertension, or smoking). Patients with CVD compared with patients with MRFs were more on insulin and less on metformin and more frequently used statins, aspirin, clopidogrel, and β-blockers. DECLARE-TIMI 58 will be followed up for a median of 4.5 years.
5.4. Primary prevention of CVD with SGLT2 inhibitors in diabetes
A new analysis of the observational study Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors (CVD-REAL)86 assessed data from more than 300,000 patients across six countries, 87% of whom did not have a history of CVD. The results showed that treatment with SGLT2 inhibitors compared with other glucose-lowering drugs reduced the rate of hospitalization for HF by 39% and death from any cause by 51% across this broad population with T2DM. The findings thus hint at a possible benefit of this drug class in the primary prevention of CVD in those with T2DM.
5.4.1. CVD-REAL 2
CVD-REAL 2 is the large international study of >40,000 patients with T2DM from Asia Pacific, the Middle East, and North America and showed that initiation of SGLT2 inhibitors compared with other glucose-lowering drugs was associated with a lower risk of a broad range of CV outcomes (all-cause death, MI, HF hospitalization, and stroke) across the wide range of patients such as all ethnicity, geographic regions and to all races.87
6. Conclusion
Given the increasing prevalence of T2DM across the populations worldwide and suboptimal control of glycemia and other CV risk factors achieved with currently available agents, the need for therapies with novel modes of action remains an important clinical priority. Of the large number of antihyperglycemic drug classes now available for patients with T2DM, none is recognized unequivocally to reduce CV events over and above any modest effects of glucose lowering itself. Metformin is considered to be CV safe and reduce CV events but has actually never been studied in a large properly designed randomized clinical trial powered to answer this specific question.
SGLT2 inhibitors are novel oral glucose-lowering agents that offer the potential to improve glycemic control with a low risk of hypoglycemia, independent of insulin secretion, offering a modest reduction in BP and body weight. Their mode of action, which is independent of endogenous insulin secretion, enables their use in any stage of T2DM. Pleiotropic effects of SGLT2 inhibitors showed the potential for its CV benefits. Results of EMPA-REG OUTCOME with empagliflozin and CANVAS trial with canagliflozin are encouraging. Data from CVD-REAL and CVD-REAL 2 clearly showed CV benefits of all available SGLT2 inhibitors in broad diabetic population, in all ethnic groups, and even in those without any prior CV events.
Conflict of interest
The author has none to declare.
References
- 1.Huxley R., Barzi F., Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sloan F.A., Bethel M.A., Ruiz D., Jr. The growing burden of diabetes mellitus in the US elderly population. Arch Intern Med. 2008;168:192–199. doi: 10.1001/archinternmed.2007.35. [DOI] [PubMed] [Google Scholar]
- 3.Inzucchi S.E., Bergenstal R.M., Buse J.B. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryden L., Grant P.J., Anker S.D. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 5.Skyler J.S., Bergenstal R., Bonow R.O. Intensive glycaemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trial; a position statement of American diabetes association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32:187–192. doi: 10.2337/dc08-9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S., Bhat J., Wang P.H. Cardiovascular effects of anti-diabetic medications in type 2 diabetes mellitus. Curr Cardiol Rep. 2013;15:327. doi: 10.1007/s11886-012-0327-1. [DOI] [PubMed] [Google Scholar]
- 7.Nam M. Clinical trial for antidiabetic drugs: FDA guidance for diabetes mellitus-evaluation of cardiovascular risk in new antidiabetic therapies. J Korean Diabetes. 2011;12:129. [Google Scholar]
- 8.Division of Metabolism and Endocrinology Products in the Center for Drug Evaluation and Research (CDER) at Food and Drug Administration . 2008. Guidance for Industry Diabetes Mellitus-evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes.https://www.fda.gov/ Available from: [Google Scholar]
- 9.Bennett W.L., Maruthur N.M., Singh S. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602–613. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scirica B.M., Bhatt D.L., Braunwald E. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 11.White W.B., Cannon C.P., Heller S.R. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 12.Fadini G.P., Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vasc Pharmacol. 2011;55:10–16. doi: 10.1016/j.vph.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Dzau V., Braunwald E. Resolved and unresolved issues in the prevention and treatment of coronary artery disease: a workshop consensus statement. Am Heart J. 1991;121:1244–1263. doi: 10.1016/0002-8703(91)90694-d. [DOI] [PubMed] [Google Scholar]
- 14.Green J.B., Angelyn Bethel M., Armstrong P.W., TECOS Study Group Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 15.Marso S.P., Gilbert H., Daniels G.H., LEADER Trial Investigators Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S.J., Leipzig R.M., Walter L.C. Incorporating lag time to benefit into prevention decisions for older adults. J Am Med Assoc. 2013;310:2609–2610. doi: 10.1001/jama.2013.282612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerich J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27:136–142. doi: 10.1111/j.1464-5491.2009.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsenic O. Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis. 2009;53:875–883. doi: 10.1053/j.ajkd.2008.12.031. 22. [DOI] [PubMed] [Google Scholar]
- 19.Rahmoune H., Thompson P.W., Ward J.M., Smith C.D., Hong G., Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54:3427–3434. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 20.Bailey C.J., Gross J.L., Pieters A., Bastien A., List J.F. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:2223–2233. doi: 10.1016/S0140-6736(10)60407-2. [DOI] [PubMed] [Google Scholar]
- 21.Chao E.C., Henry R.R. SGLT2 inhibition – a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9:551–559. doi: 10.1038/nrd3180. [DOI] [PubMed] [Google Scholar]
- 22.Saeed M.A., Narendran P. Dapagliflozin for the treatment of type 2 diabetes: a review of the literature. Drug Des Dev Ther. 2014;8:2493–2505. doi: 10.2147/DDDT.S50963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdul-Ghani M.A., Norton L., Defronzo R.A. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32:515–531. doi: 10.1210/er.2010-0029. [DOI] [PubMed] [Google Scholar]
- 24.Whalen K., Miller S., Onge E.S. The role of sodium-glucose co-transporter 2 inhibitors in the treatment of type 2 diabetes. Clin Therapeut. 2015;37:1150–1166. doi: 10.1016/j.clinthera.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Nauck M.A. Updateon developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Dev Ther. 2014;8:1335–1380. doi: 10.2147/DDDT.S50773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFronzo R.A., Hompesch M., Kasichayanula S. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care. 2013;36:3169–3317. doi: 10.2337/dc13-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sha S., Polidori D., Heise T. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metabol. 2014;16:1087–1095. doi: 10.1111/dom.12322. [DOI] [PubMed] [Google Scholar]
- 28.Ferrannini E., DeFronzo R.A. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J. 2015;36:2288–2296. doi: 10.1093/eurheartj/ehv239. [DOI] [PubMed] [Google Scholar]
- 29.Ferrannini E., Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495–502. doi: 10.1038/nrendo.2011.243. [DOI] [PubMed] [Google Scholar]
- 30.Clar C., Gill J.A., Court R., Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrannini E., Muscelli E., Frascerra S. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merovci A., Solis-Herrera C., Daniele G. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509–514. doi: 10.1172/JCI70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonner C., Kerr-Conte J., Gmyr V. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015;21:512–517. doi: 10.1038/nm.3828. [DOI] [PubMed] [Google Scholar]
- 34.Inzucchi S.E., Zinman B., Wanner C. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diabetes Vasc Dis Res. 2015;12:90–100. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ptaszynska A., Hardy E., Johnsson E., Parikh S., List J. Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med. 2013;125:181–189. doi: 10.3810/pgm.2013.05.2667. [DOI] [PubMed] [Google Scholar]
- 36.Vasilakou D., Karagiannis T., Athanasiadou E. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–274. doi: 10.7326/0003-4819-159-4-201308200-00007. [DOI] [PubMed] [Google Scholar]
- 37.Ferrannini G., Hach T., Crowe S., Sanghvi A., Hall K.D., Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1730–1735. doi: 10.2337/dc15-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridderstrale M., Andersen K.R., Zeller C. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691–700. doi: 10.1016/S2213-8587(14)70120-2. [DOI] [PubMed] [Google Scholar]
- 39.Bolinder J., Ljunggren O., Johansson L. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metabol. 2014;16:159–169. doi: 10.1111/dom.12189. [DOI] [PubMed] [Google Scholar]
- 40.Cefalu W.T., Leiter L.A., YoonKH Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes in adequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–950. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 41.Chilton R., Tikkanen I., Cannon C.P. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metabol. 2015;17:1180–1193. doi: 10.1111/dom.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cherney D.Z., Perkins B.A., Soleymanlou N. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. doi: 10.1186/1475-2840-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherney D.Z., Perkins B.A., Soleymanlou N. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type1 diabetes mellitus. Circulation. 2014;129:587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 44.Monami M., Dicembrini I., Nardini C., Fiordelli I., Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metabol. 2014;16:38–47. doi: 10.1111/dom.12175. [DOI] [PubMed] [Google Scholar]
- 45.Sinclair A., Bode B., Harris S. Efficacy and safety of canagliflozin compared with placebo in older patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. BMC Endocr Disord. 2014;14:37. doi: 10.1186/1472-6823-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin B., Koibuchi N., Hasegawa Y. Glycemic control with empagliflozin, anovel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol. 2014;13:148. doi: 10.1186/s12933-014-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banerjee S.K., McGaffin K.R., Pastor Soler N.M., Ahmad F. SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc Res. 2009;84:111–118. doi: 10.1093/cvr/cvp190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rieg T., Masuda T., Gerasimova M. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Ren Physiol. 2014;306:F188–F193. doi: 10.1152/ajprenal.00518.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taegtmeyer H., McNulty P., Young M.E. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation. 2002;105:1727–1733. doi: 10.1161/01.cir.0000012466.50373.e8. [DOI] [PubMed] [Google Scholar]
- 50.Madero M., Sarnak M.J., Wang X. Uric acid and longterm outcomes in CKD. Am J Kidney Dis. 2009;53:796–803. doi: 10.1053/j.ajkd.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ndrepepa G., Braun S., King L. Association of uric acid with mortality in patients with stable coronary artery disease. Metabolism. 2012;61:1780–1786. doi: 10.1016/j.metabol.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 52.Huang H., Huang B., Li Y. Uric acid and risk of heart failure: a systematic review and meta-analysis. Eur J Heart Fail. 2014;16:15–24. doi: 10.1093/eurjhf/hft132. [DOI] [PubMed] [Google Scholar]
- 53.Zand S., Shafiee A., Boroumand M. Serum uric acid is not an independent risk factor for premature coronary artery disease. Cardiorenal Med. 2013;4:246–253. doi: 10.1159/000355484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hach T., Gerich J., Salsali A. Empagliflozin improves glycaemic parameters and cardiovascular risk factors in patients with type 2 diabetes: pooled data from four pivotal phase III trials. Diabetes. 2013;62(Suppl. 1) LB19 [69-LB] (abstract no 94) [Google Scholar]
- 55.Rosenstock J., Aggarwal N., Polidori D. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35:1232–1238. doi: 10.2337/dc11-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monami M., Nardini C., Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metabol. 2014;16:457–466. doi: 10.1111/dom.12244. [DOI] [PubMed] [Google Scholar]
- 57.Hardy E., Ptanszynska A., de Bruin T.W.A. Changes in lipid profiles of patients with type 2 diabetes mellitus on dapagliflozin therapy. Diabetologia. 2013;(Suppl. #947):61. [Google Scholar]
- 58.Nagareddy P.R., Murphy A.J., Stirzaker R.A. Hyperglycaemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metabol. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tahara A., Kurosaki E., Yokono M. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715:246–255. doi: 10.1016/j.ejphar.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 60.Panchapakesan U., Pegg K., Gross S. Effects of SGLT2 inhibition in human kidney proximal tubular cells– renoprotection in diabetic nephropathy? PLoS One. 2013;8 doi: 10.1371/journal.pone.0054442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Osorio H., Coronel I., Arellano A. Sodium-glucose cotransporter inhibition prevents oxidative stress in the kidney of diabetic rats. Oxid Med Cell Longev. 2012;2012:542042. doi: 10.1155/2012/542042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Younis F.M., Hollander K., Mayoux E.W. Effect of prophylactic treatment with empagliflozin on cardiac function and diabetes in CRDH rats. Diabetes. 2014;63(Suppl. 1) A273 (1056-P) [Google Scholar]
- 63.Sonesson C., Johansson P.A., Johnsson E., Gause-Nilsson I. Cardiovascular effects of dapagliflozin in patients with type 2 diabetes and different risk categories: a meta-analysis. Cardiovasc Diabetol. 2016;15:37. doi: 10.1186/s12933-016-0356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zinman B., Wanner C., Lachin J.M., EMPAREG OUTCOME Investigators Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 65.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatinin patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 66.Patel A., MacMahon S., Chalmers J., ADVANCE Collaborative Group Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 67.Fitchett D., Zinman B., Wanner C. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME(R) trial. Eur Heart J. 2016;17:1526–1534. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wing R.R., Reboussin D., Lewis C.E., Look A.R.G. Intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:2358–2359. doi: 10.1056/NEJMc1312802. [DOI] [PubMed] [Google Scholar]
- 69.Cherney D.Z., Perkins B.A., Soleymanlou N. Sodium glucose cotransport-2 inhibition and intrarenal RAS activity in people with type 1 diabetes. Kidney Int. 2014;86:1057–1058. doi: 10.1038/ki.2014.246. [DOI] [PubMed] [Google Scholar]
- 70.BakerWL, Smyth L.R., Riche D.M., Bourret E.M., Chamberlin K.W., White W.B. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8:262–275. doi: 10.1016/j.jash.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 71.Lambers Heerspink H.J., de Zeeuw D., Wie L., Leslie B., List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metabol. 2013;15:853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DeFronzo R.A., Cooke C.R., Andres R., Faloona G.R., Davis P.J. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975;55:845–855. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weder A.B. Sodium metabolism, hypertension, and diabetes. Am J Med Sci. 1994;307(Suppl. 1):S53–S59. [PubMed] [Google Scholar]
- 74.Weidmann P., Ferrari P. Central role of sodium in hypertension in diabetic subjects. Diabetes Care. 1991;14:220–232. doi: 10.2337/diacare.14.3.220. [DOI] [PubMed] [Google Scholar]
- 75.Remme C.A., Wilde A.A. Late sodium current inhibition in acquired and inherited ventricular (dys)function and arrhythmias. Cardiovasc Drugs Ther. 2013;27:91–101. doi: 10.1007/s10557-012-6433-x. [DOI] [PubMed] [Google Scholar]
- 76.van Emous J.G., Nederhoff M.G., Ruigrok T.J., van Echteld C.J. The role of the Na+ channel in the accumulation of intracellular Na+during myocardial ischemia: consequences for post-ischemic recovery. J Mol Cell Cardiol. 1997;29:85–96. doi: 10.1006/jmcc.1996.0254. [DOI] [PubMed] [Google Scholar]
- 77.Liu T., Takimoto E., Dimaano V.L. Inhibiting mitochondrial Na+/Ca2+exchange prevents sudden death in a Guinea pig model of heart failure. Circ Res. 2014;115:44–54. doi: 10.1161/CIRCRESAHA.115.303062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Komoroski B., Vachharajani N., Feng Y., Li L., Kornhauser D., Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009;85:513–519. doi: 10.1038/clpt.2008.250. [DOI] [PubMed] [Google Scholar]
- 79.Kitada K., Nakano D., Ohsaki H. Hyperglycemia causes cellular senescence via a SGLT2- and p21-dependent pathway in proximal tubules in the early stage of diabetic nephropathy. J Diabetes Complicat. 2014;28:604–611. doi: 10.1016/j.jdiacomp.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferrannini E. Diabetes Care. 2016 July;39(7):1108–1114. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 81.Diamond G., Forrester J., Danzig R., Parmley W.W., Swan H.J. Haemodynamic effects of glucagon during acute myocardia linfarction with left ventricular failure in man. Br Heart J. 1971;33:290–295. doi: 10.1136/hrt.33.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murtagh J.G., Binnion P.F., Lal S., Hutchison K.J., Fletcher E. Haemodynamic effects of glucagon. Br Heart J. 1970;32:307–315. doi: 10.1136/hrt.32.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neal B., Perkovic V., Mahaffey K.W., For the CANVAS Program Collaborative Group NEJM. June 12, 2017 [Google Scholar]
- 84.Usman M.S., Siddiqi T.J., Memon M.M. Sodium-glucose co-transporter 2 inhibitors and cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25:495–502. doi: 10.1177/2047487318755531. [DOI] [PubMed] [Google Scholar]
- 85.Raz I., Mosenzon O., Bonaca M.P., DECLARE TIMI 58 Group Diabetes Obes Metabol. 2018;20:1102–1110. doi: 10.1111/dom.13217. [DOI] [PubMed] [Google Scholar]
- 86.Kosiborod M., Cavender M.A., Fu A.Z., The CVD-REAL Study Comparative effectiveness of cardiovascular outcomes in new Users of sodium-glucose Cotransporter-2 inhibitors. Circulation. 2017;136:249–259. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kosiborod M., Lam C.S.P., Kohsaka S. Lower cardiovascular risk associated with SGLT-2i in >400,000 patients: the CVD-REAL 2 study. J Am Coll Cardiol. 2018;71:2628–2639. doi: 10.1016/j.jacc.2018.03.009. [DOI] [PubMed] [Google Scholar]