Abstract
A 73-year-old woman in cardiogenic shock was referred to our hospital because of papillary muscle rupture immediately after the onset of acute myocardial infarction (MI). She had undergone emergent percutaneous coronary intervention and mitral valve replacement. Serial creatine kinase and creatine kinase MB levels indicated that she had acute phase MI on arrival. Pathological findings showed coagulative necrosis of the papillary muscle, which was characteristic evidence of the consequence of prolonged ischemia. We describe herein an unusual case of complete rupture of the posterior papillary muscle occurring immediately after the development of MI.
<Learning objective: Mechanical complications of acute myocardial infarction (AMI) such as left ventricular free wall rupture, interventricular wall rupture, and papillary muscle rupture (PMR) commonly occur 2 to 7 days after the onset of AMI. We describe a case of PMR immediately after AMI, which may cause a silent and severe ischemic event a few days before the onset of PMR. This is a rare case of PMR immediately after AMI.>
Keywords: Acute myocardial infarction, Papillary muscle rupture, Mitral valve replacement
Introduction
Papillary muscle rupture (PMR) is a rare and serious complication of acute myocardial infarction (AMI), which leads to acute mitral regurgitation, pulmonary edema, and cardiogenic shock. In cases of AMI, coagulative necrosis occurs within 24 h of initial chest pain and progresses to softening of the infarct myocardium; thus, granulation tissue and early collagen deposition support the area of infarction approximately 1 week after AMI [1]. Therefore, PMR commonly occurs 2 to 7 days after the onset of AMI [2]. We describe a rare case of PMR that happened immediately after AMI.
Case report
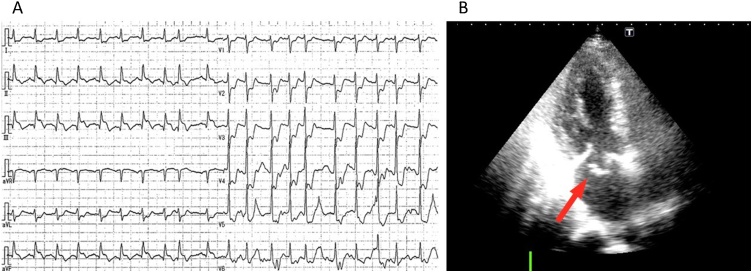
A 73-year-old woman was transferred by ambulance to our hospital for the sudden onset of chest pain and dyspnea. Her medical history included hypertension without medication and ovarian cancer for which she underwent an operation 6 years previously. Her consciousness was unclear, she had agonizing facial expressions, and her initial vital signs were as follows: temperature, 33.4 °C; blood pressure, 96/72 mmHg; heart rate, 130 beats/min; respiratory rate, 32 breaths/min; and oxygen saturation, unmeasurable using a 15-L reservoir mask. The cardiovascular examination showed an apical holosystolic murmur that was heard loudest at the apex. There was jugular venous distention of the mandible. The pulmonary examination demonstrated tachypnea with the use of accessory muscles and marked wheezing in both lung fields. The troponin I level was 6.231 ng/mL (0.00–0.04 ng/mL), but the levels of creatine kinase and creatine kinase MB were not increased (119 U/L and 14.0 U/L, respectively). The chest radiograph showed a butterfly shadow. The electrocardiogram demonstrated ST segment elevation in leads II, III, and aVF and depression in leads V2-V6 (Fig. 1A). The echocardiogram demonstrated akinesis in the inferior region and severe mitral regurgitation due to prolapse of the anterior mitral leaflet, but the left ventricular ejection fraction was preserved (76%). Left ventricular dimension was in normal rage (diastolic dimension was 43 mm and systolic dimension was 31 mm), but left atrial dimension was dilated (45 mm) (Fig. 1B and supplemental movie).
Fig. 1.
(A) Electrocardiogram showing ST-segment elevation in the inferior leads with reciprocal changes. (B) Echocardiogram showing severe mitral regurgitation with prolapse of the anterior mitral leaflet (arrow) due to papillary muscle rupture (Supplemental movie).
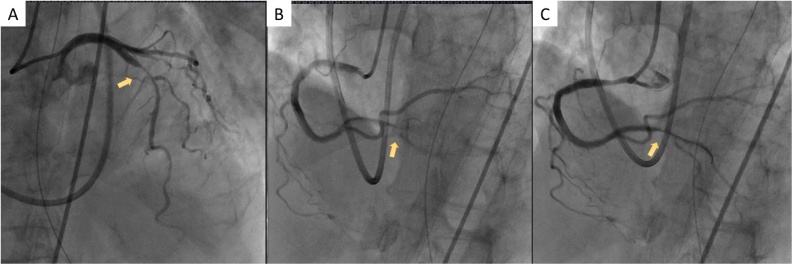
We promptly performed intubation and supported her breathing with a mechanical ventilator. She was emergently transferred to the cardiac catheterization laboratory unit. As her hemodynamic status was unstable, we promptly inserted an intra-aortic balloon pump (IABP) to stabilize it. The emergent coronary angiogram showed 99% severe stenosis with a severe flow delay (thrombolysis in myocardial infarction grade 1 flow) of the distal right coronary artery (RCA), 90% stenosis of the proximal left anterior descending artery (LAD), and collateral circulation from the septal branches to the RCA. The left circumflex artery (LCX) was intact. Balloon angioplasty was performed to treat the distal RCA lesion, and revascularization was achieved (Fig. 2).
Fig. 2.
(A) Emergent coronary angiograms showing 90% stenosis of the proximal left anterior descending artery (arrow). (B) 99% severe stenosis with severe flow delay at the distal right coronary artery (RCA) (arrow). (C) Balloon angioplasty is performed to treat the distal RCA lesion, and revascularization is achieved (arrow).
After percutaneous coronary intervention, the patient was referred for emergent mitral valve replacement with concomitant coronary artery bypass grafting. The emergency operation was started 3 h after onset, i.e. approximately 2.5 h after arrival at our hospital. Upon surgical inspection, the posterior papillary muscle was found to be completely ruptured. Mitral valve replacement was performed using a 23-mm Carpentier-Edwards PERIMOUNT biological valve (Edwards Lifesciences, Irvine, CA, USA). The left intrathoracic artery was anastomosed to the LAD, and a saphenous vein graft was anastomosed to the RCA.
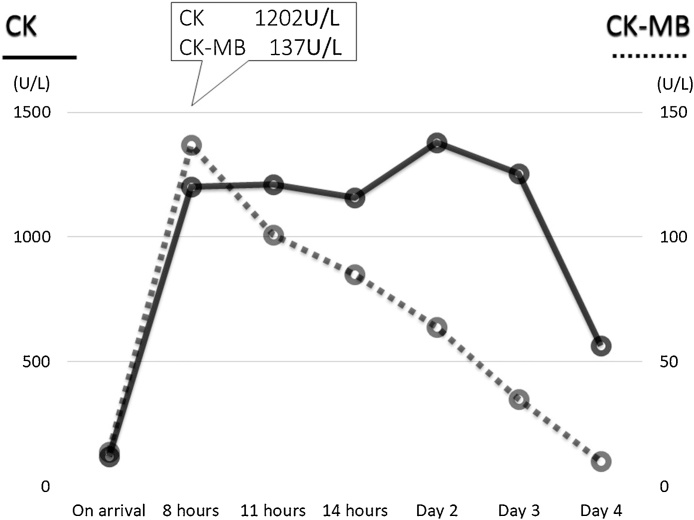
Results of serial blood tests postoperatively showed that the peak values of creatine kinase and creatine kinase MB were 1380 IU/L and 137 IU/L, respectively (Fig. 3). The patient was weaned from the IABP 37 h later and then the ventilator 41 h later. She was successfully discharged home in good condition on postoperative day 26.
Fig. 3.
The time course of creatine kinase (CK) and CK-MB.
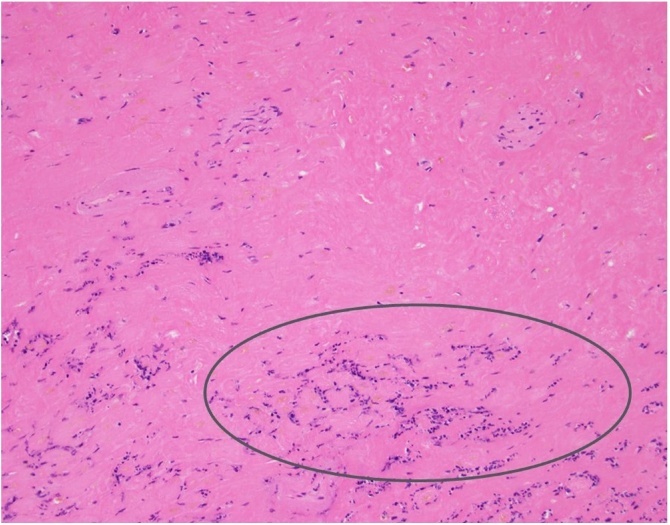
Histological examination of the posterior papillary muscle confirmed coagulative necrosis with cytological findings consistent with an infarct age of less than 7 days (Fig. 4).
Fig. 4.
Papillary muscle specimen stained with hematoxylin and eosin. Coagulative necrosis (circle) is observed in her papillary muscle.
Discussion
PMR is a rare complication (0.5–5%) that usually occurs 2 to 7 days after AMI [2]. The posterior papillary muscle tends to rupture more frequently than the anterior papillary muscle (75% vs. 25%) because of its single blood supply from the RCA or LCX. About 50% of cases of PMR have a relatively small infarction, and their ejection fraction is preserved, which leads to increased shearing stress at the rupture site. Therefore, the present case was typical PMR caused by a small posterior descending artery occlusion.
However, interestingly, in our case, PMR occurred immediately after AMI. Myocardial apoptosis has been previously observed in human acute myocardial infarction before the occurrence of coagulative necrosis. During the following 1 to 2 days, morphological features of coagulative necrosis were observed in our patient, and softening of the infarct myocardium may have progressed. However, granulation tissue and early collagen deposition developed approximately 1 week after AMI, which may have sustained the site of infarction [3].
On the basis of the pathological findings in our case, we speculate the mechanism in our patient. The case would have had a silent and severe ischemic event a few days before the onset of AMI, and coagulative necrosis may have progressed in the small posterior descending artery. Then, small inferior AMI with hyperdynamic systolic function would have occurred, and her posterior papillary muscle may not have been able to tolerate the higher shear stress.
We described a rare case of PMR immediately after AMI. The pathological findings may indicate a silent and severe ischemic event a few days before the onset of PMR.
Conflict of interest
Authors have no conflict of interest.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jccase.2018.07.009.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Veinot J.P., Gattinger D.A., Fliss H. Early apoptosis in human myocardial infarcts. Hum Pathol. 1997;28:485–492. doi: 10.1016/s0046-8177(97)90039-3. [DOI] [PubMed] [Google Scholar]
- 2.Lavie C.J., Gersh B.J. Mechanical and electrical complications of acute myocardial infarction. Mayo Clin Proc. 1990;65:709–730. doi: 10.1016/s0025-6196(12)65133-7. [DOI] [PubMed] [Google Scholar]
- 3.Schoen F.J., Mitchell R.N. Robbins and Cotran Pathologic Basis of Disease. Elsevier/Saunders; Philadelphia: 2015. Myocardial infarction; pp. 540–550. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.