Abstract
Background
European trials using procalcitonin (PCT)-guided antibiotic therapy for patients with lower respiratory tract infections (LRTIs) have demonstrated significant reductions in antibiotic use without increasing adverse outcomes. Few studies have examined PCT for LRTIs in the United States.
Methods
In this study, we evaluated whether a PCT algorithm would reduce antibiotic exposure in patients with LRTI in a US hospital. We conducted a controlled pre-post trial comparing an intervention group of PCT-guided antibiotic therapy to a control group of usual care. Consecutive patients admitted to medicine services and receiving antibiotics for LRTI were enrolled in the intervention. Providers were encouraged to discontinue antibiotics according to a PCT algorithm. Control patients were similar patients admitted before the intervention.
Results
The primary endpoint was median antibiotic duration. Overall adverse outcomes at 30 days comprised death, transfer to an intensive care unit, antibiotic side effects, Clostridium difficile infection, disease-specific complications, and post-discharge antibiotic prescription for LRTI. One hundred seventy-four intervention patients and 200 controls were enrolled. Providers complied with the PCT algorithm in 75% of encounters. Procalcitonin-guided therapy reduced median antibiotic duration for pneumonia from 7 days to 6 (P = .045) and acute exacerbation of chronic obstructive pulmonary disease (AECOPD) from 4 days to 3 (P = .01). There was no difference in the rate of adverse outcomes in the PCT and control groups.
Conclusions
A PCT-guided algorithm safely reduced the duration of antibiotics for treating LRTI. Utilization of a PCT algorithm may aid antibiotic stewardship efforts.
This clinical trial was a single-center, controlled, pre-post study of PCT-guided antibiotic therapy for LRTI. The intervention (incorporation of PCT-guided algorithms) started on April 1, 2017: the preintervention (control group) comprised patients admitted from November 1, 2016 to April 16, 2017, and the postintervention group comprised patients admitted from April 17, 2017 to November 29, 2017 (Supplementary Figure 1). The study comprised patients admitted to the internal medicine services to a medical ward, the Medical Intensive Care Unit (MICU), the Cardiac Intensive Care Unit (CICU), or the Progressive Care Unit (PCU) “step down unit”. The registration data for the trails are in the ClinicalTrials.gov database, number NCT0310910.
Keywords: antibiotic stewardship, clinical trials, lower respiratory tract infections, procalcitonin
Overuse of antibiotics for lower respiratory tract infections (LRTIs) is one of the most urgent and difficult stewardship problems in US hospitals, accounting for much of the unnecessary antibiotic use in the inpatient setting [1, 2]. Reasons include ingrained prescribing practices, patient demand for antibiotics, and a perception of antibiotics as without risk [3, 4]. The differential diagnosis for patients presenting with respiratory complaints includes infectious conditions such as pneumonia and bronchitis (viral or bacterial) and noninfectious conditions such as congestive heart failure and pulmonary embolus. As a result, diagnostic accuracy of pneumonia is notoriously poor, with up to one third of patients misclassified based on clinical and chest x-ray findings [5, 6]. Thus, physicians need effective tools to guide diagnosis and treatment of LRTI.
The characteristics of the peptide procalcitonin (PCT) make it a promising tool for distinguishing patients with bacterial infections, who might benefit from antibiotics, from those with viral infections or no infection. Procalcitonin is up-regulated in numerous tissues in response to bacterial components (lipopolysaccharide, tumor necrosis factor-α) but not viral components [7–11]. Levels of PCT increase within 4 to 6 hours of initiation of bacterial infection or intravenous endotoxin: this contrasts with increases in C-reactive protein and erythrocyte sedimentation rate, which occur after at least 24 hours [8, 12, 13].
We hypothesized that deploying PCT-guided algorithms for LRTI into clinical decision making, under the guidance of our hospital antibiotic stewardship program, would safely allow prescribers to discontinue antibiotics sooner than clinical acumen and guidelines dictate. We tested this hypothesis in a pre-post controlled intervention study of PCT-guided antibiotic therapy in patients admitted for LRTI.
MATERIALS AND METHODS
Study Design
The institutional review board approved the protocol. For the control patients, electronic medical records (EMRs) were reviewed under a waiver of consent. Written informed consent was obtained from all patients in the intervention group. A 30-day follow-up assessment using structured EMR review was conducted for both groups.
Setting and Subjects
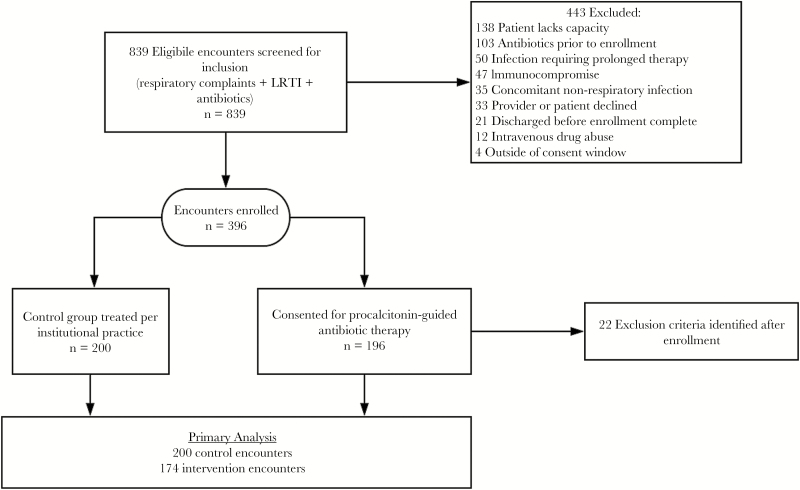
The study hospital is a 350-bed, academic, tertiary care hospital in Baltimore, Maryland, with approximately 23 500 discharges annually. Inclusion and exclusion criteria were the same for both groups and are summarized in Supplementary Table 1. Patients were eligible for inclusion if they had evidence of LRTI during the first 24 hours after arrival at the emergency department (ED) and started on antibiotics. Patients were excluded if they were immunocompromised, had a deep-seated infection, or lacked capacity. Surrogate decision makers were not allowed to consent. Patients in which exclusion criteria were identified after enrollment were removed from analysis (n = 22) (Figure 1).
Figure 1.
Patient flow of patients screened for inclusion in the trial.
A list of candidate control patients was generated by querying the EMRs for patients seen in the ED and started on antibiotics and admitted to a medicine service. All chief complaints filed to the EMRs for this group were reviewed for relevance to the respiratory system (Supplementary Table 2). Patients meeting these criteria were screened in consecutive reverse order from the day before the intervention using the same inclusion and exclusion criteria as the intervention group. Control patients were treated according to physician preference. During the intervention phase, the study team screened all admitted patients 7 days per week for study candidacy.
Interventions
Provider Education and Algorithm Distribution
Before intervention, our antibiotic stewardship program educated providers (internal medicine, clinical pharmacy, ED) regarding the evidence and rationale for PCT use in LRTI and on our study protocol. Of 186 targeted providers, 140 (65%) received education (60 via online module, 80 via in-person lecture). The PCT guidance algorithm (Figure 2) was distributed to providers, embedded in the PCT result report in the EMR, and copied to the patient’s chart after enrollment (Supplementary Figure 2).
Figure 2.
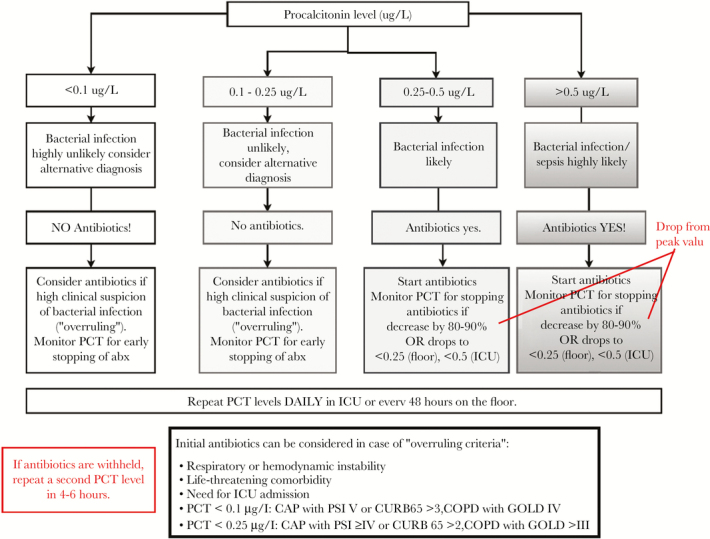
Procalcitonin (PCT)-guided algorithm distributed to providers for antibiotic discontinuation for lower respiratory tract infection. CAP, community-acquired pneumonia; COPD, chronic obstructive pulmonary disease; CURB65, confusion, uremia, respiratory rate, blood pressure, age over 65; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICU, intensive care unit.
Local Treatment Guidelines
Institutional antibiotic guidelines are distributed annually to all prescribers. For acute exacerbation of chronic obstructive pulmonary disease (AECOPD), 3−5 days of antibiotics are recommended. Recommended antibiotic durations for pneumonia are 3–5 days for patients without immunocompromise or structural lung disease, 7 days with moderate immunocompromise or structural lung disease, and 10–14 days in patients with immunocompromise or poor initial response [14]. Provider adherence to these guidelines is not routinely monitored, and antibiotic prescribing patterns for LRTI were not known before this study.
Procalcitonin Measurement and Reporting
The pre-antibiotic PCT level was measured on blood left over from initial testing in the ED. Subsequent PCT levels were timed for 48 hours after baseline for patients admitted to the floor or 24 hours after baseline for patients admitted to intensive care units (ICUs).
Procalcitonin measurements were performed by the hospital laboratory once daily, at 8:00 am on weekdays and after 3:00 pm on weekends (Figure 3). The PCT was measured using a rapid sensitive assay, with a functional assay sensitivity of 0.06 μg/L (B·R·A·H·M·S PCT sensitive KRYPTOR; Thermo Fisher Scientific, Hennigsdorf, Germany).
Figure 3.
Example time line of the timing of patient care events, study enrollment, and initial procalcitonin results. Per the study protocol, initial procalcitonin (PCT) levels were performed on leftover blood collected in the emergency department (ED) and on the hospital unit and were batch run at 8:00 am. Results for both the baseline and follow-up value were available simultaneously in a delayed fashion.
Result lists were reviewed daily by a team infectious disease (ID) pharmacist and an ID physician. The PCT levels were used only to guide antibiotic discontinuation decisions. Antibiotic discontinuation recommendations were based on previously published PCT algorithms and modified to include both floor and ICU patients. Antibiotic discontinuation was recommended if serial PCT values fell more than 80% from the peak value. In the case of a PCT result below the antibiotic stopping threshold, team members notified the treating provider via email or secure text page with a nonbinding recommendation to discontinue antibiotics. Study staff recommended antibiotic initiation in patients with high PCT values who were not on appropriate antibiotic therapy but did not intervene on subsequent high values.
Overruling of the PCT algorithm was allowed for prespecified criteria, including respiratory or hemodynamic instability, life-threatening comorbidity, ICU admission, and severe illness. Pneumonia severity was determined using the CURB-65 (confusion, uremia, respiratory rate, blood pressure, age over 65) score [15], with severe defined as a score of 3 or 4. Severity of AECOPD was determined using spirometric criteria according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification [16].
Algorithm adherence was defined as discontinuation within 48 hours after a PCT stopping threshold. Patients with override criteria on admission were considered eligible for de-escalation according to the algorithm after 24 hours, if they demonstrated clinical improvement and respiratory or hemodynamic stability.
Patient Follow Up
For patients in the intervention group, 30-day outcomes were assessed by structured EMR review. Hospital readmissions were extracted from the state-wide readmissions database [17]. A study-specific independent data and safety monitoring board monitored safety and adverse events.
Main Outcome Measures
The primary endpoint was total antibiotic duration (calendar days) per LRTI episode (within 30 days post discharge), including inpatient antibiotic administrations and outpatient prescription durations. As a secondary endpoint, antibiotic days of therapy per 1000 patient days present (DOT) was calculated using the National Healthcare Safety Network methodology [18]. No outpatient days were counted in the denominator. Measures of hospital utilization were length of stay and total antibiotic cost (calculated by multiplying the average annual hospital purchase price by the duration of treatment for each drug).
The primary safety endpoint, overall adverse events by 30 days, was a composite of new antibiotic prescription for LRTI, transfer to an ICU, death, antibiotic side effects, disease-specific complications (ie, persistence or development of new pneumonia, lung abscess, empyema, or acute respiratory distress syndrome), and Clostridium difficile infection.
Statistical Analysis
A target sample size, which included intervention and control groups of 349, was calculated using a projected reduction in antibiotic duration of 1.5 days using the outcomes observed in the ProHosp trial [11], with a plan to enroll controls at a 1:1 ratio. To accommodate a projected attrition rate of 12%, target enrollment was increased to 400.
Demographic, clinical, and admitting data and outcomes were compared between the intervention and control groups using Student t tests and Wilcoxon rank-sum test for continuous variables and χ2 or Fisher exact tests for categorical variables. The incidence rate ratio (IRR) and corresponding 95% confidence intervals (CIs) contrasting the DOT for the intervention group to the controls was also produced. Finally, the proportion of patients experiencing an adverse event, as well as hospital readmission within 30 days, were compared, and the risk difference and 95% CI between the 2 groups were estimated. Analyses were further stratified by the diagnosis documented by the admitting provider. Admitting diagnoses were categorized as pneumonia, AECOPD, or other LRTI (which encompassed acute bronchitis, asthma exacerbation, and bronchiolitis). Sensitivity analyses included comparison between PCT algorithm-adherent cases and controls. P < .05 was considered statistically significant for all comparisons. All analyses were performed using STATA version 14.1 (StataCorp, College Station, TX).
RESULTS
Patient Enrollment
During the intervention period, hospital encounters were screened daily until 200 patients were enrolled. Four records were duplicates, leaving 196 unique encounters; subsequently, 22 patients met exclusion criteria, and thus there were 174 intervention encounters for final analysis (Figure 1). For the control group, we screened 361 encounter records from the preintervention period; 161 met exclusion criteria, leaving 200 controls.
Baseline characteristics of the 2 groups were balanced, except for admitting diagnosis (Table 1). Pneumonia was a significantly greater proportion of the admitting diagnoses in the intervention than control group (74.4% vs 62.5%). Among all pneumonia patients, 47% had a CURB-65 score of ≥ 2 (intermediate or high risk), and among COPD patients, 41% had severe or very severe disease.
Table 1.
Comparison of Clinical Characteristics and Admitting Service Between Patients With LRTI Treated During the Intervention Period (PCT Group) and Control Period
| Patient Characteristics | PCT Group | Control Group | P Value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Demographics | 174 | 47 | 200 | 54 | |
| Age, mean (SD) | 63.5 (14.3) | 63.3 (14.9) | .87 | ||
| Gender | |||||
| Male | 71 | 41 | 95 | 48 | .19 |
| Female | 103 | 59 | 105 | 53 | |
| Race | .14 | ||||
| White | 127 | 73 | 148 | 74 | |
| Black | 43 | 25 | 40 | 20 | |
| Asian | 0 | 0 | 3 | 2 | |
| Multiracial | 1 | 1 | 0 | 0 | |
| Other | 3 | 2 | 9 | 5 | |
| Comorbidities | |||||
| Hypertension | 119 | 68 | 131 | 66 | .55 |
| COPD | 112 | 64 | 129 | 65 | .98 |
| COPD severity, weighted average | 3.40 (1.37) | 3.5 (1.21) | .40 | ||
| GOLD 1 (mild) | 9 | 8 | 4 | 3 | |
| GOLD 2 (moderate) | 26 | 23 | 26 | 20 | |
| GOLD 3 (severe) | 25 | 22 | 35 | 27 | |
| GOLD 4 (very severe) | 15 | 13 | 24 | 19 | |
| Unknown | 37 | 33 | 40 | 31 | |
| Current smoker | 77 | 44 | 90 | 45 | .89 |
| Diabetes | 55 | 32 | 51 | 26 | .19 |
| Congestive heart failure | 56 | 32 | 72 | 36 | .44 |
| Asthma | 25 | 14 | 19 | 10 | .15 |
| Chronic kidney disease | 45 | 26 | 33 | 17 | .03 |
| Active malignancy | 15 | 9 | 10 | 5 | .16 |
| Bronchiectasis | 8 | 5 | 4 | 2 | .16 |
| Admitting diagnosis | |||||
| Pneumonia | 130 | 75 | 125 | 63 | .04 |
| AECOPD | 36 | 21 | 64 | 32 | |
| Other LRTI | 8 | 5 | 11 | 6 | |
| CURB-65 for patients with pneumonia | |||||
| Total, mean (SD) | 1.58 (1.18) | 1.64 (1.15) | .65 | ||
| 0 | 25 | 19 | 18 | 14 | .96 |
| 1 | 46 | 34 | 47 | 37 | |
| 2 | 36 | 27 | 33 | 26 | |
| 3 | 19 | 14 | 20 | 16 | |
| 4 | 7 | 5 | 6 | 5 | |
| 5 | 2 | 2 | 2 | 2 | |
| Admitting service | .28 | ||||
| Hospitalist | 77 | 44 | 93 | 47 | |
| Internal medicine housestaff | 37 | 21 | 27 | 14 | |
| Pulmonary | 36 | 21 | 46 | 23 | |
| Medical intensive care | 15 | 9 | 17 | 9 | |
| Cardiology intensive care | 9 | 5 | 17 | 9 | |
| Admitting unit | .71 | ||||
| Floor | 110 | 63 | 118 | 59 | |
| Step down unit | 37 | 21 | 52 | 26 | |
| Intensive care unit | 27 | 16 | 30 | 15 | |
| Initial PCT values stratified by admitting diagnosis | Initial PCT value (median, IQR) | Initial PCT <0.25 (n, %) | |||
| All patients | 0.15 (0.07–0.47) | 110 (65) | |||
| Pneumonia | 0.19 (0.09–0.61) | 74 (58) | |||
| AECOPD | 0.07 (0.05– 0.17) | 29 (81) | |||
| Other LRTI | 0.05 (0.04–0.09) | 7 (88) |
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; COPD, chronic obstructive pulmonary disease; CURB-65, confusion, uremia, respiratory rate, blood pressure, age over 65; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IQR, interquartile range; LRTI, lower respiratory tract infection; PCT, procalcitonin; SD, standard deviation.
Procalcitonin Levels
Most initial PCT values (65%) were <0.25 µg/L, ie, below the threshold for recommending antibiotics (Table 1). The median time between collection and result of first PCT was 36.4 hours (interquartile range [IQR], 23.2–45.5 hours), and for subsequent tests it was 6.7 hours (IQR, 2.8–11.2).
Antibiotic Exposure and Hospital Utilization
The median antibiotic duration in the PCT group was lower than in the control group (5 vs 6 days, P = .052), total days of antibiotic therapy was significantly lower (1883 vs 2039 DOT/1000 days present; IRR, 0.92; 95% CI, 0.86–0.99), and significantly fewer patients were discharged on antibiotics (37.4% vs 55.5%, P < .001) (Table 2). A histogram of antibiotic durations is presented in Supplementary Figure 3.
Table 2.
Comparison of Antibiotic Exposure and Hospital Utilization Between Patients With LRTI Treated With Procalcitonin-Guided Antibiotic Duration (PCT Group) and Control Group
| Utilization metrics stratified by admitting diagnosis | PCT Group | Control Group | P Value | Incidence Rate Ratio (95% CI) |
|---|---|---|---|---|
| All patients | ||||
| Antibiotic duration, median (IQR), days | 5 (4) | 6 (4) | .052 | |
| Antibiotic days of therapy per 1000 patient days | 1883 | 2039 | .02 | 0.92 (0.86–0.99) |
| Discharged on antibiotics, n (%) | 65 (37.4) | 111 (55.5) | <.001 | |
| Hospital length of stay in days, median (IQR) | 3 (4) | 3 (3) | .06 | |
| Cost per case in dollars, median (IQR) | 7958 (9836) | 8897 (8983) | .06 | |
| Total variable direct cost in dollars, median (IQR) | 4822 (4765) | 4596 (4231) | .47 | |
| Total antibiotic cost in dollars, median (IQR) | 28.3 (49.3) | 31.0 (74.3) | .94 | |
| Pneumonia | ||||
| Antibiotic duration, median (IQR), days | 6 (4) | 7 (3) | .045 | |
| Antibiotic days of therapy per 1000 patient days | 2261 | 2259 | .49 | 1.05 (0.97–1.13) |
| Discharged on antibiotics, n (%) | 60 (46.2) | 78 (62.4) | .01 | |
| Hospital length of stay in days, median (IQR) | 3.5 (4) | 3 (3) | .56 | |
| Cost per case in dollars, median (IQR) | 7757 (10 261) | 9218 (9138) | .02 | |
| Total variable direct cost in dollars, median (IQR) | 4861 (4930) | 4707 (4367) | .77 | |
| Total antibiotic cost in dollars, median (IQR) | 50.9 (85.6) | 45.1 (62.1) | .93 | |
| AECOPD | ||||
| Antibiotic duration, median (IQR), days | 3 (1) | 4 (3) | .01 | |
| Antibiotic days of therapy per 1000 patient days | 788 | 1513 | <.001 | 0.52 (0.43–0.63) |
| Discharged on antibiotics, n (%) | 3 (8.3) | 28 (43.8) | <.001 | |
| Hospital length of stay in days, median (IQR) | 4 (3) | 3 (4) | .02 | |
| Cost per case in dollars, median (IQR) | 9694 (7636) | 8796 (7212) | .67 | |
| Total variable direct cost in dollars, median (IQR) | 5090 (4432) | 4741 (3937) | .41 | |
| Total antibiotic cost in dollars, median (IQR) | 8.5 (4.8) | 13.1 (12.9) | .01 | |
| Other LRTI | ||||
| Antibiotic duration, median (IQR), days | 4 (2) | 5 (3) | .61 | |
| Antibiotic days of therapy per 1000 patient days | 1440 | 2107 | .07 | 0.68 (0.44–1.05) |
| Discharged on antibiotics, n (%) | 2 (25.0) | 5 (45.5) | .36 | |
| Hospital length of stay in days, median (IQR) | 2.5 (1) | 2 (2) | .70 | |
| Cost per case in dollars, median (IQR) | 5566 (3058) | 7160 (5238) | .93 | |
| Total variable direct cost in dollars, median (IQR) | 3344 (1133) | 3530 (2715) | .77 | |
| Total antibiotic cost in dollars, median (IQR) | 11.3 (6.3) | 14.1 (11.3) | .36 |
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; CI, confidence interval; IQR, interquartile range; LRTI, lower respiratory tract infection; PCT, procalcitonin.
When stratified by admitting diagnosis, median antibiotic durations were significantly shorter in the PCT group for pneumonia (6 vs 7 days, P = .045) and AECOPD (4 vs 3 days, P < .001). Total antibiotic usage, in DOT per 1000 patient days present, was significantly lower in the PCT group than the control group for AECOPD (788 in PCT, 1513 in control) (IRR, 0.52; 95% CI, 0.43–0.63; P < .001); however, differences between groups were not significant for pneumonia (2259 vs 2360) (IRR, 1.05; 95% CI, 0.97–1.13). Apart from a significantly longer hospital stay for AECOPD cases in the PCT group than the control group (4 vs 3 days, P = .019), hospital utilization was similar in both groups.
Adverse Outcomes
There were no significant differences between PCT and control groups in rates of adverse outcomes at 30 days (Table 3). All-cause hospital readmissions at 30 days occurred in 26.5% of control patients and 22.4% of PCT patients (P = .36).
Table 3.
Comparison of Rates of Adverse Outcomes and Hospital Readmissions at 30 Days Post-Discharge Between Patients With LRTI Treated With Procalcitonin-Guided Antibiotic Duration (PCT Group) and Control Group (Data Are Presented as n,%)
| Adverse outcomes stratified by admitting diagnosis | PCT Group | Control Group | P Value | Risk Difference, % (95% CI) |
|---|---|---|---|---|
| Overall adverse outcome at 30 days | 38 (21.8) | 47 (23.5) | .702 | −0.02 (−0.10 to 0.07) |
| Posthospital antibiotic prescription for LRTI | 15 (9.6) | 22 (15.8) | .103 | −0.06 (−0.14 to 0.01) |
| ICU transfer in hospital | 11 (6.4) | 12 (6.0) | .873 | 0.00 (−0.05 to 0.05) |
| Death | 7 (4.0) | 9 (4.5) | .820 | −0.00 (−0.05 to 0.04) |
| Adverse event from antibiotics | 6 (3.5) | 6 (3.0) | .806 | 0.00 (−0.03 to 0.04) |
| Disease-specific complications | 3 (1.7) | 6 (3.0) | .422 | −0.01 (−0.04 to 0.02) |
| Clostridium difficile | 2 (1.2) | 1 (0.5) | .483 | 0.01 (−0.01 to 0.03) |
| Hospital Readmission | ||||
| Readmission in 30 days | 39 (22.4) | 53 (26.5) | .360 | −0.04 (−0.13 to 0.05) |
| Pneumonia | ||||
| Overall adverse outcome at 30 days | 31 (23.9) | 31 (24.8) | .859 | −0.01 (−0.11 to 0.10) |
| Posthospital antibiotic prescription for LRTI | 10 (8.7) | 13 (16.5) | .100 | −0.08 (−0.17 to 0.02) |
| ICU transfer | 10 (7.8) | 8 (6.4) | .675 | 0.01 (−0.05 to 0.08) |
| Death | 6 (4.6) | 7 (5.6) | .721 | −0.01 (−0.06 to 0.04) |
| Adverse effect rate from antibiotics | 6 (4.6) | 4 (3.2) | .561 | 0.01 (−0.03 to 0.06) |
| Disease-specific complications | 3 (2.3) | 6 (4.8) | .281 | −0.02 (−0.07 to 0.02) |
| C difficile | 2 (1.5) | 1 (0.8) | .585 | 0.01 (−0.02 to 0.03) |
| Hospital Readmission | ||||
| Readmission in 30 days | 28 (21.5) | 32 (25.6) | .445 | −0.04 (−0.14 to 0.06) |
| Exacerbation of COPD | ||||
| Overall adverse outcome at 30 days | 6 (16.7) | 14 (21.9) | .532 | −0.05 (−0.21 to 0.11) |
| Posthospital antibiotic prescription for LRTI | 4 (11.8) | 8 (16.0) | .586 | −0.04 (−0.19 to 0.11) |
| ICU transfer | 1 (2.9) | 3 (4.8) | .667 | −0.02 (−0.10 to 0.06) |
| Death | 1 (2.8) | 2 (3.1) | .922 | 0.00 (−0.07 to 0.07) |
| Adverse effect rate from antibiotics | 0 (0) | 2 (3.1) | .284 | −0.03 (−0.07 to 0.01) |
| Disease-specific complications | 0 (0) | 0 (0) | ||
| C difficile | 0 (0) | 0 (0) | ||
| Hospital readmission | ||||
| Readmission in 30 days | 10 (27.8) | 21 (32.8) | .601 | −0.05 (−0.24 to 0.14) |
| Other LRTI | ||||
| Overall adverse outcome | 1 (12.5) | 2 (18.2) | .737 | −0.06 (−0.38 to 0.27) |
| Post hospital antibiotic prescription for LRTI | 1 (12.5) | 1 (10.0) | .867 | 0.03 (−0.27 to 0.32) |
| ICU transfer | 0 (0) | 1 (9.1) | .381 | −0.09 (−0.26 to 0.08) |
| Death | 0 (0) | 0 (0) | ||
| Adverse effect rate from antibiotics | 0 (0) | 0 (0) | ||
| C difficile | 0 (0) | 0 (0) | ||
| Disease-specific complications | 0 (0) | 0 (0) | ||
| Hospital Readmission | ||||
| Readmission in 30 days | 1 (12.5) | 0 (0) | .228 | 0.13 (−0.10 to 0.35) |
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; LRTI, lower respiratory tract infection; PCT, procalcitonin.
Adherence With Study Algorithm
Providers adhered to antibiotic recommendations in the PCT algorithm for 119 (70%) of intervention patients. Providers discontinued antibiotics per algorithm in 16 of 27 patients (59%) who initially met override criteria. In cases of algorithm adherence, 82 patients had antibiotics discontinued during hospital admission, and 37 patients were discharged on antibiotics due to a persistently elevated PCT (Supplementary Figure 4). Providers discontinued antibiotics despite an elevated PCT in 5 patients without observed adverse events. Overall, adherence was high the first month, dropped, then slowly increased over time (Supplementary Figure 5).
Providers were less likely to follow the algorithm for patients with an admitting diagnosis of pneumonia than in AECOPD or nonpneumonic LRTI (pneumonia accounted for 93.2% of nonadherent cases and 68.5% of adherent cases, P = .005). Neither hospital service, hospital unit, nor patient severity of illness was associated with algorithm nonadherence in a univariate analysis (Supplementary Table 3). Adherence by PCT stopping criteria is presented in Supplementary Table 4.
Excess Antibiotic Days
Under optimal use conditions (test results in <1 hour, 100% provider adherence), we predicted that an additional 477 DOT could have been saved by further shortening or withholding antibiotics. When considering patients who did not meet override criteria on admission, 82 patients had 2 initial negative PCT values, accounting for 417 (87%) of the avoidable antibiotic days. In contrast, in patients with at least 1 positive PCT value who underwent PCT-guided de-escalation, only 60 additional antibiotic days (13%) could have been avoided.
DISCUSSION
In 2004, a cluster-randomized trial demonstrated that PCT-guided antibiotic therapy reduced antibiotic exposure in LRTI by 50% without harming patients [19]. Since this seminal trial, numerous trials, conducted mainly in Europe, have demonstrated the safety and efficacy of PCT-guided antibiotic therapy for LRTI. Indeed, patients treated with PCT-guided antibiotic therapy have experienced lower mortality rates than patients in control groups (8.6% vs 10.0%, P = .037), while receiving fewer days of antibiotics [20].
Although heralding great promise, PCT trials have met with mixed responses from US academic centers. Only 1 trial in the 2017 Cochrane review was conducted at a US site, and this trial excluded patients with pneumonia [20]. Critics point out that antibiotic durations in the control groups were twice those recommended in current US guidelines [21]. In addition, many US physicians favor autonomy and resist protocolization [22], which could hinder efforts to implement PCT algorithms. The ProACT trial, a randomized trial of 1656 LRTI patients in 14 US centers, was designed to address these criticisms. An intervention utilizing provider education and result reporting with interpretive guidance text did not result in a decrease in antibiotic exposure in the PCT group compared with usual care [23].
Our study demonstrates that PCT-guided antibiotic therapy can safely shorten antibiotic duration for LRTI at a US site, even with relatively short preintervention treatment durations. In our study, we observed a reduction in median treatment duration for pneumonia as well as AECOPD despite a 1.5-day delay of initial PCT results. In contrast, the ProACT trial did not observe a reduction in duration even with a rapid test-turnaround time. Several differences between our study and ProACT study may explain the differential impact.
First, the ProACT population was heavily weighted toward acute bronchitis and asthma diagnoses (63% vs 2% in our study). The enrollment of these low-acuity patients may have biased the ProACT study toward no effect, compared with our population of hospitalized patients with AECOPD and pneumonia.
Second, our experience along with other studies suggest that PCT impact improves when deployed within an institutional stewardship program [24] rather than outside of a stewardship program. Provider education and result alerting, although maximizing prescriber autonomy, may not achieve equivalent results as directive interventions. Modifying antibiotic prescribing habits requires significant trust, feedback, and 2-way communication between prescribers and stewardship teams. Our trial established trust with prescribers via strong stakeholder buy-in. The study team included the medical directors of the ICU and hospitalist services, numerous front-line providers, clinical pharmacy staff, and the antimicrobial stewardship team.
Third, the site and strategy of the ProACT trial differed from the present trial. The ProACT intervention reported PCT results and guidance text to providers in the ED, including recommendations to withhold initial antibiotics for low values. Withholding antibiotics is uncomfortable for providers who cannot follow patients postdischarge. In addition, US hospitals are subject to payment penalties if patients with suspected sepsis do not receive antibiotics within 3 hours of presentation [25]. Adherence with PCT algorithms in the ED is challenging. In contrast, our intervention occurred on inpatient units among patients who had already received a median of 36 hours of antibiotics at the time of the first result. Hence, providers may have felt more comfortable discontinuing antibiotics at this time because clinical response was evident, and patients continued under close monitoring. Our strategy mimics the timing and workflow of audit and feedback performed by many stewardship programs.
The differential findings of the ProACT trial and this current study raise the question: in which setting can PCT have the most impact? Analysis of PCT levels in our patients suggests that the greatest stewardship opportunity lies in initial withholding of antibiotics in patients with AECOPD and in patients with an admitting diagnosis of community-acquired pneumonia but have mild illness and a negative PCT. These patients likely have a noninfectious diagnosis or a viral LRTI, and antibiotics may be safely withheld. A quick PCT turnaround time would maximize this impact. Additional research in a variety of real-world settings is needed to discover optimal implementation strategies for PCT.
Provider adherence was 70% overall in our study, which is comparable to other US studies (64% in Branche et al [26], and 65% in the ProACT study [23]), but lower than the >90% adherence rates seen in European trials [11, 19]. In a univariate analysis, the main factor associated with algorithm nonadherence was an admitting diagnosis of pneumonia, although adherence in pneumonia was still relatively high (n = 89 of 130, 69%). Provider attitude survey data from the Branche et al 26] study revealed that clinical signs of bacterial pneumonia drive the decision to continue antibiotics in patients with suspected LRTI. Similar findings were reported in the United States-based ProACT trial, where the adherence rate for pneumonia was 39% compared with 82% adherence in acute bronchitis [27], suggesting that provider belief about the presence of bacterial infection is difficult to modify with a PCT result.
Our study has several limitations. The intervention was limited to internal medicine services, which limits generalizability to other specialties. Due to the before-and-after design, the control period occurred during different parts of the calendar year, which may correspond to variations in antibiotic durations due to unmeasured factors such as the acquired experience among trainees and probability of admission based on fluctuating hospital volumes. Due to the nonrandomized nature of the trial, we were unable to isolate the effect of PCT from the effects of education and active stewardship. However, the comparison of a hospital’s baseline “usual stewardship” to a stewardship-driven PCT algorithm provides a more accurate estimate of the impact of PCT introduction on overall antibiotic use in a hospital. The need for consent and once-daily test runs delayed results by 36 hours, potentially limiting the impact of our intervention.
CONCLUSIONS
Overall, our study demonstrated that PCT-guided cessation of antibiotic therapy, when undertaken as a stewardship intervention, is a safe and effective strategy to reduce antibiotic use in patients with LRTI.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Sara Cosgrove (Johns Hopkins Hospital Antibiotic Stewardship) for a critical review of the article. We also thank the laboratory faculty and staff at Johns Hopkins Bayview Medical Center for their time and effort in running the assays.
Financial support. This work was funded by B·R·A·H·M·S GmbH (ThermoFisher, Hennigsdorf, Germany), Grant Number 125346.
Potential conflicts of interest. J. T. received a grant from B·R·A·H·M·S GmbH to conduct this study. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Magill SS, Edwards JR, Beldavs ZG, et al. . Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014; 312:1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hecker MT, Aron DC, Patel NP, et al. . Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med 2003; 163:972–8. [DOI] [PubMed] [Google Scholar]
- 3. Klein EY, Martinez EM, May L, et al. . Categorical risk perception drives variability in antibiotic prescribing in the emergency department: a mixed methods observational study. J Gen Intern Med 2017; 32:1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Szymczak J. Influencing antibiotic prescribing behavior: inpatient settings. In: Presidential Advisory Council on Combating Antibiotic Resistance. Washington, DC: 2017. HHS.gov. https://www.hhs.gov/sites/default/files/szymczak-91317.pdf. [Google Scholar]
- 5. Claessens YE, Debray MP, Tubach F, et al. . Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med 2015; 192:974–82. [DOI] [PubMed] [Google Scholar]
- 6. Waterer GW. The diagnosis of community-acquired pneumonia. Do we need to take a big step backward? Am J Respir Crit Care Med 2015; 192:912–3. [DOI] [PubMed] [Google Scholar]
- 7. Assicot M, Gendrel D, Carsin H, et al. . High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993; 341:515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becker KL, Nylén ES, White JC, et al. . Clinical review 167: procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab 2004; 89:1512–25. [DOI] [PubMed] [Google Scholar]
- 9. Bouadma L, Luyt CE, Tubach F. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010; 375:463–74. [DOI] [PubMed] [Google Scholar]
- 10. Mallet E, Lanse X, Devaux AM, et al. . Hypercalcitoninaemia in fulminant meningococcaemia in children. Lancet 1983; 1:294. [DOI] [PubMed] [Google Scholar]
- 11. Schuetz P, Christ-Crain M, Thomann R, et al. . Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302:1059–66. [DOI] [PubMed] [Google Scholar]
- 12. Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med 2008; 36:941–52. [DOI] [PubMed] [Google Scholar]
- 13. Meisner M. Pathobiochemistry and clinical use of procalcitonin. Clin Chim Acta 2002; 323:17–29. [DOI] [PubMed] [Google Scholar]
- 14. Cosgrove SE, Avdic E, Dzintars K, Smith J. Antibiotic guidelines: treatment recommendations for adult inpatients. Johns Hopkins Medicine 2015. Pp. 82-90. www.insidehopkinsmedicine.org/amp. Accessed December 4, 2016. [Google Scholar]
- 15. Lim WS, van der Eerden MM, Laing R, et al. . Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vogelmeier CF, Criner GJ, Martínez FJ, et al. . Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch Bronconeumol 2017; 53:128–49. [DOI] [PubMed] [Google Scholar]
- 17. Sabatani N. Inpatient (Discharge) Dataset. In: Commission MHSCR. Baltimore, MD; Maryland Health Services Cost Review Commission; 2018. http://www.hscrc.state.md.us/Pages/data.aspx. Accessed December 10, 2017. [Google Scholar]
- 18. NHSN. Antimicrobial Use and Resistance (AUR) Module. Pp. 17-20. 2015 http://www.cdc.gov/nhsn/pdfs/pscmanual/11pscaurcurrent.pdf. Accessed February 2, 2016. [Google Scholar]
- 19. Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. . Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet 2004; 363:600–7. [DOI] [PubMed] [Google Scholar]
- 20. Schuetz P, Wirz Y, Sager R, et al. . Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2017; 10:CD007498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang DT, Angus DC, Chang CH, et al. . Design and rationale of the Procalcitonin Antibiotic Consensus Trial (ProACT), a multicenter randomized trial of procalcitonin antibiotic guidance in lower respiratory tract infection. BMC Emerg Med 2017; 17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Emanuel EJ, Pearson SD. Physician autonomy and health care reform. JAMA 2012; 307:367–8. [DOI] [PubMed] [Google Scholar]
- 23. Huang DT, Yealy DM, Filbin MR, et al. . Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med 2018; 379:236–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Broyles MR. Impact of procalcitonin-guided antibiotic management on antibiotic exposure and outcomes: real-world evidence. Open Forum Infect Dis 2017; 4:ofx213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. The Joint Commission. New Antimicrobial Stewardship Standard MM.09.01.01. In: Commission TJ, 2017. https://www.jointcommission.org/assets/1/6/New_Antimicrobial_Stewardship_Standard.pdf. Accessed September 4, 2018. [Google Scholar]
- 26. Branche AR, Walsh EE, Vargas R, et al. . Serum procalcitonin measurement and viral testing to guide antibiotic use for respiratory infections in hospitalized adults: a randomized controlled trial. J Infect Dis 2015; 212:1692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Branche AR, Walsh EE, Jadhav N, et al. . Provider decisions to treat respiratory illnesses with antibiotics: insights from a randomized controlled trial. PLoS One 2016; 11:e0152986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.