Abstract
Background:
Ameloblastoma is an uncommon, benign neoplasm of odontogenic epithelium commonly affecting the posterior mandible (almost 80%) particularly in the molar/ramus region, with occasional tumors involving the maxilla. Recently, there has been much interest generated after the reports of BRAF V600E mutations in ameloblastomas with a frequency of 46%–80% using both molecular as well as immunohistochemical (IHC) techniques. We sought to assess the presence of BRAF V600E expression in ameloblastomas in Indian patients and correlate the same with clinical behavior and histological variants by performing IHC analysis with anti-BRAF V600E antibody.
Materials and Methods:
Thirty formalin-fixed paraffin-embedded tissues of mandibular ameloblastomas were examined by anti-BRAF V600E antibody and correlated with clinicopathologic and histological parameters. Cytoplasmic staining of neoplastic epithelium was considered positive for BRAF V600E expression.
Statistical Analysis:
Data analysis was performed using Chi-square test and Student's t-test with statistical software IBM SPSS statistics 20.0.
Results:
BRAF V600E antibody showed positive expression only in 33.3% (10/30) cases. About 66.7% (4/6) recurrent cases and 63.6% (7/11) plexiform cases showed statistically significant association of P = 0.05 and P = 0.021, respectively, among cases with positive BRAF V600E immunoexpression.
Conclusion:
We report the lowest frequency (33.3%) of BRAF V600E immunoexpression in mandibular ameloblastomas in Indian population. However, a significant association of BRAF V600E-positive immunoexpression with recurrence and plexiform pattern could underline its role as a therapeutic marker for ameloblastoma.
Keywords: BRAF V600E, immunohistochemistry, mandibular ameloblastomas
INTRODUCTION
Ameloblastoma is an uncommon, benign neoplasm of odontogenic epithelium commonly affecting the posterior mandible (almost 80%) particularly in the molar/ramus region, with occasional tumors involving the maxilla.[1,2] The lesions generally advance slowly in a locally infiltrative manner and if unchecked may result in severe morbidity, rarely showing malignant behavior. The median age of presentation is approximately 36 years without any sex predilection.[2,3] Radical surgery which is presently an established treatment for ameloblastoma often results in facial disfigurement since there is a higher incidence of recurrence after conservative procedures such as enucleation and curettage.[1,3,4]
Recently, the WHO has simplified the ameloblastoma classification to ameloblastoma, unicystic ameloblastoma and extraosseous/peripheral types.[5] The year 2014 was a defining moment in our knowledge about etiopathogenesis of ameloblastoma since three independent reports identified BRAF V600E as the most prevalent mutation among recurring MAPK mutations in ameloblastoma by genomic analysis and immunohistochemistry. They reported a frequency of 63% (15/24), 64% (54/84) and 46% (13/28), respectively.[2,4,6,7] Following this, two more studies of BRAF V600E mutation in ameloblastoma reported a frequency of 80% (4/5) and 46.6% (34/73), respectively, the former using sequencing as well as BRAF V600E immunohistochemistry and latter employing only immunohistochemistry.[8,9]
V-raf murine sarcoma viral oncogene homolog B1, BRAF, an oncogene within MAPK pathway, is usually stimulated by somatic point mutation in human cancer. BRAF is a part of RAF family of serine/threonine protein kinases which are constituents of evolutionary highly conserved kinase cascade signaling downstream of RAS, which is activated by growth factors, hormones and cytokines. RAS first stimulates RAF which consecutively activates second protein kinase called MEK, which successively triggers third protein kinase ERK that synchronizes cellular response to extracellular signals. Thus, the RAS-RAF-MEK-ERK-MAP kinase pathway functions as a communicator between extracellular milieu and nucleus. Since BRAF is the most dominant activator of MAP/ERK Kinase (MEK), any dysregulation of this pathway induces tumorigenesis. The RAS/BRAF/MEK/ERK pathway is hyperactivated in ~30% of all cancers with BRAF gene mutations in almost 7% of cancers, identifying it as an important oncogene in this pathway.[10,11,12,13] BRAF gene mutation is present in cutaneous melanoma (50%), papillary thyroid cancer (46%), borderline ovarian tumor (34%), pleomorphic xanthoastrocytomas (66%), biliary tract tumor (11%), colorectal cancer (10%), nonsmall lung cell cancer (2%) and hairy cell leukemia (100%) among others.[14] Benign lesions showing BRAF mutation include melanocytic nevi,[15] melanotic neuroectodermal tumor of infancy[16] and polyps of the colon[17] as well as ameloblastic fibroma and ameloblastic fibro-odontomas.[6,18] This mutation causes critical stimulation of BRAF protein and subsequent MEK and ERK signaling, amplifying cell proliferation, survival and eventually neoplastic transformation.[2]
The predominant mutation in the BRAF gene involves thymidine to adenosine T>A transversion at exon 15 nucleotide 1799 (T1799>A), resulting in replacement of valine (V) with glutamic acid (E) at position 600 of amino acid sequence (BRAF V600E), accounting for >90% mutations in BRAF.[4,11,13,19] There is a significant evidence for activated MAPK pathway being involved in the pathogenesis of ameloblastomas.[6] The fact that BRAF V600E expression was readily detectable by immunohistochemistry has diagnostic relevance.[1]
Based on the above information, we attempted to study BRAF V600E mutation by performing BRAF V600E immunohistochemistry in mandibular ameloblastomas and correlate the same with clinical behavior and histological subtypes in Indian population.
MATERIALS AND METHODS
Sample selection
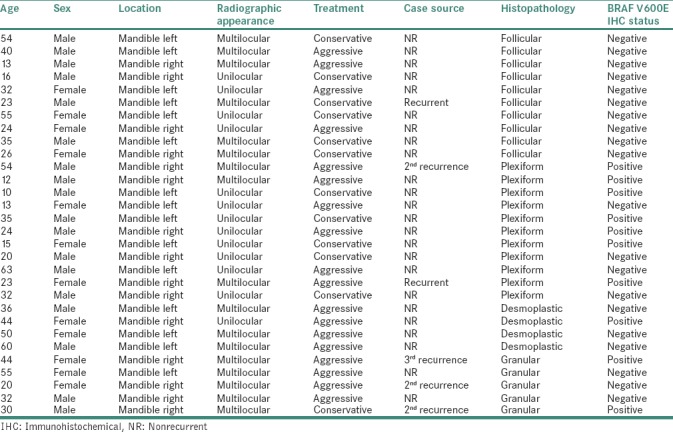
This study was approved by the Institutional Ethics Committee (EC-48/O PATH-05 ND/2016). We retrieved the formalin-fixed paraffin-embedded (FFPE) and nondecalcified tissue blocks of 30 mandibular ameloblastoma cases from 2011 to 2016 from archives of the Department of Oral Pathology. Demographic data were obtained from the patients' histopathological records [Table 1].
Table 1.
Clinical details and BRAF V600E immunohistochemical status of mandibular ameloblastomas
Immunohistochemical staining
Five micron sections were cut from nondecalcified FFPE tissue block and mounted on SuperFrost slides. Immunohistochemical (IHC) staining was carried out using polymer labeling technique. Sections were dewaxed and washed and antigen retrieval carried out in PT Link module with 1 mM ethylenediaminetetraacetic acid solution (pH 9) for 20 min. Endogenous peroxidase was blocked using 3% hydrogen peroxide in methanol at room temperature for 10 min. Immunostaining was carried out on the Dako autostainer. Sections were washed with phosphate-buffered saline (PBS) briefly and incubated with primary antibody against BRAF V600E (Tinto BRAF V600E Rabbit Monoclonal Antibody, Clone RM8, Bio SB, USA) for 60 min. Sections were washed with PBS and incubated with the EnVision polymer (Dako) for 30 min. Sections were washed with PBS. Diaminobenzidine was used as the chromogen in hydrogen peroxide for 10 min. Sections were then counterstained with Mayer's hematoxylin and mounted. Sections of papillary thyroid carcinoma treated with primary antibody served as positive control. Exclusion of primary antibody served as negative control.
Immunohistochemical analysis
Immunoreactivity was independently assessed by two authors. Cytoplasmic staining of neoplastic epithelium was considered positive for BRAF V600E expression [Figure 1].
Figure 1.
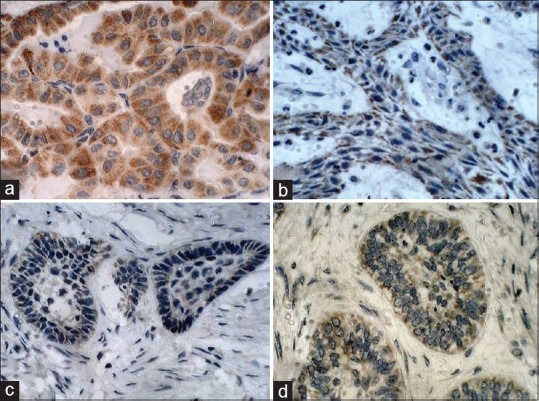
Immunohistochemical expression of BRAF V600E, ×40. (a) Positive control-papillary thyroid carcinoma, (b) plexiform variant, (c) desmoplastic variant and (d) granular cell variant of mandibular ameloblastomas
The positive staining intensity and proportion were scored with slight modification of criteria given by Reiner et al. and Barnes et al.[20,21] Intensity was scored by evaluating average intensity of entire tissue section as 0 (no staining), 1 (visible at high-power magnification, ×40), 2 (visible at low-power magnification, ×10) and 3 (visible at scanner view, ×4). The total proportion of cell staining positively at any intensity was scored by screening five fields per tissue section at random as 0 (no cell staining), 1 (when 1%–5% cells stained), 2 (when 6%–25% cells stained), 3 (when 26%–50% cells stained) and 4 (when >50% cells stained). The results of intensity and proportion of cell staining positively were combined to give “quick score” as follows 2 and 3 points = low, 4 and 5 points = intermediate and 6 and 7 points = high positivity for BRAF V600E immunoexpression.[6,9,20,21]
Statistical analysis
Chi-square analysis was used to find the significance of study parameters on categorical scale. Student's t-test (two-tailed, unpaired) was used to find the significance of study parameters on continuous scale between two groups. Level of significance was fixed at P = 0.05 and any value ≤0.05 was considered to be statistically significant. The Statistical software IBM SPSS statistics 20.0 (IBM Corporation, Armonk, NY, USA) was used for the analyses of the data.
RESULTS
We reviewed 30 cases of mandibular ameloblastoma using antibody against BRAF V600E by immunohistochemistry. Table 1 illustrates demographic data with BRAF V600E expression status.
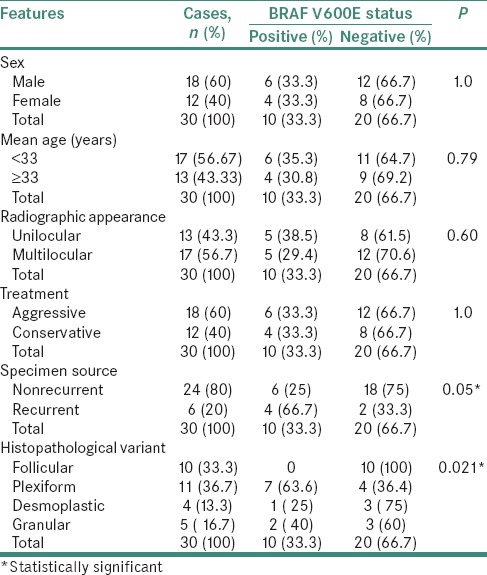
Our results showed a slight male predilection (M:F = 18:12), with a mean age of 33 years. Cases were equally distributed on the right and left sides of the mandible. Radiographically, multilocular pattern (56.7%, n = 17) was more common than unilocular (43.3%, n = 13). Aggressive treatment (60%, n = 18) was preferred over conservative therapy (40%, n = 12). Based on specimen source, majority of cases were nonrecurrent (80%, n = 24) compared to (20%, n = 6) recurrent cases. Histologically, plexiform variant (36.7%, n = 11) was most common, followed by follicular (33.3%, n = 10), granular (16.7%, n = 5) and desmoplastic (13.3%, n = 4) variety.
The present study demonstrated only 10 cases (33.3%) showing BRAF V600E-positive cytoplasmic expression in neoplastic epithelium (which included 9 cases staining strongly and 1 case with intermediate staining) whereas 20 cases (66.7%) were BRAF V600E negative.
Of these 10 positive BRAF V600E cases, recurrent source cases showed statistically significant association (P = 0.05) with BRAF V600E expression as 66.7% (4/6) of recurrent source cases were BRAF V600E positive compared to only 25% (6/24) cases positive from nonrecurrent source. Statistically significant association (P = 0.021) of BRAF V600E expression was observed on comparing histological variants of ameloblastoma as plexiform type had 63.6% (7/11) positive cases, followed by granular type with 40% (2/5) positive cases and desmoplastic type showing 25% (1/4) positive cases. Surprisingly, all cases of follicular type (10/10) were negative for BRAF V600E expression.
The present study examined five cases of granular cell ameloblastoma for BRAF V600E immunoexpression of which three were from recurrent source. Among these three recurrent cases, two expressed BRAF V600E.
Statistical analysis of clinicopathological features and BRAF V600E immunoexpression is summarized in Table 2.
Table 2.
Analysis of clinicopathological data and BRAF V600E expression in mandibular ameloblastomas
DISCUSSION
Ameloblastoma is a remarkably rare, benign epithelial odontogenic tumor which is locally invasive and highly recurrent, seldom displaying malignant behavior.[22] The current molecular approach of understanding odontogenic tumors has provided insights into their evolution and molecular pathogenesis.[12] Most of the odontogenic tumors including ameloblastomas show monoclonal pattern suggesting their origin from one altered clone of odontogenic cells.[22,23]
Since 2014, few studies on ameloblastomas have reported BRAF V600E mutations ranging from 46% to 80%.[4,6,7,8,9] Although these studies employed molecular techniques, they also evaluated the usefulness of immunohistochemistry using anti-BRAF V600E antibody in ameloblastomas and unequivocally substantiated that BRAF V600E immunopositivity significantly correlated with BRAF V600E mutation status in ameloblastomas.[4,6,7,8]
Excellent concurrence of BRAF V600E immunohistochemistry with gene mutation has been established as the presence of mutated protein can be detected at a single cell level.[6,24]
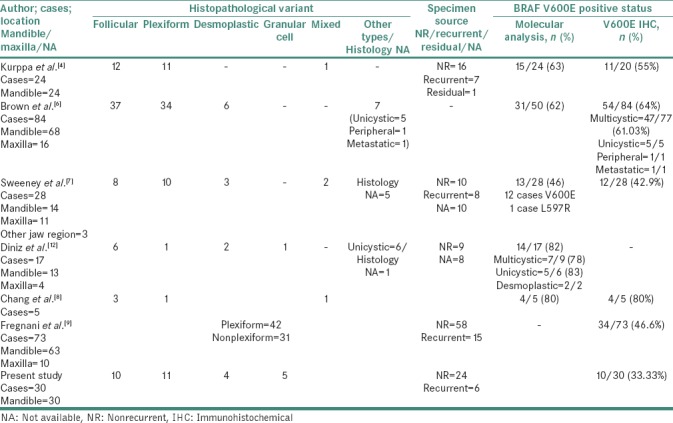
Detailed review of exclusively IHC data of ameloblastomas in previous studies revealed that 11/20 (55%) cases of Kurppa et al.,[4]47/77 (61%) cases of Brown et al.,[6]12/28 (42.9%) cases of Sweeney et al.,[7] and 4/5 (80%) cases of Chang et al.[8] were positive for BRAF V600E expression. Similar to the present study, Fregnani et al.[9] reported a positive frequency in 34/73 (46.6%) cases using only BRAF V600E immunohistochemistry. We observed a positive BRAF V600E immunoexpression in 10/30 (33.3%) cases of mandibular ameloblastomas as summarized in Table 3.
Table 3.
Previous studies of BRAF V600E frequency in ameloblastomas
Kurppa et al.[4] found no significant association of BRAF V600E mutation with age or sex, tumor histology and tumor recurrence. Brown et al.[6] observed a significant correlation of BRAF V600E mutations with younger age, but we did not obtain any significant association between age and sex with BRAF V600E expression, a finding coinciding with Kurppa et al.[4]
Most of the previous studies on BRAF V600E mutation also had majority of cases from mandibular region. Similar to Kurppa et al.,[4] we only had mandibular cases for the present study. Brown et al.[6] and Sweeney et al.[7] reported that BRAF V600E mutation showed greater predilection for the mandible. However, Fregnani et al.[9] and Diniz et al.[12] found no association of BRAF mutation and tumor location.
Fregnani et al.[9] reported a significant association of BRAF V600E expression with multilocular radiographic appearance, a finding not consistent with our results.
Contrary to Brown et al.[6] who reported that earlier recurrences were associated with BRAF-negative cases, Fregnani et al.[9] demonstrated that positive BRAF V600E immunoexpression significantly correlated with the presence of recurrences, a finding in accordance with our study.
Kurppa et al.,[4] Fregnani et al.,[9] and Diniz et al.[12] found no significant association between BRAF V600E and histological pattern. An analysis of data of Brown et al.[6] and Sweeney et al.[7] showed plexiform histology to be significantly common among BRAF-negative tumors. Chang et al.[8] reported strong association of BRAF V600E expression with follicular type of ameloblastomas. However, we found a significant correlation (P = 0.021) of BRAF V600E expression with plexiform type of ameloblastoma which is in complete contrast to findings of all previous studies.
Our results indicate the possible role of BRAF V600E immunohistochemistry as a predictive marker for mandibular ameloblastoma recurrence.
An interesting finding of the present study is the lowest frequency of BRAF V600E positive immunoexpression in mandibular ameloblastomas reported till now at 33.3% as compared to previous studies (range : 46% - 80%).[4,6,7,8,9] However, BRAF V600E immunopositivity showed a significant association with recurrent cases and plexiform histology. Granular cell ameloblastomas, second most common for BRAF V600E-positive immunoexpression, might have some yet undetermined association yet to be proven statistically significant.
Since there were no data on BRAF V600E mutation in the Indian population, we made a sincere attempt to study the same. To our surprise, we found the lowest frequency of BRAF V600E immunoexpression in our study. Previous studies have reported differing mutation frequencies ranging from 46% to 80% in different populations, and our study could represent the lowest end spectrum of BRAF V600E expression in Indian population. Only using IHC method for identification of BRAF-mutated protein in a small sample size could have resulted in low expression in Indian cohort. Cells having BRAF V600E-mutated protein below levels detectable by immunohistochemistry can also be the reason for the lowest frequency of BRAF V600E expression in our study. Furthermore, negative IHC expression probably may arise from loss of mutant antigen expression.[25] We evaluated BRAF V600E expression by stringent scoring criteria, which could be one more reason for the lowest frequency of BRAF V600E expression in our study.
The present study was an IHC study to determine BRAF V600E immunoexpression in Indian population. To the best of our knowledge, this is the first study to be carried out in Indian population. We do feel that employing molecular techniques may have probably influenced observed frequency of BRAF V600E expression in our study. Mutation at the DNA level without expression at the mRNA level is a common event, reported by the Cancer Genome Atlas studies which may have caused low BRAF V600E expression.[26] Furthermore, the possibility of ethnic and geographic variations having a role in low expression of BRAF V600E in Indian population cannot be entirely ruled out.
We want to highlight some potential limitations commonly associated with retrospective studies. Our study included only mandibular ameloblastomas as our institute receives very few maxillary, unicystic and peripheral ameloblastoma cases. Some previous studies[6,8,24] have demonstrated a perfect match of BRAF V600E immunoexpression with genetic BRAF detection, but we were unable to carry out BRAF V600E mutational analysis. Although few studies have reported discordance among the IHC data and molecular assays in melanomas,[27,28] colorectal carcinoma[26] as well as unicystic ameloblastomas,[29] the molecular characterization of ameloblastoma for BRAF status has rationalized ameloblastoma treatment using BRAF inhibitors, vemurafenib and dabrafenib,[30,31] justifying BRAF V600E as therapeutic marker.
CONCLUSION
The present study demonstrated the lowest frequency of positive BRAF V600E immunoexpression (33.3%) compared to other studies till date; hence, the dependency only on immunohistochemistry for assessing BRAF V600E mutational status in mandibular ameloblastomas remains questionable.
Based on our findings, we suggest that BRAF V600E immunopositive mandibular ameloblastoma cases should be kept under strict follow-up as there are greater chances of recurrence, especially in plexiform and granular variants.
With readily available material for BRAF V600E immunohistochemistry, extensive data on clinicopathological relation will be available for review in due course of time. Future multicentric studies with large sample size are required to substantiate the role of BRAF V600E expression as a reliable therapeutic and predictive marker for ameloblastomas.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.McClary AC, West RB, McClary AC, Pollack JR, Fischbein NJ, Holsinger CF, et al. Ameloblastoma: A clinical review and trends in management. Eur Arch Otorhinolaryngol. 2016;273:1649–61. doi: 10.1007/s00405-015-3631-8. [DOI] [PubMed] [Google Scholar]
- 2.Brown NA, Betz BL. Ameloblastoma: A Review of recent molecular pathogenetic discoveries. Biomark Cancer. 2015;7:19–24. doi: 10.4137/BIC.S29329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendenhall WM, Werning JW, Fernandes R, Malyapa RS, Mendenhall NP. Ameloblastoma. Am J Clin Oncol. 2007;30:645–8. doi: 10.1097/COC.0b013e3181573e59. [DOI] [PubMed] [Google Scholar]
- 4.Kurppa KJ, Catón J, Morgan PR, Ristimäki A, Ruhin B, Kellokoski J, et al. High frequency of BRAF V600E mutations in ameloblastoma. J Pathol. 2014;232:492–8. doi: 10.1002/path.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright JM, Vered M. Update from the 4th edition of the world health organization classification of head and neck tumours: Odontogenic and maxillofacial bone tumors. Head Neck Pathol. 2017;11:68–77. doi: 10.1007/s12105-017-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown NA, Rolland D, McHugh JB, Weigelin HC, Zhao L, Lim MS, et al. Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res. 2014;20:5517–26. doi: 10.1158/1078-0432.CCR-14-1069. [DOI] [PubMed] [Google Scholar]
- 7.Sweeney RT, McClary AC, Myers BR, Biscocho J, Neahring L, Kwei KA, et al. Identification of recurrent SMO and BRAF mutations in ameloblastomas. Nat Genet. 2014;46:722–5. doi: 10.1038/ng.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang J, Wang YP, Chiang CP. Clinicopathologic correlations of BRAF V600E mutation and BRAF V600E immunohistochemistry in ameloblastomas. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:e155. [Google Scholar]
- 9.Fregnani ER, Perez DE, Paes de Almeida O, Fonseca FP, Soares FA, Castro-Junior G, et al. BRAF-V600E expression correlates with ameloblastoma aggressiveness. Histopathology. 2017;70:473–84. doi: 10.1111/his.13095. [DOI] [PubMed] [Google Scholar]
- 10.Cantwell-Dorris ER, O'Leary JJ, Sheils OM. BRAFV600E: Implications for carcinogenesis and molecular therapy. Mol Cancer Ther. 2011;10:385–94. doi: 10.1158/1535-7163.MCT-10-0799. [DOI] [PubMed] [Google Scholar]
- 11.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 12.Diniz MG, Gomes CC, Guimarães BV, Castro WH, Lacerda JC, Cardoso SV, et al. Assessment of BRAFV600E and SMOF412E mutations in epithelial odontogenic tumours. Tumour Biol. 2015;36:5649–53. doi: 10.1007/s13277-015-3238-0. [DOI] [PubMed] [Google Scholar]
- 13.Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell. 2004;6:313–9. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Long GV, Wilmott JS, Capper D, Preusser M, Zhang YE, Thompson JF, et al. Immunohistochemistry is highly sensitive and specific for the detection of V600E BRAF mutation in melanoma. Am J Surg Pathol. 2013;37:61–5. doi: 10.1097/PAS.0b013e31826485c0. [DOI] [PubMed] [Google Scholar]
- 15.Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 16.Gomes CC, Diniz MG, de Menezes GH, Castro WH, Gomez RS. BRAFV600E mutation in melanotic neuroectodermal tumor of infancy: Toward personalized medicine? Pediatrics. 2015;136:e267–9. doi: 10.1542/peds.2014-3331. [DOI] [PubMed] [Google Scholar]
- 17.Kim MJ, Lee EJ, Chun SM, Jang SJ, Kim CH, Seo JP, et al. Pedunculated serrated polyp with histologic features of sessile serrated adenoma: A clinicopathologic and molecular study. Am J Surg Pathol. 2013;37:1039–43. doi: 10.1097/PAS.0b013e3182805696. [DOI] [PubMed] [Google Scholar]
- 18.Brunner P, Bihl M, Jundt G, Baumhoer D, Hoeller S. BRAF p.V600E mutations are not unique to ameloblastoma and are shared by other odontogenic tumors with ameloblastic morphology. Oral Oncol. 2015;51:e77–8. doi: 10.1016/j.oraloncology.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Kim BH, Kim IJ, Lee BJ, Lee JC, Kim IS, Kim SJ, et al. Detection of plasma BRAF(V600E) mutation is associated with lung metastasis in papillary thyroid carcinomas. Yonsei Med J. 2015;56:634–40. doi: 10.3349/ymj.2015.56.3.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiner A, Neumeister B, Spona J, Reiner G, Schemper M, Jakesz R, et al. Immunocytochemical localization of estrogen and progesterone receptor and prognosis in human primary breast cancer. Cancer Res. 1990;50:7057–61. [PubMed] [Google Scholar]
- 21.Barnes DM, Harris WH, Smith P, Millis RR, Rubens RD. Immunohistochemical determination of oestrogen receptor: Comparison of different methods of assessment of staining and correlation with clinical outcome of breast cancer patients. Br J Cancer. 1996;74:1445–51. doi: 10.1038/bjc.1996.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.do Canto AM, Rozatto JR, Schussel JL, de Freitas RR, Hasséus B, Braz-Silva PH, et al. Immunohistochemical biomarkers in ameloblastomas. Acta Odontol Scand. 2016;74:585–90. doi: 10.1080/00016357.2016.1224918. [DOI] [PubMed] [Google Scholar]
- 23.Gomes CC, Oliveira Cda S, Castro WH, de Lacerda JC, Gomez RS. Clonal nature of odontogenic tumours. J Oral Pathol Med. 2009;38:397–400. doi: 10.1111/j.1600-0714.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 24.Capper D, Preusser M, Habel A, Sahm F, Ackermann U, Schindler G, et al. Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol. 2011;122:11–9. doi: 10.1007/s00401-011-0841-z. [DOI] [PubMed] [Google Scholar]
- 25.Dvorak K, Aggeler B, Palting J, McKelvie P, Ruszkiewicz A, Waring P, et al. Immunohistochemistry with the anti-BRAF V600E (VE1) antibody: Impact of pre-analytical conditions and concordance with DNA sequencing in colorectal and papillary thyroid carcinoma. Pathology. 2014;46:509–17. doi: 10.1097/PAT.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estrella JS, Tetzlaff MT, Bassett RL, Jr, Patel KP, Williams MD, Curry JL, et al. Assessment of BRAF V600E status in colorectal carcinoma: Tissue-specific discordances between immunohistochemistry and sequencing. Mol Cancer Ther. 2015;14:2887–95. doi: 10.1158/1535-7163.MCT-15-0615. [DOI] [PubMed] [Google Scholar]
- 27.Thiel A, Moza M, Kytölä S, Orpana A, Jahkola T, Hernberg M, et al. Prospectiveimmunohistochemical analysis of BRAF V600E mutation in melanoma. Hum Pathol. 2015;46:169–75. doi: 10.1016/j.humpath.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Fisher KE, Cohen C, Siddiqui MT, Palma JF, Lipford EH, 3rd, Longshore JW, et al. Accurate detection of BRAF p.V600E mutations in challenging melanoma specimens requires stringent immunohistochemistry scoring criteria or sensitive molecular assays. Hum Pathol. 2014;45:2281–93. doi: 10.1016/j.humpath.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Pereira NB, Pereira KM, Coura BP, Diniz MG, de Castro WH, Gomes CC, et al. BRAFV600E mutation in the diagnosis of unicystic ameloblastoma. J Oral Pathol Med. 2016;45:780–5. doi: 10.1111/jop.12443. [DOI] [PubMed] [Google Scholar]
- 30.Heikinheimo K, Kurppa KJ, Elenius K. Novel targets for the treatment of ameloblastoma. J Dent Res. 2015;94:237–40. doi: 10.1177/0022034514560373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan S, Pollack JR, Kaplan MJ, Colevas AD, West RB. BRAF inhibitor therapy of primary ameloblastoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:518–9. doi: 10.1016/j.oooo.2016.05.017. [DOI] [PubMed] [Google Scholar]


