Abstract
Neural stem cell (NSC) grafting in conditions such as aging, brain injury, and neurodegenerative diseases promotes regeneration, plasticity and functional recovery. Recent studies have revealed that administration of NSC-derived extracellular vesicles (NSC-EVs) via non-invasive approaches can also afford therapeutic benefits. This review confers the properties and therapeutic promise of EVs secreted by NSCs. NSC-EVs enriched with specific miRNAs mediate multiple functions in physiological and pathological conditions, which include modulation of the proximate microenvironment, facilitating the entry of viruses into cells, functioning as independent metabolic units, operating as a microglial morphogen and influencing the diverse aspects of brain function in adulthood including the process of aging. Due to their anti-inflammatory, neurogenic and neurotrophic effects, NSC-EVs are also useful for treating multiple neurodegenerative diseases. Although only a few studies have demonstrated the efficacy of NSC-EVs to treat brain impairments, the promise is enormous. Moving forward, the use of well-characterized NSC-EVs generated in specific culture conditions and NSC-EVs that are engineered to carry the desired miRNAs, mRNAs and proteins have great promise for treating brain injury and neurogenerative diseases. Notably, the possibility of targeting NSC-EVs to specific neuronal types or brain regions would enable managing of diverse neurodegenerative conditions with minimal side effects.
Keywords: Exosomes, Extracellular vesicles, Hippocampus, Neural deficits, Neural Injury, Neural stem cells, Neuroinflammation, Neuroprotection, Neuroregeneration
Abbreviations: Ad5, Adenovirus type 5; ADC, Apparent diffusion coefficient; Asrgl1, Asparaginase-like protein 1; BBB, Blood brain barrier; Bmi1, B cell-specific Moloney murine leukemia virus integration site 1; CAR, Coxsackie virus and adenovirus receptor; CD, Cluster of differentiation; CNS, Central nervous system; CSF, Cerebrospinal fluid; DG, Dentate gyrus; DH, Dentate hilus; E-NSC, Enteric neural stem cell; EAAT2/GLT1, Excitatory amino acid transporter 2/glutamate transporter-1; ESC, Embryonic stem cell; ESCRT, Endosomal sorting complex required for transport; EV, Extracellular Vesicle; hESC, Human embryonic stem cell; hMSC, Human mesenchymal stem cell; hNSC, Human neural stem cell; hsa, Homo Sapiens; IFN-γ, Interferon gamma; Ifngr1, Interferon gamma receptor 1; IL-6, Interleukin 6; ILV, Intraluminal vesicle; iPSC, Induced pluripotent stem cell; LAMP1, Lysosomal-associated membrane protein 1; MCAO, Middle cerebral artery occlusion; miRNA, Micro-RNA; MSC, Mesenchymal stem cell; MVB, Multivesicular body; MVE, Multivesicular endosomes; NEC, Necrotizing enterocolitis; NSC, Neural stem/progenitor cell; SGZ, Subgranular zone; shRNA, Short hairpin RNA; siRNA, Small interfering RNA; Sox-2, Sex determining region Y-box 2; SPECT, Single photo emission chromatography; SVZ, Subventricular zone; T2W, T2-weighted sequences; TIM-4, T-cell immunoglobulin mucin protein 4; TNFα, Tumor necrosis factor alpha; TPA, Tissue plasminogen activato
Highlights
-
•
Extracellular vesicles hold a cargo comprising proteins, miRNAs, mRNAs, and DNA
-
•
Neural stem cell derived extracellular vesicles are enriched with specific miRNAs
-
•
Neural stem cell derived extracellular vesicles can function as independent metabolic units
-
•
Extracellular vesicles released from hypothalamic neural stem cells influence aging
-
•
Neural stem cell derived extracellular vesicles promote brain repair in models of stroke
1. Introduction
The developing, as well as the adult central nervous system (CNS), contain multipotent cells with unlimited self-renewal referred to as neural stem/progenitor cells (NSCs).[1,30,95] The primary NSCs are a subtype of radial glial cells that generate transit amplifying cells or intermediate progenitors through asymmetric cell division.[27,82] NSCs are also capable of undergoing symmetric divisions to produce two identical daughter cells.[14] The intermediate progenitors comprise the more restricted neuronal and glial progenitor cells displaying limited self-renewal and proliferative activity to produce differentiated neurons and glia.[21,30] NSCs make a vital contribution to the formation of the CNS during development as they generate neurons, astrocytes, and oligodendrocytes, the three major cell types in the CNS.
When the CNS development is complete with the requisite populations of neurons, astrocytes, and oligodendrocytes, NSCs stop proliferating in most brain regions and undergo terminal differentiation or acquire a quiescent state. Therefore, in the postnatal and adult periods, only a few areas in the CNS display active NSCs proficient in generating neurons, astrocytes, and oligodendrocytes. These regions, known as neurogenic regions, include the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus and the subventricular zone (SVZ) in the forebrain. Generation of neurons (also referred to as neurogenesis) continues throughout life in these regions in most mammalian species.[46,74,118] Continued neurogenesis by NSCs in the hippocampus plays a role in the maintenance of hippocampus plasticity and hippocampus-dependent learning, memory and mood function,[9,23,37,52,66] although such a role for neurogenesis in the human hippocampus is still a matter of debate.[7,99] On the other hand, neurogenesis by NSCs in the SVZ contributes to the plasticity and function of the olfactory system through continuous replacement of several types of olfactory neurons involved in the sense of smell.[68,77] Studies also suggest that SVZ neurogenesis contributes to social behavior such as mate selection and mate recognition.[1,71,83]
NSCs can also be derived from primitive pluripotent stem cells such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). NSCs expanded derived from the fetal, postnatal and the adult brain, as well as ESCs and iPSCs, have therapeutic properties, which are evident from numerous studies employing grafting of NSCs into the aged brain and the adult brain afflicted with injury or disease.[4,36,42,43,45,94,96,101,109] The beneficial effects of NSC graft appear to be mainly mediated through enhancement of regeneration, plasticity and neurogenesis, and attenuation of neuroinflammation.[13,54,88] Indeed, several clinical trials are currently progressing in different phases.[87,105,106] While NSC therapy has considerable promise for improving brain function in a variety of conditions, alternative treatments such as the use of stem cell products have received significant attention lately. One such NSC product is the extracellular vesicle (EV) released by NSCs. The EVs, released from NSCs provide therapeutic effects via modulation of the function of neurons and glia in both the local environment as well as at distant sites. In this review, we discuss EVs in general, attributes and role of NSC-derived EVs in health and disease, and the promise of NSC-derived EVs for promoting neural plasticity and neuroregeneration and improving function after injury or illness.
2. Extracellular vesicles
2.1. Definition and biogenesis of EVs
EVs are exuded membrane-encircled “arrays” of cells holding cargoes such as cytosolic proteins, mRNAs, miRNAs and even DNA.[15,44] Larger EVs, also called as microvesicles, bud off directly from the plasma membrane with a diameter ranging from 100 to 1000 nm.[104] On the other hand, smaller EVs or exosomes with a diameter ranging from 30 to 100 nm are formed in multivesicular endosomes (MVEs) within cells and then released into the extracellular space.[15] The biogenesis of MVEs may involve several parallel conduits, which are schematically illustrated in Fig. 1. The two most common paths comprise the endosomal sorting complex required for transport (ESCRT)-dependent pathway involving sphingolipids and an ESCRT-independent pathway involving tetraspanins [15]. Therefore, the same cell may release subpopulations of exosomes or exosome-like EVs with distinct cargo compositions inducing different effects on target cells.[44,56,104] Typically, the formation and release of exosomes or small EVs into the extracellular space involves the following steps. First, the inward invagination of the endosomal membrane results in the creation of intraluminal vesicles (ILVs) or exosomes [51,57]. Accumulation of multiple ILVs within an endosome forms a multivesicular body (MVB). Although some MVBs are digested through the lysosomal pathway, the other MVBs fuse with the plasma membrane and release exosomes into the extracellular space as membrane-bound vesicles with a diameter of 30–100 nm [41,57,90,108].
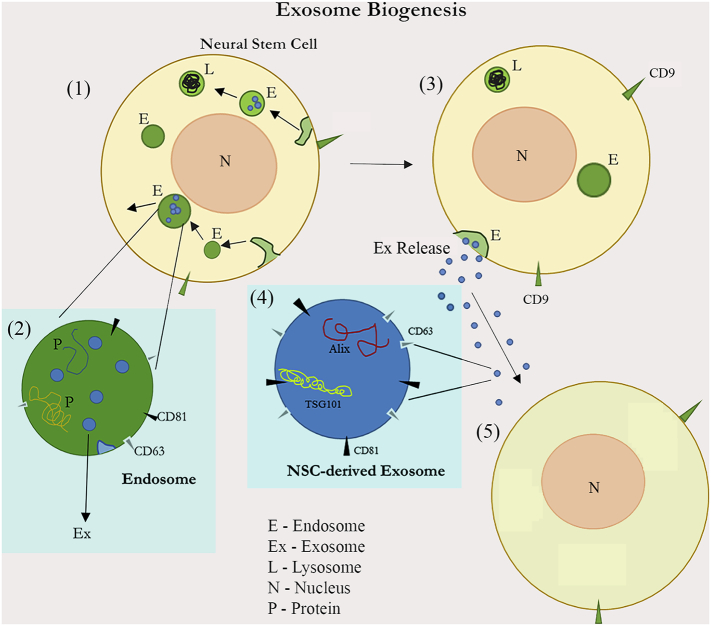
Fig. 1.
: A schematic showing the biogenesis and release of exosomes (or small extracellular vesicles) from neural stem cells into the extracellular space. First, endosomes (E) are produced through invaginations of the plasma membrane [95]. Endosomes will next generate many intraluminal vesicles or exosomes via the inward invagination of the endosomal membrane [95]. Through the ESCRT-dependent or independent pathways, exosomes are formed and packaged within endosomes using sphingolipids and tetraspanins [30]. Endosomes then either enter the lysosomal (L) route to get digested or fuse with the plasma membrane resulting in the release of exosomes (Ex Release) into the extracellular space [1]. Exosomes comprise a cargo of miRNAs, mRNAs, proteins (P) including TSG101 and Alix, and the membrane markers CD63 and CD81 [82]. The released exosomes may influence the function of neighboring as well as distant cells [27].
2.2. Structure and composition of EVs
Exosomes exhibit well-demarcated round morphology, whereas larger EVs (e.g., microvesicles) and apoptotic bodies display variable size and shape. In addition to mRNAs, miRNAs, and lipids, exosomes contain a variety of proteins within and on their plasma membrane, which allows them to identify as exosomes.[16,108] These include membrane transport and fusion proteins, tetraspanins, heat shock proteins, proteins involved in MVB biogenesis, and lipid-related proteins and phospholipases. Some proteins that can be found in exosomes include TSG101, AIP1/ALIX, 1-Integrin, the cluster of differentiation (CD) 81, CD63, ICAM-1, and MFG-E8.[16] The lipids commonly found within exosomes include cholesterol, phosphatidylserine and phosphatidylinositol, sphingomyelin, and phosphatidylcholine.[102] Likewise, exosomes also comprise mRNA as well as miRNA.[107,108]
ExoCarta has provided some interesting information compounded from all studies concerning exosomes until 2012.[72] A total of 134 exosome studies reported 4049 proteins, 1639 mRNA molecules, 58 lipid molecules, and 764 miRNA molecules. In most of these studies (~84%), exosomes were isolated via differential centrifugation method, however.[72] The guidelines for designating small EVs as exosomes are still in development. As per the Minimal Information for Studies of Extracellular Vesicles 2018 guidelines provided by the International Society for Extracellular Vesicles, the designation of EVs into different types (large EVs, small EVs including exosomes) would depend upon the method used for their recovery and protein markers present around and within its membrane. For example, a minimal requirement for designating small EVs as exosomes would require demonstration of two membrane proteins (CD63 and CD81), and several intra-vesicle proteins (TSG101, syntenin, CHMP2, and CHMP4). Thus, to define EVs obtained from a variety of available methods, one has to examine several types of tetraspanins and intra-vesicle proteins.
2.3. Function of EVs
EVs are multimodal signaling conduits, proficient in modifying the phenotype of target cells in many ways. For example, EVs can directly mediate the transfer of bioactive materials between cells, initiate signaling events at the cell surface[15] or alter the molecular composition of the extracellular milieu.[47] Also, EVs are likely long-range messengers for delivering state-dependent molecular information within and across organs because, they can travel a wide range of distances in bodily fluids such as the blood and cerebrospinal fluid.[44] Although virtually all types of cells produce and release EVs into the extracellular space or body fluids, the size, cargo, and function of EVs vary considerably between cell types.[108] Small EVs (including exosomes) are major players of the intercellular communication network [108] because they can facilitate cross-talk between cells located in distant locations.[40,108] Exosomes are also involved in antigen presentation, cell death, angiogenesis, inflammation, and coagulation.[108] Many studies have demonstrated the presence of micro-RNAs (miRNAs) within exosomes and suggest that exosomes from a group or type of cells can influence the function of another group or class of cells located either locally or distally through the delivery of miRNAs.[107]
In the CNS, secretion of small EVs including exosomes is connected to synaptic transmission as both neuronal depolarization, and glutamate release stimulates the secretion of such EVs from neurons and oligodendrocytes.[29,60] EVs are involved in the extensive cross-talk between neurons and glia, including microglia in the CNS.[86] It is believed that EVs transfer bioactive RNAs, proteins and lipids between cells during synaptic activity to regulate synaptic function, neurovascular integrity and the maintenance of myelination.[44] Furthermore, EVs secreted by neurons can transfer neuron-derived cargo to astrocytes in the brain, which may modulate the behavior of astrocytes with possible functional outcomes on synaptic activity and neurovascular integrity.[44] For example, the transfer of neuronal miR-124a-carrying EVs into astrocytes was associated with enhanced expression of excitatory amino acid transporter 2/glutamate transporter-1 (EAAT2/GLT1), a protein involved in uptake of glutamate by peri-synaptic astrocytes.[76] Neuronal EVs have also been shown to regulate brain vascular integrity through the transfer of miR-132 into vascular endothelial cells, leading to upregulated expression of the adherens junction protein Cdh5.[116] EVs derived from glial cells also mediate several functions. EVs shed from astrocytes have neurotrophic and protective roles in physiological conditions. Furthermore, EVs secreted by astrocytes in response to ischemic, oxidative and nutrient deprivation conditions carry multiple survival factors, including heat shock protein 70, synapsin-I or leukemia inhibitory factor.[11,110] Another recent study demonstrated that EVs secreted by astrocytes in response to inflammatory brain lesions regulate peripheral leukocyte response.[24] On the other hand, oligodendrocytes release EVs containing proteolipid protein, myelin proteins and proteins associated with protection against oxidative stress.[10,29,58]
However, in some pathological settings, EVs are considered as mediators of the disease. Such a phenomenon has been observed in many neurodegenerative diseases.[11] EVs, including those secreted by astrocytes and microglia, have been reported to carry specific particles from their cells of origin to other cells resulting in the propagation of pathological changes in the brain. These include beta-amyloid peptides and tau in Alzheimer's disease,[3,89] prion protein in spongiform encephalopathy,[31] α-synuclein and leucine-rich repeat kinase 2 in Parkinson's disease,[26,28] and superoxide dismutase 1 in Amyotrophic lateral sclerosis.[6] In general, microglia-derived EVs are believed to be involved actively in the spread of neuroinflammation, neurodegenerative disorders and brain cancer.[80] However, EVs have also been shown to play a role in neuroprotection because they are a part of the regeneration of peripheral nerves and repair of neuronal injuries.[49] In this context, studying the properties of EVs and exosomes released by neural stem cells has received considerable attention.
3. Properties of NSC-derived EVs
The various known properties and functions of NSC-EVs are illustrated in Fig. 2 and described in detail in sections below.
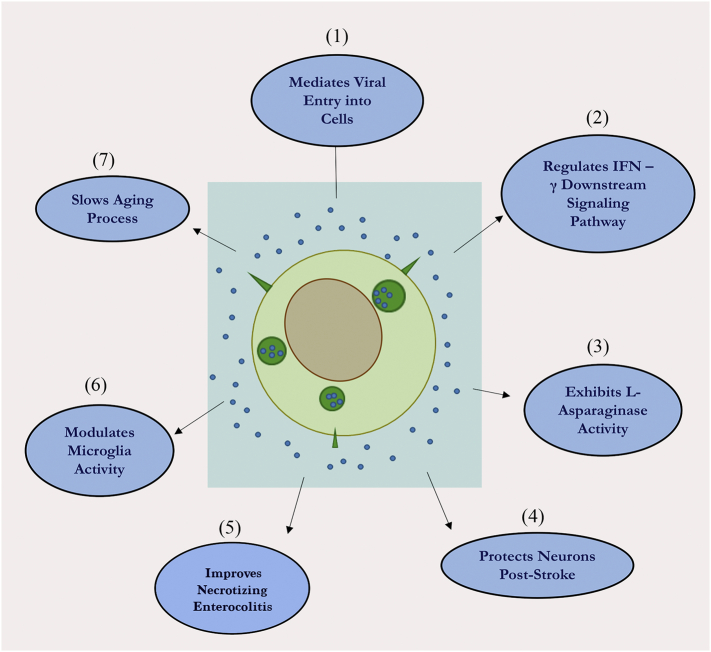
Fig. 2.
: A schematic depicting the various known functions of neural stem cell-derived extracellular vesicles (NSC-EVs). NSC-EVs have so far been found to involved in multiple activities. These include mediating the viral entry into cells via a receptor-independent fashion [95], regulating specific downstream signaling pathways in target cells [30], and functioning as independent metabolic units via L-asparaginase activity [1]. Moreover, NSC-EVs have been shown to promote neuroprotection in the mouse and porcine models of stroke [82] facilitating recovery after necrotic enterocolitis [27] and acting as a microglial morphogen to modulate microglia activity during brain development [14]. Additionally, NSC-EVs have been shown to control the process of aging through specific EVs released from the hypothalamus [21].
3.1. NSC-derived EVs are enriched with specific miRNAs
Stevanato and colleagues characterized the miRNA content of EVs derived from a conditionally immortalized human neural stem cell (hNSC) line, which is an hNSC line employed in clinical trials for stroke and critical limb ischemia.[100] The exosomes, isolated from the spent media of hNSC line cultures via ultracentrifugation, were positive for CD63 and CD81 with a modal size ranging from 86 to 95 nm. Through next-generation sequencing technology, this study found 113 miRNAs in hNSC-derived exosomes in comparison to 446 in hNSCs. The five most exosomal enriched miRNAs in these exosomes comprised Homo sapiens (hsa)-miR-1246, hsa-miR-4488, hsa-miR-4508, hsa-miR-4492, and hsa-miR-4516.[100] Notably, through the quantification of miRNA stoichiometry of a selected miRNA, this study revealed that hNSC exosomal preparations could transfer a specific miRNA at a functional level sufficient to mediate a biological effect. Some of the miRNAs enriched in hNSC exosomes have significance for regeneration. For instance, miR-1246, also found in embryonic stem cells, targets p53[78,121] and is involved in cell growth and apoptosis.[65,115] Furthermore, miR-4488, also found in mesenchymal stem cells, is involved in accelerating skeletal muscle regeneration.[81] Overall, this study suggested that hNSC-derived exosomal miRNAs have physiological importance and are suitable for therapeutic applications.
3.2. NSC-derived EVs communicate with the microenvironment
Cossetti and others showed that adult mouse NSCs communicate with the microenvironment via EVs.[18] Focusing on cytokine-regulated pathways that sort proteins and mRNAs into EVs, this study showed a significant modification of RNA and protein cohorts in EVs secreted by cells under both pro- and anti-inflammatory cytokine treatment conditions. Specifically, proinflammatory cytokine signaling in NSCs activated signal transduction along the interferon gamma (IFN-γ)-Stat1 pathway, which resulted in the export of specific components of this pathway to EVs. Thus, highly specific induction of (IFN-γ) pathway in NSCs after exposure to proinflammatory cytokines could be mirrored in EVs. Furthermore, intercellular transfer of IFN-γ bound to interferon gamma receptor 1 (Ifngr1) on the surface of EVs activated Stat1-dependent signaling in target NIH 3 T3 cells.[18] However, both endogenous Stat1 and Ifngr1 in target cells were found to be critical for the sustained activation of Stat1 signaling by NSC-derived EVs. Additionally, this study showed that EVs entering target cells do not bind quickly to lysosomal-associated membrane protein 1 (LAMP1) implying that EVs entering target cells do not undergo immediate degradation, which facilitated activation of signaling pathways by newly introduced EVs.
3.3. NSC-derived EVs are involved in viral entry into cells
A study by Sims and associates suggested that NSC-derived exosomes mediate entry of viruses into cells via a receptor-independent fashion.[98] In this study, the specific role of exosomes in mediating the entry of adenovirus type 5 (Ad5) into Coxsackie virus and adenovirus receptor (CAR)-deficient cells was examined. The choice of CAR-deficient cells is based on earlier observations that Ad5 has minimal entry to these cells. Addition of NSC-derived exosomes expressing CD63 and CD81 into cultures of CAR-deficient cells and Ad5 resulted in an enhanced entry of Ad5 into CAR-deficient cells. Additional analysis suggested the role of T-cell immunoglobulin mucin protein 4 (TIM-4, a phosphatidylserine receptor) in NSC-derived exosomes for mediating viral entry, as Ad5 can bind to TIM-4 through interaction with anionic phospholipids.[5] Consistent with this idea, inactivation of TIM-4 function through a specific antibody blocked the exosome-mediated Ad5 entry into CAR-deficient cells. Thus, exosomes secreted by NSCs and likely other neural cells appear to be involved in virus trafficking in the brain.
3.4. NSC-derived EVs can function as independent metabolic units
A recent study has ascertained the competence of both human and mouse NSC-derived EVs to utilize and generate metabolites by using metabolomics and functional analyses approach.[47] The EVs produced from NSCs exhibited markers such as Pdcd6ip, Tsg101, CD63, and CD9. Furthermore, NSC-EVs displayed L-asparaginase activity catalyzed by the enzyme asparaginase-like protein 1 (Asrgl1), in contrast to lack of such activity in the NSC conditioned medium or the EV-deprived NSC supernatants. Interestingly, both mouse and human NSCs package Asrgl1 into their EVs. The study also found that the utilization and discharge of metabolites was primarily a result of intrinsic enzymatic activity in EVs. Asparagine was the highest consumed metabolite whereas aspartate and glutamate were the two metabolites released by EVs. They also found that Asrgl1 activity was selective for asparagine and was devoid of glutaminase activity. Thus, NSC-EVs can function as independent metabolic units, which can alter the concentrations of critical nutrients in the milieu, and thereby affect the physiology of cells in the neighborhood. Indeed, several studies have been shown that distinct cell types producing EVs can act as metabolic regulators.[32,92,125] Aspartate released from EVs can also play a supportive role in cells' bioenergetics.[47,103]
3.5. EVs released from hypothalamic NSCs influence the process of aging
A study has uncovered that the process of aging is prompted by the lack of specific miRNA release by EVs generated from postnatal mouse hypothalamic NSCs.[122] NSCs displaying markers such as nestin, Sox-2 (sex determining region Y-box 2) and Bmi1 (B cell-specific Moloney murine leukemia virus integration site 1) persist in the hypothalamic third ventricle region in adulthood but their loss later in life seemed to accelerate aging processes.[64,122] Such links were evidenced by several observations.[122] First, the effect of ablation of hypothalamic NSCs in adulthood on the aging process was measured. Ablation of NSCs was accomplished via injection of lentiviruses expressing herpes simplex virus thymidine kinase-1 controlled by the Sox-2 promoter. Subsequent application of Ganciclovir killed ~70% NSCs in the hypothalamic wall through conversion of Ganciclovir into a toxin by thymidine kinase-1, without affecting the surrounding hypothalamic neurons. Such selective ablation of NSCs eventually resulted in multiple premature age-related changes, including accentuated decreases in muscle endurance, coordination, treadmill performance, sociality, novel object recognition and spatial learning ability.[122] These animals also had a shortened lifespan. An alternative method of NSC ablation via injections of lentiviruses expressing diphtheria toxic receptor controlled by Sox-2 promoter into the hypothalamic third ventricle of middle-aged mice followed diphtheria toxin treatment resulted in similar effects.
Moreover, transplantation of postnatal hypothalamic NSCs into the mediobasal hypothalamic nucleus of middle-aged mice slowed down the process of aging and lifespan extension.[122] However, transplantation of astrocytes or mesenchymal stem cells did not induce any beneficial effects on aging. The donor cells were genetically engineered to stably express dominant-negative IκBα and green fluorescent protein, to facilitate the survival of NSCs and other cells in the inflammatory milieu of the aging hypothalamus. Such an approach was based on the concept that cells expressing dominant-negative IκBα are resilient to NF-κB-mediated inflammation and hence survive well in an inflammatory microenvironment.[64]
Additional studies demonstrated that mouse hypothalamic NSCs control the process of aging by releasing EVs carrying a specific miRNA cargo into the cerebrospinal fluid.[122] In one experiment, exosome secretion by hypothalamic NSCs was inhibited through co-injection of lentivirus containing Sox-2 promoter-driven Cre and lentivirus containing Cre-dependent shRNA against Rab27a (a molecule involved in exosome secretion) into the hypothalamic third ventricle of middle-aged mice. Such inhibition resulted in impairments in several aspects of physiology. In another experiment, the effect of the direct release of EVs generated from postnatal hypothalamic NSCs into the brain of middle-aged mice was examined. EV treated mice displayed reduced age-related changes, in comparison to vehicle-treated mice exhibiting many age-related disorders. Overall, this study demonstrated that hypothalamic NSC derived EVs play a vital role in the process of aging. Since hypothalamic NSCs decline with aging, the overall concentration of specific miRNAs coming from hypothalamic NSC-EVs decreases considerably, which leads to the process of aging. On the other hand, transplantation of healthy hypothalamic NSCs into the aging brain maintains the concentration of miRNAs at optimal levels through the release of specific EVs, which leads to successful aging and lifespan extension. Thus, aging speed seemed to be controlled by EVs released by hypothalamic NSCs.
3.6. EVs released from SVZ-NSCs act as microglial morphogen
A recent study has demonstrated that EVs expressing CD63, CD9, and Alix, secreted by mouse subventricular zone (SVZ)-NSCs, specifically target microglia and influence their morphology and function in the postnatal nervous system.[79] This was evident from the reduced density of extracellular CD9-GFP+ EVs derived from SVZ-NSCs with the influx of microglia into the SVZ. Subventricular zone (SVZ) is one of the few canonical neurogenic regions in the perinatal brain maintaining active NSC activity in a neurogenic niche.[67] NSC activity in this region produces neuroblasts which migrate along the rostral migratory stream into the olfactory bulb where they renew olfactory granule neurons, which facilitates continuous adaptation to environmental olfactory cues.[69] The neonatal SVZ is also enriched with a band of microglia, a type of glia involved in the phagocytic activity and hence referred to as resident macrophages in the nervous system. Microglia are generated from myeloid cells, which originate in the yolk sac and migrate to the developing brain during the mid-gestation period.
Small RNA sequencing demonstrated highly enriched miRNAs (miR-9 and Let-7) within EVs released from SVZ-NSCs. Members from each of these miRNA families have roles in regulating microglia morphology and physiology.[59,62,117,123] Consistent with this concept, transplantation of EVs derived from NSCs resulted in increased numbers of CD11b + rounded, non-stellate microglia. However, transplantation of EVs pre-treated with UV resulted in much fewer CD11b + microglia, implying that the cargo released from EVs (e.g., miRNAs) is influencing microglial morphology. Indeed, transplantation of synthetic Let-7 miRNA-transfected exosomes into the SVZ of neonatal mice induced microglia to acquire a rounder appearance, which implied that miRNAs in the EVs secreted by SVZ-NSCs modulate microglial morphology. Furthermore, the uptake of EVs generated from SVZ-NSCs resulted in robust re-wiring of transcriptional networks in microglia. These networks were associated with inflammatory response, defense response to virus, neutrophil chemotaxis, positive regulation of cytokine regulation and I-kappa B kinase/NF-kB signaling. Furthermore, transcriptional changes corresponded to enhanced cytokine release, particularly IL-6 and TNFα, implying that miRNAs released by SVZ-NSC EVs function as a morphogen-associated molecular pattern to control microglia. Additionally, injection of conditioned media from exosome treated microglia into the lateral ventricle regulated proliferation of SVZ-NSCs, akin to the regulation of NSC proliferation by microglia reported in earlier studies.[19,33] Collectively, the above results implied that NSC-derived EVs have a role in neurodevelopment.
4. Therapeutic efficacy of NSC-derived EVs for treating neurological disorders
Like EVs from any other cells, NSC-derived exosomes carry a cargo of miRNAs, mRNAs, proteins, and lipids. However, the composition of NSC-derived EVs and targeting behavior after intravenous or intranasal administration is likely to be different from EVs derived from other stem cells or different types of somatic cells. It appears that NSC-derived EVs contain miRNAs and proteins relevant for neural regeneration, neuroprotection and neural plasticity.[100] Furthermore, studies have shown that NSC-derived EVs can act as independent metabolic units and hypothalamic NSC-derived seem to play a role in the aging process.[47,122] Therefore, exogenous application of NSC-derived EVs into the adult brain in both normal and disease conditions can influence the microenvironment and signaling pathways positively, which would likely lead to an improved function of neurons and glia. Considering these, there is a significant interest to utilize NSC-derived exosomes for treating a variety of neurological disorders. However, only a few studies have been published so far.
4.1. Advantages of using NSC-EVs over NSCs
There are several advantages of using NSC-derived exosomes over NSCs themselves. First, a large number of EVs can be targeted to diverse regions of the brain through an intranasal administration [70]. Second, unlike cell therapy, the probability for developing a tumor or malignant transformation after EV administration is virtually zero because they are not nucleated cells, cannot replicate and quickly disintegrate after releasing the cargo.[73] Third, intravenous or intranasal administration of EVs is unlikely to lead to thrombosis (obstruction of small blood vessels), as EVs are tiny vesicles having the ability to cross the blood-brain barrier as well as pass-through endothelial cells through transcytosis.[53] EVs also have low immunogenicity.[25] With the advent of new techniques, it is also feasible to generate therapeutic vesicles using large-scale cellular factories.[34] Moreover, since EVs are stable for extended periods of time at –80 °C and several weeks at 4 °C, they can be stored and transported easily. However, EVs need to be fully characterized for consistency in their composition and biological effects prior to clinical translation to avoid any undesired effects, as stem-cell derived EVs may exhibit some toxic features in some conditions.[63] Thus, EVs providing cell-free therapies could change the way medicine is perceived and could save many lives without significant side effects.
4.2. NSC-derived EVs promote brain repair in a mouse model of stroke
Stroke is among the primary cause of mortality and morbidity. The only treatment available for stroke is systemic thrombolysis with tissue plasminogen activator (TPA). However, such treatment is limited to ischemic stroke only and can be employed in only a small percentage of stroke patients as it causes adverse effects when administered at deferred time points after stroke.[97] Currently, a drawn-out series of rehabilitation with a lifetime process of clinical support is the norm for most stroke survivors. Therefore, alternative therapies that are non-invasive and promising to repair the stroke-afflicted brain are required. From this perspective, a few studies analyzing the efficacy of NSC-derived EVs in stroke models have considerable significance. While multiple studies have shown the efficacy of MSC-derived EVs or cardiosphere-derived cell EVs for moderating stroke-induced neurodegeneration, neuroinflammation and neurological deficits,[20,61,75,84,114] studies on the usefulness of NSC-derived EVs for treating stroke have been scarce but have shown very robust positive results.
A study by Webb and colleagues suggested that intravenous administration of NSC-derived EVs after thromboembolic stroke in the mouse can improve the cellular, tissue, and functional outcomes in middle-aged mice.[112] In this study, EVs collected from human embryonic stem cell (hESC) derived NSCs were used, which were less than 200 nm in diameter and expressed markers such as CD63 and CD81. To ascertain the targeting of EVs, indium-111 labeled EVs were injected 1 h after thromboembolic middle cerebral artery occlusion. Animals were imaged at 1 and 24 h after the injection of EVs using a single photo emission chromatography (SPECT), which demonstrated that EVs reach the infarcted hemisphere by 1 h and get cleared by 24 h.[112] Using T2-weighted sequences (T2W) and ex vivo Q-ball MRI, this study also found significantly decreased tissue loss in NSC-EV treated animals compared to controls. Moreover, administration of EVs generated from NSCs had positive effects on motor function when examined through beam walk and hanging wire tests. Additionally, increased time with a novel object also implied improved episodic memory in animals received EVs after stroke. Since stroke leads to chronic neurodegeneration via a localized neuroimmune response opening the blood-brain barrier (BBB),[12,38] microglial activity was also examined in this study. NSC-EV administration promoted a conducive milieu for polarization of microglia into an M2-phenotype, which likely promoted debris clearance and reduced chronic inflammation. Overall, this study showed that administration of EVs generated hESC-derived EVs is efficacious for providing neuroprotection and alleviating motor and memory impairments and chronic inflammation.[112]
4.3. NSC-derived EVs promote functional recovery in a porcine model of stroke
After seeing the beneficial effects in the mouse model, the same group investigated the efficacy of EVs derived from hESC-NSCs in a porcine model.[111] In this study, ischemic stroke was induced through a permanent middle cerebral artery occlusion (MCAO), and EVs were administered intravenously at 2, 14, and 24 h after stroke. In this study, EV treatment after stroke induction was neuroprotective and resulted in significant improvements at the tissue and functional levels. Administration of NSC-EVs eliminated intracranial hemorrhage in ischemic lesions, decreased cerebral lesion volume and brain swelling. A day after stroke, pigs receiving EVs displayed a reduced percent change in apparent diffusion coefficient (ADC) values when compared with the control group.[111] NSC-EV treatment also preserved white matter integrity with increased corpus callosum fractional anisotropy values when examined 84 days after stroke with MRI. These results suggested that EV treatment after stroke promoted neuroprotection, which was evident from reduced loss of cerebral tissue, decreased swelling, and preservation of white matter integrity.
Furthermore, administration of EVs preserved normal exploratory behavior and motor activity in pigs after stroke. A faster recovery was also evident in pigs receiving EVs when examined at seven days after stroke. These positive results have considerable significance because of the use of a larger animal model in this study. Additionally, EVs from hESC-NSCs did not cause any negative immune responses. Overall, this study demonstrated that administration of NSC-derived EVs after stroke could provide significant neuroprotection and preserve motor function.
4.4. Efficacy of NSC-derived EVs for reducing the severity of enterocolitis
Another recent study has investigated the efficacy of EVs derived from neonatal enteric NSCs (E-NSCs) with EVs derived from amniotic fluid NSCs, amniotic fluid mesenchymal stem cells (MSCs), and bone marrow-derived MSCs using a model of necrotizing enterocolitis (NEC).[73] Increasing doses of EVs proportionately decreased the incidence of NEC. Furthermore, animals receiving EVs after NEC also displayed significantly less intestinal injury compared to the vehicle group. While EVs derived from all sources reduced the incidence of NEC in this study by 11–27%, the overall effect was somewhat higher with E-NSCs. Thus, NSC-derived EVs have promise for treating NEC. However, the mechanisms by which EVs reduced NEC is not examined in this study.
5. Conclusions and future perspectives
The field of EVs is currently one of the fast-moving areas in basic science and translational research.[48,85] However, the properties of different types of EVs (e.g., EVs versus exosomes) from a particular cell type and differences between EVs generated from different kinds of cells (e.g., MSC vis-à-vis NSC) in various conditions remain to be explored.[113] In this review, we specifically focused on the properties and therapeutic potential of NSC-derived EVs. NSC-derived EVs enriched with specific miRNAs likely mediate multiple functions in physiological and pathological conditions. EVs secreted from NSCs can modulate the immediate microenvironment. For instance, pro- and anti-inflammatory conditions in the microenvironment can lead to the production of distinct types of EVs with a specific composition of RNAs and proteins required for modulating the milieu. Also, EVs entering target cells do not get digested quickly to facilitate the activation of signaling pathways in target cells.[18]
NSC-derived exosomes also mediate the entry of viruses into cells via a receptor-independent fashion;[98] however, it is unclear whether exosomes secreted by other neural cells (neurons and glia) also have this property. Nonetheless, after a viral infection of the brain, exosomes secreted by NSCs can effectively spread the disease to multiple regions of the brain. This finding provides an avenue for controlling viral infection in the brain by blocking the production of exosomes from endogenous NSCs. NSC-EVs can also function as independent metabolic units,[47] which facilitates modulation of concentrations of critical nutrients in the milieu to influence the function of cells in both physiological and pathological conditions, depending upon the cargo released by EVs. Moreover, EVs released from NSCs in different regions might be influencing diverse aspects of brain function. For example, EVs released from NSCs in the hypothalamus control the process of aging.[122] Since hypothalamic NSCs get depleted with aging, it may be possible in the future to treat aging by repeated intranasal administration of EVs secreted from human hypothalamic NSCs in culture. NSC-derived EVs can also act as microglial morphogen,[79] which might have implications for neuroinflammatory conditions in the adult brain.
It is plausible that NSC-derived EVs are involved in modulating the activated microglia in chronic inflammatory conditions into M2 phenotype. Indeed, previous studies have shown that grafting of NSCs in prototypes of Alzheimer's disease, traumatic brain injury, hypoxic-ischemic injury, spinal cord injury and stroke resulted in reduced inflammation.[8,31,55,91,101,119,124] It has been shown that grafted NSCs ameliorate chronic CNS inflammation through several mechanisms. NSCs can reprogram the infiltrating inflammatory monocyte-derived cells into antiinflammatory myeloid cells via secretion of TGF-β2.[22] NSCs can also modulate succinate levels in the cerebrospinal fluid, resulting in reduced mononuclear phagocyte infiltration and secondary CNS damage.[88] NSC grafting can also enhance neurogenesis in the aged brain22 and improve cognitive and mood function in the injured brain.[42,94] All of these effects may be mediated mainly or partially through the release of EVs by the transplanted NSCs. Indeed, a recent study using next-generation sequencing has shown that hNSC-derived EVs promote stroke recovery via modulation of gene expression.[120] Nonetheless, focused studies on reactive astrocytes, activated microglia and pro- and anti-inflammatory cytokines in inflamed regions of the brain following intranasal administration of NSC-derived EVs are needed to confirm these possibilities. Direct grafting of NSC-EVs to the inflamed regions may not be required as intranasally administered EVs quickly target multiple regions of the brain including those afflicted with acute inflammation.[70]
Furthermore, due to their anti-inflammatory, neurogenic and neurotrophic effects, intravenous or intranasal administration of NSC-derived EVs are likely useful for treating multiple neurodegenerative diseases and neurological disorders. These include conditions such as dementia, Alzheimer's disease, Huntington's disease, Parkinson's disease and Amyotrophic lateral sclerosis, stroke, traumatic brain injury, multiple sclerosis and major depressive disorder. Indeed, studies have shown that intravenous administration of hESC-derived EVs can positively modulate stroke-induced injury and recovery in both mouse and porcine models.[111,112] NSC-derived EVs have also shown efficacy for treating necrotizing enterocolitis in animal models.[73] While only a few studies have been published about the effectiveness of NSC-EVs to treat brain dysfunction so far, the promise seems to be enormous.
Consequently, a large number of studies on NSC-EVs are currently in progress in distinct neurodegenerative/neurological disease models. Thorough understanding of the composition of EVs and the development of advanced approaches to modulate the cargo of EVs to include the desired miRNAs and proteins would further enhance the therapeutic efficacy of EVs for different conditions afflicting the CNS. For example, Alzheimer's disease may be treated with EVs comprising miRNAs and proteins that help in clearing amyloid beta plaques, provide neuroprotection, enhance neurogenesis, reduce hyperphosphorylation of tau and enhance the phagocytic role of microglia would be of tremendous value. The cargo in EVs may include neprilysin for clearing plaques,[50] brain-derived neurotrophic factor for providing neuroprotection,[93] and catalase for combating oxidative stress.[35]
In summary, the use of well-characterized EVs secreted by NSCs in specific culture conditions and/or NSC-derived EVs that are engineered to carry the desired miRNAs, mRNAs and proteins has great promise for treating multiple neurogenerative diseases and neurological conditions. Mainly, non-invasive therapeutic approaches using NSC-EVs are likely to be more efficacious than NSCs because EVs can easily cross the BBB following intravenous or intranasal administration. For example, neurons can be specifically targeted to receive a functional siRNA through systemic injection of engineered exosomes.[2,17,39] In the future, the possibility of targeting EVs to specific neuronal types or region in the brain would facilitate treating distinct neurodegenerative conditions with minimal side effects. For example, specific EVs may be engineered to target dopaminergic neurons in Parkinson's disease, medium-sized spiny interneurons in Huntington's disease, and motor neurons in amyotrophic lateral sclerosis. However, several hurdles remain to be overcome before the widespread clinical application of EVs. The challenges include finding an apt method for purification of EVs that is scalable, compatible with CGMP guidelines, does not alter the biological activity of EVs, and efficient for providing a homogenous population of EVs containing similar cargo of miRNAs, proteins and lipids.
Funding
This work was supported by grants from the Department of Defense (CDMRP W81XWH-14-1-0558 to A.K.S.), and the Department of Veterans Affairs (VA Merit Award grant I01BX000883 and VA-BLR&D Research Career Scientist award, 1IK6BX003612 to A.K.S.). The funders had no role in study design, data collection, data analysis, interpretation, writing of the report.
Conflicts of interest
The authors declared that there is no conflict of interest.
Author contributions
AV: Literature search, discussion of findings in original research articles and preparation of the first draft of manuscript; RU: Literature search, discussion of findings in original research articles, feedbacks to the manuscript text, reference list preparation, and manuscript revision; AKS: Literature search, discussion of findings in original research articles, manuscript writing and editing, manuscript revision, and preparation of the final version of the manuscript.
References
- 1.Adams K.V., Morshead C.M. Neural stem cell heterogeneity in the mammalian forebrain. Prog Neurobiol. 2018;170:2–36. doi: 10.1016/j.pneurobio.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Erviti L., Seow Y., Yin H., et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 3.Asai H., Ikezu S., Tsunoda S., et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015;18:1584–1593. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assinck P., Duncan G.J., Plemel J.R., et al. Myelinogenic plasticity of oligodendrocyte precursor cells following spinal cord contusion injury. J Neurosci. 2017;37:8635–8654. doi: 10.1523/JNEUROSCI.2409-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balakireva L., Schoehn G., Thouvenin E., et al. Binding of adenovirus capsid to dipalmitoyl phosphatidylcholine provides a novel pathway for virus entry. J Virol. 2003;77:4858–4866. doi: 10.1128/JVI.77.8.4858-4866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basso M., Pozzi S., Tortarolo M., et al. Mutant copper-zinc superoxide dismutase (SOD1) induces protein secretion pathway alterations and exosome release in astrocytes: implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis. J Biol Chem. 2013;288:15699–15711. doi: 10.1074/jbc.M112.425066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boldrini M., Fulmore C.A., Tartt A.N., et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22:589–599. doi: 10.1016/j.stem.2018.03.015. [e5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braccioli L., Vervoort S.J., Adolfs Y., et al. FOXP1 promotes embryonic neural stem cell differentiation by repressing Jagged1 expression. Stem Cell Rep. 2017;9:1530–1545. doi: 10.1016/j.stemcr.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun S.M., Jessberger S. Adult neurogenesis: mechanisms and functional significance. Development. 2014;141:1983–1986. doi: 10.1242/dev.104596. [DOI] [PubMed] [Google Scholar]
- 10.Budnik V., Ruiz-Canada C., Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17:160–172. doi: 10.1038/nrn.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhuri A.D., Dastgheyb R.M., Yoo S.W., et al. TNFalpha and IL-1beta modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death Dis. 2018;9:363. doi: 10.1038/s41419-018-0369-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J., Venkat P., Zacharek A., et al. Neurorestorative therapy for stroke. Front Hum Neurosci. 2014;8:382. doi: 10.3389/fnhum.2014.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Z., Zhu W., Cao K., et al. Anti-inflammatory mechanism of neural stem cell transplantation in spinal cord injury. Int J Mol Sci. 2016;17:E1380. doi: 10.3390/ijms17091380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung T.H., Rando T.A. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colombo M., Raposo G., Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 16.Conde-Vancells J., Rodriguez-Suarez E., Embade N., et al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res. 2008;7:5157–5166. doi: 10.1021/pr8004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper J.M., Wiklander P.B., Nordin J.Z., et al. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov Disord. 2014;29:1476–1485. doi: 10.1002/mds.25978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cossetti C., Iraci N., Mercer T.R., et al. Extracellular vesicles from neural stem cells transfer IFN-gamma via Ifngr1 to activate Stat1 signaling in target cells. Mol Cell. 2014;56:193–204. doi: 10.1016/j.molcel.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham C.L., Martinez-Cerdeno V., Noctor S.C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33:4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham C.J., Redondo-Castro E., Allan S.M. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cereb Blood Flow Metab. 2018;38:1276–1292. doi: 10.1177/0271678X18776802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis A.A., Temple S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature. 1994;372(6503):263–266. doi: 10.1038/372263a0. [DOI] [PubMed] [Google Scholar]
- 22.De Feo D., Merlini A., Brambilla E., et al. Neural precursor cell-secreted TGF-beta2 redirects inflammatory monocyte-derived cells in CNS autoimmunity. J Clin Invest. 2017;127:3937–3953. doi: 10.1172/JCI92387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng W., Aimone J.B., Gage F.H. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickens A.M., Tovar Y.R.L.B., Yoo S.W., et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10 doi: 10.1126/scisignal.aai7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Andaloussi S., Lakhal S., Mager I., et al. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev. 2013;65:391–397. doi: 10.1016/j.addr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Emmanouilidou E., Melachroinou K., Roumeliotis T., et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Englund C., Fink A., Lau C., et al. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraser K.B., Rawlins A.B., Clark R.G., et al. Ser(P)-1292 LRRK2 in urinary exosomes is elevated in idiopathic Parkinson's disease. Mov Disord. 2016;31:1543–1550. doi: 10.1002/mds.26686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frohlich D., Kuo W.P., Fruhbeis C., et al. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130510. doi: 10.1098/rstb.2013.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gage F.H., Temple S. Neural stem cells: generating and regenerating the brain. Neuron. 2013;80:588–601. doi: 10.1016/j.neuron.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 31.Gao M., Yao H., Dong Q., et al. Neurotrophy and immunomodulation of induced neural stem cell grafts in a mouse model of closed head injury. Stem Cell Res. 2017;23:132–142. doi: 10.1016/j.scr.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Garcia N.A., Moncayo-Arlandi J., Sepulveda P., et al. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovasc Res. 2016;109:397–408. doi: 10.1093/cvr/cvv260. [DOI] [PubMed] [Google Scholar]
- 33.Gebara E., Sultan S., Kocher-Braissant J., et al. Adult hippocampal neurogenesis inversely correlates with microglia in conditions of voluntary running and aging. Front Neurosci. 2013;7:145. doi: 10.3389/fnins.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gimona M., Pachler K., Laner-Plamberger S., et al. Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int J Mol Sci. 2017;18:E1190. doi: 10.3390/ijms18061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Godoy M.A., Saraiva L.M., de Carvalho L.R.P., et al. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. J Biol Chem. 2018;293:1957–1975. doi: 10.1074/jbc.M117.807180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldman S.A. Stem and progenitor cell-based therapy of the central nervous system: hopes, hype, and wishful thinking. Cell Stem Cell. 2016;18:174–188. doi: 10.1016/j.stem.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goncalves J.T., Schafer S.T., Gage F.H. Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell. 2016;167:897–914. doi: 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 38.Gronberg N.V., Johansen F.F., Kristiansen U., et al. Leukocyte infiltration in experimental stroke. J Neuroinflammation. 2013;10:115. doi: 10.1186/1742-2094-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haney M.J., Klyachko N.L., Zhao Y., et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hannafon B.N., Ding W.Q. Intercellular communication by exosome-derived microRNAs in cancer. Int J Mol Sci. 2013;14:14240–14269. doi: 10.3390/ijms140714240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hattiangady B., Shetty A.K. Neural stem cell grafting counteracts hippocampal injury-mediated impairments in mood, memory, and neurogenesis. Stem Cells Transl Med. 2012;1:696–708. doi: 10.5966/sctm.2012-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hattiangady B., Shuai B., Cai J., et al. Increased dentate neurogenesis after grafting of glial restricted progenitors or neural stem cells in the aging hippocampus. Stem Cells. 2007;25:2104–2117. doi: 10.1634/stemcells.2006-0726. [DOI] [PubMed] [Google Scholar]
- 44.Holm M.M., Kaiser J., Schwab M.E. Extracellular vesicles: multimodal envoys in neural maintenance and repair. Trends Neurosci. 2018;41:360–372. doi: 10.1016/j.tins.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Huang H., Young W., Chen L., et al. Clinical cell therapy guidelines for neurorestoration (IANR/CANR 2017) Cell Transplant. 2018;27:310–324. doi: 10.1177/0963689717746999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ihrie R.A., Alvarez-Buylla A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70:674–686. doi: 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iraci N., Gaude E., Leonardi T., et al. Extracellular vesicles are independent metabolic units with asparaginase activity. Nat Chem Biol. 2017;13:951–955. doi: 10.1038/nchembio.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Izadpanah M., Seddigh A., Ebrahimi Barough S., et al. Potential of extracellular vesicles in neurodegenerative diseases: diagnostic and therapeutic indications. J Mol Neurosci. 2018;66:172–179. doi: 10.1007/s12031-018-1135-x. [DOI] [PubMed] [Google Scholar]
- 49.Kalani A., Tyagi N. Exosomes in neurological disease, neuroprotection, repair and therapeutics: problems and perspectives. Neural Regen Res. 2015;10:1565–1567. doi: 10.4103/1673-5374.165305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katsuda T., Tsuchiya R., Kosaka N., et al. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013;3:1197. doi: 10.1038/srep01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katzmann D.J., Babst M., Emr S.D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 52.Kempermann G., Song H., Gage F.H. Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol. 2015;7:a018812. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim D.K., Nishida H., An S.Y., et al. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci U S A. 2016;113:170–175. doi: 10.1073/pnas.1522297113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kokaia Z., Lindvall O. Sensors of succinate: neural stem cell grafts fight neuroinflammation. Cell Stem Cell. 2018;22:283–285. doi: 10.1016/j.stem.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 55.Koutsoudaki P.N., Papastefanaki F., Stamatakis A., et al. Neural stem/progenitor cells differentiate into oligodendrocytes, reduce inflammation, and ameliorate learning deficits after transplantation in a mouse model of traumatic brain injury. Glia. 2016;64:763–779. doi: 10.1002/glia.22959. [DOI] [PubMed] [Google Scholar]
- 56.Kowal J., Arras G., Colombo M., et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8):E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kowal J., Tkach M., Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Kramer-Albers E.M., Bretz N., Tenzer S., et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons? Proteomics Clin Appl. 2007;1:1446–1461. doi: 10.1002/prca.200700522. [DOI] [PubMed] [Google Scholar]
- 59.Kumar M., Sahu S.K., Kumar R., et al. MicroRNA let-7 modulates the immune response to mycobacterium tuberculosis infection via control of A20, an inhibitor of the NF-kappaB pathway. Cell Host Microbe. 2015;17:345–356. doi: 10.1016/j.chom.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Lachenal G., Pernet-Gallay K., Chivet M., et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011;46:409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Lapchak P.A., Boitano P.D., de Couto G., et al. Intravenous xenogeneic human cardiosphere-derived cell extracellular vesicles (exosomes) improves behavioral function in small-clot embolized rabbits. Exp Neurol. 2018;307:109–117. doi: 10.1016/j.expneurol.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 62.Lehmann S.M., Kruger C., Park B., et al. An unconventional role for miRNA:let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci. 2012;15:827–835. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- 63.Lener T., Gimona M., Aigner L., et al. Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper. J Extracell Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li J., Tang Y., Cai D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012;14:999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li W., Wu Y.F., Xu R.H., et al. miR-1246 releases RTKN2-dependent resistance to UVB-induced apoptosis in HaCaT cells. Mol Cell Biochem. 2014;394:299–306. doi: 10.1007/s11010-014-2108-1. [DOI] [PubMed] [Google Scholar]
- 66.Lieberwirth C., Pan Y., Liu Y., et al. Hippocampal adult neurogenesis: its regulation and potential role in spatial learning and memory. Brain Res. 1644;2016:127–140. doi: 10.1016/j.brainres.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lim D.A., Alvarez-Buylla A. The adult ventricular-subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb Perspect Biol. 2016;8 doi: 10.1101/cshperspect.a018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Livneh Y., Adam Y., Mizrahi A. Odor processing by adult-born neurons. Neuron. 2014;81(5):1097–1110. doi: 10.1016/j.neuron.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 69.Lledo P.M., Valley M. Adult olfactory bulb neurogenesis. Cold Spring Harb Perspect Biol. 2016;8:a018820. doi: 10.1101/cshperspect.a018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Long Q., Upadhya D., Hattiangady B., et al. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci U S A. 2017;114 doi: 10.1073/pnas.1703920114. (E3536-E45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mak G.K., Weiss S. Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat Neurosci. 2010;13(6):753–758. doi: 10.1038/nn.2550. [DOI] [PubMed] [Google Scholar]
- 72.Mathivanan S., Fahner C.J., Reid G.E., et al. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCulloh C.J., Olson J.K., Wang Y., et al. Treatment of experimental necrotizing enterocolitis with stem cell-derived exosomes. J Pediatr Surg. 2018;53:1215–1220. doi: 10.1016/j.jpedsurg.2018.02.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ming G.L., Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moon G.J., Sung J.H., Kim D.H., et al. Application of mesenchymal stem cell-derived extracellular vesicles for stroke: biodistribution and MicroRNA study. Transl Stroke Res. 2018 doi: 10.1007/s12975-018-0668-1. Oct 19 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 76.Morel L., Regan M., Higashimori H., et al. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem. 2013;288:7105–7116. doi: 10.1074/jbc.M112.410944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moreno M.M., Linster C., Escanilla O., et al. Olfactory perceptual learning requires adult neurogenesis. Proc Natl Acad Sci U S A. 2009;106:17980–17985. doi: 10.1073/pnas.0907063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morin R.D., O'Connor M.D., Griffith M., et al. Application of massively parallel sequencing to microRNA profiling and discovery in human embryonic stem cells. Genome Res. 2008;18:610–621. doi: 10.1101/gr.7179508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morton M.C., Neckles V.N., Seluzicki C.M., et al. Neonatal subventricular zone neural stem cells release extracellular vesicles that act as a microglial morphogen. Cell Rep. 2018;23:78–89. doi: 10.1016/j.celrep.2018.03.037. [DOI] [PubMed] [Google Scholar]
- 80.Murgoci A.-N., Cizkova D., Majerova P., et al. Brain-cortex microglia-derived exosomes: nanoparticles for glioma therapy. Chemphyschem. 2018;19:1205–1214. doi: 10.1002/cphc.201701198. [DOI] [PubMed] [Google Scholar]
- 81.Nakamura Y., Miyaki S., Ishitobi H., et al. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589:1257–1265. doi: 10.1016/j.febslet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 82.Noctor S.C., Flint A.C., Weissman T.A., et al. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 83.Oboti L., Schellino R., Giachino C., et al. Newborn interneurons in the accessory olfactory bulb promote mate recognition in female mice. Front Neurosci. 2011;5:113. doi: 10.3389/fnins.2011.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Otero-Ortega L., Laso-Garcia F., Gomez-De Frutos M.D., et al. White matter repair after extracellular vesicles administration in an experimental animal model of subcortical stroke. Sci Rep. 2017;7 doi: 10.1038/srep44433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Otero-Ortega L., Laso-Garcia F., Gomez-De Frutos M., et al. Role of exosomes as a treatment and potential biomarker for stroke. Transl Stroke Res. 2018 doi: 10.1007/s12975-018-0654-7. Aug 13 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 86.Paolicelli R.C., Bergamini G., Rajendran L. Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience. 2018;S0306-4522:30254–30259. doi: 10.1016/j.neuroscience.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 87.Parmar M. Towards stem cell-based therapies for Parkinson's disease. Development. 2018;145 doi: 10.1242/dev.156117. [DOI] [PubMed] [Google Scholar]
- 88.Peruzzotti-Jametti L., Bernstock J.D., Vicario N., et al. Macrophage-derived extracellular succinate licenses neural stem cells to suppress chronic neuroinflammation. Cell Stem Cell. 2018;22:355–368. doi: 10.1016/j.stem.2018.01.020. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rajendran L., Honsho M., Zahn T.R., et al. Alzheimer's disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Raposo G., Nijman H.W., Stoorvogel W., et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Riemann A., Reime S., Wollny P., et al. Expression of microRNAs in fibroblasts and macrophages is regulated by hypoxia-induced extracellular acidosis. Adv Exp Med Biol. 2018;1072:207–211. doi: 10.1007/978-3-319-91287-5_33. [DOI] [PubMed] [Google Scholar]
- 92.Ronquist K.G., Ek B., Stavreus-Evers A., et al. Human prostasomes express glycolytic enzymes with capacity for ATP production. Am J Physiol Endocrinol Metab. 2013;304:E576–E582. doi: 10.1152/ajpendo.00511.2012. [DOI] [PubMed] [Google Scholar]
- 93.Schindowski K., Belarbi K., Buee L. Neurotrophic factors in Alzheimer's disease: role of axonal transport. Genes Brain Behav. 2008;7(Suppl. 1):43–56. doi: 10.1111/j.1601-183X.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shetty A.K. Hippocampal injury-induced cognitive and mood dysfunction, altered neurogenesis, and epilepsy: can early neural stem cell grafting intervention provide protection? Epilepsy Behav. 2014;38:117–124. doi: 10.1016/j.yebeh.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shetty A.K. In: Neural Stem Cells in Health and Disease. Shetty A.K., editor. 2015. Introduction: neural stem cells in health and disease; pp. 1–20. [Google Scholar]
- 96.Shetty A.K., Hattiangady B. Grafted subventricular zone neural stem cells display robust engraftment and similar differentiation properties and form new neurogenic niches in the young and aged hippocampus. Stem Cells Transl Med. 2016;5:1204–1215. doi: 10.5966/sctm.2015-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shetty A.K., Jin K. Neural Stem Cells in Health and Disease. 2015. Neural stem cell activity and neurogenesis after stroke; pp. 133–150. [Google Scholar]
- 98.Sims B., Gu L., Krendelchtchikov A., et al. Neural stem cell-derived exosomes mediate viral entry. Int J Nanomedicine. 2014;9:4893–4897. doi: 10.2147/IJN.S70999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sorrells S.F., Paredes M.F., Cebrian-Silla A., et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stevanato L., Thanabalasundaram L., Vysokov N., et al. Investigation of content, stoichiometry and transfer of miRNA from human neural stem cell line derived exosomes. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stonesifer C., Corey S., Ghanekar S., et al. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol. 2017;158:94–131. doi: 10.1016/j.pneurobio.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Subra C., Laulagnier K., Perret B., et al. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89:205–212. doi: 10.1016/j.biochi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 103.Sullivan L.B., Gui D.Y., Hosios A.M., et al. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell. 2015;162:552–563. doi: 10.1016/j.cell.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Szatanek R., Baj-Krzyworzeka M., Zimoch J., et al. The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci. 2017;18:E1153. doi: 10.3390/ijms18061153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takahashi J. Stem cells and regenerative medicine for neural repair. Curr Opin Biotechnol. 2018;52:102–108. doi: 10.1016/j.copbio.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 106.Tang Y., Yu P., Cheng L. Current progress in the derivation and therapeutic application of neural stem cells. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Valadi H., Ekstrom K., Bossios A., et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 108.Vlassov A.V., Magdaleno S., Setterquist R., et al. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 109.Waldau B. The vascular stem cell niche: roadmap for transplanted neural progenitor cells during environmental enrichment? Neural Regen Res. 2015;10(8):1204–1245. doi: 10.4103/1673-5374.162692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang S., Cesca F., Loers G., et al. Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J Neurosci. 2011;31:7275–7290. doi: 10.1523/JNEUROSCI.6476-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Webb R.L., Kaiser E.E., Jurgielewicz B.J., et al. Human neural stem cell extracellular vesicles improve recovery in a Porcine model of ischemic stroke. Stroke. 2018;49:1248–1256. doi: 10.1161/STROKEAHA.117.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Webb R.L., Kaiser E.E., Scoville S.L., et al. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl Stroke Res. 2018;9:530–539. doi: 10.1007/s12975-017-0599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Willms E., Cabanas C., Mager I., et al. Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol. 2018;9:738. doi: 10.3389/fimmu.2018.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xin H., Li Y., Cui Y., et al. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu J.F., Yang G.H., Pan X.H., et al. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0114627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu B., Zhang Y., Du X.F., et al. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res. 2017;27:882–897. doi: 10.1038/cr.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yao H., Ma R., Yang L., et al. MiR-9 promotes microglial activation by targeting MCPIP1. Nat Commun. 2014;5:4386. doi: 10.1038/ncomms5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yao J., Mu Y., Gage F.H. Neural stem cells: mechanisms and modeling. Protein Cell. 2012;3:251–261. doi: 10.1007/s13238-012-2033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang G., Chen L., Chen W., et al. Neural stem cells alleviate inflammation via neutralization of IFN-gamma negative effect in ischemic stroke model. J Biomed Nanotechnol. 2018;14:1178–1188. doi: 10.1166/jbn.2018.2568. [DOI] [PubMed] [Google Scholar]
- 120.Zhang G., Chen L., Guo X., et al. Comparative analysis of microRNA expression profiles of exosomes derived from normal and hypoxic preconditioning human neural stem cells by next generation sequencing. J Biomed Nanotechnol. 2018;14:1075–1089. doi: 10.1166/jbn.2018.2567. [DOI] [PubMed] [Google Scholar]
- 121.Zhang H.G., Grizzle W.E. Exosomes and cancer: a newly described pathway of immune suppression. Clin Cancer Res. 2011;17:959–964. doi: 10.1158/1078-0432.CCR-10-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y., Kim M.S., Jia B., et al. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017;548:52–57. doi: 10.1038/nature23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang G.Y., Wang J., Jia Y.J., et al. MicroRNA-9 promotes the neuronal differentiation of rat bone marrow mesenchymal stem cells by activating autophagy. Neural Regen Res. 2015;10:314–320. doi: 10.4103/1673-5374.143439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Q., Wu H.H., Wang Y., et al. Neural stem cell transplantation decreases neuroinflammation in a transgenic mouse model of Alzheimer's disease. J Neurochem. 2016;136:815–825. doi: 10.1111/jnc.13413. [DOI] [PubMed] [Google Scholar]
- 125.Zhao H., Yang L., Baddour J., et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5 doi: 10.7554/eLife.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]