Abstract
Background
Several studies have reported microRNAs (miRNAs) could regulate the placental development, though the role and mechanism of miRNAs in the development of non-diabetic macrosomia (NDFMS) remains unclear.
Methods
To identify the aberrantly expressed key miRNAs in placenta of NDFMS, we employed a strategy consisting of initial screening with miRNA microarray and further validation with quantitative RT-PCR assay (qRT-PCR). In vitro cellular model and a mouse pregnancy model were used to delineate the functional effects of key miRNA on proliferation, invasion, and migration.
Findings
miR-141-3p was identified as the key miRNA with expression level significantly higher in placentas of NDFMS compared with those from normal controls. Overexpressed miR-141-3p in HTR-8/SVneo cells contributed to increased cell proliferation, invasion, and migration. miR-141-3p inhibition in HTR-8/SVneo cells resulted in decreased cell proliferation and invasion. Significantly increased infant birth weight was observed in late pregnancy of C57BL/6J mice treated with miR-141-3p agomir. However, no significant difference was found in early pregnancy of C57BL/6J mice treated with miR-141-3p agomir.
Interpretation
miR-141-3p could stimulate placental cell proliferation to participate in the occurrence and development of NDFMS.
Keywords: Birth weight, Macrosomia, microRNAs, miR-141-3p, Placenta
Research in context.
Evidence before this study
Fetal macrosomia is associated with an increased risk of adverse outcomes for mother and long-term health problems of the infant. Previous studies confirmed a variety of miRNAs with abnormal expression in placental tissues are related to fetal growth and development. However, miRNAs expression pattern in placenta of non-diabetic macrosomia (NDFMS) and the underlying mechanism is still unclear.
Added value of this study
miR-141-3p was identified as the key miRNA, which expression level was significantly higher in placentas of NDFMS compared with those from normal controls. Overexpressed miR-141-3p in HTR-8/SVneo cells resulted in increased cell proliferation, invasion, and migration. miR-141-3p inhibition in HTR-8/SVneo cells resulted in decreased cell proliferation and invasion. Significantly increased of infant birth weight was observed in late pregnancy of C57BL/6J mice treated with miR-141-3p agomir. miR-141-3p could stimulate placental cell proliferation to participate in the occurrence and development of NDFMS.
Implication of all the available evidence
These findings reveal an important role of miR-141-3p in development of NDFMS, which may provide new therapeutic approaches for NDFMS.
Alt-text: Unlabelled Box
1. Introduction
Macrosomia is a common perinatal complication of pregnancy defined as infant's birth weight of 4000 g or greater at term. There has been an increased incidence of macrosomia in recent decades. Though various interventions for the management of diabetic macrosomia, there is no clear consensus on antepartum prediction and management of non-diabetic macrosomia (NDFMS) and its accurate diagnosis is only made retrospectively [1]. It is known that fetal macrosomia increases risk of adverse outcomes in mother and long-term health problems in the infant [2,3]. However, the potential causes and mechanisms of NDFMS need further studies.
Neonatal birth weight is associated with a variety of factors, including genetic factors, gestational nutrition, endocrine, and placental function, etc. [[4], [5], [6]]. The placenta acts as a bridge between mother and fetus, and can also protect the fetus from the maternal immune system attack and secrete pregnancy-related hormones and growth factors [7]. Therefore, placental development and abnormal function may have an important impact on the growth and development of the fetus [[8], [9], [10]]. Previous studies reported that genes dysregulation in placentas played an important role in the pathology of fetal growth restriction and macrosomia. Sabri et al. found 338 and 41 genes were significantly dysregulated in the growth-restricted and macrosomic placentas through gene expression microarray analysis [11]. Yuan et al. reported that Leptin (LEP) and a leptin-binding modulator (SIGLEC6) were significantly downregulated in macrosomia [11], which has been confirmed relating to proliferation on myometrial cells and some cancer cells, such as breast cancer [12]. Besides, several studies have shown that microRNAs (miRNAs) participate in all stages of embryonic development and pregnancy [13,14]. Previous studies confirmed a variety of miRNAs with abnormal expression in placental tissues are related to fetal growth and development [[15], [16], [17], [18]]. However, miRNAs expression pattern in placenta of NDFMS and the underlying mechanism is still unclear.
In order to investigate the expression pattern of miRNAs in placental tissues of NDFMS, we employed a strategy consisting of initial screening by miRNA microarray and further validation by quantitative RT-PCR assay. Furthermore, we also studied the mechanism of key miRNA using the trophoblast HTR-8/SVneo cell line and mouse pregnancy model.
2. Materials and methods
2.1. Human material samples and ethics
A total of 91 participants (44 non-diabetes macrosomia pregnant women and 47 normal controls) who delivered in Changzhou Maternal and Child Health Hospital between September 2014 to June 2015 were included. All participants in our study were primigravida. Inclusion criteria of NDFMS: 1. Full-term birth (≥37 weeks and < 42 weeks), birth weight ≥ 4000 g; 2. Normal blood glucose and oral glucose tolerance test (OGTT) result, negative urine sugar; 3. Without pregnant complications (gestational hypertension, placental abruption, placenta previa, intrahepatic cholestasis of pregnancy, infectious diseases, and other pregnancy complications). Inclusion criteria of control: 1. Full-term birth (≥37 weeks and < 42 weeks), birth weight < 4000 g and ≥ 2500 g; 2. Normal blood glucose and oral glucose tolerance test (OGTT) result, negative urine sugar; 3. Without pregnant complications (gestational hypertension, placental abruption, placenta previa, intrahepatic cholestasis of pregnancy, infectious diseases, and other pregnancy complications).
After removal of the fetal membranes, three pieces of the placental tissues with 1 × 1 × 1 cm3 size were randomly taken from the center of the maternal side. Then, these tissues were washed using sterile saline and immediately frozen in liquid nitrogen, and then stored at −80 °C. The study was approved by the Ethics Committee of Nanjing Medical University, and all pregnant women signed the informed consent before participation.
2.2. RNA extraction, miRNA microarray analysis, and quantitative real-time PCR (qRT-PCR)
Total RNA from placental tissues and cultured cells were extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. About 50 mg placental tissues were kept in TRIzol for RNA extraction. Ultrasonic cell crusher was used for tissue homogenate (working for 3 s, pause for 3 s with 70% energy about three minutes). Then, total RNA was extracted according to the manufacturer's instructions. To prevent RNA degradation, all steps were performed on ice. The concentration and purity of RNA were measured using NanoDrop® ND-1000 (samples with A260/A280 ratio > 1·8 and < 2·0 were stored at −80 °C until use), while its integrity was assessed using electrophoresis with 1·5% denaturing agarose gels. RNA was reverse transcribed using the specific Megaplex RT primers (Life Technologies). Thereafter, we screened the expression level of 754 miRNAs using the TaqMan® Array Human MicroRNA A + B Cards Set v3.0 (Life Technologies) on a 7900 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Data were analyzed by DataAssist v3.0 (Life Technologies). The amount of target miRNAs was normalized relative to the amount of RNU6B (U6) (△Ct = Ctsample−CtU6).
For qRT-PCR, PrimeScript RT reagent Kit (Takara, Tokyo, Japan) was used to reverse transcribe RNA sample to cDNA (Takara RR037A), and PCR analysis was performed using SYBR® Premix Ex Taq™ according to the manufacturer's instructions. The quantity of specific RNA for target genes in each sample was estimated by qRT-PCR using ABI Prism 7900HT (Applied Biosystems, Foster City, CA, USA). The expression of each miRNA relative to U6 was calculated using the 2−△△Ct method. All the reactions were run in triplicate to control for PCR variation. The primer sequences for qRT-PCR are shown in Table S1.
2.3. Cell culture and transfection
The human placenta trophoblast cell line HTR-8/SVneo was cultured in RPMI 1640 medium containing 10% fetal bovine serum and 10% double antibiotic (100 U/ml penicillin and 100 U/ml streptomycin) at 37 °C in a 5% CO2 cell incubator. When the cells were in the logarithmic growth phase, they were transfected with miR-141-3p mimic, inhibitor or negative control (GenePharma, Shanghai, China) using transfection reagent Lipofectamine 2000 reagent (Invitrogen, CA, USA) according to the manufacturer's instructions (5 μl Lipofectamine 2000 reagent per 6-well plate).
2.4. Cell proliferation assay
HTR-8/SVneo cells were seeded in 96-well plates at a density of 1 × 103 cells/well. After transfection for 24 h and 48 h, cell proliferation ability was measured by using the Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan). Using culture medium as the blank control, we measured the absorbance at a wavelength of 450 nm by TECAN infinite M200 Multimode microplate reader (Tecan, Mechelen, Belgium). The experiments were repeated thrice independently.
2.5. Cell cycle analysis
We collected the treated cells seeded in 6-well plates, washed with cold PBS, fixed in cold 70% ethanol overnight at −20 °C. Propidium iodide (PI) (Sigma, MO, USA) was used to stain the fixed cells for 30 min at room temperature under dark condition. Cell cycle distribution of stained cells was analyzed with FACS Calibur Flow Cytometer afterward, and the fraction of the cell cycle was measured by Modfit LT version 3.0 software (Verity Software House, Toshan, ME, USA).
2.6. Apoptosis assay
We harvested the treated cells seeded in 6-well plates with a density of 1 × 103 cells/well, washed twice with PBS, and cells were processed using Annexin V-FITC Kit (BD Pharmingen, NJ, USA) in the dark for 15 min at room temperature. The cells were analyzed using FACS Calibur Flow Cytometer immediately after incubated with Annexin V-FITC.
2.7. Cell invasion analysis
The treated cells were transferred to the transwell chamber with 100 μl empty RPMI 1640 medium, while the 24-well plate contained 600 μl complete medium as the container of the transwell chamber. After 24 h, the transwell chamber was transferred to 95% methanol to fix cells for 30 min, then stained by crystal violet for 30 min, and washed twice with PBS. After drying, we captured the pictures by light microscope. Then, by direct cell counting, we used the cell number in vision as a measure.
2.8. Cell migration analysis
We used 10 μl pipette tip to draw a straight line in each hole of 6-well plate with treated cells and washed twice with PBS. Next, the BioSation IM-Q live cell workstation was used to record the migration situation of cells to the middle real-timely and capture dynamic change graphs. Then we used Image-Pro Plus software to analyze the pictures, which take the horizontal distance of cells migrated as the quantitative indicator.
2.9. C57BL/6J mice feeding and experiment
We used miR-141-3p agomir (microON™ miR-141-3p agomir) (RiboBio, Guangzhou, China) to construct miR-141-3p overexpression model in placental tissues via the tail vein, and used microON™ agomir negative control treated controls. Eight-week-old pregnant C57BL/6J mice were divided into two groups: early pregnant group (treated: control = 5: 5) and late pregnant group (treated: control = 7: 5). Early pregnant group mice were treated twice at pregnant day 5 and day 8, while late pregnant group mice were treated twice at pregnant day 14 and 17, and they were sacrificed afterwards at pregnant day 18. Fetal weight, body height, placental weight, and placental diameter were measured. Besides, we treated some non-pregnant mice twice with 3-day interval (number of treated: control = 4: 5), followed by sacrificed at 10 days after the second treatment, and the weight fluctuation was observed (The results of effect on body weight and food intake were shown in Fig. S1). This study was carried out strictly in accordance with the international standards on animal welfare and the guidelines of the Institute for Laboratory Animal Research of Nanjing Medical University.
2.10. Statistical analysis
Data were analyzed by SPSS 18.0 (SPSS Inc., Chicago, IL, USA) and presented with GraphPad Prism 5.01 (GraphPad Software Inc., San Diego, CA, USA). If data were in normal distribution, the student's t-test was used to compare two groups. Otherwise, the Mann-Whitney U-test was used. All results are expressed as mean ± standard error (SE) without special instructions. P < 0·05 was considered statistically significant. P < 0·05: ⁎, P < 0·01: ⁎⁎, P < 0·001: ⁎⁎⁎.
3. Results
3.1. Clinical data
Clinical characteristics of the study population are summarized in Table 1. NDFMS and normal controls were matched by maternal age, pre-pregnant body mass index, gestational weeks and infant gender distribution. The birth weight of neonates and weight gain during pregnancy were significantly higher in NDFMS compared with that in normal controls (P < 0·05).
Table 1.
Clinical characteristics of the study population.
| Characteristic | Total (n = 91) | Control (n = 47) | NDFMS (n = 44) | P-Value |
|---|---|---|---|---|
| Maternal age (years) | 26·42 ± 2·87 | 26·15 ± 0·36 | 26·70 ± 0·48 | NS |
| Gestational age (weeks) | 39·71 ± 1·01 | 39·70 ± 0·15 | 39·72 ± 0·15 | NS |
| BMI before pregnancy (kg/m2) | 20·71 ± 3·32 | 20·05 ± 0·33 | 21·43 ± 0·62 | NS |
| Placental weight (g) | 672·36 ± 15·03 | 587·77 ± 14·66 | 762·73 ± 19·08 | < 0·001 |
| Weight gain during pregnancy (kg) | 18·21 ± 4·77 | 16·60 ± 0·59 | 19·97 ± 0·75 | < 0·001 |
| Birth weight (g) | 3764·68 ± 58·63 | 3309·57 ± 47·01 | 4250·82 ± 41·41 | < 0·001 |
| Infant gender, n (%) | NS | |||
| Male | 43 (47) | 23 (49) | 20 (45) | |
| Female | 48 (53) | 24 (51) | 24 (55) |
Values are mean ± SD. BMI, body mass index. NS, not significant.
3.2. Expression pattern of miRNAs in placentas of NDFMS
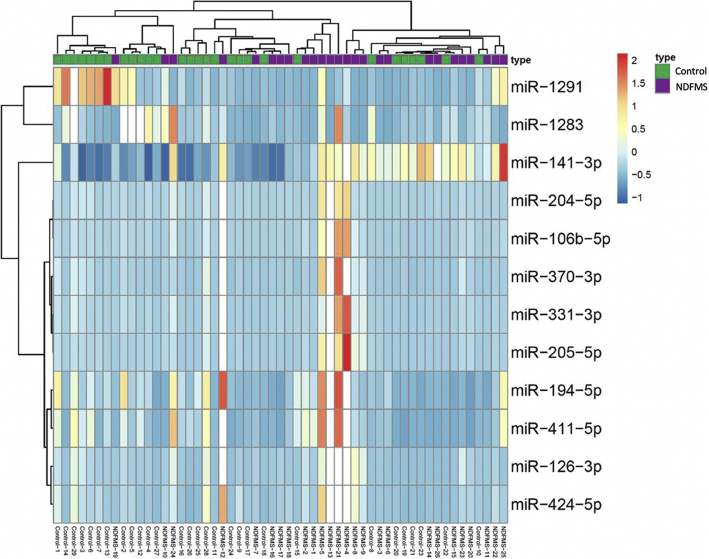
We randomly selected 18 cases of NDFMS and 18 normal controls, and pooled RNA samples of the 18 cases and 18 controls, respectively and subjected them to miRNA microarray screening. A total of 754 miRNAs were detected by miRNA microarray, and 264 and 318 miRNAs could be detected in placental tissues of normal controls and NDFMS, respectively (Ct < 30). One hundred and thirty-three miRNAs showed dysregulated in NDFMS placentas (fold change ≥2), including 63 up-regulated and 70 down-regulated (Table S2). There were 21 miRNAs that expressed four times higher in NDFMS group (miR-523-3p, miR-519a-3p, miR-518f-3p, miR-331-3p, miR-204-5p, miR-106b-5p, miR-574-3p, miR-518a-3p, miR-370-3p, miR-518c-3p, miR-205-5p, miR-141-3p, miR-126-3p, miR-424-5p, miR-194-5p, miR-411-5p, miR-376c-3p, miR-200b-3p, miR-374-5p, miR-200c-3p, and miR-199a-3p), and 11 miRNAs expressed eight times lower compared with normal controls (miR-1290, miR-1227-3p, miR-660-5p, miR-1275, miR-320b, miR-584-5p, miR-34b-3p, miR-656-3p, miR-15a-5p, miR-1291, and miR-1283).
3.3. qRT-PCR validation of miRNAs in placentas
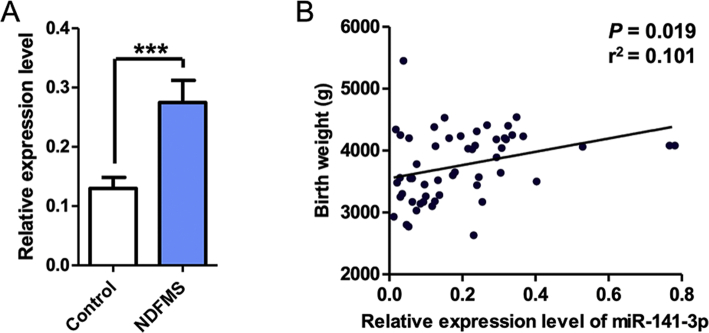
Considering the bioinformatics analysis results, species conservatism and feasibility, we selected twelve miRNAs (miR-331-3p, miR-204-5p, miR-106b-5p, miR-370-3p, miR-205-5p, miR-141-3p, miR-126-3p, miR-424-5p, miR-194-5p, miR-411-5p, miR-1291, and miR-1283) (Table S3) for further validation using qRT-PCR in the remaining placental samples (26 of NDFMS, and 29 of normal controls). The twelve miRNAs listed in Table S3 are highly conservative and homologous between human and mouse, thus which have a strong practical significance in the following in vitro and in vivo experiments. The results showed that the expression levels of miR-331-3p, miR-106b-5p, miR-370-3p, miR-205-5p, miR-141-3p, miR-126-3p, miR-424-5p, and miR-411-5p were statistically different between NDFMS and normal controls. However, only miR-141-3p was significant with false discovery rate (FDR) adjustment (Table S4, Fig. 1). Additionally, the infant birth weight gradually increased along with the elevated expression of miR-141-3p (P = 0·019, r2 = 0·101) (Fig. 2B). Therefore, miR-141-3p was determined as the key miRNA for further functional analyses (Fig. 2A).
Fig. 1.
Heat map of miRNA expression levels in placental tissue screened out by miRNA microarray in NDFMS compared with controls.
Fig. 2.
The expression level of miR-141-3p in placental tissues and the correlation with infant birth weight. (A) The expression level of miR-141-3p in NDFMS placental tissues compared with normal controls. (B) The correlation between the expression level of miR-141-3p and infant birth weight.
3.4. Effects of miR-141-3p on proliferation, apoptosis, invasion, and migration in HTR-8/SVneo
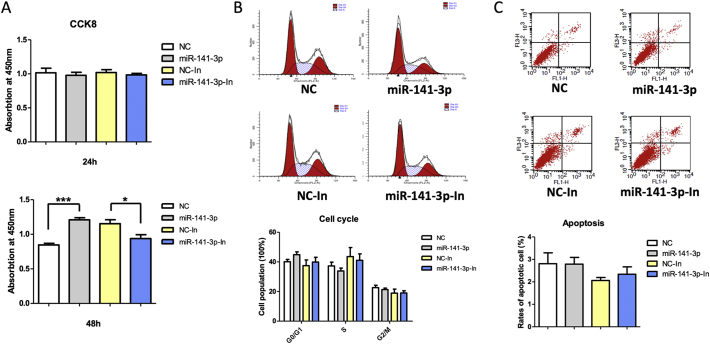
Cell proliferation, cell cycle, apoptosis, invasion, and migration assays were performed with HTR-8/SVneo cells transfected with miR-141-3p mimic or inhibitor. Cell proliferation was significantly increased in HTR-8/SVneo cells transfected with miR-141-3p mimic and significantly decreased in HTR-8/SVneo cells transfected with miR-141-3p inhibitor at 48 h (Fig. 3A), whereas no significant difference was found at 24 h (Fig. 3A). Additionally, no significant differences of cell apoptosis and cell cycle were found in miR-141-3p mimic-treated or inhibitor-treated group compared with negative controls (Fig. 3B, C).
Fig. 3.
Effects of miR-141-3p on proliferation, cell cycle, and apoptosis of HTR-8/SVneo cells. (A) The proliferation ability of HTR-8/SVneo transfected with miR-141-3p mimic or inhibitor at 24 and 48 h detected by CCK8. (B) The cell cycle of HTR-8/SVneo transfected with miR-141-3p mimic or inhibitor at 24 h detected by flow cytometry. (C) Cell apoptosis of HTR-8/SVneo transfected with miR-141-3p mimic or inhibitor at 24 h detected by flow cytometry.
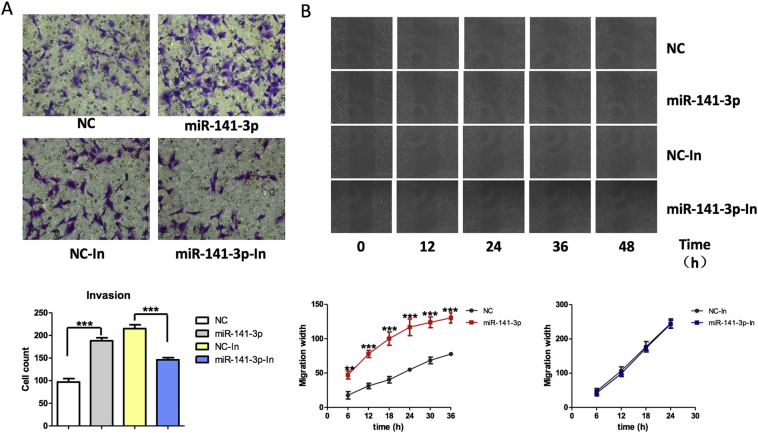
Overexpressed miR-141-3p in HTR-8/SVneo cells resulted in increased cell invasion and migration (Fig. 4A, B). miR-141-3p inhibition in HTR-8/SVneo cells resulted in decreased cell invasion (Fig. 4A). However, no significant difference of cell migration was found in HTR-8/SVneo cells treated with miR-141-3p inhibitor (Fig. 4B).
Fig. 4.
Effects of miR-141-3p on invasion, and migration of HTR-8/SVneo cells. (A) The invasion ability of HTR-8/SVneo transfected with miR-141-3p mimic or inhibitor detected by crystal violet staining in transwell. (B) The migration ability of HTR-8/SVneo transfected with miR-141-3p mimic or inhibitor detected by BioSation IM-Q live cell workstation.
3.5. Roles of miR-141-3p in birth weight of C57BL/6J mice
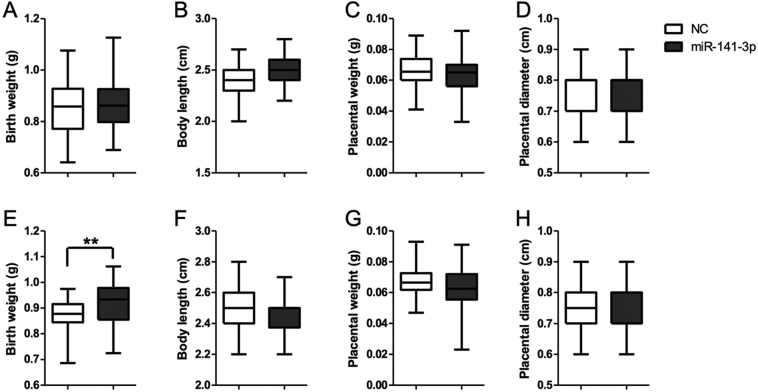
To confirm the role of miR-141-3p in the development of NDFMS, we constructed miR-141-3p overexpression mouse pregnancy models at early pregnancy and at late pregnancy (Fig. S2). There was no significant difference of birth weight between the treated group and control group in early pregnancy (Fig. 5A, B, C, D), while the birth weight of mice in the treated group was significantly higher than that of controls in late pregnancy (P = 0·006) (Fig. 5E, F, G, H). In addition, no statistically significant difference of placental weight, placental diameter, and body length of offspring was observed between the treated group and control group either in early pregnancy or in late pregnancy (Fig. 5).
Fig. 5.
The effects of miR-141-3p treated at early pregnancy and late pregnancy on offspring and placenta of pregnant C57BL/6J mice. (A) Birth weight of offspring at early pregnancy. (B) Body length of offspring at early pregnancy. (C) Placental weight at early pregnancy. (D) Placental diameter at early pregnancy. (E) Birth weight of offspring at late pregnancy. (F) Body length of offspring at late pregnancy. (G) Placental weight at late pregnancy. (H) Placental diameter at late pregnancy.
4. Discussion
From the miRNA microarray data, we found many miRNAs were dysregulated in placenta of NDFMS (Table S2). miR-17, miR-19a, miR-20a were up-regulated, which is consistent with the finding of Li et al. in NDFMS [18]. In contrast to previous studies in macrosomia [15,17], we found miR-483-3p and miR-21 were down-regulated in microarray screening results. The inconsistent results may be attributed to the differences of samples size and detection methods. Besides, these miRNAs were only examined in the microarray with no further verification in large sample size. Among all the miRNAs expression, we found only the miR-141-3p expression level was significantly increased in NDFMS placental tissues, suggesting aberrant expression of miR-141-3p may be related to the occurrence of NDFMS. miR-141-3p is a member of the miR-200 family, which is highly conservative in vertebrates [19]. The study of miR-200 family in rodent animal model is common while its regulating in human fetal growth and development of population-based is rare [[20], [21], [22]]. Yao et al. [22] found miR-200 family expression in the uterus and offspring (F2 generation) brain tissues was altered when the parental generations (F0) exposed to stress during pregnancy. Chim et al. [23] detected known 157 miRNAs by miRNA microarray and found 17 miRNAs were 10 times higher in placental tissues than in maternal blood, which could not be detected in maternal blood after delivery. miR-141, miR-149, miR-299-5p, and miR-135b were the four most abundant miRNAs in the placenta, of which the expression level of miR-141 in maternal blood significantly increased in the third trimester [23]. Prior investigations have documented miR-141-3p participates in the process of cell proliferation, especially dysregulated in a variety of cancers, including exerting a growth-promoted activity in ovarian cancer [24], cervical cancer [25], and a growth-suppressive activity in hepatocellular carcinoma [26]. Similarly, it also showed different effects on fetal growth. Tang et al. found miR-141 up-regulated in fetal growth restriction (FGR) by down-regulating its target genes [27]. Baker et al. reported low maternal folate status is associated with higher incidence of small for gestation age (SGA) infants, with elevated level of miR-141-3p in placental tissues [28]. Interestingly, they also found that trophoblast proliferation was increased in low maternal folate status, to some extend that is consistent with our results i.e. miR-141-3p elevates trophoblast proliferation. It is possible that the elevated miR-141-3p expression is a compensatory increase in the placenta of SGA and FGR. But the specific in-depth mechanism demands further research to clarify in the future.
The cell experiments suggest miR-141-3p may participate in the development of NDFMS by affecting the proliferation and invasion of trophoblast cells. Ospina-Prieto et al. [29] suggested the expression level of miR-141 in HTR-8/SVneo cells was significantly lower than that in normal and preeclampsia placenta. The expression level of miR-141 in normal placental tissues was 42,394 times higher than that in HTR-8/SVneo cells. Morales-Prieto et al. [30] also found the expression levels of miR-141 in all placental lineage cells were low, suggesting that the basal level of miR-141 in human chorionic trophoblast cells was very low, which may explain that why the proliferation of HTR-8/SVneo cells treated by miR-141-3p inhibitor did not decrease as expected. Moreover, further studies are still needed to confirm this conclusion.
Because miR-141-3p affected proliferation and invasion ability of HTR-8/SVneo cells, we constructed the miR-141-3p overexpression model of the placenta in early pregnancy and late pregnancy of C57BL/6J mice to verify the role of miR-141-3p in the development of NDFMS. The miR-141-3p agomir reagent from Guangzhou RiboBio can be maintained for about three days, so as to ensure the treatment at early pregnancy will not be maintained until late pregnancy, which ensured the accuracy of modeling. Interestingly, we found that the fetal birth weight of the treated group was significantly higher only in the late pregnant group. Therefore, we conclude that miR-141-3p may affect the proliferation at late pregnancy by participating in the occurrence of NDFMS, rather than by influencing the invasion at early pregnancy.
Previous researches have demonstrated the role of miR-141 in fetal growth [29,31]. Ospina-Prieto et al. [29] found miR-141 was highly expressed in placental tissues of preeclampsia, and overexpression of miR-141 enhanced the invasion ability of HTR-8/SVneo cells, which is consistent with our findings. They did not find any significant changes of cell proliferation ability in HTR-8/SVneo cells after 24 h of transfection with either miR-141-mimic or miR-141-inhibitor. This result is also consistent with our findings that no significant difference of cell proliferation was found in HTR-8/SVneo cells after 24 h of transfection. However, we found cell proliferation was significantly higher in the HTR-8/SVneo cells transfected with miR-141-3p mimic at 48 h. Morales-Prieto et al. [32] demonstrated that the reduction of miR-141 caused by leukemia inhibitory factors inhibited the proliferation of choriocarcinoma cell line JEG-3. In addition, the study team also found miR-141 expression level in placental tissues at the late pregnancy was higher than the early pregnancy [32]. This may be due to placenta grows fastest along with the growth of fetus in late pregnancy, which also suggested miR-141 in placenta is related to proliferation function and confirmed the rationality of our results.
A limitation of this study is the lack of follow-up mechanism of how miR-141-3p promotes the proliferation of trophoblasts. It is confirmed that miR-141-3p has many target genes [24,33] and miRNA can regulate target genes via partial complementary binding to the 3'-UTR of mRNAs [34]. For example, miR-141-3p targets FOXA2 to foster the growth, invasion, and tumorigenesis of cervical cancer cells [25]. Another study showed miR-141 targeting PLAG1 to participant FGR [27]. We speculated that there are one or more specific target genes of miR-141-3p to regulate the proliferation of trophoblasts in NDFMS and follow-up experiments are required to confirm. Besides, it is well known that there was excess nutrient transfer to the baby through placenta in macrosomia, therefore effect of miR-141-3p on nutrient transporters can't be ruled out. Future studies are required to reveal the specific mechanism of miR-141-3p participating in the occurrence of NDFMS.
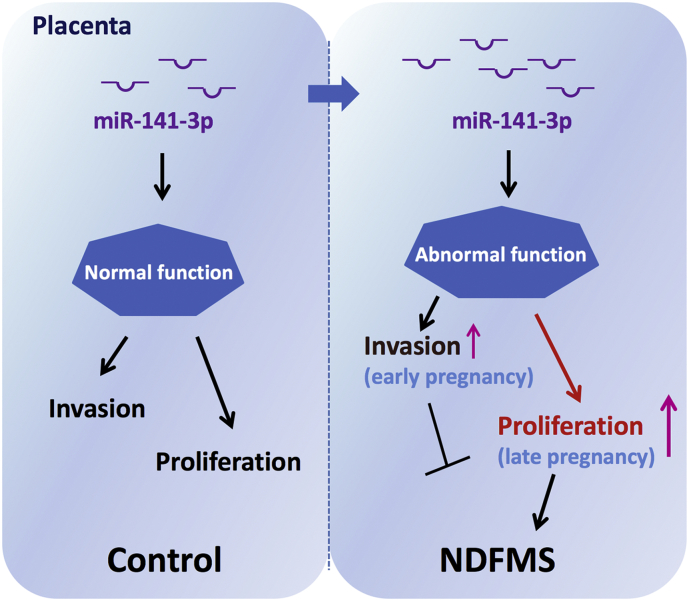
In conclusion, aberrant high expression level of miR-141-3p may contribute to the pathogenesis of NDFMS through regulating trophoblast proliferation in late pregnancy as shown in Fig. 6. Our study provided a foundation for the explanation of miRNA changes related to NDFMS and expanded the current understanding of its pathogenesis. Future studies are needed to illustrate the exact molecular mechanism of miR-141-3p in NDFMS.
Fig. 6.
A model was presented for miR-141-3p mediated NDFMS. Up-regulation of miR-141-3p in placenta contributes to the pathogenesis of NDFMS through regulating trophoblast proliferation in late pregnancy.
Acknowledgments
Acknowledgements
This work was supported by the National Natural Science Foundation of China (814012138), Jiangsu Provincial Medical Youth Talent (QNRC2016110), “333 Project” Science Research Project of Jiangsu Province (BRA2016197), “six big talent peak” Project of Jiangsu Province (WSN-283), Clinical Medicine Center Project of Nantong City (HS2016005), Jiangsu Overseas Visiting Scholar Program for University Prominent Young & Middle-aged Teachers and Presidents, and the Priority Academic Program for the Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine).
Funding sources
This work was supported by the National Natural Science Foundation of China (814012138), Jiangsu Provincial Medical Youth Talent (QNRC2016110), “333 Project” Science Research Project of Jiangsu Province (BRA2016197), “six big talent peak” Project of Jiangsu Province (WSN-283), Clinical Medicine Center Project of Nantong City (HS2016005), Jiangsu Overseas Visiting Scholar Program for University Prominent Young & Middle-aged Teachers and Presidents, and the Priority Academic Program for the Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests
The authors declare that they have no competing interests.
Author contributions
W.W., Q.T., L.C., D.G., and H.J. conceived and designed the experiment. D.G., H.J., Y.C., J.Y., Z.F., J.L., and X.H. performed the experiments. D.G., X.W., and W.W. performed the bioinformatic analysis. W.W., Q.T., L.C., Y.X., and X.W. contributed materials/analysis tools. D.G., W.W., Q.T., L.C., Y.X., and X.W. wrote and revised the manuscript. All authors read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.11.002.
Contributor Information
Liping Chen, Email: jichen0816@163.com.
Qiuqin Tang, Email: t19871004@sina.com.
Wei Wu, Email: wwu@njmu.edu.cn.
Appendix A. Supplementary data
Supplementary material
References
- 1.Pundir J., Sinha P. Non-diabetic macrosomia: An obstetric dilemma. J Obstet Gynaecol. 2009;29(3):200–205. doi: 10.1080/01443610902735140. [DOI] [PubMed] [Google Scholar]
- 2.Linder N., Lahat Y., Kogan A., Fridman E., Kouadio F., Melamed N. Macrosomic newborns of non-diabetic mothers: Anthropometric measurements and neonatal complications. Arch Dis Child Fetal Neonatal Ed. 2014;99(5):F353–F358. doi: 10.1136/archdischild-2013-305032. [DOI] [PubMed] [Google Scholar]
- 3.Koyanagi A., Zhang J., Dagvadorj A., Hirayama F., Shibuya K., Souza J.P. Macrosomia in 23 developing countries: An analysis of a multicountry, facility-based, cross-sectional survey. Lancet. 2013;381(9865):476–483. doi: 10.1016/S0140-6736(12)61605-5. [DOI] [PubMed] [Google Scholar]
- 4.Shi P., Yang W., Yu Q., Zhao Q., Li C., Ma X. Overweight, gestational weight gain and elevated fasting plasma glucose and their association with macrosomia in chinese pregnant women. Matern Child Health J. 2014;18(1):10–15. doi: 10.1007/s10995-013-1253-6. [DOI] [PubMed] [Google Scholar]
- 5.Marjonen H., Auvinen P., Kahila H., Tsuiko O., Koks S., Tiirats A. 10:80. 2018. rs10732516 polymorphism at the IGF2/H19 locus associates with genotype-specific effects on placental DNA methylation and birth weight of newborns conceived by assisted reproductive technology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winterbottom E.F., Fei D.L., Koestler D.C., Giambelli C., Wika E., Capobianco A.J. GLI3 links environmental arsenic exposure and human fetal growth. EBioMedicine. 2015;2(6):536–543. doi: 10.1016/j.ebiom.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossant J., Cross J.C. Placental development: Lessons from mouse mutants. Nat Rev Genet. 2001;2(7):538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 8.Longtine M.S., Nelson D.M. Placental dysfunction and fetal programming: the importance of placental size, shape, histopathology, and molecular composition. Semin Reprod Med. 2011;29(3):187–196. doi: 10.1055/s-0031-1275515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilekis J.V., Tsilou E., Fisher S., Abrahams V.M., Soares M.J., Cross J.C. Placental origins of adverse pregnancy outcomes: Potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am J Obstet Gynecol. 2016;215(1 Suppl):S1–S46. doi: 10.1016/j.ajog.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian F.Y., Wang X.M., Xie C., Zhao B., Niu Z., Fan L. Placental surface area mediates the association between FGFR2 methylation in placenta and full-term low birth weight in girls. Clin Epigenetics. 2018;10:39. doi: 10.1186/s13148-018-0472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabri A., Lai D., D'Silva A., Seeho S., Kaur J., Ng C. Differential placental gene expression in term pregnancies affected by fetal growth restriction and macrosomia. Fetal Diagn Ther. 2014;36(2):173–180. doi: 10.1159/000360535. [DOI] [PubMed] [Google Scholar]
- 12.Yuan H.J., Sun K.W., Yu K. Leptin promotes the proliferation and migration of human breast cancer through the extracellular-signal regulated kinase pathway. Mol Med Rep. 2014;9(1):350–354. doi: 10.3892/mmr.2013.1786. [DOI] [PubMed] [Google Scholar]
- 13.Niu Z.R., Han T., Sun X.L., Luan L.X., Gou W.L., Zhu X.M. MicroRNA-30a-3p is overexpressed in the placentas of patients with preeclampsia and affects trophoblast invasion and apoptosis by its effects on IGF-1. Am J Obstet Gynecol. 2018;218(2) doi: 10.1016/j.ajog.2017.11.568. [249.e1-.e12] [DOI] [PubMed] [Google Scholar]
- 14.Murphy M.S., Casselman R.C., Tayade C., Smith G.N. Differential expression of plasma microRNA in preeclamptic patients at delivery and 1 year postpartum. Am J Obstet Gynecol. 2015;213(3) doi: 10.1016/j.ajog.2015.05.013. [367.e1–9] [DOI] [PubMed] [Google Scholar]
- 15.Li J., Fu Z., Jiang H., Chen L., Wu X., Ding H. IGF2-derived miR-483-3p contributes to macrosomia through regulating trophoblast proliferation by targeting RB1CC1. Mol Hum Reprod. 2018;24(9):444–452. doi: 10.1093/molehr/gay027. [DOI] [PubMed] [Google Scholar]
- 16.Higashijima A., Miura K., Mishima H., Kinoshita A., Jo O., Abe S. Characterization of placenta-specific microRNAs in fetal growth restriction pregnancy. Prenat Diagn. 2013;33(3):214–222. doi: 10.1002/pd.4045. [DOI] [PubMed] [Google Scholar]
- 17.Jiang H., Wu W., Zhang M., Li J., Peng Y., Miao T.T. 34(9) 2014. Aberrant upregulation of miR-21 in placental tissues of macrosomia; pp. 658–663. (J Perinatol). [DOI] [PubMed] [Google Scholar]
- 18.Li J., Chen L., Tang Q., Wu W., Gu H., Liu L. The role, mechanism and potentially novel biomarker of microRNA-17-92 cluster in macrosomia. Sci Rep. 2015;5:17212. doi: 10.1038/srep17212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paterson E.L., Kazenwadel J., Bert A.G., Khew-Goodall Y., Ruszkiewicz A., Goodall G.J. Down-regulation of the miRNA-200 family at the invasive front of colorectal cancers with degraded basement membrane indicates EMT is involved in cancer progression. Neoplasia (New York, NY) 2013;15(2):180–191. doi: 10.1593/neo.121828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haraguchi H., Saito-Fujita T., Hirota Y., Egashira M., Matsumoto L., Matsuo M. MicroRNA-200a locally attenuates progesterone signaling in the cervix, preventing embryo implantation. Mol Endocrinol (Baltimore, Md) 2014;28(7):1108–1117. doi: 10.1210/me.2014-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jimenez P.T., Mainigi M.A., Word R.A., Kraus W.L., Mendelson C.R. miR-200 regulates endometrial development during early pregnancy. Mol Endocrinol (Baltimore, Md) 2016;30(9):977–987. doi: 10.1210/me.2016-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao Y., Robinson A.M., Zucchi F.C., Robbins J.C., Babenko O., Kovalchuk O. Ancestral exposure to stress epigenetically programs preterm birth risk and adverse maternal and newborn outcomes. BMC Med. 2014;12:121. doi: 10.1186/s12916-014-0121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chim S.S., Shing T.K., Hung E.C., Leung T.Y., Lau T.K., Chiu R.W. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem. 2008;54(3):482–490. doi: 10.1373/clinchem.2007.097972. [DOI] [PubMed] [Google Scholar]
- 24.Mak C.S., Yung M.M., Hui L.M., Leung L.L., Liang R., Chen K. MicroRNA-141 enhances anoikis resistance in metastatic progression of ovarian cancer through targeting KLF12/Sp1/survivin axis. Mol Cancer. 2017;16(1):11. doi: 10.1186/s12943-017-0582-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J.H., Zhang Z., Du M.Z., Guan Y.C., Yao J.N., Yu H.Y. microRNA-141-3p fosters the growth, invasion, and tumorigenesis of cervical cancer cells by targeting FOXA2. Arch Biochem Biophys. 2018;657:23–30. doi: 10.1016/j.abb.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Lou G., Dong X., Xia C., Ye B., Yan Q., Wu S. Direct targeting sperm-associated antigen 9 by miR-141 influences hepatocellular carcinoma cell growth and metastasis via JNK pathway. J Exp Clin Cancer Res. 2016;35:14. doi: 10.1186/s13046-016-0289-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Q., Wu W., Xu X., Huang L., Gao Q., Chen H. miR-141 contributes to fetal growth restriction by regulating PLAG1 expression. PLoS One. 2013;8(3):e58737. doi: 10.1371/journal.pone.0058737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker B.C., Mackie F.L., Lean S.C., Greenwood S.L., Heazell A.E.P., Forbes K. Placental dysfunction is associated with altered microRNA expression in pregnant women with low folate status. Mol Nutr Food Res. 2017;61(8) doi: 10.1002/mnfr.201600646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ospina-Prieto S., Chaiwangyen W., Herrmann J., Groten T., Schleussner E., Markert U.R. MicroRNA-141 is upregulated in preeclamptic placentae and regulates trophoblast invasion and intercellular communication. Transl Res. 2016;172:61–72. doi: 10.1016/j.trsl.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Morales-Prieto D.M., Chaiwangyen W., Ospina-Prieto S., Schneider U., Herrmann J., Gruhn B. MicroRNA expression profiles of trophoblastic cells. Placenta. 2012;33(9):725–734. doi: 10.1016/j.placenta.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Li D., Li J. Association of miR-34a-3p/5p, miR-141-3p/5p, and miR-24 in Decidual Natural Killer Cells with Unexplained Recurrent Spontaneous Abortion. Med Sci Monitor. 2016;22:922–929. doi: 10.12659/MSM.895459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morales-Prieto D.M., Schleussner E., Markert U.R. Reduction in miR-141 is induced by leukemia inhibitory factor and inhibits proliferation in choriocarcinoma cell line JEG-3. Am J Reprod Immunol (New York, NY: 1989) 2011;66(Suppl. 1):57–62. doi: 10.1111/j.1600-0897.2011.01037.x. [DOI] [PubMed] [Google Scholar]
- 33.Choi S.K., Kim H.S., Jin T., Hwang E.H., Jung M., Moon W.K. Overexpression of the miR-141/200c cluster promotes the migratory and invasive ability of triple-negative breast cancer cells through the activation of the FAK and PI3K/AKT signaling pathways by secreting VEGF-A. BMC Cancer. 2016;16:570. doi: 10.1186/s12885-016-2620-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granados-Lopez A.J., Ruiz-Carrillo J.L., Servin-Gonzalez L.S., Martinez-Rodriguez J.L., Reyes-Estrada C.A., Gutierrez-Hernandez R. Use of mature miRNA strand selection in miRNAs families in cervical cancer development. Int J Mol Sci. 2017;18(2) doi: 10.3390/ijms18020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material