Abstract
For generations, blood has been the medium of choice for diagnosing most diseases and conditions. The reason for this is mainly the limitations of technology. The concept of oral diagnostics is preferred to more invasive methods. In recent years, it has become evident that the salivary constituents become detectably altered in response to certain disease states. Even so, what is most impressive is that salivary biomarkers not only arise in correlation with oral disorders but also those of distal tissues and organs. This suggests that oral fluids may represent a substantial reservoir of molecular and microbial information capable of communicating the onset or presence of disease throughout the body. An initiative of the National Institute of Dental and Craniofacial Research created a roadmap to achieve these goals whereby, with the use of oral fluids as the diagnostic medium, it would become possible to scrutinize the health and/or disease status of patients. The real promise of salivary analysis use is the ability of the patient or clinician to directly and continuously assess disease status, progression and therapeutic efficacy. The sensitive analysis may even allow presymptomatic diagnosis. There are five major diagnostic alphabets available in saliva namely, proteins, messenger RNAs, micro-RNAs (mi-RNAs), metabolic compounds and microbes which offer substantial advantages for salivary diagnostics because, the state of the disease may be associated with detectable changes in one, but not all, dimensions. Recently, the Salivaomics Knowledge Base (SKB) has been established by aligning the salivary biomarker discovery. The SKB constitutes data repository, management system and web resource fabricated to support human salivary proteomics, transcriptomics, miRNA, metabolomics and microbiome research.
Keywords: Metabolomics, microbiome, microRNA, proteomics, salivaomics, transcriptomics
INTRODUCTION
The diagnosis of systemic diseases in routine circumstances is through:
Patient-reported symptoms
Examination and a medical history obtained by a physician or other medical professional
Chemical analysis of blood and/or urine samples.
The patient's samples are typically sent to a remote, clinical diagnostic laboratory for determination of the levels of a broad series of markers including ions, antibodies, hormone levels and a variety of disease-specific biomarkers.[1]
For generations, blood has been the medium of choice for diagnosing most diseases and conditions. The reason for this is mainly the limitations of technology.
Despite the regular screenings and check-ups, many diseases are undetected until a late phase where morbid symptoms become apparent. To overcome these challenges, researchers are unravelling biomarkers. These biomarkers include genetic material (e.g., deoxyribonucleic acid [DNA] and RNA) and protein molecules that reflect the current physiological state of an individual and hence help scientists to better understand the underlying cause of a disease.[2] Biomarkers in the blood are commonly measured in micrograms, which are one-millionths of a Gram.
Saliva is a biological fluid (bio-fluid) that could be a source of biochemical data capable of detecting certain diseases and so would be useful for novel approaches to prognosis, laboratory or clinical diagnosis and monitoring and management of patients with both oral and systemic diseases. Besides, it is easily collected and stored and ideal for early detection of disease as it contains specific soluble biological markers (biomarkers).[3]
The various chemical components of saliva include water, inorganic compounds (ions), organic compounds (nonproteins and lipids), protein/polypeptides and hormones, and an array of analytes.[4]
Salivary diagnostics is a developing field with the growing appreciation of saliva as it reflects:
Tissue fluid levels of natural substances and a large variety of molecules introduced for therapeutic, dependency, or recreational purposes
Emotional status from high anxiety to low-down blues, from mania to depression
Hormonal status
Immunological status and responsiveness
Neurological effects
Nutritional and metabolic influences.[5]
Saliva contains multiple biomarkers which make it useful for multiplexed assays that are being developed as point-of-care (POC) devices, rapid tests, or in more standardized formats for centralized clinical laboratory operations. It involves NanoBiochip technology that does fluid processing to detect the pH, local electrolytes, metal cations, chemical environment, sugar, toxins, antibodies and proteins. POC contains a modular and miniaturized sensor system, universal analyzer with functional integrated mechanical/optical interfaces and flexible microchip architecture which caters to the future needs of researchers.[6]
Salivary diagnostics is a dynamic field that is being incorporated as part of disease diagnosis, clinical monitoring and for making important clinical decisions for patient care.[1]
Although the discovery of discriminatory biomarkers in oral fluids epitomizes an almost incomparable breakthrough in clinical and translational science, continuation in this area is required to credentiate saliva as an acceptable diagnostic medium, an endpoint with the potential to bring about a paradigm shift in the discipline of molecular diagnostics.[3]
Besides, biomarkers in saliva are commonly measurable in picograms, one trillionth of a Gram, or nanogram quantities, one billionth of a Gram and so have to be very meticulously identified and assessed.
Also, as studies show that salivary parameters such as salivary flow rate, salivary viscosity, salivary pH and salivary buffering capacity were lower in subjects with high dental caries. Hence, it is recommended that salivary testing be a part of routine diagnosis when treating patients with[7,3] high risk of dental caries with saliva-based caries activity tests such as, saliva-based caries activity tests include Lactobaccilus colony count test, Snyder test, reductase test, Buffer capacity test, Fosdick calcium dissolution test, Streptococcus mutans adherence method and S. mutans dip slide test.[6]
The proposed markers for periodontal disease activity in saliva include proteins of host origin (i.e., enzymes and immunoglobulins), phenotypic markers (epithelial keratins), host cells, hormones, bacteria and bacterial products, volatile compounds and ions.[6,8]
Thus, two prerequisites exist before the goal of standard deviations can be achieved:
Identification of specific biomarkers associated with a health or disease state
The development of technologies that can discriminate between the biomarkers.
Although the utility and advantages of saliva as a screening tool for cystic fibrosis has been identified in the early 1960s, its full diagnostic potential was discovered three decades later when studies revealed distinct advantages of saliva over serum. Like serum, saliva also contains hormones, antibodies, growth factors, enzymes, microbes and their products.[2] Today, since technology is at a point where the minute quantities are readily detectable using a number of methodologies, including next-generation sequencing, proteomics, mass spectrometry, genome-wide association studies and other screening techniques so, the advantages of salivary testing are now obvious and the improved efficiency and accuracy of genomic and proteomic biomarkers are turning salivary diagnostics into a clinical and commercial reality. Biomarkers in saliva have been identified for a wide variety of diseases and conditions. In the near future, salivary samples may be able to distinguish between bacterial and viral infections and detect recent heart attacks, traumatic brain injury, even lung and gastric diseases [Table 1].
Table 1.
Role of saliva in systemic diseases
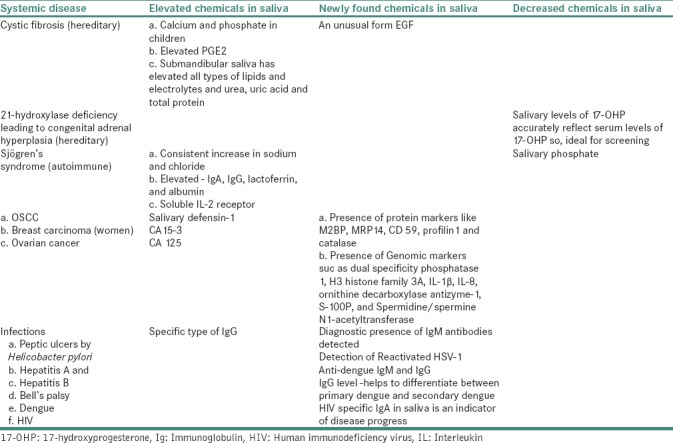
The concept of oral diagnostics is preferred to more invasive methods and gets substantiated by the success of the oral thermometer to detect fever which has totally replaced its predecessor, the rectal thermometer.[3,8]
However, the ability to accurately assess biomarkers in samples obtained from the oral cavity depends on the biochemical nature of the marker, the source and type of sample being taken, and the mechanism by which the marker enters the oral cavity. The most widely used type of oral sample is a swab that collects a DNA sample, particularly in forensic odontology and more recently in single nucleotide polymorphisms analyses for mutations associated with specific diseases.[1]
Recently, new methods have emerged to collect saliva based on modifications to the traditional expectoration techniques. Oragene is commonly used sophisticated technique wherein preservation buffers are used to protect the integrity of the sample until processing and extraction take place. This is the most commonly used technique. Other methods of saliva expectoration include Saligene, Oracol and Verofy [Table 1]. Saligene is an alternative technique which is based on split in a cup technique. The technique utilizes a collection tube into which saliva is expectorated to a predetermined volume following which a plunger is used to cap the tube. This pressure releases the buffer into the specimen, and then it is sent for further processing. Oracol is based on saliva collection through an absorbent foam swab which picks up 1 mL of saliva. Oracol is used in salivary diagnosis of measles, human immunodeficiency virus, hepatitis A and B, mumps and rubella. Verofy is a unique method which utilizes high-quality immunochromatographic strips for delivery of immediate results.[6]
Advantages of the use of saliva for biomarker detection:[9]
It has real-time diagnostic values
Safer for health professionals than blood sampling
Ease of obtaining multiple samples
Collection and screening can be done at home
It is noninvasive, easy and inexpensive
It has minimal risk of cross-contamination
More economical sampling, shipping and storage
Manipulation required during diagnostic procedures is less as compared to that for serum
There is the commercial availability of screening assay
Saliva does not clot unlike blood.
Limitations[9]
Levels of certain markers in saliva are not always a reliable reflection of the levels of these markers in serum
Salivary composition can be influenced by the method of collection and degree of stimulation of salivary flow
Changes in salivary flow rate may affect the concentration of salivary markers and also their availability due to changes in salivary pH
Variability in salivary flow rate is expected between individuals and in the same individual under different conditions
In addition, many serum markers can reach whole saliva in an unpredictable way (i.e., gingival crevicular fluid flow and through oral wounds). These parameters will affect the diagnostic usefulness of many salivary constituents
Furthermore, certain systemic disorders, numerous medications and radiation may affect salivary gland function and consequently the quantity and composition of saliva
Whole saliva also contains proteolytic enzymes derived from the host and oral microorganisms. These enzymes can affect the stability of certain diagnostic markers. Some molecules are also degraded during intracellular diffusion into saliva.
An initiative of the National Institute of Dental and Craniofacial Research (NIDCR) has created a roadmap to achieve these goals through the use of oral fluids as the diagnostic medium to scrutinize the health and/or disease status of patients. This is an ideal opportunity to optimize state-of-the-art saliva-based biosensors for salivary biomarkers that discriminate between diseases.[10]
In recent years, it has become evident that the salivary constituents become detectably altered in response to certain disease states. Even so, what is most impressive is that salivary biomarkers not only arise in correlation with oral disorders but also those disorders of distal tissues and organs. This suggests that oral fluids may represent a substantial reservoir of molecular and microbial information capable of communicating the onset or presence of disease throughout the body. Today, a growing number of studies are focused on elucidating and developing saliva-based biomarkers indicative of both local and systemic diseases.[3]
Some systemic diseases affect salivary glands directly or indirectly and may influence the quantity and quality of saliva. These characteristic changes may contribute to the diagnosis and early detection of these diseases. Systemic disorders that may affect salivary glands and saliva are given below.[8,11]
DIAGNOSTIC USES OF SALIVA IN SYSTEMIC DISEASES
Glands and saliva
Due to its diverse biological functions, salivary testing is rapidly growing as a practical and reliable means in clinics and research to recognize early signs of systemic illness and exposure to risk factors. Owing to the wide-spread use and growing acceptability of saliva as a diagnostic tool, various investigators have focused their efforts to establish its clinical usefulness. The data have proved to be of significant utility for researchers, health-care professionals and community health program personals to detect and monitor diseases and to improve the general health of the public.[12]
The real promise of salivary analysis use is the ability of the patient or clinician to directly and continuously assess disease status, progression and therapeutic efficacy. The sensitive analysis may even allow presymptomatic diagnosis. Thus, microfluidic and microelectromechanical systems and nanoelectrofluidic systems for salivary diagnostics have been under development for at least the last decade. Microfluidics have the advantage of using low sample and reagent volumes; microelectronics facilitate the development of miniaturized chairside and handheld devices suitable for use in the dental clinic and home in the absence of specialized laboratory facilities. In combination, they promise to provide simultaneous and rapid measurement of multiple biomarkers coupled with data storage and transmission.[13]
The process of biomarker discovery is through definitive validation and approval. Biomarkers of oral and systemic diseases are discovered using one or more of the “omics” libraries. Verified biomarkers are subjected to increasing scrutiny and larger independent cohorts until they reach a pivotal multicenter validation. Salivaomics includes proteomics, epigenomics, metabolomics, immunomics, microbiome and transcriptomics.[3]
There are five major diagnostic alphabets available in saliva namely, proteins, messenger RNAs (mRNAs), micro-RNAs (mi-RNAs), metabolic compounds and microbes which offer substantial advantages for salivary diagnostics because, the state of the disease may be associated with detectable changes in one, but not all, dimensions. The salivary biomarkers are also classified based on the mechanism of action.[4]
Salivary proteins and proteomics
In 2004, the NIDCR provided funding to comprehensively decipher, catalog and annotate the human salivary proteome. These efforts revealed the salivary proteome as a sizeable collection of up to 1,166 diverse proteins, the majority of which are synthesized and subsequently secreted into the oral cavity by the acinar cells of the salivary gland. It could be indicative of both local and distant diseases.
To evaluate the capacity of salivary proteomic entities as diagnostic metrics, scientists frequently employ traditional laboratory techniques including:
Liquid chromatography
Gel and capillary electrophoresis
Nuclear magnetic resonance
Mass spectrography
Immunoassays
Lectin probe analysis and
2-dimensional gel electrophoresis coupled with mass spectrometry.[3]
More than 2300 minor proteins or peptides are present in saliva defining the first salivary biomarker alphabet, although, the proteomic content of saliva was found to be only 30% that of blood. The most plentiful proteins include α-amylase, albumin, cystatins, hystatins, secretory-immunoglobulin A, lactoferrin, mucins, lysozymes, proline-rich proteins, statherin and transferrin which together account for more than 98% of the total salivary proteins.
Functional use of salivary proteins
Salivary proteins are involved in a number of metabolic pathways, including amino acid-related metabolism, carbohydrate metabolism, energy metabolism, as well as glycan biosynthesis and metabolism. Therefore, the sequential amino acid in a protein provides a link between the proteins and their respective coding genes through genetic code, and the protein complement of the genome is known as the proteome.[4]
The presence of these salivary proteomic markers in low concentration plays a major role in the discrimination of diseases. Researchers have reported unique proteomic profiles indicative of oral cancer, cystic fibrosis, endocrinological and connective tissue disorders, dental caries, periodontitis and even breast carcinoma in saliva.[7,3]
The analysis of the salivary proteomes may reveal morbidity signatures in the early stage and monitor disease progression.
Proteomic studies of human saliva constitute four major salivary families of specific secretory proteins that differ significantly from other host defence salivary proteins, as the former group has specific functions in the oral environment. The aim of the proteomic study of saliva from mammals is to define the protein complex of whole human saliva in healthy controls, the contribution of the different glands, the alterations related to pathological conditions, either systemic or restricted to the oral cavity, to understand the functions of each protein component in the oral cavity, to characterize new peptides and proteins displaying biological activity.[14]
Four major salivary families of specific secretory proteins
Proline-rich proteins (PRPs), statherins, histatins and cystatins (can be distinguished in five cystatins of salivary origin (S, S1, S2, SA, SN) and two others common to various body fluids (C and D). They are multifunctional proteins playing various roles in the oral environment. Cystatin C, was un-detectable in saliva).[4]
The difficulties encountered in the study of the peptidome (the complete set of peptides encoded by a particular genome or present within a particular cell type) are due to the high genetic polymorphisms, complicated by individual insertions/deletions and alternative splicing to the complex posttranslational maturations including different proteolytic cleavages and glycosylation, phosphorylation and sulfation processes.[4]
Transcriptomics
Composed of coding (mRNA) and noncoding RNAs (small nucleolar RNAs, mi-RNAs, i.e., miRNAs and small nuclear RNAs), the transcriptome makes a significant source of potentially relevant diagnostic information with highly specific discriminatory indicators [Table 2].
Table 2.
Classification of salivary biomarkers depending on the level of action at molecular level
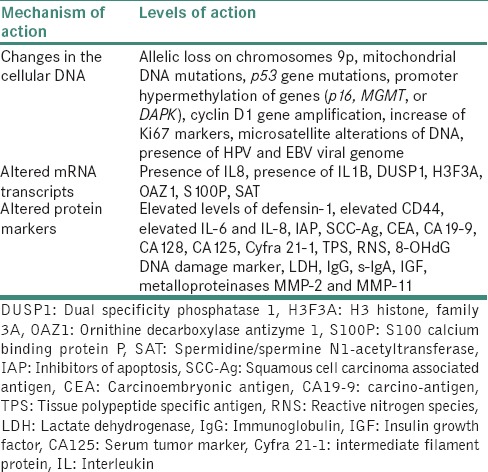
Whole saliva as well as saliva supernatant harbor both miRNAs and total RNA. Investigators probing the salivary transcriptome have identified over 1,000 miRNAs and more than 3,000 species of mRNAs in the human saliva, out of which 180 are common between different normal participants constituting the normal salivary transcriptome core.
In addition to the combined approach of transcriptomics and proteomics, miRNA constitutes the third diagnostic alphabet in saliva. This is because any dysregulation in expressing these miRNA will adversely affect the cell growth and acts like a tumor suppressor or oncogene in many cancers. In oral cancer, miRNAs have been shown to affect cell proliferation, apoptosis and even chemotherapy resistance in oropharyngeal squamous cell carcinomas (OSCC) patients, miRNAs have also been observed in OSCC to be epigenetically regulated by DNA methylation.[7,4]
Salivary metabolome
The collection of small molecules present in cells, tissues, organs and bio-fluids is known as metabolome, and the study of metabolome is metabolomics. The metabolome validates the parallel assessment of a group of endogenous and exogenous metabolites, including lipids, amino acids, peptides, nucleic acids, organic acids, vitamins, thiols and carbohydrates and is a valuable tool for discovering biomarkers, monitoring physiological status and making proper treatment decisions. For example, taurine and piperidine are considered as the oral cancer-specific diagnostic marker [Table 3].[4]
Table 3.
Salivary steroid hormones as biomarkers

Salivary microbiome
Oral microbiome is constituted by proportionately a minute number of bacterial phyla, of which the commonly reported abundant phyla are Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria and Fusobacteria. The majority of interindividual variation has been personated because of diversity at the species or strain level. Streptococcus is routinely observed to be the dominant genus in the healthy oral microbiome, and commonly Prevotella, Veillonella, Neisseria and Haemophilus dominate an individual's oral microbiome. Variation is also observed in the microbial community composition of biofilms at each intraoral habitat (e.g., teeth surface, lateral and dorsal surface of the tongue) most likely reflecting the different surface properties and microenvironments. Many individuals shelter only about 75–100 predominant species of bacteria which are known to inhabit the oral cavity from the reported 700–1,200 bacterial species that reside in the mouth.[4]
The identification of salivary biomarkers for oral, pancreatic, lung and gastric cancers along with Sjögren's syndrome, diabetes and periodontal disease is being pursued by researchers. Gene chip arrays and quantitative polymerase chain reaction denote the most common methodologies for generating diagnostic values.[3]
Recently, the Salivaomics Knowledge Base (SKB) has been established by aligning the salivary biomarker discovery. The SKB constitutes data repository, management system and web resource fabricated to support human salivary proteomics, transcriptomics, miRNA, metabolomics and microbiome research. The SKB provides the first web resource dedicated to salivary “omics” studies. This comprises the major data and information required to explore the biology, diagnostic potentials, pharmacoproteomics, and pharmacogenomics of human saliva.[4]
Employing saliva to monitor the health and disease state of an individual is a highly desirable goal for health promotion and health-care research. However, only recently has there been a growing appreciation of saliva as a mirror of the body which can reflect virtually the entire spectrum of normal and disease state and so an interest in saliva diagnostics is evolving.[5]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Malamud D. Saliva as a diagnostic fluid. Dent Clin North Am. 2011;55:159–78. doi: 10.1016/j.cden.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Javaid MA, Ahmed AS, Durand R, Tran SD. Saliva as a diagnostic tool for oral and systemic diseases. J Oral Biol Craniofac Res. 2016;6:66–75. doi: 10.1016/j.jobcr.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poornima G, Mahesh Kumar TS. Genomic alphabets of saliva as a biomarker in oral cancer. J Indian Acad Oral Med Radiol. 2017;29:300–5. [Google Scholar]
- 4.Ar P, Gulati A, Mehta D, Sugandhan S. Diagnostic applications of saliva in dentistry. Int J Clin Pediatr Dent. 2009;2:7–13. doi: 10.5005/jp-journals-10005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshizawa JM, Schafer CA, Schafer JJ, Farrell JJ, Paster BJ, Wong DT, et al. Salivary biomarkers: Toward future clinical and diagnostic utilities. Clin Microbiol Rev. 2013;26:781–91. doi: 10.1128/CMR.00021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarano E, Fiorita A, Picciotti PM, Passali GC, Calò L, Cabras T, et al. Proteomics of saliva: Personal experience. Acta Otorhinolaryngol Ital. 2010;30:125–30. [PMC free article] [PubMed] [Google Scholar]
- 7.Schafer CA, Schafer JJ, Yakob M, Lima P, Camargo P, Wong DT. Saliva diagnostics: Utilizing oral fluids to determine health status. Monogr Oral Sci. 2014;24:88–98. doi: 10.1159/000358791. [DOI] [PubMed] [Google Scholar]
- 8.Devi TJ. Saliva – A potential diagnostic tool. [Last accessed on 2018 Sep 19];IOSR J Dent Med Sci. 2014 13:52–7. Available from: http://www.iosrjournals.org . [Google Scholar]
- 9.Sindhu S, Jagannathan N. Saliva: A cutting edge in diagnostic procedures. J Oral Dis. 2014;2014:8. [Google Scholar]
- 10.Madalli VB, Basavarradi SM, Burde K. Saliva – A diagnostic tool. IOSR J Dent Med Sci. 2013;11:96–9. [Google Scholar]
- 11.Arunkumar S, Arunkumar JS, Burde KN, Shakunthala GK. Developments in diagnostic applications of saliva in oral and systemic diseases – A comprehensive review. J Sci Innov Res. 2014;3:372–87. [Google Scholar]
- 12.Shah FD, Begum R, Vajaria BN, Patel KR, Patel JB, Shukla SN, et al. Areview on salivary genomics and proteomics biomarkers in oral cancer. Indian J Clin Biochem. 2011;26:326–34. doi: 10.1007/s12291-011-0149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor JJ. Protein biomarkers of periodontitis in saliva (Review article) ISRN Inflamm. 2014;2014:18. doi: 10.1155/2014/593151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittal S, Bansal V, Garg S, Atreja G, Bansal S. The diagnostic role of saliva a review. J Clin Exp Dent. 2011;3:e314–20. [Google Scholar]


