Abstract
Progressive encephalomyelitis with rigidity and myoclonus (PERM) is an autoimmune disorder involving the brainstem and spinal cord and is sometimes associated with thymoma. We encountered a 75-year-old woman with typical PERM features, glycine receptor antibody, and thymoma. Her neurologic symptoms improved after thymectomy, but she unexpectedly developed anasarca with massive pleural effusions and hypoalbuminemia and finally succumbed to death. The autopsy showed edema and mononuclear infiltration in the pleura but no neuropathological findings typical of PERM. Effective treatment of PERM can reverse the neuropathological signs of encephalomyelitis. The autoimmune nature of anasarca is possible but not proven.
Keywords: progressive encephalomyelitis with rigidity and myoclonus, anti-glycine receptor antibody, anasarca, pleural effusion, edema, systemic fluid retention
Introduction
Progressive encephalomyelitis with rigidity and myoclonus (PERM) is an autoimmune disorder characterized by progressive brainstem symptoms, rigidity of the trunk and extremities, and myoclonus or hyperekplexia. PERM is associated with antibodies against the glycine receptor α1 subunit (GlyR-Abs) and against glutamic acid decarboxylase (GAD-Abs). It has been considered to be on the continuum of stiff person syndrome (1-10). Some patients with PERM have had thymomas and have responded to thymectomy along with immunotherapy (4,9). Many of these cases were presented in the largest published review of this disorder (8).
Before the recognition of specific antibodies and the use of immunotherapy, the disease was often fatal. The neuropathology in such cases included perivascular cuffing in the hippocampus, basal ganglia, brainstem, and cerebellum; microglial nodules around hippocampal pyramidal neurons and cerebellar Purkinje cells; and neuronal loss in the anterior horn of the spinal cord. These findings supported the concept of an autoimmune mechanism of the disease (1,5).
We herein report an autopsy case of GlyR-Ab-positive PERM for a patient with a thymoma. The patient recovered after plasma exchange and thymectomy, but subsequently developed anasarca of unknown cause and died after suffering a cerebral infarction.
Case Report
A 75-year-old woman [mistakenly reported as male; Table 4, fifth patient (8)] presented with subacute progressive difficulty in walking and rigidity of the lower extremities. She demonstrated occasional jerky movements, elicited by the touching of her face. She was hospitalized elsewhere and underwent screening blood tests and magnetic resonance imaging (MRI) of the brain and spinal cord. All test results were unremarkable. She subsequently developed dysarthria, dysphagia, and autonomic dysfunction with urinary retention, constipation, hyperhidrosis, hypersalivation, and sinus tachycardia. Upper respiratory tract obstruction developed acutely, requiring tracheostomy. She was then transferred to our hospital for the further evaluation and management. Her history notably included diabetes, chronic thyroiditis, and hypertension.
She was alert and oriented. On a neurologic examination, she had bilateral horizontal and upward gaze nystagmus, dysphagia, dysarthria, difficulty opening her mouth, hyperactive deep tendon reflexes of the jaw and upper extremities, mild rigidity and spasticity in both legs, and a positive Babinski sign on the right. Bulbar signs included bilaterally impaired movement of the soft palate and laryngeal muscles and weakness and a severely limited range of tongue movement. Jerky movements of the neck and trunk, sometimes triggered by touching of the patient's face, were observed and considered consistent with myoclonus, although they were too rare to be recorded electrophysiologically. Laryngoscopy revealed moderate bilateral vocal cord paralysis with slight adduction at rest (Fig. 1a). Serologic testing revealed positive results for GlyR-Abs (1:350), GAD-Abs (45,000 U/mL), and thyroid peroxidase antibodies (TPO-Abs) (306 U/mL). However, tests for anti-Hu, Yo, Ri, Tr, amphiphysin, CRMP-5 IgG, P/Q- and N-type calcium channels, AQP4, and ganglioside antibodies were all negative. Anti-gephyrin antibodies were not examined. There was no pleocytosis or an elevated protein level in the cerebrospinal fluid (CSF), but GlyR-Abs (1:40) and GAD-Abs (350 U/mL) were present. Repeat brain MRI including gadolinium enhancement and MR angiography revealed no abnormalities. Enhanced computed tomography (CT) revealed a 6-cm mediastinal mass, suggestive of a thymoma (Fig. 1d). Blink reflex, brainstem auditory evoked potential, and electroencephalography were all unremarkable.
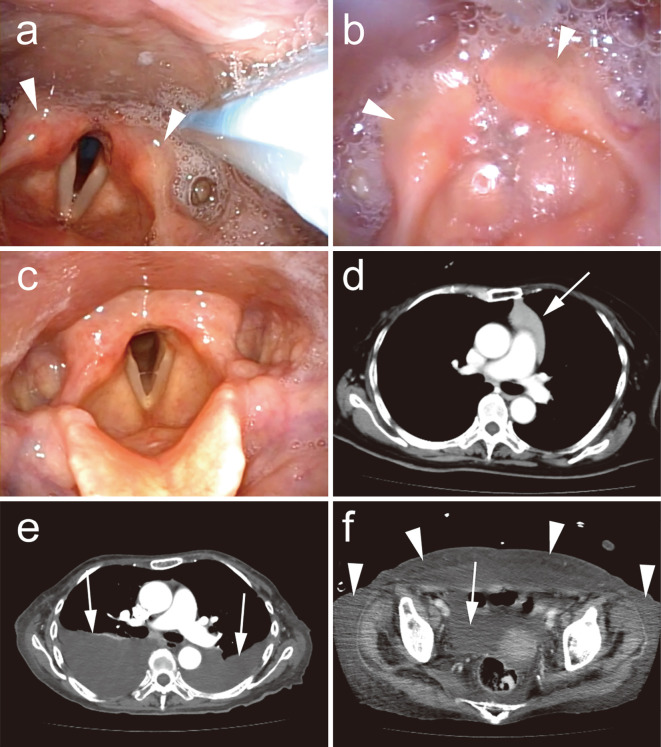
Figure 1.
Progressive encephalomyelitis with rigidity and myoclonus associated with thymoma, with subsequent generalized edema. a) Laryngoscopy immediately after transfer to our hospital showing moderate bilateral vocal cord paralysis with mild pharyngeal edema (arrowheads) and hypersalivation. b) Bilateral vocal cord paralysis with median fixation developed subsequently. Edema (arrowheads) and hypersalivation are still present. c) After thymectomy, the vocal cord paralysis resolved completely. d) Thoracic enhanced computed tomography (CT) showing a thymoma (arrow). e) Chest CT five months after thymectomy, showing massive bilateral pleural effusions (arrows). f) Abdominal CT showing ascites (arrow) and subcutaneous edema (arrowheads).
During the hospital course, the patient's vocal cord paralysis worsened, resulting in median fixation of the cords (Fig. 1b). The other signs, such as rigidity of the extremities, were generally unchanged or mildly improved. A course of plasma exchange resulted in partial improvement of the leg rigidity and spasticity, but that rigidity and spasticity worsened again a few days later. However, the transient response to treatment was consistent with an immunologic etiology.
The constellation of subacute progressive rigidity, myoclonus, brainstem dysfunction, autonomic symptoms, and presence of GlyR-Abs suggested a diagnosis of PERM. For a previously reported case of a patient with PERM with thymoma and GlyR-Abs, there was marked recovery after thymectomy (4). Therefore, we resected our patient's thymoma. There was no evidence of invasion of the tumor into the surrounding organs. On a pathologic examination, the resected tumor was diagnosed as a type B1 thymoma. All of the patient's neurologic symptoms markedly improved over the subsequent four months. She was able to walk and eat without assistance and was discharged from rehabilitation six months after the onset of her symptoms. Follow-up laryngoscopy showed recovery to an almost normal state (Fig. 1c). The GlyR-Ab titer was markedly reduced in the CSF (from 1:40 before treatment to 1:5 at 6 months after thymectomy) and slightly reduced in the serum (from 1:400 to 1:300). In contrast, the GAD-Ab and TPO-Ab titers remained high after thymectomy in both the CSF and serum.
Soon after discharge, the patient noted the subacute development of mild edema in her legs. Subsequently, she developed dyspnea and was hospitalized. CT revealed massive bilateral pleural effusions, edema of the abdominal wall, and mild ascites (Fig. 1e and f). The patient had hypoalbuminemia, but there was no evidence of kidney dysfunction. The serum creatinine level was normal, and there was no proteinuria or hematuria. Anemia and hypothyroidism were also excluded as causes of the edema. Liver function test results were normal, and transthoracic echocardiography showed a normal ventricular function. Observations from upper and lower gastrointestinal endoscopy were unremarkable. Thoracentesis yielded a mildly exudative pleural effusion [serum total protein 5.8 g/dL, pleural fluid protein 2,837 mg/dL, serum lactate dehydrogenase (LDH) 262 U/L, pleural fluid LDH 175 U/L]. Repeated cultures did not reveal any signs of infection. Cytology, carcinogenic embryonic antigen, and adenosine deaminase investigations were negative. Bilateral pleurodesis was performed, but the effect was limited. The patient subsequently had a major cerebral infarction in the territory of the right middle cerebral artery that was thought to be due to dehydration. She had recurrent aspiration pneumonia, sometimes accompanied by bronchospasm and upper airway obstruction, and again required tracheostomy. However, the neurologic symptoms previously associated with PERM did not recur. She ultimately succumbed to pneumonia and cardiac failure 10 months after developing fluid retention. An autopsy was performed.
Autopsy
On a gross examination, there were bilateral clear, yellow pleural effusions of 450 mL on the right and of 200 mL on the left; a clear, yellow pericardial effusion (60 mL); and mildly cloudy, light-yellow ascites (1,100 mL). A microscopic examination of the pleura showed nonspecific edema of the submesothelial tissue, with a mild mononuclear cell infiltrate (Fig. 2a and b), in addition to thickening caused by pleurodesis (not shown). The peritoneum and epicardium similarly had mild edema and exhibited a slight mononuclear infiltrate (Fig. 2c-e). Organizing pneumonia was present bilaterally. There was laryngeal edema, and a moderate mononuclear infiltrate was observed in the submucosal interstitial spaces (Fig. 2f-h). The trachea had a similar moderate mononuclear infiltrate of the submucosal interstitial spaces and around the tracheal glands. A 1-cm submucosal gastric tumor was found just beneath the mucosa near the pyloric ring. An immunohistochemical analysis (Supplementary material) indicated that the tumor was positive for CD34 and DOG-1 but negative (or only focally weakly positive) for c-kit, alpha smooth muscle actin, S100, and Desmin, all findings that are compatible with a diagnosis of gastrointestinal stromal tumor (GIST) (11) (Fig. 3a-f). The tumor was also positive for platelet-derived growth factor receptor (PDGFR) alpha and beta (12,13) (Fig. 3g and h). An examination of the myocardium, liver, kidneys and other organs revealed no remarkable findings.
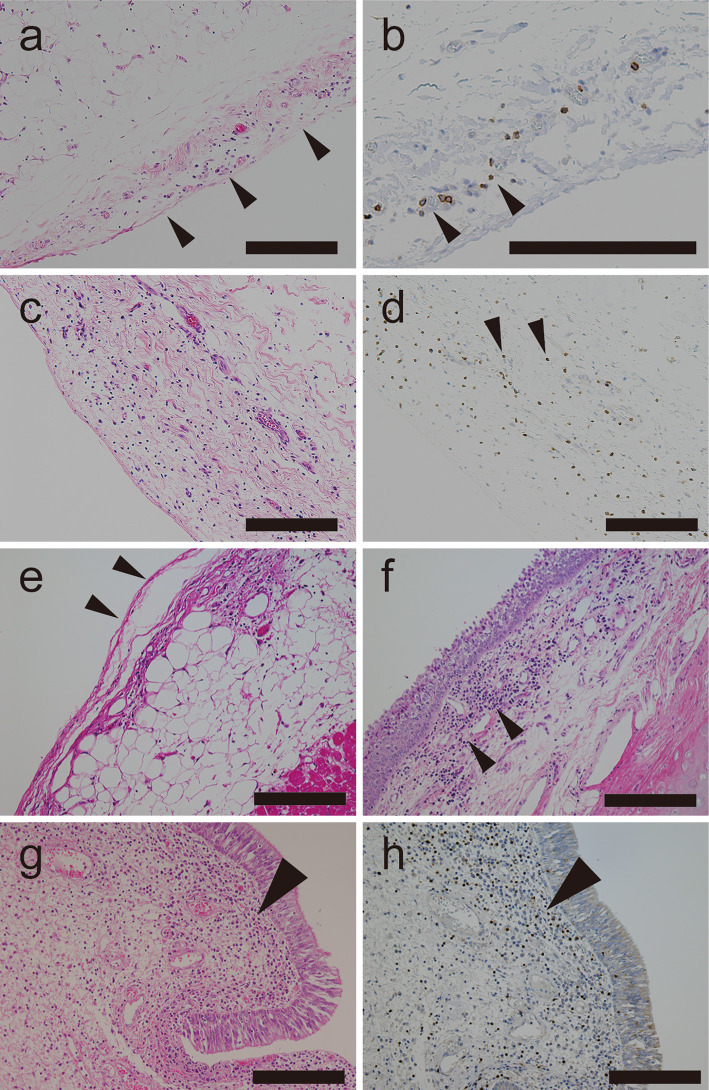
Figure 2.
A postmortem examination of a case of progressive encephalomyelitis with rigidity and myoclonus that resolved after thymectomy. The patient subsequently developed generalized edema of unknown cause. a, b) Pleura showing mild submesothelial edema (arrowheads in a) and a mononuclear infiltrate [Hematoxylin and Eosin (H&E) staining]. CD3-positive cells on immunohistochemistry (arrowheads in b). c, d) Mild edema and mononuclear infiltrate in the peritoneum (H&E staining), with some CD3-positive cells (arrowheads in d). e) Epicardium (arrowheads) with mild interstitial edema. f) Epiglottis showing a moderate interstitial mononuclear infiltrate (arrowheads). g, h) Vocal folds showing a moderate interstitial infiltrate (H&E staining) (arrowhead in g) with some CD3-positive cells (arrowhead in h). Scale bar: a-h 200 μm
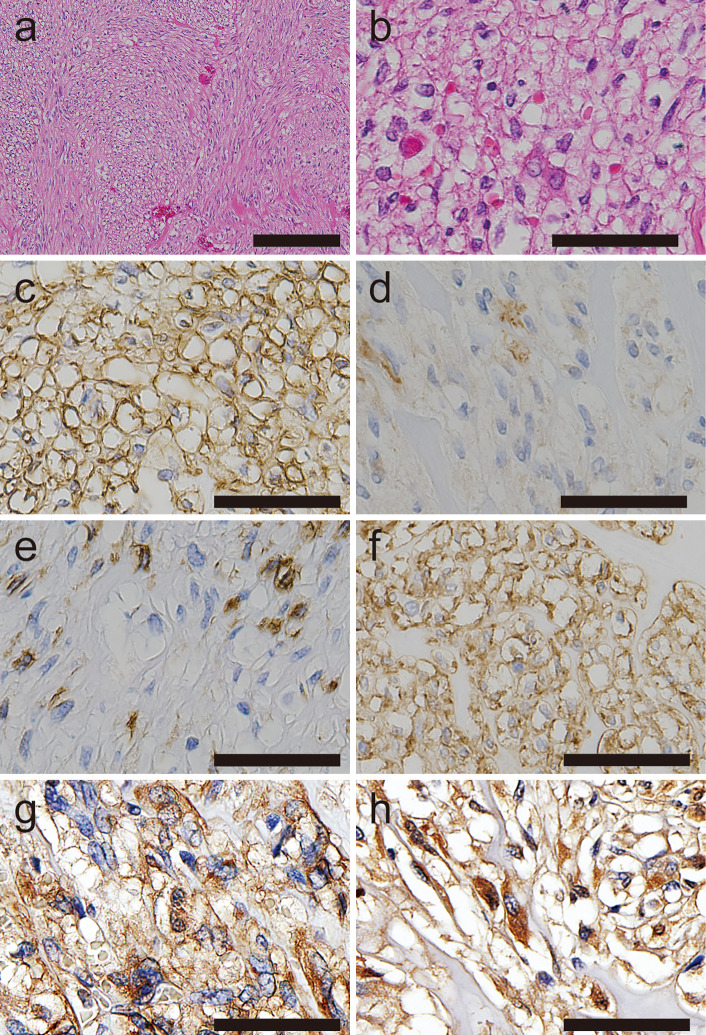
Figure 3.
Immunostaining of a submucosal tumor compatible with gastrointestinal stromal tumor. a, b) Epithelioid and spindle-shaped tumor cells (Hematoxylin and Eosin staining). c-e) Tumor cells positive for CD34 (c) but only trace-positive for c-kit (d) and alpha smooth muscle actin (e). f-h) Tumor cells positive for DOG-1 (f) and heterogeneously positive for PDGFR alpha with a dot-like cytoplasmic pattern (g), and for PDGFR beta with a cytoplasmic pattern (h). Scale bar: a 200 μm; b-h 50 μm
The brain weight was 1,045 g before fixation. Samples were fixed with 10% formalin and embedded in paraffin, and 6-μm-thick sections of the right cerebrum, midbrain, pons, medulla oblongata, right cerebellum, and spinal cord were prepared. The sections were stained with Hematoxylin and Eosin and Klüver-Barrera stains. For immunohistochemistry, we used antibodies to CD3, CD4, CD20, CD68, and IgG (Supplementary material). An old infarction in the right middle cerebral artery territory, with accompanying Wallerian degeneration, was observed. There were no findings typical of PERM, such as perivascular cuffing, microglial nodules, or anterior horn neuronal loss (Fig. 4a-c). Mild mononuclear infiltrates positive for CD3 were occasionally found around the vessels in the brainstem (Fig. 4d).
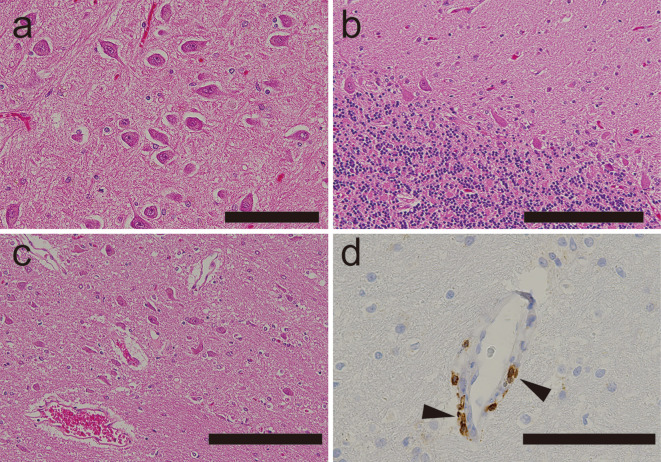
Figure 4.
A neuropathological examination of a patient’s brain in a case of progressive encephalomyelitis with rigidity and myoclonus that resolved after thymectomy. a-c) Hippocampus (a), cerebellum (b), and brainstem (c) showing no evidence of microglial nodules or perivascular cuffing (Hematoxylin and Eosin staining). d) Immunohistochemistry revealing occasional CD3-positive cells around vessels in the pons (arrowheads) and other brainstem structures. Scale bar: a, d 100 μm; b, c 200 μm
Discussion
Our patient's PERM resolved clinically following thymectomy, and an autopsy revealed no persisting neuropathological evidence of the disease. Resection of the thymoma led to the improvement of the neurologic symptoms typical of PERM, as well as a reduction in the GlyR-Ab titers. However, the patient unexpectedly developed fluid retention of unknown cause, which has not previously been reported in association with PERM. The edema and effusions progressed, but the neurologic symptoms of PERM did not recur. The severe fluid appears to have been a phenomenon distinct from PERM. The patient had developed upper airway obstruction as part of her PERM that later resolved along with the other neurologic findings. The obstruction developed again after her cerebral infarction, when she had repeated episodes of aspiration pneumonia, so we could not completely exclude the possibility of some residual vocal cord paralysis from her original disorder.
The postmortem examination revealed none of the typical neuropathological findings of PERM, suggesting that they had disappeared in response to thymectomy, although the mild perivascular mononuclear infiltrate may have been residual evidence of PERM. This is consistent with the idea that PERM is an autoimmune disorder that responds to immunomodulation therapy.
Regarding the pathogenesis of the patient's generalized edema, the exudative pleural effusion and hypoalbuminemia without apparent kidney, liver, or gastrointestinal tract dysfunction may suggest capillary leakage. The autopsy findings of nonspecific interstitial edema and mild mononuclear infiltrates in the pleura and peritoneum suggest a possible autoimmune etiology. One hypothesis is that PDGFR alpha expression from the unsuspected GIST might have triggered an autoimmune reaction against PDGFRs that damaged the vascular integrity and led to generalized edema. In chronic myeloid leukemia, treatment with tyrosine kinase inhibitors targeting the activated tyrosine kinase Bcr-Abl can cause marked fluid retention, including pleural effusion. This effect may be because these drugs bind an off-target kinase (PDGFR beta expressed in pericytes), perhaps triggering an autoimmune reaction (14-17). PDGFR alpha is mutated in about 5% of GISTs that are negative for c-kit mutations and therefore has constitutive kinase activity. Since PDGFR alpha and beta can function as a heterodimer, it is conceivable that an autoimmune reaction to PDGFRs might occur in the presence of a GIST expressing a mutant PDGFR alpha. This might block and suppress the PDGFRs, affecting pericytes that express PDGFR beta, and thus influence the vascular integrity (18). Knockout of PDGF-B and PDGFR beta in mice was lethal to embryos, which showed microaneurysms and severe edema (19). Although there have been a few case reports of paraneoplastic syndromes associated with GIST, including membranous nephropathy, bilateral sixth cranial nerve palsy, hypoglycemia, or hypercalcemia (20-25), no cases of GIST-associated fluid retention in the absence of renal dysfunction have been reported. Further investigations are needed to verify the hypothesis regarding PDGFR and whether or not this could account for similar findings in cases of GIST as well as PERM.
The patient's initial bilateral vocal cord paralysis appeared to be a bulbar symptom of PERM, which may give further insight into the pathogenesis of PERM. The possible role of GlyR-Abs might be to block glycinergic neurons identified in the brainstem, thalamus, hypothalamus, cerebellum, and other areas, potentially allowing brainstem dysfunction with bulbar symptoms to occur (26). Congenital bilateral vocal cord paralysis has been experimentally shown to be associated with impaired glycine neurotransmission (27,28). This raises the possibility that the bilateral vocal cord paralysis in our patient with PERM might have been due to impaired glycine neurotransmission induced by GlyR-Abs above the motoneuron level. In addition, it might be interesting to examine anti-gephyrin antibodies in such PERM cases, as gephyrin is another protein component involved in inhibitory synapses, and its autoantibodies have been reported in a case of paraneoplastic stiff person syndrome (29). The acute upper airway obstruction that our patient experienced suggests the need to be alert to this possibility as a clinical manifestation of PERM, as it might require prompt life-saving treatment.
Conclusions
We herein reported a case of PERM in which the patient's neurologic findings successfully resolved after thymectomy and did not recur. The cause of her subsequent generalized edema, preceding her death from a cerebral infarction and aspiration pneumonia, remains unknown. Despite massive pleural effusions, a postmortem examination revealed only mild submesothelial swelling and a mild, nonspecific mononuclear infiltrate in the pleura. A neuropathological investigation revealed none of the typical findings of PERM, consistent with the clinical resolution of the patient's neurologic symptoms prior to the onset of the severe fluid retention. The possible autoimmune nature of her generalized edema is intriguing but could not be definitively proven in this case.
The authors state that they have no Conflict of Interest (COI).
Supplementary Material
Antibodies used in a case of progressive encephalomyelitis with rigidity and myoclonus.
Acknowledgement
We thank Prof. Angela Vincent in the Nuffield Department of Clinical Neurosciences, John Radcliffe Hospital, for providing the GlyR-Ab titers in serum and CSF. We are also grateful to Prof. Susumu Kusunoki in the Department of Neurology, Kinki University School of Medicine, for analyzing the anti-ganglioside antibodies and Prof. Toshiyuki Takahashi in the Department of Neurology, Tohoku University, for evaluating the anti-AQP4 antibodies.
References
- 1. Barker RA, Revesz T, Thom M, Marsden CD, Brown P. Review of 23 patients affected by the stiff man syndrome: clinical subdivision into stiff trunk (man) syndrome, stiff limb syndrome, and progressive encephalomyelitis with rigidity. J Neurol Neurosurg Psychiatry 65: 633-640, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hutchinson M, Waters P, McHugh J, et al. Progressive encephalomyelitis, rigidity, and myoclonus: a novel glycine receptor antibody. Neurology 71: 1291-1292, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Mas N, Saiz A, Leite MI, et al. Antiglycine-receptor encephalomyelitis with rigidity. J Neurol Neurosurg Psychiatry 82: 1399-1401, 2011. [DOI] [PubMed] [Google Scholar]
- 4. Clerinx K, Breban T, Schrooten M, et al. Progressive encephalomyelitis with rigidity and myoclonus: resolution after thymectomy. Neurology 76: 303-304, 2011. [DOI] [PubMed] [Google Scholar]
- 5. Turner MR, Irani SR, Leite MI, Nithi K, Vincent A, Ansorge O. Progressive encephalomyelitis with rigidity and myoclonus: glycine and NMDA receptor antibodies. Neurology 77: 439-443, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iizuka T, Leite MI, Lang B, et al. Glycine receptor antibodies are detected in progressive encephalomyelitis with rigidity and myoclonus (PERM) but not in saccadic oscillations. J Neurol 259: 1566-1573, 2012. [DOI] [PubMed] [Google Scholar]
- 7. McKeon A, Martinez-Hernandez E, Lancaster E, et al. Glycine receptor autoimmune spectrum with stiff-man syndrome phenotype. JAMA Neurol 70: 44-50, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carvajal-González A, Leite MI, Waters P, et al. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain 137: 2178-2192, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alexopoulos H, Akrivou S, Dalakas MC. Glycine receptor antibodies in stiff-person syndrome and other GAD-positive CNS disorders. Neurology 81: 1962-1964, 2013. [DOI] [PubMed] [Google Scholar]
- 10. Martinez-Hernandez E, Arino H, McKeon A, et al. Clinical and immunologic investigations in patients with stiff-person spectrum disorder. JAMA Neurol 73: 714-720, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miettinen M, Lasota J. Gastrointestinal stromal tumors-definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 438: 1-12, 2001. [DOI] [PubMed] [Google Scholar]
- 12. Rossi G, Valli R, Bertolini F, et al. PDGFR expression in differential diagnosis between KIT-negative gastrointestinal stromal tumours and other primary soft-tissue tumours of the gastrointestinal tract. Histopathology 46: 522-531, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Agaimy A, Otto C, Braun A, Geddert H, Schaefer IM, Haller F. Value of epithelioid morphology and PDGFRA immunostaining pattern for prediction of PDGFRA mutated genotype in gastrointestinal stromal tumors (GISTs). Int J Clin Exp Pathol 6: 1839-1846, 2013. [PMC free article] [PubMed] [Google Scholar]
- 14. Kim KW, Shinagare AB, Krajewski KM, et al. Fluid retention associated with imatinib treatment in patients with gastrointestinal stromal tumor: quantitative radiologic assessment and implications for management. Korean J Radiol 16: 304-313, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masiello D, Gorospe G 3rd, Yang AS. The occurrence and management of fluid retention associated with TKI therapy in CML, with a focus on dasatinib. J Hematol Oncol 2: 46, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quintas-Cardama A, Kantarjian H, O'Brien S, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol 25: 3908-3914, 2007. [DOI] [PubMed] [Google Scholar]
- 17. De Lavallade H, Punnialingam S, Milojkovic D, et al. Pleural effusions in patients with chronic myeloid leukaemia treated with dasatinib may have an immune-mediated pathogenesis. Br J Haematol 141: 745-747, 2008. [DOI] [PubMed] [Google Scholar]
- 18. Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21: 193-215, 2011. [DOI] [PubMed] [Google Scholar]
- 19. Hellström M, Gerhardt H, Kalén M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 153: 543-554, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guirgis HM, Holcombe RF. A case of advanced gastrointestinal stromal tumor (GIST) presenting with paraneoplastic syndrome. J Clin Oncol 22 (14_suppl): 4242, 2004. [Google Scholar]
- 21. Takane K, Midorikawa Y, Yamazaki S, et al. Gastrointestinal stromal tumor with nephrotic syndrome as a paraneoplastic syndrome: a case report. J Med Case Reports 8: 108, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guiteau J, Fanucchi M, Folpe A, Staley CA 3rd, Kooby DA. Hypoglycemia in the setting of advanced gastrointestinal stromal tumor. Am Surg 72: 1225-1230, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Cimic A, Pastan SO, Bijol V. Membranous nephropathy associated with gastrointestinal stromal tumour: a case report. NDT Plus 2: 306-308, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calviño J, Adeva M, Sobrido M-J. Membranous nephropathy, leiomyoma and autoimmune myasthenia: more than a coincidence? Clin Kidney J 5: 562-565, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsikrikas S, Manolakopoulos S, Deutsch M, et al. Unusual combination of paraneoplastic manifestations in a patient with metastatic gastrointestinal stromal tumor (GIST). Scand J Gastroenterol 43: 1012-1015, 2008. [DOI] [PubMed] [Google Scholar]
- 26. Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev 84: 1051-1095, 2004. [DOI] [PubMed] [Google Scholar]
- 27. Holinger LD, Holinger PC, Holinger PH. Etiology of bilateral abductor vocal cord paralysis: a review of 389 cases. Ann Otol Rhinol Laryngol 85: 428-436, 1976. [DOI] [PubMed] [Google Scholar]
- 28. Berkowitz RG, Sun QJ, Pilowsky PM. Congenital bilateral vocal cord paralysis and the role of glycine. Ann Otol Rhinol Laryngol 114: 494-498, 2005. [DOI] [PubMed] [Google Scholar]
- 29. Butler MH, Hayashi A, Ohkoshi N, et al. Autoimmunity to gephyrin in stiff-man syndrome. Neuron 26: 307-312, 2000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antibodies used in a case of progressive encephalomyelitis with rigidity and myoclonus.