Abstract
Context:
Salivary analytes may be used as biomarkers for translational and clinical applications. Heat shock proteins (Hsps) are ubiquitous, highly conserved proteins found in all prokaryotic and eukaryotic species. Hsp27, a low molecular weight protein, may act as a salivary biomarker. Leukoplakia is the most common oral potentially malignant disorder and various salivary biomarkers such as interleukin-6, 8, tumor necrosis factor-α and MMPs have been detected in it. Oral leukoplakia presents clinically as homogenous and nonhomogenous forms; the microscopic pattern ranges from simple epithelial hyperplasia to carcinoma in situ.
Aims:
This study aims to detect salivary Hsp27 in oral leukoplakia by enzyme-linked immunosorbent assay (ELISA) and to correlate its expression pattern with histopathology.
Materials and Methods:
A total of 45 cases had constituted the study group. Salivary Hsp27 levels were assessed by ELISA in histopathologically confirmed cases of oral leukoplakia and were compared with that of healthy volunteers.
Statistical Analysis:
Mann–Whitney U-test and Spearman's correlation coefficient were used for the detection of Hsp27 and its correlation with mean absorbance levels.
Results:
The mean absorbance values had shown elevated expression of Hsp27 in oral leukoplakia when compared to that in healthy volunteers.
Conclusions:
The present study had shown elevated expression of salivary Hsp27 in oral leukoplakia which could be attributed to altered redox potential.
Keywords: Enzyme-linked immunosorbent assay, epithelial dysplasia, heat shock protein 27, oral leukoplakia, saliva, tobacco
INTRODUCTION
Oral mucosa is exposed to various irritants, due to which the alterations noticed are hypertrophy, hyperplasia, atrophy, altered growth (dysplasia) and so on; one such alteration may be epithelial dysplasia.[1,2] Lesions with such a dysplastic pattern appear clinically as a white and/or a red lesion; leukoplakia, erythroplakia and oral submucous fibrosis are a few to mention.
Among various oral potentially malignant disorders (OPMDs), oral leukoplakia appears to be common in clinical practice. The recent definition of oral leukoplakia goes as: “A white plaque of questionable risk having excluded (other) known diseases/disorders that carry no increased risk for cancer.”[3]
Oral leukoplakia exhibits wide variation in its clinical presentation, ranging from slightly elevated gray or gray-white plaques/corrugated white patches to nodular lesions. Homogenous and nonhomogenous forms are the two main clinical forms of oral leukoplakia.[4,5] The microscopic picture of oral leukoplakia may be categorized as nondysplastic and dysplastic types. The nondysplastic type exhibits the features of surface hyperkeratosis with or without epithelial hyperplasia and there is no evidence of epithelial dysplasia. The dysplastic type of oral leukoplakia shows mild, moderate or severe degrees of epithelial dysplasia; sometimes carcinoma in situ.[1,2,4]
The genetic and epigenetic factors have been implicated in the causation of oral leukoplakia;[6] one important epigenetic factor is tobacco, either in smoked/smokeless form. Following the use of tobacco, chronic inflammatory process sets in. It is well known that cytokines and other inflammatory mediators play an important role in carcinogenesis. Interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) may act as promoters of carcinogenesis. Patients with oral leukoplakia exhibit increased production of IL-6 and TNF-α which have been detected in salivary samples.[7] In addition, metal ions (secondary to tobacco smoking) induce increased chaperone-like activity of the heat shock proteins (Hsps).[8]
Hsps are ubiquitous, highly conserved proteins that aid in protein assembly, stabilization, folding and translocation of oligomeric proteins.[9,10] Among various Hsp subtypes, Hsp27/HspB1 belongs to a family of the small molecular weight type. Hsp27 has a key role in the elevation of the cells' resistance to oxidative stress, antiapoptotic pathway, tumor progression and metastasis.[8,9,10,11,12,13]
In addition to IL-6, TNF-α and many more molecules, few members of Hsps such as Hsp60, Hsp70 and Hsp 90 have been detected in the saliva.[14,15]
“Salivaomics” (constellation of technologies to explore different molecules found in saliva) includes proteomics, metabolomics, transcriptomics, genomics, epigenomics and microbiota through polymerase chain reaction assays, mass spectrometry, Raman spectroscopy and next-generation sequencing.[16]
Enzyme-linked immunosorbent assay (ELISA) is a simpler technique to assess specific protein in the given salivary/serum sample and is of three types – indirect, sandwich and competitive.[17]
Considering the noninvasive nature of the salivary sample collection, biomarker analysis by a reliable and an economical tool (ELISA) in a more common oral lesion/OPMD (oral leukoplakia), coupled with thorough review of scientific English literature prompted the authors to investigate the role of salivary Hsp27 in patients with oral leukoplakia.
The present study was first of its kind to detect salivary Hsp27 in patients with oral leukoplakia by ELISA and had further correlated its expression pattern with the histopathology of oral leukoplakia.
MATERIALS AND METHODS
The study sample had comprised a prospective group of healthy volunteers and oral leukoplakia patients. The study participants were selected from the Outpatient Department (Oral Medicine and Radiology) of DAPM R V Dental College and Hospital, Bengaluru. A total of 45 cases (29 normal and 16 leukoplakic) were included, whose age had ranged from 23 to 60 years. In the control group, one was male and 28 were female; in the leukoplakic group, all 16 participants were male. Clinically diagnosed patients of oral leukoplakia were considered.[18] Written informed consent was obtained from all the study participants. Clearance from the institutional review board was obtained. As part of the study protocol, complete blood cell count was performed for all, to check systemic health. Participants with normal hematological parameters were only included. Study individuals were subjected to oral prophylaxis, were prepared for saliva collection and lesional biopsy was performed. All known oral foci of infection were removed before saliva collection. Clinically diagnosed [Figure 1] and histopathologically confirmed cases of oral leukoplakia (nondysplastic [Figure 2] and dysplastic forms) were included for the present study.
Figure 1.
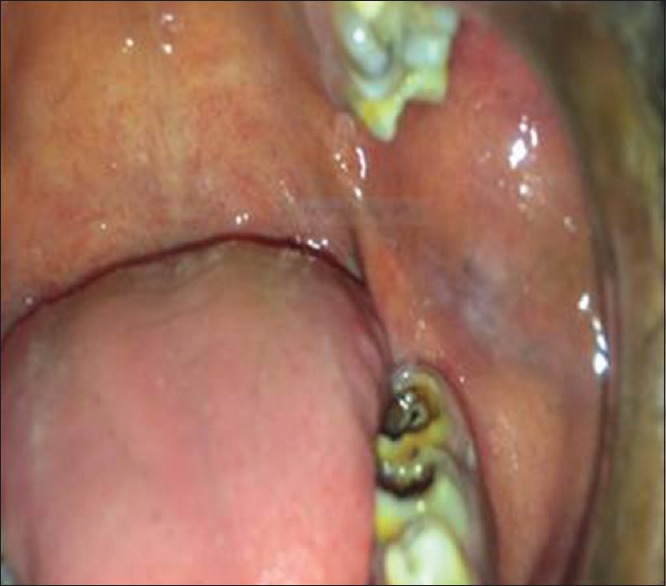
Clinical photo showing white patch along with area of pigmentation on the left buccal mucosa suggestive of homogenous leukoplakia
Figure 2.
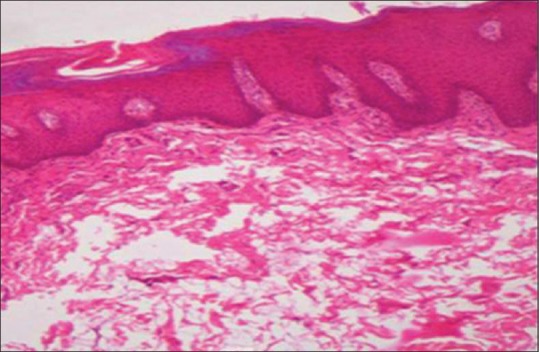
Photomicrograph showing hyperkeratotic lesion with epithelial hyperplasia (H&E stain, ×100)
The total study period was 15 months.
Strict aseptic precautions were followed wherever required. One week after oral prophylaxis, the study participants were called for the collection of saliva samples during early hours of the day. The participants were asked to refrain from eating or drinking for at least 1 h before the collection of saliva. The study participants were given drinking water and were asked to rinse their mouth well. Rinsing was for about 2–3 min. Ten minutes after this oral rinse, the participants were asked to spit whole saliva into a sterile Falcon tube (50 ml capacity). The participants were refrained from speech and were instructed to drop the head down, hold for a while and were asked to spit the saliva into the tube provided. Patients were asked to spit into the collection tube about once a minute for up to 10 min. About 1.0–1.5 ml of saliva was collected from every study individual. The participants were reminded not to cough up mucus/phlegm during saliva collection.
Collected salivary samples were centrifuged at 1200 rpm, stored in deep freezer at −20°C and were subjected to Sandwich ELISA procedure. ELISA was carried out at SKANDA life Sciences Private Limited, Bengaluru.
The principle of sandwich ELISA procedure involves attachment of a capture antibody to solid phase support. Samples containing known or unknown antigen were added in a matrix or buffer that had minimized attachment to the solid phase. An enzyme-labeled antibody was then added for detection [Figures 3 and 4]. ORIGENE Hsp27 ELISA kit (Sentier Laboratories Private Limited, Bengaluru) was used for the present study. At the end of the procedure, the mean absorbance and Hsp27(pg/ml) values were calculated for both control and leukoplakic samples. A curve was plotted for both the groups and the correlation of mean absorbance and mean Hsp27 were calculated for both the groups.
Figure 3.
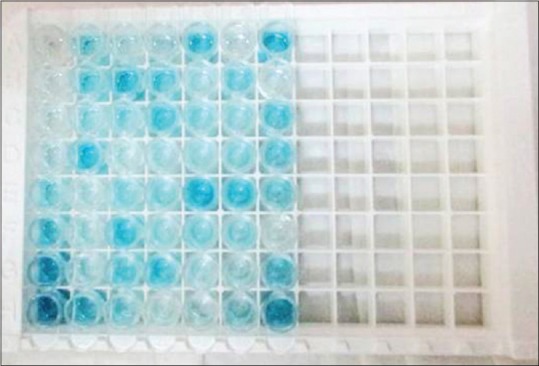
Salivary samples were blue before addition of stop solution
Figure 4.
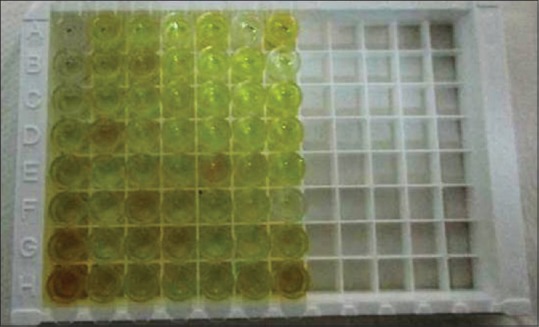
Salivary samples had turned yellow after addition of stop solution
From the same study (leukoplakia group) participants, an incisional/excisional biopsy was done depending on the size of the leukoplakic lesion; the specimen was subjected to routine tissue processing with paraffin wax as embedding medium using the standard protocol. The hematoxylin and eosin staining was carried out.[19] The stained tissue sections were reviewed under the research microscope. The WHO (2005) guidelines were used for grading epithelial dysplasia.[20] Two oral pathologists had graded the slides independently. Any difference of opinion was discussed and a mutual consensus was arrived at. Photomicrographs were captured using Prog Res software. The results were tabulated for further analyses.
Mann–Whitney U-test and Spearman's correlation coefficient were used for the statistical analyses.
RESULTS
The study was conducted on 45 participants (29 healthy volunteers and 16 leukoplakic patients). In leukoplakic group, 14 were homogeneous type and 2 cases were diagnosed as nonhomogeneous form.
After routine tissue processing of biopsied specimens, the hematoxylin and eosin stained sections were observed under research microscope. The WHO guidelines (2005) were considered for grading epithelial dysplasia. The histopathological evaluation had revealed nondysplastic (hyperkeratosis with hyperplastic epithelium) features for 11 cases and dysplastic pattern for 5 cases.
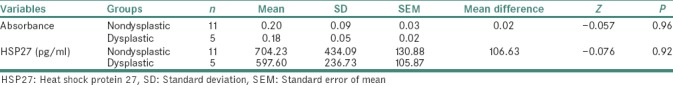
ELISA of salivary samples from the study participants had shown mean absorbance values and mean Hsp27 (pg/ml) values, to be more in leukoplakic patients when compared to that of healthy volunteers. The values obtained for the correlation between absorbance and Hsp27 (pg/ml), between control and leukoplakia groups were statistically significant [Tables 1 and 2]. The mean absorbance values and mean Hsp27 (pg/ml) were more in nondysplastic cases when compared to that in dysplastic cases. The mean difference between nondysplastic and dysplastic forms of oral leukoplakia was not statistically significant [Table 3].
Table 1.
Comparison of mean absorbance and heat shock protein 27 (pg/ml) between oral leukoplakia and control group using Mann–Whitney U-test
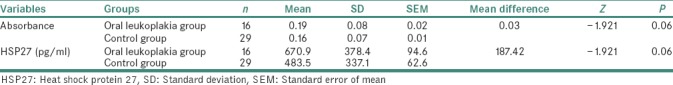
Table 2.
Correlation between absorbance and heat shock protein 27 (pg/ml) in oral leukoplakia and control group using Spearman's correlation test
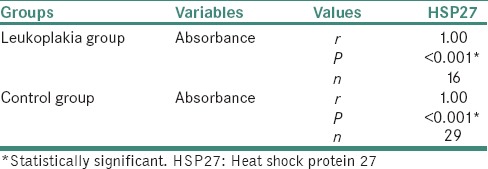
Table 3.
Comparison of mean values of the study variables between the nondysplastic and dysplastic cases within oral leukoplakia group using Mann–Whitney U-test
DISCUSSION
Oral mucosa is exposed to various irritants, due to which altered growth/differentiation occurs; one such alteration could be epithelial dysplasia.[1,2] Oral leukoplakia, a potentially malignant disorder, is a relatively common entity and its global prevalence has been estimated as 2.60%.[21] The leukoplakic lesions may exhibit features of epithelial dysplasia.[1,2]
Chronic habit of tobacco consumption is the main etiological factor for oral leukoplakia.[1] Generation of inflammatory mediators (secondary to tobacco usage) will increase over time and could be detected in patients with oral leukoplakia. Such salivary biomarkers would be IL-6, IL-8, TNF-α, MMPs and so on;[7,8] among these, Hsp27 was chosen for the present study.
Hsps are ubiquitous, highly conserved proteins that play an important role in protein assembly and stabilization. Hsps are broadly divided into low molecular weight and high molecular weight types. Hsp27, a member of subfamily of small Hsps, shows its expression in various tissues of the body, cytoplasm of the cell and in saliva.[8,9,10] Hsp27 has not been studied in saliva of oral leukoplakia, hence it was considered for the present study. To study salivary Hsp27, ELISA was chosen as it is a simpler procedure that detects a specific salivary protein with descent amount of accuracy. The advantages of using ELISA over other molecular methods would be – cost-effectiveness, good amount of sensitivity and specificity.[17]
Although Hsp27 has been studied by very few, in tissue sections of oral leukoplakia, there are no reported studies in scientific English literature on salivary Hsp27 expression in oral leukoplakia. The present study was first of its kind to detect Hsp27 in the saliva of oral leukoplakia patients.
The present study had considered 29 healthy volunteers (control group) and 16 oral leukoplakic patients – a total of 45 study participants. In the control group, one was male and 28 were females. In the leukoplakic group, all 16 participants were males. The age of the study participants had fallen between 23 and 60 years. All the study participants were from prospective group and the total study period was 15 months. Within this period, selection and preparation of the patients, hematological examination, collection and analysis of salivary samples, histopathological evaluation of the biopsied samples were carried out. On histopathological examination, out of 16 leukoplakic cases, 11 were nondysplastic and 5 were dysplastic types. The saliva collection from the healthy individuals and from oral leukoplakic patients, was in Falcon tubes (1–1.5 ml), during early hours of the day. The salivary samples were centrifuged and were stored in the deep freezer (−20°C). The technique of Sandwich ELISA was performed with the saliva samples, and the absorbance and Hsp27 (pg/ml) values were detected for both control and leukoplakic groups. There were elevated absorbance levels and elevated Hsp27 expression levels in oral leukoplakic patients when compared to that of healthy volunteers. The mean absorbance and mean Hsp27 (pg/ml) expression levels were higher in nondysplastic cases when compared to that of dysplastic cases of leukoplakic patients [Tables 1–3].
The reasons for the elevated expression of Hsp27 levels in saliva of oral leukoplakia patients might be attributed to the generation of reactive oxygen species (ROS) and reactive nitrogen species from the tobacco products that in turn could have resulted in upregulation of Hsp27.
Oral leukoplakic lesions exhibit chronic inflammatory reaction secondary to tobacco usage. Various inflammatory mediators induce the generation of free radicals with the subsequent cell injury and cell death, causing tissue damage. Under the conditions of cellular stress, Hsps come to the rescue of the cells as they have antioxidant potential.
The potential means of oxidative stress would be Cu2+ and ascorbate-mediated generation of ROS.
Chronic exposure to tobacco smoke results in metal ion induced increase in the chaperone-like activity of the small Hsps. Being a small Hsp, Hsp27 confers cytoprotection against the generation of ROS by binding to Cu2+ with close to picomolar affinity, inhibiting the Cu2+-ascorbate-induced generation of ROS.[8,9,10,11]
The antiapoptotic mechanism and/or the regulatory role of Hsp27 in cell proliferation and differentiation could be attributed to excessive proliferation of oral epithelial cells in nondysplastic leukoplakic lesions of the present study.
Of course, a small sample in the present study might have influenced the difference in the expression levels of Hsp27 between nondysplastic and dysplastic types of oral leukoplakic cases.
Since there are no similar studies to compare, few reported studies on Hsp27 expression at the tissue level in some oral lesions have been considered.
Leonardi et al. had evaluated Hsp27 expression in normal oral mucosa, oral premalignant epithelial lesions and oral squamous cell carcinoma. Hsp27 had shown stronger expression in basal and parabasal layers of normal oral epithelium. In potentially malignant lesions, the Hsp27 expression was low. In oral squamous cell carcinoma, both low and high Hsp27 expression levels were noticed. Thus, Hsp27 was downregulated in poorly differentiated areas and was upregulated in highly differentiated regions. These observations had inferred that downregulation of Hsp27 in dysplasia could impair the protective mechanism against mutagenesis triggered by various environmental factors and thus promoting the malignant transformation of oral epithelial dysplasia.[11]
In the present study, salivary Hsp27 levels were more in nondysplastic oral leukoplakic cases when compared to that in dysplastic group of oral leukoplakia.
In another study, the expression of Hsp-27, 60 and 70 was studied in normal oral mucosa, oral leukoplakia and in oral squamous cell carcinoma. The immunohistochemical analysis had shown Hsp-27, 60 and 70 to be definitely positive in oral squamous cell carcinoma cells, whereas Hsp-27 and 70 were definitely positive in oral leukoplakia. In normal oral epithelium, only Hsp60 was definitely positive. The changes in expression of Hsp-70 and 27 have been implicated in the tumorigenesis of oral squamous epithelium.[12]
In the present study, Hsp27 values were more in salivary samples of oral leukoplakic patients when compared to that of healthy volunteers.
Siqueira et al. had evaluated the expression of Hsp27 in normal salivary glands and in pleomorphic adenoma tissue samples by ELISA. Hsp27 was expressed by all the normal salivary gland tissue samples and only half of the pleomorphic adenoma tissues had shown the positive expression for Hsp27. In addition, Hsp27 expression levels had correlated positively with the Bcl2/Bax mRNA ratio, thereby suggesting an antiapoptotic effect.[22]
On the contrary, the present study had shown elevated Hsp27 levels in saliva of diseased group (oral leukoplakia) when compared to that of healthy volunteers.
The present study was an initial attempt to assess the salivary expression of Hsp27 in oral leukoplakic patients. Further, age-gender and site-matched control studies on oral leukoplakia are desirable to confirm the expression of salivary Hsp27. Thorough check of the systemic health of the participants with removal of all possible oral foci of infection is to be considered to know the role played by Hsp27, by assessing its expression levels solely attributable to altered oral mucosa. Needless to say that, larger sample size with long-term follow-up is mandatory to correlate the salivary expression of Hsp27 in oral leukoplakia cases. Expression of salivary Hsp27 may also be compared with that of tissue in oral leukoplakic lesions. Salivary and tissue Hsp27 may be assessed in pre- and postinterventional phase of oral leukoplakia and may be extended to other OPMDs, as well as correlating with their malignant transformation potential.
CONCLUSIONS
Oral leukoplakia is the most common OPMD and various salivary biomarkers such as IL-6, 8 and TNF-α have been detected in it. The present study was first of its kind to detect Hsp27 in the saliva of oral leukoplakia patients, wherein the expression of Hsp27 levels was elevated when compared to that of healthy volunteers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Suchetha (Professor and HOD), Department of Periodontics, DAPM RV Dental College and Hospital, Bengaluru, for her kind cooperation toward the study. Special thanks to Sentier laboratories Private Limited, Bengaluru, for providing ORIGENE Human Hsp27 kit and the scientific team of Research and Development section of Skanda Life Sciences, Bengaluru, for carrying out ELISA; many thanks to Dr. Santosh Kumar for carrying out statistical analysis. The authors would express token of gratitude to postgraduates: Dr. Amrita S, Dr. Garima P, Dr. Lipika K, Dr. Roopa P G, Dr. Deepa K K and Dr. Azam M for their help; special thanks to Mrs. Jaya, Mrs. Netravati and Mr. Chandrashekhar, for technical work.
REFERENCES
- 1.Neville BW, Damn DD, Allen CM, Bouquot JE. Oral and Maxillofacial Pathology. 3rd ed. Philadelphia, PA: Saunders; 2009. pp. 388–97. [Google Scholar]
- 2.Madhura MG, Kansal L, Kumar BV, Bhavana VS. Algorithm to reduce subjectivity in grading oral epithelial dysplasia - A preliminary study. J Adv Clin Res Insights. 2016;3:112–7. [Google Scholar]
- 3.Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36:575–80. doi: 10.1111/j.1600-0714.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 4.Sivapathasundharam B. Shafer's Text Book of Oral Pathology. 8th ed. India: Elsevier; 2016. pp. 138–42. [Google Scholar]
- 5.Kramer IR, Pindborg JJ, Bezroukov V, Infirri JS. Guide to epidemiology and diagnosis of oral mucosal diseases and conditions. World Health Organization. Community Dent Oral Epidemiol. 1980;8:1–26. doi: 10.1111/j.1600-0528.1980.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 6.Lingen MW, Pinto A, Mendes RA, Franchini R, Czerninski R, Tilakaratne WM, et al. Genetics/epigenetics of oral premalignancy: Current status and future research. Oral Dis. 2011;17(Suppl 1):7–22. doi: 10.1111/j.1601-0825.2011.01789.x. [DOI] [PubMed] [Google Scholar]
- 7.Brailo V, Vucićević-Boras V, Cekić-Arambasin A, Alajbeg IZ, Milenović A, Lukac J, et al. The significance of salivary interleukin 6 and tumor necrosis factor alpha in patients with oral leukoplakia. Oral Oncol. 2006;42:370–3. doi: 10.1016/j.oraloncology.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Bakthisaran R, Tangirala R, Rao ChM. Small heat shock proteins: Role in cellular functions and pathology. Biochim Biophys Acta. 2015;1854:291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Hemmingsen SM, Woolford C, van der Vies SM, Tilly K, Dennis DT, Georgopoulos CP, et al. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988;333:330–4. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- 10.Vos MJ, Hageman J, Carra S, Kampinga HH. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry. 2008;47:7001–11. doi: 10.1021/bi800639z. [DOI] [PubMed] [Google Scholar]
- 11.Leonardi R, Pannone G, Magro G, Kudo Y, Takata T, Lo Muzio L, et al. Differential expression of heat shock protein 27 in normal oral mucosa, oral epithelial dysplasia and squamous cell carcinoma. Oncol Rep. 2002;9:261–6. [PubMed] [Google Scholar]
- 12.Tekkeşin MS, Mutlu S, Aksakalli N, Olgac V. Expression of heat shock proteins 27, 60 and 70 in oral carcinogenesis: An immunohistochemical study. Tűrk Onkol Derg. 2011;26:115–20. [Google Scholar]
- 13.Kapranos N, Kominea A, Konstantinopoulos PA, Savva S, Arletaris S, Vandoros G, et al. Expression of the 29-kDa heat shock protein (HSP27) in gastric carcinomas and adjacent normal, metaplastic and dysplastic gastric mucosa, and its prognostic significance. J Cancer Res Clin Oncol. 2002;128:426–32. doi: 10.1007/s00432-002-0357-y. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee S, Damle SG, Sharma AK. Salivary heat shock proteins and their interactions with oral microenvironment. Inflamm Cell Signal. 2014;1:e101. [Google Scholar]
- 15.Fábián TK, Gáspár J, Fejérdy L, Kaán B, Bálint M, Csermely P, et al. Hsp70 is present in human saliva. Med Sci Monit. 2003;9:BR62–5. [PubMed] [Google Scholar]
- 16.Wang X, Kaczor-Urbanowicz1 KE, Wong DT. Salivary biomarkers in cancer detection. Med Oncol. 2017;34:7. doi: 10.1007/s12032-016-0863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Overview of ELISA. [Last accessed on 2016 Mar 04]. Available from: http://www.Thermoficher.com/in .
- 18.van der Waal I. Oral leukoplakia, the ongoing discussion on definition and terminology. Med Oral Patol Oral Cir Bucal. 2015;20:e685–92. doi: 10.4317/medoral.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suvarna SK, Layton C, Bancroft JD. Bancroft's Theory and Practice of Histological Techniques. 7th ed. China: Churchill Livingstone Elsevier; 2013. pp. 173–9. [Google Scholar]
- 20.Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization Classification of Tumours: Pathology and Genetics of Head and Neck Tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 21.Petti S. Pooled estimate of world leukoplakia prevalence: A systematic review. Oral Oncol. 2003;39:770–80. doi: 10.1016/s1368-8375(03)00102-7. [DOI] [PubMed] [Google Scholar]
- 22.Siqueira EC, Souza FT, Diniz MG, Gomez RS, Gomes CC. Hsp27 (HSPB1) differential expression in normal salivary glands and pleomorphic adenomas and association with an increased Bcl2/Bax ratio. Tumour Biol. 2015;36:213–7. doi: 10.1007/s13277-014-2634-1. [DOI] [PubMed] [Google Scholar]


