Abstract
Introduction:
Oral cancer is a multistep process involving foul play of proto-oncogenes that induce cell proliferation, inactivation of tumor suppressor gene and cessation of programmed cell death. Among various proto-oncogenes, the nature and behavior of Bcl-2 and c-Myc in oral precancerous/cancerous lesions were obscured which require further assessment for better understanding of etiology, treatment and prognosis.
Aim:
The aim of the study is to assess the expression of Bcl-2 and c-Myc in oral epithelial dysplasia and oral squamous cell carcinoma (OSCC).
Materials and Methods:
This retrospective study of 70 (oral dysplasia [30], OSCC [30] and normal gingiva [10]) is immunohistochemically assessed for Bcl-2 and c-Myc for distribution, intensity, percentage of positive cells, localization and immunoreactive scores using ImageJ software.
Results:
Bcl-2 showed 60% and 37% positivity within dysplasia and OSCC, respectively (P = 0.03); c-Myc showed 87% and 90% positivity within dysplasia and OSCC, respectively. In OSCC, c-Myc showed moderate intensity (P = 0.04). Average percentage of positive cells expressing c-Myc and Bcl-2 increased proportionally within grades of dysplasia (P = 0.000 and P = 0.008, respectively), whereas in OSCC, only c-Myc showed significant expression (P = 0.021). Localization of c-Myc was seen in the nucleus among OSCC (P = 0.01). c-Myc and Bcl-2 showed moderate immunoreactivity in dysplasia (P = 0.005 and P = 0.013, respectively), whereas in OSCC, moderate immunoreactivity of c-Myc (P = 0.05) was observed.
Conclusion:
Variable expression of c-Myc and Bcl-2 reveals that these proteins act in synergism in early phases of carcinogenesis, whereas in later stages, due to the diminished activity of Bcl-2, c-Myc interacts incoordination with other oncogenes contributing to tumor progression.
Keywords: Antiapoptotic protein, Bcl-2, c-Myc, dysplasia and oral squamous cell carcinoma, immunohistochemistry, nuclear transcription factor
INTRODUCTION
Cancer is a heterogeneous disease with complex molecular alteration that includes the genetic and epigenetic changes. Oral squamous cell carcinoma (OSCC) is the 8th most common oral malignancy constituting 90% of all oral malignancies,[1] with higher incidence in South Asian countries. About 80% of oral carcinomas are preceded by oral potentially malignant disorders, most often, persistent leukoplakia or oral submucous fibrosis.[2]
Proliferation, apoptosis and differentiation are fundamental aspects of tumor biology.[3] Several oncogenes are involved in initiation and progression of cancer. Proto-oncogenes such as c-Myc and Bcl-2 categorized as genes regulating cellular proliferation and genes evading apoptosis are documented in tumor progression. c-Myc gene located on chromosome 8q21 is a nuclear transcription factor involved in cell proliferation, differentiation and apoptosis. It is implicated in activation of cyclin-dependent kinases facilitating transcription, therefore promoting cell proliferation.[4] Bcl-2 is an antiapoptotic gene on chromosome 18, which maintains membrane integrity of mitochondria and involved in apoptotic pathway. It evades apoptosis and facilitates cell survival.[5]
Cellular oncogenes, c-Myc and Bcl-2, have a parallel effect in progression of tumor. Several studies documented the synergistic mechanism of Bcl-2 and c-Myc in the abrogation of apoptosis and facilitating abnormal proliferation of the cell.[6,7] Literature contains limited data regarding these proteins and their coexpression in oral dysplasia and OSCC. However, it is unclear whether the overexpression of these genes can be identified as molecules to forecast the aggressiveness, prognosis and be useful in treatment. Thus, the present study is an effort to unveil the unclear aspects of these proteins in oral dysplasia and OSCC.
MATERIALS AND METHODS
This retrospective study constituting 70 cases of 10% neutral-buffered formalin-fixed, paraffin-embedded blocks was retrieved from the archives of department of oral pathology, which comprised n = 10 cases of normal gingiva, n = 30 cases of oral dysplasia (10 – mild, 10 –moderate and 10 – severe) and n = 30 cases of OSCC (10 – well, 10 – moderate, 10 – poor). The set samples were further segregated demographically by age, gender, lesion site and habit history from the records.
About 3-μm thick sections were deparaffinized in xylene, rehydrated in grades of alcohol and washed. Antigen retrieval was done using Tris-ethylenediaminetetraacetic acid (EDTA) buffer for Bcl-2 (pH-9) and EDTA buffer for c-Myc (pH-8) in a pressure cooker. The cooker is left to cool toward room temperature, and the slides were washed with phosphate-buffered saline (PBS). Endogenous peroxidases were blocked by treating with peroxide block for 10 min. Sections were incubated with primary rabbit monoclonal antibodies that include Bcl-2 oncoprotein (PathnSitu; clone: EP36) and c-Myc oncoprotein (PathnSitu; clone: EP124) for 1 h. Target binder was used to enhance the uptake of secondary antibody for 20 min followed by incubation with secondary antibody poly HRP (PolyExcel HRP/DAB Detection System, Universal Kit, PathnSitu) for 15 min. After every single step, slides were washed with PBS 2 times. Each wash should be done for 5 min. Finally, sections were incubated with DAB for 3 min in dark, washed in water, counterstained with Harris hematoxylin and mounted.
Tonsil for Bcl-2 and carcinoma of stomach for c-Myc were considered as positive control. For negative control, the sections were treated with PBS instead of primary antibody. Resident lymphocytes positive for Bcl-2 and c-Myc were considered as internal controls. One positive control and one negative control were included with each batch of immunostained sections. Two investigators observed the sections under light microscope and evaluated using ImageJ software (developed at national institutes of health by Wayne Rasband, Bethesda, Maryland, USA). Observer variability was eliminated by achieving common consensus.
The expression of Bcl-2 and c-Myc was evaluated for distribution, intensity, percentage of positive cells, localization and immunoreactive scores. The authors of the present study modified Allred's semi-quantitative immunoreactive score to give a common score to all the lesions. The immunoreactive score was obtained by adding the qualitative and quantitative indices.
Staining evaluation was done by selecting ten representative fields randomly at 40× high-power magnification for quantification and localization of stain. Sections with <50% positive cells were considered focal and >50% as diffuse when viewed under lower magnification. Both nuclear and cytoplasmic localizations were considered as positive for both the antibodies. Qualitative assessment was done by evaluating the staining intensity which was classified into four groups: 0 – no staining, 1 – weak, 2 – moderate and 3 – strong. Quantitative analysis included the extent of staining and was classified as follows: (1) <25% of epithelial cells demonstrated positivity, (2) 25%–50% of cells demonstrated positivity, (3) 50%–75% of cells demonstrated positivity and (4) >75% of cells demonstrated positivity. A final immunoreactive score was established by adding qualitative and quantitative indices. According to this final score, the cases were divided into four groups: 0 – negative, 1–3 – weak, 4–5 – moderate.
Statistical analysis
The statistical analysis was performed with the use of SPSS software, (IBM Inc, Chicago, Illinois, USA)version 16. Pearson Chi-square test was used for comparing the staining pattern, intensity and localization and immunoreactivity of the Bcl-2 and c-Myc in study groups. One-way ANOVA test and Kruskal–Wallis test were used for comparing average number of positive cells in different study groups. Tukey's honestly significant difference test was used for multiple comparisons among grades of different study groups. P < 0.05 was considered statistically significant.
RESULTS
Out of 70 cases, 49 (70%) were males and 21 (30%) were females within an age range of 20–80 years. Predominant cases reported in the 5th–7th decade. The most common site affected by oral dysplasia is buccal mucosa 22 (69%), whereas tongue 13 (76.5%) is the most common site of involvement by OSCC. Smoking is the consistent habit observed in both the study groups.
Dysplasia showed 87% and 60% positivity for c-Myc and Bcl-2, respectively. OSCC showed 90% and 36.6% positivity for c-Myc and Bcl-2, respectively. The difference is statistically significant for c-Myc and Bcl-2 (P = 0.085 and P = 0.034, respectively).
Moderate intensity of c-Myc and Bcl-2 staining was detected in all the three study groups. Comparative analysis was done using Pearson Chi-square test which revealed statistical significance only for Bcl-2 with P = 0.010.
c-Myc and Bcl-2 showed exponential increase in positive cells with grades of dysplasia and OSCC (P = 0.000 and P = 0.042, respectively), with highest percentage in severe dysplasia (95.26% and 94.33%, respectively).
In spite of variations in immunolocalization, c-Myc and Bcl-2 were chiefly detected in cytoplasm of control as well as the study groups. Statistically significant correlation was achieved using Pearson Chi-square test with P = 0.000 and P = 0.015, respectively [Graph 1].
Graph 1.
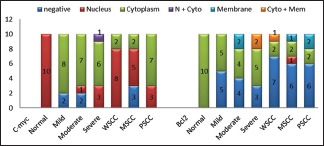
Comparative localization of c-Myc and Bcl-2 between control and study groups
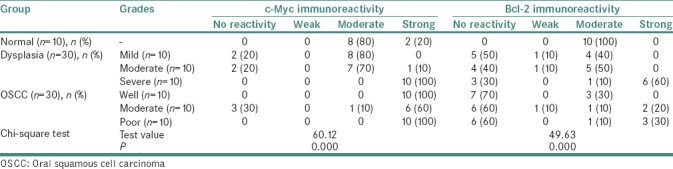
Bcl-2 exhibited moderate immunoreactivity among all the study groups (P = 0.000). c-Myc showed varied immunolabeling from moderate to strong reactivity in different study groups [P = 0.000; Table 1].
Table 1.
Comparative immunoreactive score of c-Myc and Bcl-2 between control and study groups
DISCUSSION
Apoptosis, an active cellular process occurring both physiologically and pathologically, is responsible for normal turnover of adult tissue and of malignant tumors.[8] c-Myc and Bcl-2 are the proteins involved in the regulation of apoptosis and cell proliferation. Any aberrations in normal integrated molecular network amid cell proliferation and apoptosis result in “oncogenic nexus” that drive toward growth and metastasis of tumor.[9] These aberrations become strong indicators of cancerous transformation as well as cancers. OSCCs account for 30% of all cancers in India with 5-year survival rate below 20% when cervical metastasis is present.[10] The present study also showed male predominance with peak incidence in the 5th–7th decade of life which was in accordance with the literature.[11]
In India, 50% of OSCC are seen affecting the buccal mucosa, whereas it accounts for 5% in Western countries.[10] In the present study, most of the cases are associated with smoking and buccal mucosa is the most common site affected by OSCC.
OSCC most often arises from oral potentially malignant disorders. Epithelial dysplasia is the gold standard for the identification of potentially malignant disorders.[12] In spite of extensive research on many molecular markers, still there are lacunae for reliable predictive molecular markers in OSCC. The present study focuses to test the hypothesis that whether the cellular oncogenes, c-Myc and Bcl-2, can be identified as molecules to forecast the aggressiveness and prognosis.
Tumor initiation results from the absence of checkpoints in cell cycle and genomic instability caused by telomere dysfunction. Telomerase gets activated in order to stabilize the length of chromosome after every cell division. Human telomerase reverse transcriptase is the catalytic subunit of telomerase which is regulated by cellular transcriptional activator c-Myc.[4,13,14] Myc proteins activate cyclin-dependent kinases facilitating transcription and consequently promoting cell proliferation.[4] Thus, dysregulation of c-Myc causes deregulated DNA synthesis, thereby uncontrolled cellular proliferation and malignant transformation.[4,13,14]
Bcl-2 (B-cell lymphoma/leukemia-2) gene is an integral membrane protein first discovered in B-cell lymphomas. Radiation, cytotoxic drugs and reactive oxygen species disrupt mitochondrial membrane releasing cytochrome c which induces apoptosis. Bcl-2 inhibits such apoptosis by preserving membrane integrity.[15,16] Bcl-2 overexpression increases the longevity of the cell and thus provides chance for further accumulation of other mutations.[7]
The present study showed 87% and 90% positivity of c-Myc within dysplasia and OSCC, respectively. Papakosta et al.[13] (77%) and Segura et al.[17] (87%) studies had similar proportions of positivity in dysplasia, while studies of Sakai et al.[18] (100%), Scott et al.[8] (98.57%) and Ozdek et al.[6] (91.8%) supported the present observations within OSCC.[6,8,13,18]
Bcl-2 showed 60% and 37% positivity within dysplasia and OSCC, respectively (P = 0.034). The results of the present study in dysplasia showed similarity with Sudha and Hemavathy[2] (100%) and Soares et al.[3] (70%). This study showed a decline in Bcl-2 expression in OSCC. This probably suggests that Bcl-2 expression presents in early stages of carcinogenesis and gets diminished once the tumor is established which renders Bcl-2 redundant.[5,8] Lymphokines, transforming growth factor-β, p53, retinoids and phorbol esters are few contributing factors to be considered that influence the expression of which can thereby downregulate or upregulate Bcl-2.[19] Scott et al.[8] (35%), Singh et al.[5] (25%), Ozdek et al.[6] (36%) and Soares et al.[3] (25%) also reported studies with diminished Bcl-2 positivity.[3,5,6,8]
The present study showed variable intensities of c-Myc and Bcl-2 within dysplasia and OSCC. Moderate intensity predominated in both the study groups that disclose a higher concentration gradient and an increased activity of both genes throughout the phases of carcinogenesis. When c-Myc and Bcl-2 were compared, only severe dysplasia showed moderate-to-strong intensity of c-Myc (P = 0.026). This suggests that c-Myc is more dysregulated in higher grades of dysplasia.
Basal, few parabasal layer cells of mild and moderate dysplasia and all the strata of severe dysplasia [Figure 1a] expressed Bcl-2 positivity. Most of the cells in mild and moderate dysplasia were devoid of Bcl-2 expression. This reflects that Bcl-2 expression decreases with terminal differentiation and presence of Bax protein (dominant inhibitor of Bcl-2).[5,20]
Figure 1.
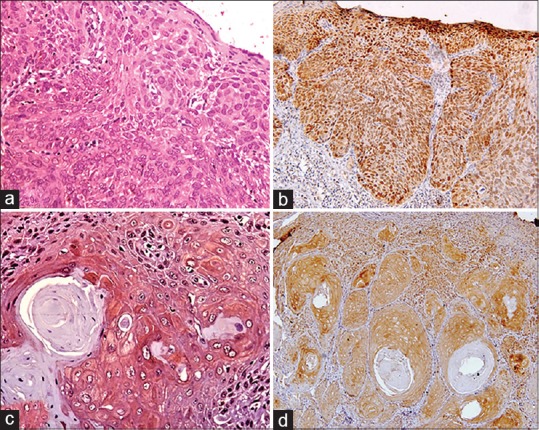
(a) Mild-to-moderate Bcl-2 expression localized in cytoplasm of severe dysplastic epithelium (×40). (b) Moderate-to-strong c-Myc expression localized in nucleus of severe dysplastic epithelium (×40). (c) Tumor islands of well-differentiated squamous cell carcinoma showing Bcl-2 expression at the periphery and no expression at the center (×40). (d) Tumor islands of well-differentiated squamous cell carcinoma showing c-Myc expression at the periphery and no expression at the center (×40)
The present study showed proportional increase in c-Myc expressing cells among grades of dysplasia and OSCC (P = 0.000 and P = 0.021, respectively). In dysplasia, c-Myc positivity proportionally involved the strata of the epithelium in accordance with grade [Figure 1b]. Papakosta et al. and Bhattacharya et al. studies are in harmony with the result of the present study.[13,21] Baral et al. showed proportional increase in c-Myc expression in grades of OSCC.[22] Eversole and Sapp showed parallel results in precancerous and early cancerous lesions.[23] In the present study, among grades of OSCC, well-differentiated and poorly differentiated squamous cell carcinoma showed increased c-Myc expression compared to moderately differentiated squamous cell carcinaoma (P = 0.021). The likelihood of such result would be the variation in tumor location and its interaction with the surrounding environment and activation of tumor suppressor genes such as MM-1 might have antagonized c-Myc in such tumors.[13]
Tumor islands with central keratinization showed Bcl-2 positivity at the periphery [Figure 1c], but those without central keratinization showed positivity both at the periphery and in the center.[20] This suggests that Bcl-2 expression is decreased in terminally differentiated cells. Majority of cases in the present study disclosed preponderance of Bcl-2 expressing cells in dysplasia and OSCC (P = 0.000 and P = 0.036, respectively). A significant difference was noticed when dysplasia and OSCC were compared for Bcl-2 due to decline in Bcl-2 activity in many of the OSCC cases (P = 0.000).
Entire tumor cell population in poorly differentiated squamous cell carcinoma (PSCC) showed Bcl-2 expression indicating that these cells are not undergoing terminal differentiation and have acquired resistance to apoptosis. The present study revealed many Bcl-2-negative PSCC cases disclosing them as highly anaplastic tumors.[20,24] Suri, Chen et al. and Muzio et al. studies are consistent with this study.[20,25,26] Difference in the expression patterns may be because of the caspase cleavage of Bcl-2 protein ultimately leading to degradation of the protein in such tumors.[20,24,27]
The same pattern of distribution was observed in the tumor cells with respect to c-Myc [Figure 1d]. In addition to cellular proliferation, c-Myc also plays a role in inhibiting cellular differentiation. In vitro downregulation of c-Myc gene causes increased cellular differentiation in several cell types.[8] Results of the present study showed decreased c-Myc expression in terminally differentiated cells. Most of the cases in the present study showed greater proportion of cells expressing c-Myc in dysplasia and OSCC (P = 0.003 and 0.423, respectively) and also between grades of dysplasia and OSCC (P = 0.003). Bcl-2 expression in severe dysplasia was significantly greater than in PSCC, which reflects downregulation of Bcl-2 in relation to terminal differentiation[3,5](P = 0.042). These findings were consistent with Singh et al., Soares et al. and Tete et al. studies.[3,5]
C-terminal membrane insertion domain of Bcl-2 targets the protein to intracellular membranes of mitochondria, endoplasmic reticulum and nuclear membrane.[5] Proteins that lack such transmembrane domain are localized in the cytoplasm but still retain antiapoptotic property.[28] Variable subcellular localization patterns of Bcl-2 [Figure 2a] were observed of which cytoplasmic expression was predominant in majority of cases of dysplasia and OSCC [Graph 1]. Several other studies were consistent with the present study.[3,5,7,8]
Figure 2.
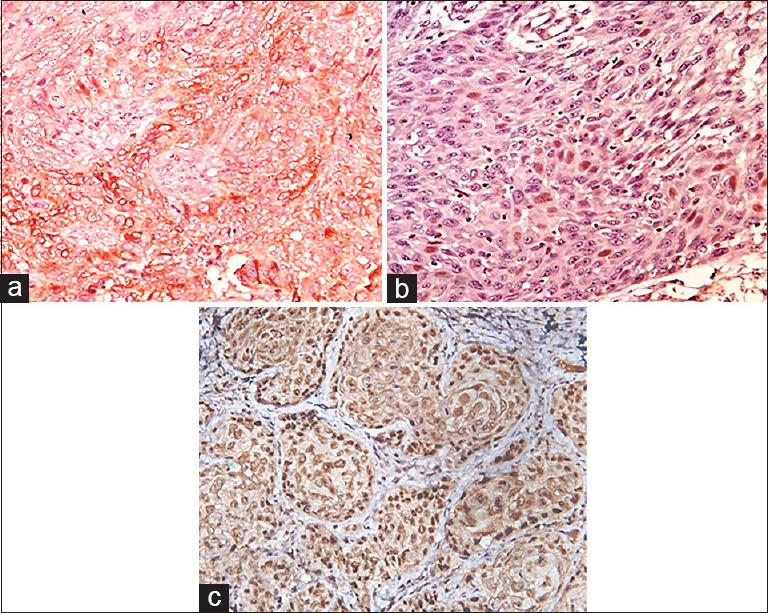
(a) Tumor cells of poorly differentiated squamous cell carcinoma showing cytoplasmic and membrane Bcl-2 expression (×40). (b) Tumor islands of moderately differentiated squamous cell carcinoma showing nuclear Bcl-2 expression (×40). (c) Tumor islands of moderately differentiated squamous cell carcinoma showing nuclear c-Myc expression (×40)
Interestingly, one case of MSCC disclosed nuclear positivity [Figure 2b and Table 2]. Nuclear Bcl-2 positivity was reported in limited OSCC cases. Kannan et al. observed similar results of strong nuclear positivity in 23% of OSCC cases.[29] Cytoplasmic Bcl-2 prevents apoptosis, whereas nuclear Bcl-2 inhibits activation of transcription factor and nuclear factor-κB, thereby inducing apoptosis rather than preventing it.[28,30]
Table 2.
Bcl-2 in nucleus
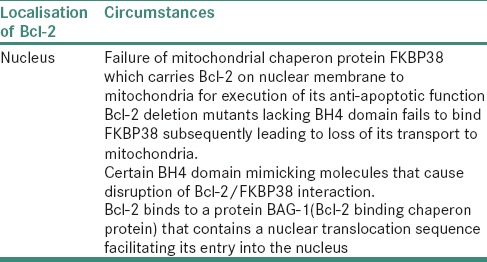
The present study showed variable localization patterns of c-Myc among which cytoplasmic expression dominated in majority of dysplasia cases, whereas nuclear expression predominated in grades of OSCC. Ectopic expression of c-Myc is sufficient for induction of cell-cycle progression; thus, cytoplasmic c-Myc promotes proliferation and further inhibits cellular differentiation.[4,17,18] Eversole and Sapp reported consistent outcomes in cancerous lesions.[23]
Literature revealed no studies differentiating cytoplasmic and nuclear localization of c-Myc in dysplasia. Pérez-Sayáns et al. and Sakai et al. studies on nuclear and cytoplasmic localization were the only documented reports differentiating the staining localization of c-Myc in OSCC.[4,18] The present study also differentiated nuclear and cytoplasmic localization of c-Myc among grades of OSCC (P = 0.012) [Graph 1]. Nuclear positivity dominated in majority of the cases which showed larger nuclei and pleomorphism [Figure 2c] which reflects that subcellular localization has an effect on biology of neoplastic cells. Segura et al. showed similar findings of nuclear overexpression in 73% of OSCC.[17] Cytoplasmic staining of c-Myc has a better survival when compared to the nuclear staining that has higher proliferation and is thus aggressive.[31]
Overall immunoreactivity of the lesions for both the proteins disclosed moderate immunoreactivity in dysplasia (P = 0.005 and P = 0.013, respectively), whereas OSCC showed strong immunoreactivity with c-Myc and moderate-to-strong reactivity with Bcl-2 (P = 0.000 and P = 0.029, respectively). Substantial results were obtained when grades of dysplasia and OSCC were compared with control group with both the markers (P = 0.000 and P = 0.000) [Table 1]. Bcl-2 immunoreactivity was more in dysplasia and had diminished activity in OSCC, whereas c-Myc immunoreactivity had an exponential increase from dysplasia to OSCC.
CONCLUSION
The authors concluded that these proteins act in synergism in early phases of carcinogenesis, whereas in later stages, due to diminished activity of Bcl-2, c-Myc interacts in coordination with other oncogenes contributing to tumor progression. Bcl-2 can be used as indicator of early carcinogenesis and its expression is inversely proportional to degree of differentiation of OSCC. Subcellular localization of Bcl-2 and c-Myc alters the functional, morphological and biological activity of the tumors.
Accumulating evidence from the study enroutes the great challenge to explore anti-Bcl-2 and anti-c-Myc agents to best utilize at specific grade of the lesion to curb the process of tumorigenesis. Several promising inhibitors of Bcl-2 and pan Bcl-2 inhibitors are developed to achieve antitumor domino effect [Table 3]. They induce death of the tumor cells by inhibiting the Bcl-2 pathway.[32] c-Myc inhibitors, certain small molecule ligands, have been developed for therapeutic approach in the prevention of tumor advancement by targeting transcription and downregulating c-Myc gene [Table 3].[33] In spite of advanced novel treatment strategies, still there is an urge for the development of panel of molecular inhibitors that would bring about antitumor effect in multiple prospects to alleviate the suffering of cancer patients.
Table 3.
Bcl-2 and c-Myc inhibitors

Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Coutinho-Camillo CM, Lourenço SV, Nishimoto IN, Kowalski LP, Soares FA. Expression of Bcl-2 family proteins and association with clinicopathological characteristics of oral squamous cell carcinoma. Histopathology. 2010;57:304–16. doi: 10.1111/j.1365-2559.2010.03621.x. [DOI] [PubMed] [Google Scholar]
- 2.Sudha VM, Hemavathy S. Role of Bcl-2 oncoprotein in oral potentially malignant disorders and squamous cell carcinoma: An immunohistochemical study. Indian J Dent Res. 2011;22:520–5. doi: 10.4103/0970-9290.90286. [DOI] [PubMed] [Google Scholar]
- 3.Soares FD, Barroso DR, Balassiano KZ, da Silva LE, da Fonseca EC, Lourenço S. Immunohistochemistry for Ki-67, Bcl2 and p53 in oral leukoplakias differentiates noncancerous from cancerous states. Appl Can Res. 2013;33:173–9. [Google Scholar]
- 4.Pérez-Sayáns M, Suárez-Peñaranda JM, Pilar GD, Barros-Angueira F, Gándara-Rey JM, García-García A, et al. What real influence does the proto-oncogene c-myc have in OSCC behavior? Oral Oncol. 2011;47:688–92. doi: 10.1016/j.oraloncology.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Singh BB, Chandler FW, Jr, Whitaker SB, Forbes-Nelson AE. Immunohistochemical evaluation of bcl-2 oncoprotein in oral dysplasia and carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:692–8. doi: 10.1016/s1079-2104(98)90037-3. [DOI] [PubMed] [Google Scholar]
- 6.Ozdek A, Sarac S, Akyol MU, Sungur A, Yilmaz T. C-myc and bcl-2 expression in supraglottic squamous cell carcinoma of the larynx. Otolaryngol Head Neck Surg. 2004;131:77–83. doi: 10.1016/j.otohns.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Sierra A, Castellsague X, Escobedo A, Moreno A, Drudis T, Fabra A, et al. Synergistic cooperation between c-myc and bcl-2 in lymph node progression of T1 human breast carcinomas. Breast Cancer Res Treat. 1999;54:39–45. doi: 10.1023/a:1006120006471. [DOI] [PubMed] [Google Scholar]
- 8.Scott N, Martin I, Jack AS, Dixon MF, Quirke P. Genes mediating programmed cell death: An immunohistochemical study of bcl-2, c-myc and p53 expression in colorectal neoplasia. Clin Mol Pathol. 1996;49:M151–8. doi: 10.1136/mp.49.3.m151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De P, Carlson JH, Leyland-Jones B, Dey N. Role of “oncogenic nexus” of CIP2A in breast oncogenesis: How does it work? Am J Cancer Res. 2015;5:2872–91. [PMC free article] [PubMed] [Google Scholar]
- 10.Lunde ML, Roman E, Warnakulasuriya S, Mehrotra R, Laranne J, Vasstrand EN, et al. Profiling of chromosomal changes in potentially malignant and malignant oral mucosal lesions from South and South-East Asia using array-comparative genomic hybridization. Cancer Genomics Proteomics. 2014;11:127–40. [PubMed] [Google Scholar]
- 11.Tyagi N, Tyagi R. Squamous cell carcinoma (well differentiated): A case report. J Dent Oral Hyg. 2013;5:31–4. [Google Scholar]
- 12.Liu W, Bao ZX, Shi LJ, Tang GY, Zhou ZT. Malignant transformation of oral epithelial dysplasia: clinicopathological risk factors and outcome analysis in a retrospective cohort of 138 cases. Histopathology. 2011;59:733–740. doi: 10.1111/j.1365-2559.2011.03938.x. [DOI] [PubMed] [Google Scholar]
- 13.Papakosta V, Vairaktaris E, Vylliotis A, Derka S, Nkenke E, Vassiliou S, et al. The co-expression of c-myc and p53 increases and reaches a plateau early in oral oncogenesis. Anticancer Res. 2006;26:2957–62. [PubMed] [Google Scholar]
- 14.Silva TC, Leal MF, Calcagno DQ, de Souza CR, Khayat AS, dos Santos NP, et al. HTERT, MYC and TP53 deregulation in gastric preneoplastic lesions. BMC Gastroenterol. 2012;12:85. doi: 10.1186/1471-230X-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzifi F, et al. The Role of BCL2 Family of Apoptosis Regulator Proteins in Acute and Chronic Leukemias. Adv Hematol. 2012;2012:524308. doi: 10.1155/2012/524308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Portt L, Norman G, Clapp C, Greenwood M, Greenwood MT. Anti-apoptosis and cell survival: a review. Biochim Biophys Acta. 2011;1813:238–59. doi: 10.1016/j.bbamcr.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Segura S, Rozas-Muñoz E, Toll A, Martín-Ezquerra G, Masferrer E, Espinet B, et al. Evaluation of MYC status in oral lichen planus in patients with progression to oral squamous cell carcinoma. Br J Dermatol. 2013;169:106–14. doi: 10.1111/bjd.12303. [DOI] [PubMed] [Google Scholar]
- 18.Sakai H, Kawano K, Okamura K, Hashimoto N. Immunohistochemical localization of c-myc oncogene product and EGF receptor in oral squamous cell carcinoma. J Oral Pathol Med. 1990;19:1–4. doi: 10.1111/j.1600-0714.1990.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 19.Loro LL, Johannessen AC, Vintermyr OK. Loss of BCL-2 in the progression of oral cancer is not attributable to mutations. J Clin Pathol. 2005;58:1157–62. doi: 10.1136/jcp.2004.021709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suri C. The immunohistochemical evaluation of expression of Bcl2 in different grades of oral squamous cell carcinoma. J Clin Diagn Res. 2009;3:1891–9. [Google Scholar]
- 21.Bhattacharya N, Roy A, Roy B, Roychoudhury S, Panda CK. MYC gene amplification reveals clinical association with head and neck squamous cell carcinoma in Indian patients. J Oral Pathol Med. 2009;38:759–63. doi: 10.1111/j.1600-0714.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- 22.Baral R, Patnaik S, Das BR. Co-overexpression of p53 and c-myc proteins linked with advanced stages of betel- and tobacco-related oral squamous cell carcinomas from Eastern India. Eur J Oral Sci. 1998;106:907–13. doi: 10.1046/j.0909-8836.1998.eos106502.x. [DOI] [PubMed] [Google Scholar]
- 23.Eversole LR, Sapp JP. C-myc oncoprotein expression in oral precancerous and early cancerous lesions. Eur J Cancer B Oral Oncol. 1993;29B:131–5. doi: 10.1016/0964-1955(93)90035-d. [DOI] [PubMed] [Google Scholar]
- 24.Loro LL, Vintermyr OK, Liavaag PG, Jonsson R, Johannessen AC. Oral squamous cell carcinoma is associated with decreased Bcl-2/bax expression ratio and increased apoptosis. Hum Pathol. 1999;30:1097–105. doi: 10.1016/s0046-8177(99)90229-0. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Kayano T, Takagi M. Dysregulated expression of Bcl-2 and bax in oral carcinomas: Evidence of post-transcriptional control. J Oral Pathol Med. 2000;29:63–9. doi: 10.1034/j.1600-0714.2000.290203.x. [DOI] [PubMed] [Google Scholar]
- 26.Lo Muzio L, Mignogna MD, Pannone G, Rubini C, Grassi R, Nocini PF, et al. Expresssion of bcl-2 in oral squamous cell carcinoma: An immunohistochemical study of 90 cases with clinico- patholigcal correlations. Oncology Reports. 2003;10:285–91. doi: 10.3892/or.10.2.285. [DOI] [PubMed] [Google Scholar]
- 27.Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, et al. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–8. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 28.Hoetelmans R, van Slooten HJ, Keijzer R, Erkeland S, van de Velde CJ, Dierendonck JH, et al. Bcl-2 and bax proteins are present in interphase nuclei of mammalian cells. Cell Death Differ. 2000;7:384–92. doi: 10.1038/sj.cdd.4400664. [DOI] [PubMed] [Google Scholar]
- 29.Kannan K, Latha PN, Shanmugam G. Expression of Bcl-2 oncoprotein in Indian oral squamous cell carcinomas. Oral Oncol. 1998;34:373–6. doi: 10.1016/s1368-8375(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 30.Portier BP, Taglialatela G. Bcl-2 localized at the nuclear compartment induces apoptosis after transient overexpression. J Biol Chem. 2006;281:40493–502. doi: 10.1074/jbc.M606181200. [DOI] [PubMed] [Google Scholar]
- 31.Geisler JP, Geisler HE, Manahan KJ, Miller GA, Wiemann MC, Zhou Z, et al. Nuclear and cytoplasmic c-myc staining in endometrial carcinoma and their relationship to survival. Int J Gynecol Cancer. 2004;14:133–7. doi: 10.1111/j.1048-891x.2004.14027.x. [DOI] [PubMed] [Google Scholar]
- 32.Vogler M. Targeting BCL2-proteins for the treatment of solid tumours. Adv Med 2014. 2014 doi: 10.1155/2014/943648. 943648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen BJ, Wu YL, Tanaka Y, Zhang W. Small molecules targeting c-myc oncogene: Promising anti-cancer therapeutics. Int J Biol Sci. 2014;10:1084–96. doi: 10.7150/ijbs.10190. [DOI] [PMC free article] [PubMed] [Google Scholar]


