Abstract
We present a unique case of disseminated Leishmaniasis in an HIV patient. Two different Leishmania species were identified by genomic sequencing in both bone marrow and skin. The Leishmania infection could be suppressed but not cured, despite a high dose of amphotericin B of nearly 65 g over more than 6 years.
Keywords: Leishmaniasis, HIV-coinfection, amphotericin B, CD4 cell count, relapse
Leishmania–HIV coinfection is considered a major threat to the lives of HIV-infected patients [1]. It can present as a cutaneous, muco-cutaneous, or visceral Leishmaniasis depending on the species. Those who are severely immunocompromised with CD4 T-cell counts <200 cells/μL may present with atypical features. Managing such patients is a challenge [2]. We present a case of combined muco-cutaneous and visceral Leishmaniasis with 2 different Leishmania species requiring repeated high doses of amphotericin B for more than 6 years to be controlled but without cure.
CASE REPORT
Our case was a 40-year-old Omani male, an intravenous drug user with a history of extended travel in the Mediterranean region in 1989; he traveled to Oman and southern Yemen, where he stayed for 30 days, then crossed the Red Sea to the Suez Canal. From there, he had different stops in European countries including the United Kingdom, France, Italy, Portugal, Spain, and Gibraltar; he stayed for at least 20 to 30 days in each country. In 1990, he visited Pakistan for 4 weeks.
He presented with oral candidiasis and cystic brain lesions in August 2010. He was found to be HIV-positive with cerebral toxoplasmosis. His initial HIV viral load was 300 000 copies/mL with a CD4 T-cell count of 6 cells/μL. He was started on efavirenz 600 mg, zidovudine 600 mg, and lamivudine 300 mg once daily and received pneumocystis carinii pneumonia (PCP) prophylaxis. He was coinfected with hepatitis C virus, genotype 1B, for which interferon and ribavirin treatment was started. He failed therapy due to noncompliance, but later, in 2016, he responded well to ledipasvir/sofosbuvir treatment.
In 2012, he presented with 5 nodular skin lesions, each <1 cm, brownish to purple in color, involving the forehead, fingers, and left lower limb. A skin biopsy from the forehead showed macrophages in the dermis loaded by Leishman Donovan bodies, and cutaneous Leishmaniasis (CL) was diagnosed. The biopsy was negative for HHV-8, dysplasia, and malignancy.
He was managed initially with topical paromomycin, but a few months later, his condition progressed to disseminated muco-cutaneous (MCL) and visceral Leishmaniasis (VL) with new nodular lesions on the limbs, ears, and nose, with nasal bridge destruction, or “saddle nose.”
He had hepatosplenomegaly and an inguinal lymph node of 1.2 × 1.0 cm confirmed on computed tomography. The patient had pancytopenia, with a hemoglobin level of 7.3 g/dL, thrombocytopenia of 30 × 10^9 /L, leukopenia of 1.3 × 10^9 WBC/L, and neutropenia of 0.3 × 10^9/L.
A bone marrow aspirate and biopsy showed Leishmania parasites that were also seen in the inguinal lymph node and gastric biopsies.
Compliance was an issue. His antiretroviral therapy (ART) regimen was changed to emtricitabine 200 mg, tenofovir disoproxil fumarate (DF) 300 mg, and efavirenz 600 mg once daily. In 2015, his HIV viral load was suppressed, and his absolute CD4 count stabilized to between 30 and 80 cells/μL (Table 1).
Table 1.
Doses of Liposomal Amphotericin B and Patient’s Virological and Immunological Response
| Year | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|---|---|---|---|
| Highest CD4 T-cell count, cells/μL | 6 | 169 | 108 | 305 | 288 | 76 | 67 | 49 | |
| HIV viral load, copies/mL | 316 018 | 30–109 | 0–1 367 727 | 88–3479 | <20–545 285 | 0–568 | <20 | <20 | |
| Total dose of liposomal amphotericin B, g | 0 | 0 | 0 | 3.5 | 13.3 | 1.95 | 14.7 | 19.8 | 10.5 |
The table demonstrates the highest CD4 T-cell count for our patient per year, along with the range of the HIV viral load during that year. It also shows the total dose of liposomal amphotericin B the patient received each year.
The patient was managed initially with liposomal amphotericin B, 5 mg/kg for 14 days, and was placed on a maintenance dose of 5 mg/kg every 3 weeks for 3 to 5 days. During the first year, he showed a good response but then experienced frequent relapses. Compliance to the ART and to the Leishmaniasis treatment was an issue. Several regimens were used to control the relapses, including a combination of liposomal amphotericin and fluconazole to enable shortening treatment duration and prolonging the intervals; however, all were unsuccessful. With subsequent improvement in ART compliance, his viral load was persistently undetectable. However, the CD4 T-cell count remained below 80 cells/uL. In 2017, he was started on paromomycin orally 500 mg twice a day but did not tolerate it. Currently, he is on a high maintenance dose of liposomal amphotericin B 5–7.5 mg/kg for 5 days every 3 weeks. He is responding well except if he misses doses.
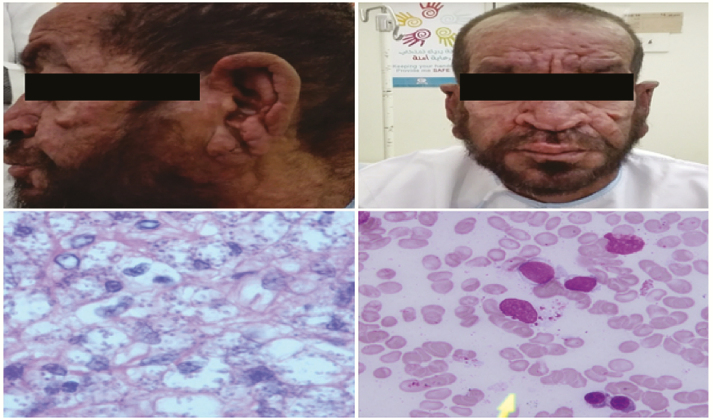
The latest bone marrow biopsy performed in 2016 showed sparse Leishmania parasites; 2 skin biopsies in 2016 and 2017 showed abundant Leishmania parasites (Figure 1).
Figure 1.
A and B, Cutaneous lesions with nodules and destruction of the nasal bridge. C, Skin biopsy with multiple Leishmania parasites (hematoxylin and eosin stain, magnification ×600). D, Bone marrow aspirate with sparse Leishmania parasites (Giemsa stain, magnification ×600).
Genomic Testing of Leishmania Species
One bone marrow and 2 skin biopsy specimens tested positive in the Leishmania genus-specific real-time polymerase chain reaction assay [3]. The Leishmania species was determined by amplification of the HSP70 (N-terminal fragment) and mini-exon loci and sequencing [4]. Sequence analysis demonstrated the presence of a mixture of DNA of L. major and L. tropica in the bone marrow specimen, which could be explained by either an infection by a hybrid species or by a simultaneous infection by 2 distinct Leishmania species. Subsequent analysis of 2 skin biopsy specimens demonstrated the presence of L. tropica only, which excludes the possibility that the patient was infected by a single hybrid species, as the skin biopsy specimens should have contained this hybrid species as well. Therefore, the patient was simultaneously infected by 2 Leishmania species: L. major and L. tropica.
DISCUSSION
Leishmaniasis is a protozoal infection transmitted by the bite of female Phlebotomine sand fly. Amastigotes replicate in the macrophages of the host, causing muco-cutaneous or visceral Leishmaniasis depending on the Leishmania species [5].
Genomic sequencing of species isolated from the bone marrow and the skin of our patient showed 2 different species, L. tropica and L. major, and the patient probably had Leishmania infection before becoming infected with HIV. Although an infection with both these Leishmania species normally results in cutaneous Leishmaniasis, in severely immunocompromised patients, both Leishmania species can disseminate, which we believe happened in this patient. Dissemination of “cutaneous Leishmania species” has been previously described in immune-compromised patients, but to the best of our knowledge, this is the first case in which 2 cutaneous Leishmania species could be detected in bone marrow [3, 4].
Oman is a nonendemic area for Leishmaniasis with only sporadically reported cases in mainly the 1980s and 1990s. The main species involved were L. tropica and L. infantum [6].
L. tropica and L. major are reported to cause CL in Yemen, Pakistan, Egypt, and Morocco. In the 1990s, France, Italy, Portugal, and Spain reported the most cases of Leishmania–HIV coinfection in the Mediterranean region, predominantly L. infantum, but with introduction of ART in 1997, these reported cases decreased drastically [7].
Leishmaniasis reactivation can occur many years after initial infection in immune-compromised patients, which was seen in our patient, who was probably infected 22 years before developing clinical Leishmaniasis [5, 7].
In severely immunocompromised HIV-infected individuals with CD4 T-cell counts <200 cells/uL, atypical presentations of cutaneous Leishmaniasis and visceralization are common [5, 7, 8]. Our patient had lymphadenopathy, hepatosplenomegaly, and gut and bone marrow involvement.
Serological tests for Leishmaniasis have low sensitivity in HIV patients and are therefore less reliable [5, 7, 8]. Finding amastigotes in bone marrow or spleen tissue is considered a highly sensitive method for the diagnosis of VL [8], and we did not perform serology.
Management of VL-HIV coinfection is challenging. In HIV patients, higher relapse rates and treatment failure occur more frequently, especially in severely immunocompromised individuals with CD4 T-cell counts <200 cells/uL [5, 7, 8].
The World Health Organization (WHO) has recommended liposomal amphotericin B with a total cumulative dose of 20–40 mg/kg (equal to 2 g in our patient) as the preferred agent to treat VL in HIV-coinfected patients [7–9]. Despite having good cure rates ranging from 42% to 85%, frequent relapses are seen in HIV-infected patients [9]. This patient responded to high-dose liposomal amphotericin B 5–7.5 mg/kg but needed frequent, repeated administration daily for 5 days every 3 weeks to maintain the status quo.
The patient received far more amphotericin B than recommended in the WHO guideline [9], with an estimated 15–20 g per year, and altogether he has received close to 65 g over the past 6 years. The latest Infectious Diseases Society of America (IDSA) guidelines recommend follow-up treatment in immunocompromised patients with relapse but do not state for how long or with which doses [10].
Combination therapies with pentamidine, paromomycin, fluconazole, or allopurinol are recommended in cases with multiple relapses. This approach can lead to better response and reduced relapse rates [7–9] but has higher toxicity profiles [9]; our patient tolerated amphotericin B well.
Several studies have demonstrated that miltefosine is a well-tolerated drug even in long-term use for treatment of VL-HIV coinfection, with better safety profiles [7–9, 11], but we do not have access to miltefosine. A combination of amphotericin B and miltefosine is a possibility in cases like this patient.
Response to treatment depends on the virulence of the causative Leishmania species [8, 11], and most of previous studies were carried out in Europe and Ethiopia involving L. infantum and L. donovani, respectively [8, 13]. More studies are needed to assess the efficacy of those drugs in patients infected with other Leishmania species. The IDSA guidelines recommend genotyping of the Leishmania species, and treatment efficacy may vary depending on the Leishmania genotype; however, treatment recommendations do not consider different genotypes [10].
Despite starting ART and having good viral suppression, the patient failed to have good immunological recovery. Visceral Leishmaniasis hinders immunological recovery and increases HIV viral replication. This is attributed to sharing the same immunological mechanism involving dendritic cells, specific T-helper type I cells, and macrophages [5, 7, 8, 11]. Starting ART can reduce the incidence of VL-HIV coinfection and the rate of relapses, but relapses can still occur with an improved CD4 count and an undetectable HIV viral load [7, 11, 12, 14].
Our patient failed to maintain immunological recovery and is probably going to require lifelong treatment with amphotericin B if more efficient treatment of his Leishmania infection is not available.
Acknowledgments
We thank Florence Robert-Gangneux for help and assistance with the isolation of the nucleic acids from the biopsy specimens. We also thank Christophe Ravel from the French Reference Center of Leishmaniasis (University of Montpellier) for his help with the hypothesis of the hybrid.
Potential conflicts of interest . All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pagliano P, Esposito S. Visceral leishmaniosis in immunocompromised host: an update and literature review. J Chemother 2017; 29:261–6. [DOI] [PubMed] [Google Scholar]
- 2. Ponte-Sucre A, Gamarro F, Dujardin JC, et al. . Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl Trop Dis 2017; 11:e0006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wortmann G, Sweeney C, Houng HS, et al. . Rapid diagnosis of leishmaniasis by fluorogenic polymerase chain reaction. Am J Trop Med Hyg 2001; 65:583–7. [DOI] [PubMed] [Google Scholar]
- 4. Van der Auwera G, Ravel C, Verweij JJ, et al. . Evaluation of four single-locus markers for Leishmania species discrimination by sequencing. J Clin Microbiol 2014; 52:1098–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lindoso JA, Cunha MA, Queiroz IT, Moreira CH. Leishmaniasis-HIV coinfection: current challenges. HIV AIDS (Auckl) 2016; 8:147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alvar J, Vélez ID, Bern C, et al. ; WHO Leishmaniasis Control Team Leishmaniasis worldwide and global estimates of its incidence. PLoS One 2012; 7:e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Monge-Malio B, Norman FF, Cruz I, et al. . Visceral Leishmaniasis and HIV coinfection in the Mediterranean region. PLoS Negl Trop Dis 2014; 8:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Griensven J, Carrillo E, López-Vélez R, et al. . Leishmaniasis in immunosuppressed individuals. Clin Microbiol Infect 2014; 20:286–99. [DOI] [PubMed] [Google Scholar]
- 9. Monge-Malio B, Lopez -Vélez R. Treatment options for visceral leishmaniasis and HIV coinfection. AIDS Rev 2016; 18:32–43. [PubMed] [Google Scholar]
- 10. Aronson N, Herwaldt BL, Libman M, et al. . Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis 2016; 63:1539–57. [DOI] [PubMed] [Google Scholar]
- 11. Alvar J, Aparicio P, Aseffa A, et al. . The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev 2008; 21:334–59, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cota GF, de Sousa MR, Rabello A. Predictors of visceral leishmaniasis relapse in HIV-infected patients: a systematic review. PLoS Negl Trop Dis 2011; 5:e1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diro E, Ritmeijer K, Boelaert M, et al. . Long-term clinical outcomes in visceral leishmaniasis/human immunodeficiency virus–coinfected patients during and after pentamidine secondary prophylaxis in ethiopia: a single-arm clinical trial. Clin Infect Dis 2017; 66:444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alemayehu M, Wubshet M, Mesfin N. Magnitude of visceral leishmaniasis and poor treatment outcome among HIV patients: meta-analysis and systematic review. HIV AIDS (Auckl) 2016; 8:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]