Abstract
Neuronal intranuclear inclusion disease (NIID) is a rare neurodegenerative disease with marked variety in its clinical manifestations. While characteristic neuroimaging and skin biopsy findings are important clues to the diagnosis, autopsy studies are still important for confirming the exact disease features. We herein report the case of a patient who received an antemortem diagnosis of familial NIID with dementia-dominant phenotype that was later confirmed by an autopsy. Our report is the first to document a case of autopsy-confirmed NIID involving both cognitive impairment and sensorimotor neuropathy.
Keywords: neuronal intranuclear inclusion disease, autopsy, cognitive impairment, sensorimotor neuropathy
Introduction
Neuronal intranuclear inclusion disease (NIID) is a rare progressive neurodegenerative disorder characterized by the presence of eosinophilic, ubiquitin/p62-positive intranuclear inclusions in various organs, including the nervous system. The neurological symptoms of NIID include cerebellar ataxia, parkinsonism, peripheral neuropathy, autonomic dysfunction, cognitive dysfunction, and retinopathy (1). Familial and sporadic forms of NIID have been reported, with the age of onset varying between infancy and the late 60s (2-4). The clinical heterogeneity of NIID long complicated its antemortem diagnosis until Sone et al. reported the presence of eosinophilic intranuclear inclusion bodies in skin biopsy samples and high-intensity lesions in the cortico-medullary junction on diffusion-weighted images (DWI) as characteristic findings (5,6). Since these reports, the diagnosis of NIID has been mostly made only by magnetic resonance imaging (MRI) or a skin biopsy (7,8); only two autopsy studies with an antemortem diagnosis describing familial cases of NIID with neuropathy and a sporadic case with encephalopathy-like symptoms have been reported to date (9,10). These cases showed various neurological symptoms and signs presumed to be a part of NIID. However, since this disease entity has yet to be fully established genetically or biochemically, an autopsy and full neuropathological examination are required to attribute those symptoms to this disease. Indeed, there has been no report on the autopsy findings with an antemortem diagnosis of familial case of NIID with dementia-dominant phenotype.
We herein report the clinical and autopsy findings of a patient who had familial NIID and various neurological symptoms, including cognitive decline and neuropathy. A clinical diagnosis was made based on a skin biopsy and MRI findings, and the pathological diagnosis was later confirmed by an autopsy. Our case clarifies the pathological findings of familial NIID with dementia-dominant phenotype and suggests that the heterogeneous clinical features can be attributed to a single disease entity.
Case Report
The patient was a 79-year-old woman who experienced the onset of postural hand tremor at 66 years of age. At 74 years of age, she developed memory disturbance and apathy, and at 75 years of age, she developed urinary incontinence, nyctalopia, and occasional daytime solemnness. A neurological examination at 75 years of age revealed resting and postural bilateral tremor in the upper extremities, cerebellar limb ataxia, distal muscular atrophy, and decreased tendon reflexes. Her Wechsler Adult Intelligence Scale-III Full-Scale IQ, Verbal IQ and Performance IQ scores were 54, 63, and 52, respectively.
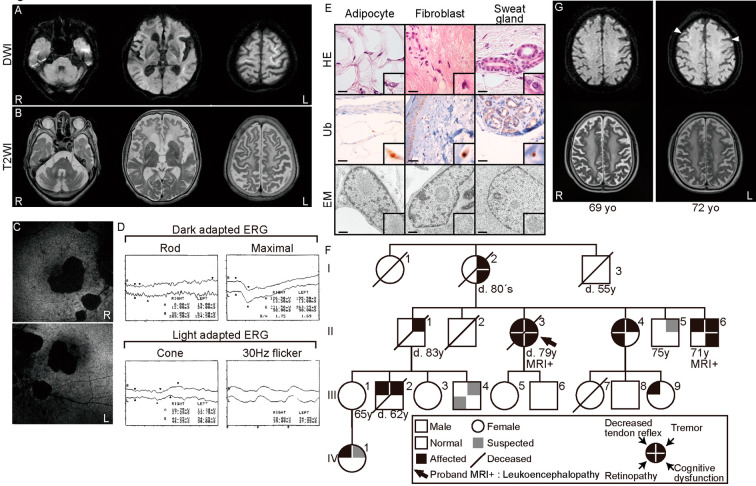
The patient experienced functional ileus of unknown origin at 78 years of age. At 79 years of age, she scored 6/30 on the Mini-Mental State Examination. Brain MRI revealed diffuse high-intensity lesions in the bilateral white matter with a frontal-predominant distribution on T2-weighted imaging (T2WI), and linearly-shaped high-intensity lesions of U-fibers on DWI, which retrospectively had been identified at 75 years of age and showed progression at this time (Fig. 1A and B). Auto-fluorescence imaging of the optic fundus revealed retinochoroidal atrophy (Fig. 1C), and electroretinography (ERG) showed attenuated cone and rod responses, suggesting cone-rod dystrophy (Fig. 1D). A nerve conduction study showed reduced conduction velocities and amplitudes (motor: left median nerve 37.3 m/s, 3.5 mV; ulnar nerve 44.7 m/s, 8.6 mV; tibial nerve 28.9 m/s, 9.4 mV. Sensory: median nerve 38.1 m/s, 11.7 μV; ulnar nerve 38 m/s, 37.9 μV; sural nerve 29.1 m/s, 0.57 μV), indicative of both demyelinating and axonal neuropathy. A skin biopsy revealed eosinophilic, ubiquitin-positive intranuclear inclusions in adipocytes, fibroblasts, and sweat gland cells, which consisted of tubulo-filamentous material on electron microscopy (Fig. 1E), strongly indicating a diagnosis of NIID.
Figure 1.
The clinical presentation of the proband and the other family members. (A, B) Magnetic resonance imaging showing linearly-shaped high-intensity lesions on diffusion-weighted images (A) and frontal-predominant leukoencephalopathy on T2-weighted images (B). (C) Fundus autofluorescence imaging showing fundus atrophy. (D) Electroretinography showing attenuated cone and rod responses. (E) Pathological skin biopsy findings. Hematoxylin and Eosin (H&E) staining, immunohistochemical staining for ubiquitin (Ub), and an electron microscopy (EM) image are shown. Sections include sweat gland cells, fibroblasts, and adipocytes. Insets are magnified views of nuclei with inclusion bodies. Scale bar=20 µm (H&E staining, Ubiquitin), 500 nm (EM). (F) Family genealogy for four generations. The arrow indicates the autopsied patient. (G) Magnetic resonance imaging of family member II-6 at 69 and 72 years of age. Diffusion-weighted images (upper panel) and T2-weighted images (lower panel) are shown. Linearly-shaped high-intensity lesions (arrowheads) appeared at 72 years of age.
Nine family members across four generations had exhibited partial symptoms and laboratory test abnormalities resembling the patient, including two MRI-confirmed cases of leukoencephalopathy with linearly-shaped DWI high-intensity lesion (Fig. 1F and G) indicative of autosomal dominant inheritance. The numbers of disease-associated CAG repeats on the polyglutamine diseases, including Huntington's disease, dentatorubural pallidoluysian atrophy (DRPLA), Machado-Joseph disease (MJD), and spinocerebellar ataxias (type 1, 2, 6, 8, 12, and 17), were within normal ranges in the proband. The numbers of CGG repeats in the 5'-untranslated region of FMR1 were 31 and 35 on each allele (normally 5-44). The patient died of aspiration pneumonia at 79 years of age, and an autopsy was performed.
Autopsy findings
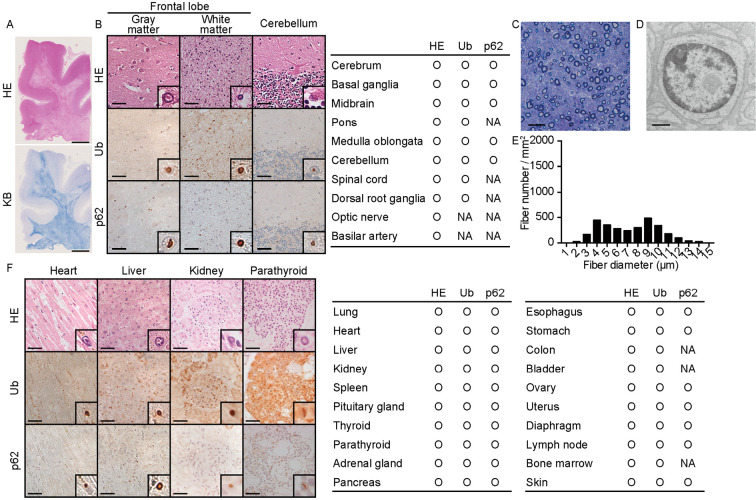
Her brain weight was 1,053 g, and a macroscopic observation showed frontal-dominant cortical atrophy and cerebellar atrophy. Spongiosis of the white matter adjacent to the cerebral cortex were present predominantly in the frontotemporal lobe, consistent with the MRI findings (Fig. 2A). In these lesions, eosinophilic intranuclear inclusions with p62 and ubiquitin immunoreactivity were primarily observed in swollen astrocytes as well as in neurons (Fig. 2B). The distribution of spongiosis corresponded with linearly-shaped high-intensity lesions on DWI. In the cerebellum, the inclusion bodies were observed mainly in Bergman glial cells and Purkinje cells. In the sural nerve, the density of myelinated fibers was mildly reduced (3,016 MF/mm2). The predominant loss of small fibers was observed, and unmyelinated fibers were also depleted (Fig. 2C and E). Inclusions were identified in Schwann cells (Fig. 2D), potentially accounting for the decreased nerve conduction velocity. Inclusions were detected in all other organs examined (Fig. 2F). The densities of the nuclei with Ub-positive inclusions were approximately 10/mm2, 75/mm2, 120/mm2, and 160/mm2 in the heart, liver, kidney, and parathyroid, respectively. Based on the pathological findings, a diagnosis of NIID was pathologically confirmed.
Figure 2.
Pathological autopsy findings of the proband. (A) Low-magnification image of the frontal lobe. Hematoxylin and Eosin (H&E) staining (upper) and Klüver-Barrera (KB, lower) staining results are shown. Scale bar=5 mm. (B) Representative pathological findings for the central and peripheral nervous systems. H&E staining and immunohistochemical staining results for ubiquitin and p62 are shown. The table provides a summary of the pathological findings. Insets are the magnified views of nuclei with inclusion bodies. Scale bar=50 µm. (C-E) Pathological findings of the distal sural nerve. Transverse semi-thin sections show the loss of myelinated fibers (C, E). (D) EM image of a Schwann cell nucleus with an inclusion body. Scale bar=20 µm (C), 1 µm (D). (F) Representative pathological findings in the visceral organs. H&E staining and immunohistochemical staining results for ubiquitin and p62 are shown. Insets are the magnified views of nuclei with inclusion bodies. The densities of the nuclei with Ub-positive inclusions were approximately 10/mm2, 75/mm2, 120/mm2, and 160/mm2 in the heart, liver, kidney, and parathyroid, respectively. Scale bar=50 µm. The table provides a summary of the pathological findings. NA: not available
Discussion
Sone et al. (10) proposed clinical phenotype subgroups of NIID; sporadic or familial dementia-dominant group, and familial weakness-dominant group. Yokoi et al. (9) reported the autopsy findings of sporadic NIID showing subacute progression of impaired consciousness, and Sone et al. (10). also reported the autopsy findings of three familial NIID cases with neuropathy; however, the autopsy findings of the familial dementia-dominant group have not been described. Our case was an autopsy case of a familial dementia-dominant patient. Despite the varied clinical manifestations, all of the forms of NIID showed the same pathological findings as the intranuclear eosinophilic inclusions in every visceral organ and nervous system, attributing the heterogeneous clinical features to a single disease entity.
In the present case, one of the patient's siblings exhibited cognitive impairment (II-6) and frontal-predominant leukoencephalopathy on T2WI. Initial MRI at 69 years of age did not detect any DWI abnormalities; however, linearly-shaped high-intensity lesions appeared at 72 years of age (Fig. 1G). Therefore, while persistent high-intensity U-fiber lesions on DWI are presumably a specific feature of NIID (6), these lesions might appear in more advanced stages or specific periods of the disease, whereas frontal-predominant leukoencephalopathy might be a more sensitive and early diagnostic indicator.
In conclusion, we presented an autopsy case of NIID with the presence of both cognitive impairment and sensorimotor neuropathy. The autopsy findings of familial NIID with the dementia-dominant phenotype is a missing piece key to establishing the disease entity of pathologically endorsed NIID; the findings from the present case are therefore important. Various neurological symptoms and signs among the family members of our patient and autopsy findings suggest that NIID can manifest with a broad range of neurological symptoms with significant between-patient variation.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Lindenberg R, Rubinstein LJ, Herman MM, Haydon GB. A light and electron microscopy study of an unusual widespread nuclear inclusion body disease. A possible residuum of an old herpesvirus infection. Acta Neuropathol 10: 54-73, 1968. [DOI] [PubMed] [Google Scholar]
- 2. Schuffler MD, Bird TD, Sumi SM, Cook A. Familial neuronal disease presenting as intestinal pseudoobstruction. Gastroenterology 75: 889-898, 1978. [PubMed] [Google Scholar]
- 3. Kimber TE, Blumbergs PC, Rice JP, et al. Familial neuronal intranuclear inclusion disease with ubiquitin positive inclusions. J Neurol Sci 160: 33-40, 1998. [DOI] [PubMed] [Google Scholar]
- 4. Zannolli R, Gilman S, Rossi S, et al. Hereditary neuronal intranuclear inclusion disease with autonomic failure and cerebellar degeneration. Arch Neurol 59: 1319-1326, 2002. [DOI] [PubMed] [Google Scholar]
- 5. Sone J, Tanaka F, Koike H, et al. Skin biopsy is useful for the antemortem diagnosis of neuronal intranuclear inclusion disease. Neurology 76: 1372-1376, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Sone J, Kitagawa N, Sugawara E, et al. Neuronal intranuclear inclusion disease cases with leukoencephalopathy diagnosed via skin biopsy. J Neurol Neurosurg Psychiatry 85: 354-356, 2014. [DOI] [PubMed] [Google Scholar]
- 7. Sasaki T, Hideyama T, Saito Y, Shimizu J, Maekawa R, Shiio Y. Neuronal intranuclear inclusion disease presenting with recurrent cerebral infarct-like lesions. Neurol Clin Neurosci 3: 185-187, 2015. [Google Scholar]
- 8. Toyota T, Huang Z, Nohara S, et al. Neuronal intranuclear inclusion disease manifesting with new-onset epilepsy in the elderly. Neurol Clin Neurosci 3: 238-240, 2015. [Google Scholar]
- 9. Yokoi S, Yasui K, Hasegawa Y, et al. Pathological background of subcortical hyperintensities on diffusion-weighted images in a case of neuronal intranuclear inclusion disease. Clin Neuropathol 35: 375, 2016. [DOI] [PubMed] [Google Scholar]
- 10. Sone J, Mori K, Inagaki T, et al. Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain 139: 3170-3186, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]