Abstract
Background
Individuals with human immunodeficiency virus (HIV) face elevated cardiovascular disease (CVD) risk. There are limited data regarding the application of the American College of Cardiology/American Heart Association (ACC/AHA) cholesterol guidelines in HIV compared with non-HIV patients.
Methods
Human immunodeficiency virus-infected and demographically similar control patients were assessed for statin recommendation status by ACC/AHA and the National Cholesterol Education Program Adult Treatment Program III (ATPIII), indication for statin recommendation, actual statin prescription, and CVD event. Outcomes were atherosclerotic CVD for ACC/AHA and coronary heart disease for ATPIII.
Results
In a clinical care cohort of 1394 patients infected with HIV, 38.6% (538 of 1394) of patients were recommended for statin therapy by the ACC/AHA guidelines compared with 20.1% (280 of 1394) by the ATPIII guidelines. Of those recommended for statin therapy, actual statin prescription rates were 42.8% (230 of 538) for ACC/AHA and 66.4% (186 of 280) for ATPIII. Among patients infected with HIV with an incident CVD event during follow-up, statin therapy was recommended for 59.2% (42 of 71) of patients by ACC/AHA and 35.2% (25 of 71) by ATPIII, versus 71.6% (141 of 197) by ACC/AHA and 43.1% (85 of 197) by ATPIII in the control group.
Conclusions
In an HIV clinical care cohort, the ACC/AHA cholesterol guidelines recommend a higher proportion of patients for statin therapy and identify an increased proportion of patients with a CVD event compared with ATPIII. However, 40% of patients with a CVD event would not have been recommended for statin therapy by ACC/AHA, compared with 29% for controls. This gap in identification of patients infected with HIV at high CVD risk underscores the need for HIV-specific cardiovascular prevention strategies.
Keywords: atherosclerosis, cardiovascular disease, HIV, myocardial infarction, statin
Human immunodeficiency virus (HIV)-infection confers a 1.5- to 2-fold increased risk of cardiovascular disease (CVD) [1–3]. Among other risk reduction strategies, statin therapy may reduce CVD risk in patients infected with HIV. Statins have been associated with decrease in cholesterol levels, CVD-related inflammation and immune activation, and mortality rates among HIV-infected groups [4–8]. However, it remains unknown which patients infected with HIV will benefit from statin therapy and whether strategies to identify patients as eligible for statin therapy developed for the general population are applicable in the setting of HIV.
In 2013, the American College of Cardiology/American Heart Association (ACC/AHA) released new cholesterol guidelines [9] to replace the National Cholesterol Education Program Adult Treatment Program III (ATPIII) [10]. The ACC/AHA cholesterol guidelines differ from ATPIII in that low-density lipoprotein (LDL) thresholds to determine statin eligibility are largely eliminated and a new risk score with an expanded outcome definition of CVD (coronary heart disease [CHD] or stroke) is used as a major criterion for statin eligibility [11]. Although the guidelines both outline criteria for treating dyslipidemia to prevent a cardiovascular event, the guidelines utilize different endpoints; the ACC/AHA guidelines use a broader endpoint of atherosclerotic CVD (including myocardial infarction, stroke, or coronary death) versus an endpoint of CHD (including myocardial infarction or coronary death) for ATPIII. Studies indicate that in the general population, an additional 11% of patients would be newly recommended for statin therapy by the ACC/AHA guidelines, and that this increase would largely be driven by older patients with an elevated risk score [12].
Application of the ACC/AHA cholesterol guidelines to HIV populations remains limited. When applied to women infected with HIV, who make up a small proportion of the HIV-infected population in the United States, these guidelines may recommend statins to fewer HIV-infected women [13]. However, whether a similarly increased number of all patients infected with HIV would be recommended for statin therapy under the new guidelines has not been explored relative to a control population of all patients infected with HIV. Moreover, it is not known whether application of the ACC/AHA guidelines would result in improved identification of patients who ultimately experience a CVD event and would benefit from preventative therapy with a statin.
We had 2 primary objectives. In an HIV clinical care cohort, we sought to (1) compare the proportion of HIV-infected patients recommended for statin use by the ACC/AHA guidelines versus ATPIII and (2) determine how many patients who incurred a CVD event would have been recommended for statin use by general population guidelines, comparing HIV to control patients. We used the broader outcome of CVD to reflect the most recent ACC/AHA guidelines.
METHODS
Data Source
We conducted a retrospective clinical care cohort study of 1394 patients infected with HIV in the Partners HIV Cohort. The cohort comprised patients followed in hospital-based or affiliated outpatient clinics at Brigham and Women’s Hospital or Massachusetts General Hospital, 2 urban tertiary care hospitals in the Partners HealthCare System. The cohort was initially developed by querying the Research Patient Data Registry, a centralized clinical data registry containing comprehensive demographic and clinical information for over 4.7 million patients. A control group was generated from the same data source and individually matched to patients in the HIV cohort on the basis of age, gender, and race (5 controls: 1 case). The study was approved by Partners Human Research Committee.
Eligibility Criteria
Patients were eligible for inclusion in the study if they had 3 or more encounters assigned International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 042 and V08 or associated electronic health record (EHR) codes and available data to generate a CVD risk score (ATPIII and ACC/AHA) between January 1, 2006 and December 31, 2008. A definition of 3 or more ICD codes has been demonstrated in a validation study in this cohort to have a sensitivity of 89% and specificity of 100% for identifying HIV infection. Patients under 18 years old or with a prior diagnosis of CHD at start of observation were excluded. Start of observation was defined as the date on which the risk score was generated and occurred for each individual patient in a 3-year window ending December 31, 2008. Patients were followed through the earliest of CVD event, death, last encounter, or December 31, 2011.
Ascertainment of Covariates
Traditional CVD risk factors have been validated and included hypertension (ICD-9-CM code 401), diabetes mellitus (ICD-9-CM code 250), and dyslipidemia (ICD-9-CM code 272) [14]. Blood pressure data were obtained through EHR health maintenance-coded field data and by query of free text notes. Total and high-density lipoprotein cholesterol were obtained from laboratory data. Smoking status was obtained through a combination of coded smoking status from the EHR and a natural language processing-based algorithm applied to free text notes [15]. Use of statins and antihypertensive medications was obtained from inpatient and outpatient prescription medication data. Human immunodeficiency virus-related measures obtained from the EHR included use of antiretroviral therapy (ART), CD4 cell counts, and HIV ribonucleic acid level.
Ascertainment of Outcomes
In the analyses addressing the first objective, the primary outcome was statin recommendation according to either ACC/AHA or ATPIII, as of the observation start date for each individual patient. The outcome for the ACC/AHA guideline is atherosclerotic CVD (defined as myocardial infarction, stroke, or coronary death), and the outcome for the ATPIII guideline is CHD (defined as myocardial infarction or coronary death). In the analyses addressing the second objective, the primary outcome was CVD event during the observation period defined by ICD-9-CM codes 410–414 and 433–434. Ascertainment of these outcomes have been validated in our prior publications [16–19]. Coronary death was determined using EHR data.
Statistical Analysis
Baseline characteristics were compared using χ2 tests for categorical variables and Kruskal-Wallis tests for continuous variables. Recommendation for statin use by ATPIII was assessed using risk category (including CHD, CHD risk equivalent, or CHD risk factors and calculating 10-year Framingham Risk Score [FRS] if indicated). Recommendation for statin use by ACC/AHA was assessed as per guideline-defined statin benefit groups and calculating the atherosclerotic CVD (Pooled Cohorts Equation) risk score if indicated. Reason for statin recommendation according to each cholesterol guideline was established for all study participants. Cardiovascular disease event rates were calculated. Analyses were performed for the HIV group and repeated for the control group. All analyses were performed using STATA (Release 13; StataCorp LP, College Station, TX), and all tests were 2-sided with statistical significance defined as P < .05.
RESULTS
Demographic and Clinical Characteristics
The HIV group included 1394 patients with median follow-up of 4.7 years. The control group included 6141 patients with a shorter median follow-up of 3.9 years compared with patients infected with HIV (P < .001). Demographic and clinical characteristics for the patients infected with HIV are summarized in Table 1 overall and by gender. The cohorts were initially matched on age, gender, and race, but some differences in demographics were present after application of inclusion criteria. Women comprised 26.9% of the HIV cohort versus 36.3% for the control cohort. Rates of some traditional CVD risk factors tended to be lower in the HIV group versus controls for dyslipidemia (53.1% vs 56.2%, P = .037) and hypertension (30.9% vs 44.1%, P < .001), but smoking was more prevalent among patients infected with HIV (45.3% vs 27.8%, P < .001). There was no difference in the rates of diabetes between HIV-infected group and controls (16.4% vs 17.4%, P = .366). In the HIV group, diabetes was more common among women than men (21.3% vs 14.6%, P = .003). In contrast, a higher proportion of HIV-infected males were smokers (47.3% vs 40.0%, P = .015) (Table 1). A higher proportion of controls were on statin therapy compared with the HIV group (33.7% vs 22.0%, P < .001).
Table 1.
Demographic and Clinical Characteristics of HIV-Infected Patientsa and Controls
| Characteristics | HIV | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Female | Male | P Value | All | Female | Male | P Value | b P Value | |
| N | 1394 | 375 | 1019 | 6141 | 2227 | 3914 | |||
| Age, mean (SD) | 46 (9.0) | 44 (9.6) | 46 (8.7) | .002 | 47 (10.4) | 46 (11.8) | 48.3 (9.43) | <.001 | <.001 |
| Race, N (%) | <.001 | <.001 | <.001 | ||||||
| White | 734 (53) | 126 (34) | 608 (60) | 2826 (46) | 759 (34) | 2067 (53) | |||
| Black | 360 (26) | 156 (42) | 204 (20) | 1448 (24) | 735 (33) | 713 (18) | |||
| Hispanic | 220 (16) | 69 (18) | 151 (15) | 1505 (25) | 603 (27) | 902 (23) | |||
| Other | 33 (2) | 15 (4) | 18 (2) | 155 (3) | 69 (3) | 86 (2) | |||
| Unknown | 47 (3) | 9 (2) | 38 (4) | 207 (3) | 61 (3) | 146 (4) | |||
| Hypertension, N (%) | 431 (31) | 120 (32) | 311 (31) | .596 | 2707 (44) | 840 (38) | 1867 (48) | <.001 | <.001 |
| Diabetes, N (%) | 229 (16) | 80 (21) | 149 (15) | .003 | 1071 (17) | 377 (17) | 694 (18) | .426 | .366 |
| Dyslipidemia, N (%) | 740 (53) | 197 (53) | 543 (53) | .802 | 3449 (56) | 1039 (47) | 2410 (62) | <.001 | .037 |
| Smoking, N (%) | 632 (45) | 150 40) | 482 (47) | .015 | 1709 (28) | 535 (24) | 1174 (30) | <.001 | <.001 |
| Statin Use, N (%) | 306 (22) | 74 (20) | 232 (23) | .225 | 2068 (34) | 540 (24) | 1528 (39) | <.001 | <.001 |
| ART, N (%) | 1050 (75) | 278 (74) | 772 (76) | .532 | - | - | - | - | |
| CD4, mean (SD) | 507 (305.3) | 519 (301.4) | 503 (306.8) | .394 | - | - | - | - | |
| Nadir CD4, mean (SD) | 269 (235.6) | 250 (225.0) | 276 (238.9) | .076 | - | - | - | - | |
| HIV RNA <400, N (%) | 839 (75) | 204 (68) | 635 (78) | .001 | - | - | - | - | |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; RNA, ribonucleic acid; SD, standard deviation.
aAll data are N (%) unless otherwise indicated. CD4 count data were available for 1254 patients (326 women, 928 men). Viral load data were available for 1115 patients (300 women, 815 men).
bComparing HIV-infected group to controls.
Recommended and Actual Statin Use
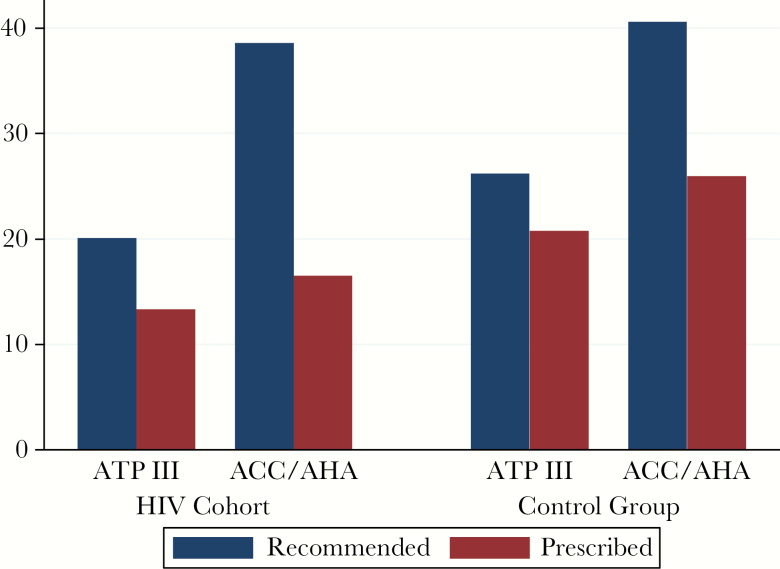
Statin recommendations under the 2 guidelines and actual statin prescriptions are presented in Figure 1 for the HIV and control groups. Overall, patients infected with HIV were less likely to be prescribed statin therapy compared with controls (22.0% vs 33.7%, P < .001). Statins were recommended for more patients infected with HIV under ACC/AHA (38.6%; 95% confidence interval [CI], 36.1%–41.2%) than ATPIII (20.1%; 95% CI, 18.1%–22.3%). In the HIV group, statins were prescribed for 66.4% (95% CI, 60.7%–71.7%) of those meeting ATPIII and 42.8% (95% CI, 38.6%–47.0%) of those meeting ACC/AHA guidelines.
Figure 1.
Statin recommendation status and prescriptions by guideline. Percentages of patients recommended for statin therapy by each guideline are shown for both human immunodeficiency virus (HIV) and control groups. Among those recommended statin therapy by each guideline, the percentage of patients who were prescribed statin therapy is shown. ACC/AHA, American College of Cardiology/American Heart Association; ATPIII, Adult Treatment Panel III.
Statin Recommendation Status and Prescriptions
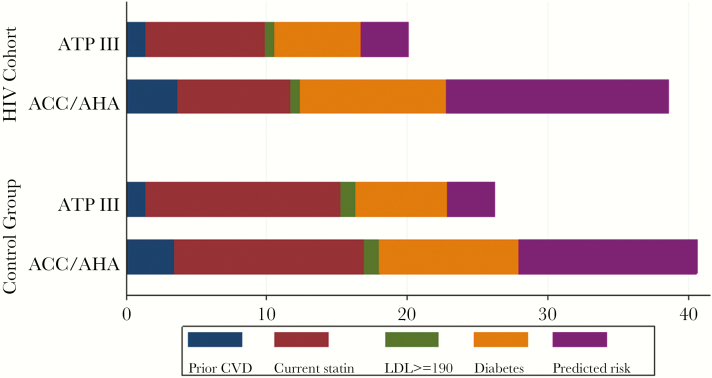
The indications for statin recommendation among the HIV and control groups according to each guideline are shown in Figure 2. Among all, an increased proportion of patients met criteria for statin use due to predicted risk of an event under ACC/AHA compared with ATPIII (40.2% vs 25.1%, P < .001).
Figure 2.
Indications for statin recommendation by guideline. Indications for statin recommendation are shown for Adult Treatment Panel III (ATPIII) and American College of Cardiology/American Heart Association (ACC/AHA) cholesterol guidelines for the human immunodeficiency virus (HIV) and control groups, with the length of the bars indicating the percentage of overall patients recommended for statin by indication. For ACC/AHA, the prior cardiovascular disease (CVD) category includes patients with atherosclerotic CVD (ASCVD) and the diabetes category includes patients with diabetes and low-density lipoprotein (LDL) ≥70. For ATPIII, the prior coronary heart disease (CHD) category includes patients with CHD or CHD risk equivalents as defined by ATPIII (symptomatic carotid artery disease, peripheral artery disease, and abdominal aortic aneurysm) and with LDL ≥100 and the diabetes category includes patients with diabetes and LDL ≥100. For both guidelines, current statin group refers to patients who were already on statin therapy at the time of the observation period. The predicted risk category reflects statin recommendation based on calculated predicted ASCVD risk ≥7.5% for ACC/AHA and statin recommendation based on a combination of CHD risk factors and calculated predicted CHD risk by the Framingham Risk Score for ATPIII.
Statin Recommendation Status Among Patients With Cardiovascular Disease Events
A total of 71 CVD events occurred in the HIV group, and 197 events occurred in the control group. Table 2 presents CVD event rates (ICD-9-CM 410–414 and 433–434) by statin recommendation status for both groups. Of 71 patients with events observed in the HIV group, 42 (59.2%) met criteria for statin use under ACC/AHA versus 25 (35.2%) under ATPIII. Of 197 patients with events observed in the control group, 71.6% (141 of 197) met ACC/AHA criteria for statin use and 43.1% (85 of 197) met ATPIII criteria. Results were similar when the outcome of CHD was used (data not shown).
Table 2.
Statin Recommendation Status Among Patients With CVD Eventsa
| HIV | Controls | |||||
|---|---|---|---|---|---|---|
| Guideline | N | Events | Rateb | N | Events | Rateb |
| ATPIII | 280 | 25 | 19.4 (13.1–28.7) | 1612 | 85 | 13.3 (10.8–16.4) |
| ACC/AHA | 538 | 42 | 17.6 (13.0–28.8) | 2493 | 141 | 14.5 (12.3–17.1) |
| Both | 271 | 24 | 19.2 (12.9–28.7) | 1553 | 82 | 13.2 (10.7–16.5) |
| Neither | 847 | 28 | 8.0 (5.5–11.6) | 3589 | 53 | 4.2 (3.2–5.5) |
| Overall | 1394 | 71 | 12.0 (9.5–15.1) | 6141 | 197 | 8.8 (7.6–10.1) |
Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; ATP, Adult Treatment Panel; CDV, cardiovascular disease; HIV, human immunodeficiency virus; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
aOutcome is defined as CVD (ICD-9-CM codes 410–414 or 433–434).
bPer 1000 person years.
DISCUSSION
In this retrospective clinical care cohort study conducted in the modern ART era, we showed that an increased proportion of patients were recommended to be on a statin when applying the ACC/AHA cholesterol guidelines compared with ATPIII and that the ACC/AHA guidelines identified a higher proportion of patients who would experience a future CVD event. Despite this increase in recommended statin use, however, over 40% of HIV-infected patients who experienced a CVD event would not have been identified as statin-eligible at baseline by the ACC/AHA guidelines. This contrasts with only 29% of control patients with a CVD event who would not have been identified as statin-eligible. Our findings suggest that although the ACC/AHA guidelines may identify more HIV-infected patients as statin-eligible, the newer cholesterol guidelines still perform suboptimally in HIV patients in terms of identifying patients at high cardiovascular risk who merit preventative therapy.
Individuals infected with HIV face increased rates of noncommunicable chronic diseases including CVD, often at younger ages [20, 21]. However, the mechanism of CVD in HIV is thought to differ from that in non-HIV-infected individuals, with prominent contributions from novel risk factors related to HIV-associated inflammation and immune activation [22–26]. Whether widespread CVD guidelines developed for the general population are applicable in the setting of HIV remains unknown, because the guidelines were developed based on traditional risk profiles. The ACC/AHA cholesterol guidelines specifically cite HIV-infected individuals as a group in whom recommendations may not apply and further research is needed [9]. In the current study, we sought to assess the impact of the ACC/AHA cholesterol guidelines in individuals infected with HIV. In comparison to the longstanding ATPIII guidelines and using a matched control group from the same background population as a comparator group, we compared recommended and actual statin use and assessed the ability of general population cholesterol guidelines to identify high-risk individuals who would experience a future CVD event.
Statins have the potential to reduce CVD risk in patients infected with HIV via effects on lipids or anti-inflammatory effects. Data have demonstrated many beneficial effects of statins in individuals infected with HIV, including decrease in high-risk morphology coronary plaque, decreases in cholesterol levels, in CVD-related inflammation and immune activation, and in mortality rates [4–8, 25, 27, 28]. However, whether strategies to identify statin-eligible patients developed for the general population are applicable in the setting of HIV remains unknown. The Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE trial) [29] will provide valuable insight into the extent to which statins are a robust CVD risk reduction method in HIV-infected individuals at low to moderate CVD risk. In the present study, we investigate current patterns of use and applicability of cholesterol guidelines in HIV to assess whether these paradigms are likely to need to be adapted for use in HIV.
The ACC/AHA released new cholesterol guidelines in 2013 to update the longstanding ATPIII guidelines [30]. In contrast to ATPIII, which used LDL thresholds to determine statin eligibility, the new guidelines delineate 4 at-risk groups to consider for initiation of statin therapy. A new CVD risk score (the Pooled Cohorts Equations) introduced as part of new CVD risk prediction guidelines [11] concurrently released by the ACC/AHA forms the basis for one of the statin risk categories in the new cholesterol guidelines. It is notable that the outcome for the ACC/AHA risk score, which is a key determinant of the cholesterol guidelines, encompasses both CHD and stroke (CVD) and is broader than the outcome used in the risk scores used in ATPIII (CHD). Data from the National Health and Nutrition Examination Surveys (NHANES) indicate that an additional 11% of patients would be newly recommended for statin therapy by the ACC/AHA guidelines, and that this increase would largely be driven by older patients with an elevated CVD risk score [12]. In the current study, we similarly show that an increased proportion of patients infected with HIV would be newly recommended for statin therapy by ACC/AHA versus ATPIII, at a rate nearly doubled (39% vs 20%), similar to observations reported by others [13, 31, 32]. Moreover, the increase in the proportion recommended for statin comparing ACC/AHA to ATPIII was relatively greater in the HIV compared with the control group (19 percentage point increase in statin recommendation among patients infected with HIV compared with 15-point increase among controls). In our cohort, as in the NHANES-based general population estimate, the increase in recommended statin use was driven by the predicted risk score category.
In our study, actual statin prescription rates in patients identified as eligible for statins were lower in the HIV group than in the control group despite similar proportions recommended for statins. Statin use in our cohort was comparable to reported statin use among other HIV-clinical cohorts [33], including among European cohorts [34]. However, in a small study of 155 HIV-infected patients in a primary care setting, there was no difference in statin recommendation by either ACC/AHA or ATPIII [35]. Suboptimal adherence to CVD prevention guidelines is consistent with prior reports of low aspirin prescription rates for primary and secondary prevention [18, 34, 36] and low treatment rates for traditional cardiovascular risk factors among individuals infected with HIV [37–39]. In a study assessing compliance with cholesterol guidelines among male veterans infected with HIV, 23% of eligible individuals were not receiving statins; these results are in alignment with our results showing underutilization of statin therapy, with 33% eligible by ATPIII not receiving treatment [40]. The relatively low rates of statin use in HIV we observed during the ATPIII treatment era may reflect competing priorities of advanced HIV and opportunistic infection management, concern for possible drug interactions, lower prioritization of chronic disease management in HIV clinic settings, or lack of evidence for clear transportability of general population guidelines to HIV.
Despite the increase in the proportion of HIV-infected patients recommended for statins by ACC/AHA, more than 40% of HIV-infected patients who experienced a CVD event would not have been recommended for statin therapy under this guideline. Although this unrecognized, at-risk group is smaller than that for ATPIII, it is notably larger than that for non-HIV control patients from the same population (29% of patients with CVD events would not have been recommended for a statin by ACC/AHA in the control group). This gap in statin recommendation for HIV patients who subsequently experience a CVD event is consistent with a recent study indicating that 74% of HIV patients with proven high-risk coronary plaque were not recommended statin therapy under ACC/AHA for primary prevention [41]. The failure to recommend a statin for a large proportion of HIV-infected patients who would experience a CVD event demonstrates the inadequacy of applying general population guidelines to HIV-infected populations, a group with a discrete risk factor profile comprising novel risk factors not captured in general population guidelines.
Our study has several limitations. There is possible incomplete ascertainment of primary exposure (statin) or CVD outcome data. We anticipate medication data to be robust because the timing of this study corresponded with widespread adoption of mandatory EHR prescribing. Given the possibility of missing exposure or outcome data, we included a non-HIV control group from the same healthcare system that would be anticipated to have similar rates of incomplete data as an internal control. We do not anticipate that incomplete capture of outcome events would differ by HIV status. We calculated ACC/AHA risk scores for those in the 20–39 year age group because patients infected with HIV experience CVD at younger ages, but we acknowledge the limited evidence for widespread use of statin therapy in this age group. Furthermore, we did not conduct a time-to-event analysis. Because results reflect practice from a single center versus pooled data across several centers, the reported prescribing rates may not necessarily apply to all clinical settings. For instance, differences in health insurance may have influenced statin prescriptions in our cohort. Finally, it is possible that statin prescription rates in HIV may vary over time, reflecting changes in practice patterns.
CONCLUSIONS
In summary, our findings highlight that the ACC/AHA cholesterol guidelines are likely to be less applicable in the setting of HIV than in the general population, with more than 40% of HIV-infected individuals who experienced a CVD event not recommended for statin therapy by these guidelines. Notably, this group of patients who incurred events but were not identified as statin-eligible was larger for the HIV group compared with the control group, suggesting that factors specific to HIV infection that are not reflected in the guidelines may mediate the development of atherosclerosis in HIV [42, 43]. Our finding that less than 50% of patients recommended for statin therapy were prescribed statins during the ATPIII era suggests that patients with traditional, modifiable CVD risk factors may not have been receiving appropriate guideline-recommended preventive therapy. Our findings underscore an urgent need for ongoing research on CVD risk stratification and prevention strategies in HIV.
Acknowledgments
We are grateful to Dr. Shawn Murphy (Massachusetts General Hospital Laboratory of Computer Science) and the Partners HealthCare Research Patient Data Registry group for facilitating use of their database and to Dr. Fran Cook for comments on overall study design, data analysis, and review of an earlier version of the manuscript.
Financial support. This work was funded by National Institutes of Health (NIH) Grants 5R21HL122138-02 and 5R01HL132786-02 (to V. A. T.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Triant VA. HIV infection and coronary heart disease: an intersection of epidemics. J Infect Dis 2012; 205(Suppl 3):S355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Triant VA. Cardiovascular disease and HIV infection. Curr HIV/AIDS Rep 2013; 10:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lang S, Lacombe JM, Mary-Krause M, et al. . Is impact of statin therapy on all-cause mortality different in HIV-infected individuals compared to general population? Results from the FHDH-ANRS CO4 cohort. PLoS One 2015; 10:e0133358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silverberg MJ, Leyden W, Hurley L, et al. . Response to newly prescribed lipid-lowering therapy in patients with and without HIV infection. Ann Intern Med 2009; 150:301–13. [DOI] [PubMed] [Google Scholar]
- 6. Rasmussen LD, Kronborg G, Larsen CS, et al. . Statin therapy and mortality in HIV-infected individuals; a Danish nationwide population-based cohort study. PLoS One 2013; 8:e52828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eckard AR, McComsey GA. The role of statins in the setting of HIV infection. Curr HIV/AIDS Rep 2015; 12:305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eckard AR, Jiang Y, Debanne SM, et al. . Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis 2014; 209:1156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stone NJ, Robinson JG, Lichtenstein AH, et al. . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S1–45. [DOI] [PubMed] [Google Scholar]
- 10. Expert Panelon Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–97. [DOI] [PubMed] [Google Scholar]
- 11. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S49–73. [DOI] [PubMed] [Google Scholar]
- 12. Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr, et al. . Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014; 370:1422–31. [DOI] [PubMed] [Google Scholar]
- 13. Todd JV, Cole SR, Wohl DA, et al. . Underutilization of statins when indicated in HIV-seropositive and seronegative women. AIDS Patient Care STDS 2017; 31:447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr 2009; 51:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Regan S, Meigs JB, Grinspoon SK, Triant VA. Determinants of smoking and quitting in HIV-infected individuals. PLoS One 2016; 11:e0153103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Triant VA, Perez J, Regan S, et al. . Cardiovascular risk prediction functions underestimate risk in HIV infection. Circulation 2018; 137:2203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chow FC, Regan S, Zanni MV, et al. . Elevated ischemic stroke risk among women living with HIV infection. AIDS 2018; 32:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suchindran S, Regan S, Meigs JB, et al. . Aspirin use for primary and secondary prevention in human immunodeficiency virus (HIV)-infected and HIV-uninfected patients. Open Forum Infect Dis 2014; 1:ofu076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chow FC, He W, Bacchetti P, et al. . Elevated rates of intracerebral hemorrhage in individuals from a US clinical care HIV cohort. Neurology 2014; 83:1705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith CJ, Ryom L, Weber R, et al. . Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–8. [DOI] [PubMed] [Google Scholar]
- 21. Mills EJ, Bärnighausen T, Negin J. HIV and aging–preparing for the challenges ahead. N Engl J Med 2012; 366:1270–3. [DOI] [PubMed] [Google Scholar]
- 22. Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis 2012; 205(Suppl 3):S375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep 2012; 9:139–47. [DOI] [PubMed] [Google Scholar]
- 24. Grinspoon SK. Cardiovascular disease in HIV: traditional and nontraditional risk factors. Top Antivir Med 2014; 22:676–9. [PMC free article] [PubMed] [Google Scholar]
- 25. Lo J, Lu MT, Ihenachor EJ, et al. . Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2015; 2:e52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Subramanian S, Tawakol A, Burdo TH, et al. . Arterial inflammation in patients with HIV. JAMA 2012; 308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moore RD, Bartlett JG, Gallant JE. Association between use of HMG CoA reductase inhibitors and mortality in HIV-infected patients. PLoS One 2011; 6:e21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toribio M, Fitch KV, Sanchez L, et al. . Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. AIDS 2017; 31:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gilbert JM, Fitch KV, Grinspoon SK. HIV-related cardiovascular disease, statins, and the REPRIEVE Trial. Top Antivir Med 2015; 23:146–9. [PMC free article] [PubMed] [Google Scholar]
- 30. Stone NJ, Robinson JG, Lichtenstein AH, et al. . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2889–934. [DOI] [PubMed] [Google Scholar]
- 31. Levy ME, Greenberg AE, Magnus M, et al. . Evaluation of statin eligibility, prescribing practices, and therapeutic responses using ATP III, ACC/AHA, and NLA dyslipidemia treatment guidelines in a large urban cohort of HIV-infected outpatients. AIDS Patient Care STDS 2018; 32:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clement ME, Park LP, Navar AM, et al. . Statin utilization and recommendations among HIV- and HCV-infected veterans: a cohort study. Clin Infect Dis 2016; 63:407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mitka M. Exploring statins to decrease HIV-related heart disease risk. JAMA 2015; 314:657–9. [DOI] [PubMed] [Google Scholar]
- 34. De Socio GV, Ricci E, Parruti G, et al. . Statins and aspirin use in HIV-infected people: gap between European AIDS Clinical Society guidelines and clinical practice: the results from HIV-HY study. Infection 2016; 44:589–97. [DOI] [PubMed] [Google Scholar]
- 35. Elsamadisi P, Cha A, Kim E, Latif S. Statin use with the ATP III guidelines compared to the 2013 ACC/AHA guidelines in HIV primary care patients. J Pharm Pract 2017; 30:64–9. [DOI] [PubMed] [Google Scholar]
- 36. Burkholder GA, Tamhane AR, Salinas JL, et al. . Underutilization of aspirin for primary prevention of cardiovascular disease among HIV-infected patients. Clin Infect Dis 2012; 55:1550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lichtenstein KA, Armon C, Buchacz K, et al. . Provider compliance with guidelines for management of cardiovascular risk in HIV-infected patients. Prev Chronic Dis 2013; 10:E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reinsch N, Neuhaus K, Esser S, et al. . Are HIV patients undertreated? Cardiovascular risk factors in HIV: results of the HIV-HEART study. Eur J Prev Cardiol 2012; 19:267–74. [DOI] [PubMed] [Google Scholar]
- 39. Okeke NL, Chin T, Clement M, et al. . Coronary artery disease risk reduction in HIV-infected persons: a comparative analysis. AIDS Care 2016; 28:475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Clement ME, Park LP, Navar AM, et al. . Statin utilization and recommendations among HIV- and HCV-infected veterans: a cohort study. Clin Infect Dis 2016; 63:407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zanni MV, Fitch KV, Feldpausch M, et al. . 2013 American College of Cardiology/American Heart Association and 2004 Adult Treatment Panel III cholesterol guidelines applied to HIV-infected patients with/without subclinical high-risk coronary plaque. AIDS 2014; 28:2061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tripathi A, Liese AD, Winniford MD, et al. . Impact of clinical and therapeutic factors on incident cardiovascular and cerebrovascular events in a population-based cohort of HIV-infected and non-HIV-infected adults. Clin Cardiol 2014; 37:517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Palella FJ Jr, Phair JP. Cardiovascular disease in HIV infection. Curr Opin HIV AIDS 2011; 6:266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]