Abstract
Biliary endoscopic sphincterotomy (EST) refers to the cutting of the biliary sphincter and intraduodenal segment of the common bile duct following selective cannulation, using a high frequency current applied with a special knife, sphincterotome, inserted into the papilla. EST is either used solely for the treatment of diseases of the papilla of Vater, such as sphincter of Oddi dysfunction or to facilitate subsequent therapeutic biliary interventions, such as stone extraction, stenting, etc. It is a prerequisite for biliary interventions, thus every practitioner who performs endoscopic retrograde cholangiopancreatography needs to know different techniques and the clinical and anatomic parameters related to the efficacy and safety of the procedure. In this manuscript, we will review the indications, contraindications and techniques of biliary EST and the management of its complications.
Keywords: Complication, Indication, Biliary endoscopic sphincterotomy, Endoscopic retrograde cholangiopancreatography
Core tip: Biliary endoscopic sphincterotomy (EST) is an essential procedure of the endoscopic retrograde cholangiopancreatography (ERCP) for the treatment and palliation of a wide spectrum of biliary and papillary diseases. It has some contraindications and complications. The physicians who interested in ERCP must learn the equipments, techniques and the management of the complications of biliary EST. We herein widely review the indications, contraindications and techniques of biliary EST and the management of its complications.
INTRODUCTION
Owing to the use of magnetic resonance cholangiopancreatography and endoscopic ultrasonography with high accuracy in diagnosis, endoscopic retrograde cholangiopancreatography (ERCP) has nowadays become a therapeutic procedure for various pancreaticobiliary diseases. Biliary endoscopic sphincterotomy (EST) is either used solely for the treatment of diseases of the papilla of Vater, such as sphincter of Oddi dysfunction (SOD) or to facilitate subsequent therapeutic biliary interventions, such as stone extraction, stenting, etc. In other words, it is a prerequisite for biliary interventions, thus every practitioner who performs ERCP needs to know different techniques and the clinical and anatomic parameters related to the efficacy and safety of the procedure. In this manuscript, we will review the indications, contraindications and techniques of biliary EST and the management of its complications.
INDICATIONS OF BILIARY EST
Biliary EST refers to the cutting of the biliary sphincter and intraduodenal segment of the common bile duct following selective cannulation, using a high frequency current applied with a special knife, sphincterotome, inserted into the papilla. It was first introduced in Germany and Japan in 1974[1,2]. Since then, it has been used for the treatment and palliation of a wide spectrum of biliary and papillary diseases including bile duct stones, benign and malignant biliary strictures, SOD and bile leaks, etc (Table 1). In addition, it may be used for facilitating diagnostic procedures such as transpapillary bile duct biopsy, papillary tumor biopsy and insertion of a cholangioscope.
Table 1.
Indications of biliary endoscopic spincterotomy
| Extraction of choledocholithiasis and/or intrahepatic stones |
| Treatment of benign biliary/papillary strictures |
| Palliation of malignant biliary strictures |
| Treatment of SOD |
| Treatment of bile leaks |
| Gall bladder drainage |
| Others: Biliary parasites, Sump syndrome, choledochocele |
SOD: Sphincter of Oddi dysfunction.
CONTRAINDICATIONS OF BILIARY EST
The contraindications of biliary EST include general contraindications of ERCP, such as unstable or uncooperative patient and those specific to EST: Uncorrected coagulopathy and inability to orient cutting wire of the sphincterotome towards the axis of the common bile duct. Under these circumstances, alternative methods, such as balloon dilation should be considered.
Biliary EST is a high risk procedure for bleeding. Platelet count and international normalized ratio (INR) should be checked before the procedure. Platelet and fresh frozen plasma transfusions should be done in order to increase the platelet count to > 50000/mm3 and decrease INR to < 1.5. Discontinuation of antiplatelet or anticoagulant therapies should be decided by considering the risk of procedural hemorrhage versus thrombosis due to discontinuation. Aspirin monotherapy is safe in EST[3,4]. P2Y12 receptor antagonists, such as clopidogrel, and warfarin should be stopped 5 days before EST in patients with a low risk of thrombosis. Direct oral anticoagulants, such as dabigatran, rivaroxaban, apixaban and edoxaban should be stopped at least 48 h before EST. Practitioners should refer to guidelines for discontinuation of antiplatelet therapy, management of dual antiplatelet drugs and initiation of bridging anticoagulant therapy in patients with a high risk of thrombosis[4].
Biliary EST is not contraindicated in patients with chronic diseases such as cirrhosis and chronic renal failure. Literature about the safety of ERCP in cirrhotic patients is confusing. Prat et al[5] reported a high rate of mortality (12.5%) and morbidity (34.5%) during the first month after biliary EST in 52 cirrhotic patients. A large retrospective multicenter study in patients with cirrhosis reported a significantly increased risk of post-ERCP pancreatitis (PEP) (12.8%) and cardiopulmonary adverse events (3.5%) after biliary EST[6]. Moreover, a recent large nationwide database study including 2155 cirrhotic patients found a significantly increased risk of bleeding and PEP after biliary EST[7].
Several studies reported hemodialysis as an independent risk factor for EST bleeding, however, this is not accepted by the major endoscopy society guidelines[3,8,9]. Platelet dysfunction due to uremic toxins, accumulation of heparin due to decreased renal clearance, alterations in the vessel wall, anemia and adverse effects of the hemodialysis procedure are the possible mechanisms. Platelet function may be improved with desmopressin, estrogens and hemodialysis before the procedure.
EQUIPMENTS SPECIFIC TO BILIARY EST
Sphincterotome and electrosurgical unit are the equipments specific to EST.
Sphincterotome
Sphincterotome is a catheter with a cutting wire at its distal end. Design of sphincterotomes differs in length and characteristic of cutting wire, length and diameter of tip (part of the catheter extending beyond the distal end of the cutting wire) and number of lumens. They are categorized into 3 types: Pull, push and needle-knife.
Pull-type sphincterotomes have a steel cutting wire inside a Teflon catheter. When the wire is tightened from the handle of the sphincterotome, it is pulled away from the catheter, flexing the tip of the catheter upward. This upward motion of the tip is important since it facilitates cannulation by orienting the tip of the sphincterotome towards the biliary sphincter while maintaining the contact of the cutting wire with papilla. Cutting wire is connected to an electrode connector of a monopolar electrosurgical unit on the handle and functions as a knife when current is applied. Cutting wires are mostly monofilament in configuration and the length of the exposed part at the distal side of the sphincterotome varies between 15-35 mm[10]. There are certain technical implications of using different lengths of cutting wire. Sphincterotomes with a short cutting wire, 15-20 mm, are easily controlled and do not lead to a large cut when inserted too deep into the bile duct, however, they have a tendency to orient towards 2-3 o’clock. In pediatric patients with a narrow duodenum, it may be necessary to use sphincterotomes with a short cutting wire while performing ERCP using adult duodenoscopes, as the distance between the duodenoscope and papilla is short. Sphincterotomes with longer cutting wires, 25-30 mm, are more likely to orient towards the biliary sphincter. However, as all of the cutting wire should be out of the endoscope with only a small part of it inside the papilla during sphincterotomy, it requires more effort to take and maintain a faraway position while using them. In addition, this difficulty in positioning may lead to unintentional thermal injury of the overhanging duodenal folds due to contact with the proximal part of the cutting wire, which may be overcome by using a sphincterotome that is insulated on the proximal part of the cutting wire.
The distal outer diameter of the sphincterotomes is mostly between 4.4-6 Fr. Sphincterotomes with a thinner outer diameter (3.9-4 Fr) or tapered tip may facilitate cannulation in patients with a small papilla[10]. However, they require smaller guidewires and may lead to more tissue trauma during cannulation attempts. The length of the tip of sphincterotomes varies between 3 mm and 20 mm. Smaller-tipped sphincterotomes may facilitate cannulation, as they are more easily oriented towards the bile duct axis when the cutting wire is tightened. Longer-tipped ones may facilitate cannulation in patients with juxtapapillary diverticula. There are also some rotatable sphincterotomes, which make it possible to change the axis of cannulation or EST. They may be useful in patients with an unusually oriented papilla or Billroth II gastrectomy.
Sphincterotomes also vary depending on the number of lumens. Currently, there are sphincterotomes with a single-lumen for cutting wire, double-lumen for cutting wire and guidewire insertion or triple-lumen with an additional lumen for contrast injection. Some sphincterotomes have a short wire design which decreases the time to exchange the instruments and the risk of wire loss. They also allow manipulation of the guidewire by the operator[11].
Push-type sphincterotomes have a different cutting wire design. Tightening the cutting wire pushes it out to form a bow and orient towards 5 to 6 o’clock. Therefore, they are useful in patients with Billroth II gastrectomy. Sigmoid shaped sphincterotomes can also be used for EST in patients with Billroth II gastrectomy.
A needle knife sphincterotome consists of an outer Teflon catheter with an inner retractable cutting wire. The length of the cutting wire is 3-5 mm. In majority of the cases, they are used for pre-cut sphincterotomy to gain access to the underlying bile duct when standard cannulation methods fail. They may also be used for EST over a plastic stent inserted into the common bile duct in patients with a difficult or altered anatomy (Figure 1A-E).
Figure 1.
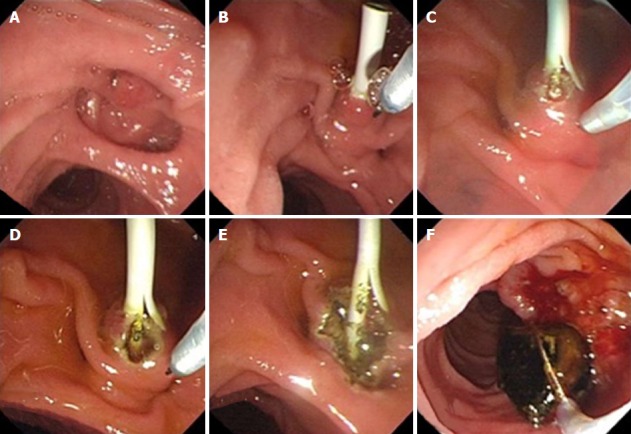
Needle knife sphincterotome was used for endoscopic sphincterotomy over a plastic stent inserted into the common bile duct in a patient with an altered anatomy. A: A juxtapapillary diverticula is seen in a patient with Billroth II gastrectomy; B: A 7Fr-plastic stent is inserted to the common bile duct after cannulation; C-E: Sphincterotomy is performed step wise with a needle knife sphincterotome over the plastic stent; F: Common bile duct stone is extracted with a basket catheter.
Electrosurgical unit
Electrosurgical generators deliver electrical current in 3 forms: pure cut, coagulation or mixed current. There are two modes of mixed current: the blended mode which delivers cutting and coagulating currents together in one waveform and an alternating mode, which is managed by an intrinsic software and delivers cutting and coagulation currents one after another in short bursts (ex, endocut or pulsecut mode). In a meta-analysis comparing pure versus mixed electrosurgical current for endoscopic biliary sphincterotomy, pure cut current was associated with more episodes of bleeding[12]. A retrospective analysis revealed a significantly lower frequency of endoscopically observed mild bleeding after EST with mixed current in alternating mode (endocut) compared to blended mode[13]. A small retrospective observational study in cirrhotic patients also reported a lower frequency of EST bleeding in patients who underwent sphincterotomy with mixed current in the alternating mode (pulsecut), compared to blended mode[14]. Mixed current in alternating mode may also be associated with fewer episodes of uncontrolled cutting (zipper), since software applies a constant voltage. Most studies revealed similar incidence of post-ERCP pancreatitis in patients who underwent EST by using pure cutting or mixed current[12,15]. ESGE recommends to use a mixed current in alternating mode for EST[16].
TECHNIQUES OF BILIARY EST
Standard sphincterotomy
It is necessary to cannulate the bile duct before therapeutic biliary interventions. After cannulation, a cholangiography is obtained initially to diagnose the biliary pathology and determine the following interventions to be made. As most of the ERCPs are done for therapeutic purposes, EST is required in almost all cases, unless contraindicated. After deep cannulation, the sphincterotome is slowly withdrawn until 1/4 to 1/3 of its cutting wire remains inside the papilla. Later on, the tip of the sphincterotome is bowed in order to contact the cutting wire with the roof of the papilla and orient it towards the biliary sphincter between 11-13 o’clock, at the same time. Based on our experience, we think that endoscopists should comply with the following precautions in order to decrease the sphincterotomy related complications: First, duodenoscope should be in such a position that endoscopist can clearly see the starting point, direction and upper border of sphincterotomy at the same time, to make sure that sphincterotomy is proceeding in the right direction. Second, sphincterotome should not compress the overlying tissue but cut with small contacts in order to prevent zipper cut (Figure 2). Third, it not always possible to orient the sphincterotome towards the axis of the bile duct in the short duodenoscope position. In such cases, some additional manuevers, such as rotating the scope to the left with simultaneous advancement to a long or semi-long position (Figure 3) or gentle shortening with simultaneous left rotation must be done for correct positioning.
Figure 2.
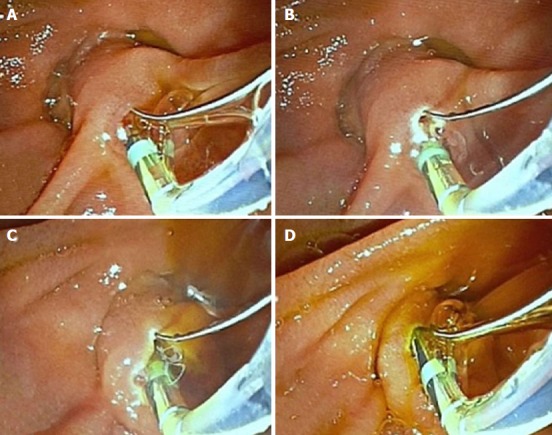
Precautions for decreasing the sphincterotomy related complications. A: Sphincterotomy is started with a standard sphincterotome after biliary cannulation; B: Extended towards 11 o’clock; C: Biliary drainage is noticed; D: Sphincterotomy is finished near the roof of the papilla.
Figure 3.
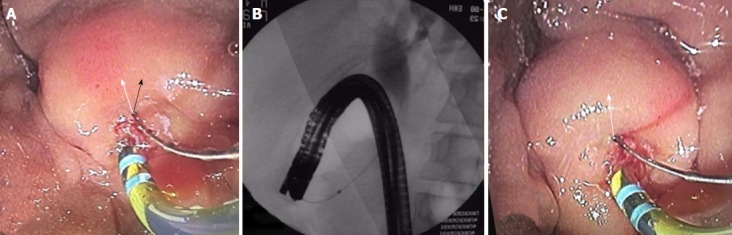
Rotating the scope to the left with simultaneous advancement to a long or semi-long position must be done for correct positioning. A: Sphincterotome is oriented towards the wrong direction (black arrow) in the short endoscope position; B: Endoscope is pushed to the long position; C: Sphincterotome is oriented towards the right direction (white arrow).
After a proper position is attained, it is prudent to check the position of the cutting wire once again before starting sphincterotomy. There should be no more than 5 mm cutting wire inside the papilla. Otherwise, tissue resistance may increase which leads to an uncontrolled large cut (zipper) and thermal injury of the surrounding tissues, hence pancreatitis. During sphincterotomy, contact with the roof of the papilla is maintained by several ways in the short position: upward lifting of the tip of the sphincterotome with elevator, slightly pulling back the duodenoscope, tipping up the proximal end of the duodenoscope or counterclockwise rotation of the duodenoscope. EST can be extended along the bile duct axis upto the junction between the intraduodenal part of the bile duct and duodenal wall. Sometimes, this superior margin of sphincterotomy can not be clearly identified. In this case, sphincterotomy should be terminated if a fully bowed sphincterotome that is pulled back from the bile duct into the duodenum does not bulge the roof of the papilla, lumen of the bile duct is completely visible or bowed sphincterotome slides easily through the orifice. The size of sphincterotomy can be classified into 3 groups: Small, medium or large. Sphincterotomy extending upto the transverse hood is defined as a small EST, whereas large EST extends upto the superior margin of the intramural bile duct. There is not a standard size of incision appropriate for all patients. Size should be determined individually according to the indication of sphincterotomy. Diameter of the distal common bile duct and technical difficulties encountered during sphincterotomy also play a role in determining the size of sphincterotomy due to safety concerns. For example, a small EST may be enough to insert a stent for palliation in a patient with a malignant biliary stricture, whereas extraction of large stones necessitates a large EST. If there is a difficulty in maintaining appropriate position while extending sphincterotomy, e.g. in a patient with periampullary diverticula and the patient needs a large sphincterotomy, it may be safer to terminate EST early and switch to balloon dilation of the papilla (Figure 4A-D). Finally, it is safe to extend a previous EST (re-EST). However, there is a trend towards a higher risk of hemorrhage when re-EST is performed early after the primary one, due to increase in the vascularity[17].
Figure 4.
It may be safer to terminate endoscopic sphincterotomy early and switch to balloon dilation of the papilla in patients with periampullary diverticula. A: Papilla is located on the edge of the diverticulum at 7 o’clock; B: A small sphincterotomy could be made because of difficulty in maintaining appropriate position; C: Papilla is dilated with a 12-mm balloon, D: A notch on the balloon is seen.
Precut sphincterotomy
Precut biliary EST refers to the techniques used to cut the papillary mucosa and biliary sphincter in order to expose the underlying bile duct and gain access to it when selective cannulation fails. There are several types of biliary pre-cut sphincterotomy techniques, such as transpancreatic septotomy, needle knife papillotomy and needle knife fistulotomy.
Transpancreatic biliary sphincterotomy (septotomy): Transpancreatic biliary sphincterotomy (Goff technique) was first reported by Goff[18] in 1995. In this technique, when the guidewire is unintentionally inserted into the main pancreatic duct, standard sphincterotome is placed in the pancreatic duct and gently bowed in order to direct its cutting wire towards 11 o’clock, to cut the septum between the pancreatic duct and bile duct A small cut (< 5 mm) is made with short bursts. Afterwards, the sphincterotome is withdrawn and re-directed into the incision site to cannulate the bile duct with the guidewire (Figure 5A-D). If the bile duct cannot be cannulated at the first attempt, the cut can be extended to a maximum of two times. If the bile duct still cannot be cannulated after two extensions, the technique is terminated due to the risk of exceeding the upper boundary of septum between the pancreatic and bile duct, leading to perforation. ESGE recommends the insertion a prophylactic pancreatic stent after the procedure[16].
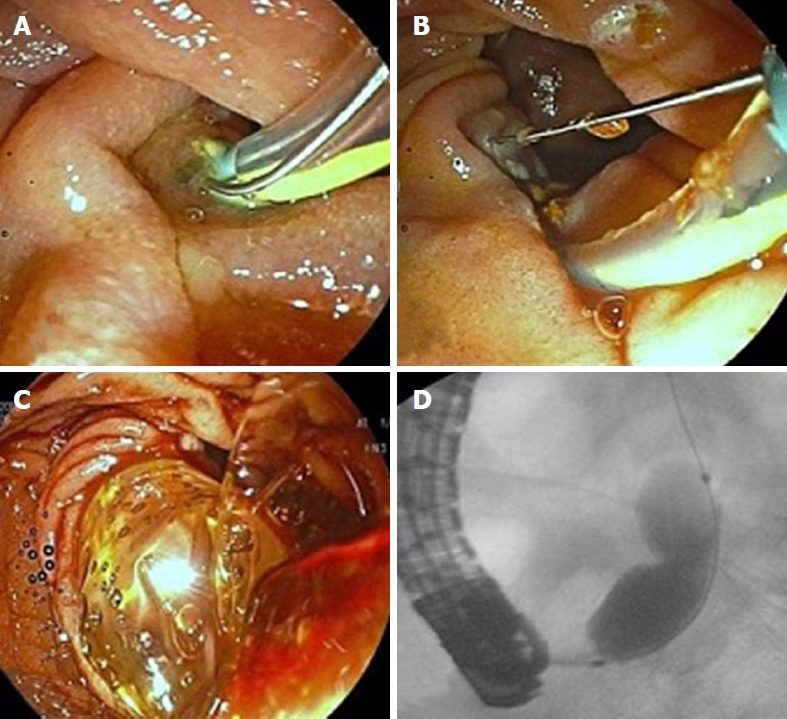
Figure 5.
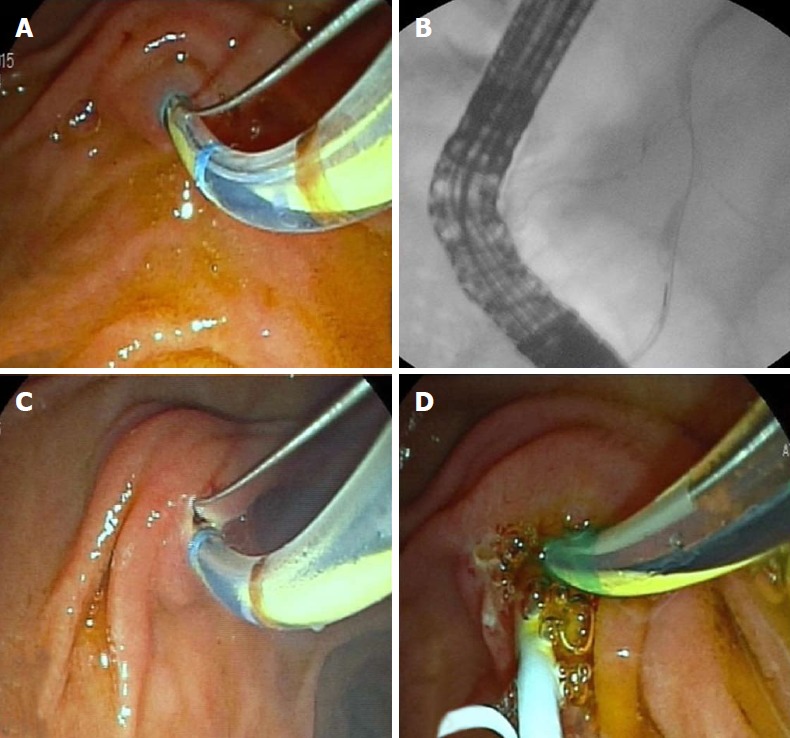
Transpancreatic biliary sphincterotomy. A: Pancreatic duct is unintentionally cannulated during biliary cannulation attempts; B: Guidewire is inserted to the pancreatic duct; C: Cutting wire of the sphincterotome is oriented towards 11 o’clock to cut the septum between the pancreatic duct and bile duct; D: Bile duct is cannulated after a 5-Fr plastic stent is inserted to the pancreatic duct for post-endoscopic retrograde cholangiopancreatography pancreatitis prophylaxis.
In this technique, sphincterotome should enter the pancreatic channel a certain amount and its cutting wire, after proper bowing and positioning, should contact with the septum. The shorter the nose of the sphincterotome, the easier it is to attain this contact. There are sphincterotomes, called no nose sphincterotomes, produced specifically for this purpose. Sometimes, precut sphincterotomy is initiated with Goff technique but it is not possible to contact the septum. In such cases, endoscopists can switch to a needle knife precut sphincterotomy if biliary sphincter has become visible. Goff technique is mostly preferred in patients with a small papilla or difficult anatomy if unintentional insertion of a guidewire into the pancreatic duct occurs. However, only experts should perform it[16].
Needle knife papillotomy: In this technique, after an en-face position to papilla is attained, a 5-10 mm incision is made stepwise with a needle knife, starting at the upper margin of the papillary orifice and extending towards the biliary sphincter at 11-13 o’clock. The first incision should be deep enough to ablate the papillary mucosa and reach the underlying biliary sphincter. Needle knife should be kept in motion during mucosal incision to prevent thermal injury to the papillary orifice. Subsequently, biliary sphincter and the bile duct are incised to cannulate the bile duct. When the endoscopist decides that the incision of the bile duct is accomplished by recognizing a hole in the bile duct and/or bile leakage, cannulation can be attempted through this hole using a needle knife or a standard sphincterotome. If cannulation is achieved, sphincterotomy is extended using a standard sphincterotome most of the time. In cases where the anatomy is unfavorable for extension with a standard sphincterotome, a plastic stent may be inserted to the common bile duct following cannulation and sphincterotomy can be extended over it with a needle knife. If cannulation cannot be achieved, pre-cutting can be extended upto the upper boundary of the intraduodenal bile duct or made deeper with a needle knife. Endoscopists should be cautious during these procedures and try to do their best to recognize the layers of concern in order to prevent perforation. If cannulation is still unsuccessful after extending and/or deepening the incision, or the anatomy is obscured due to development of edema etc., endoscopists should not hesitate to terminate the procedure. The procedure can be repeated after 48-72 h when edema has subsided. Cannulation can mostly be achieved easily in the second session if the pre-cut has been made in the correct direction.
Needle knife fistulotomy: There are two techniques of needle knife fistulotomy. In the first technique, incision is started a few milimeters above the papillary orifice and extended upwards along the bile duct axis in a stepwise fashion. In the second technique, incision is started between the middle and upper portion of the roof of the papilla and proceeded either in the upward or downward direction, without involving the papillary orifice. The advantage of the second technique is the decreased risk of thermal injury to the pancreatic duct. A meta-analysis revealed that the rate of PEP was significantly lower after fistulotomy, compared to other precut techniques[19]. In addition, needle knife fistulotomy can be preferred when the papillary orifice is obstructed with tumor or stone.
According to our experience, it is easier and safer to perform needle knife papillotomy and fistulotomy in close position with the papilla. At this position, the length of the sphincterotome outside the duodenoscope is less, so it can be more easily controlled and its contact with the papilla can be maintained with the elevator or upward motion of the duodenoscope. Otherwise, the risk of insufficient EST, bleeding and perforation may increase.
Precut techniques are used when standard cannulation techniques fail. Persistent cannulation attempts using standard techniques increase trauma to the papilla and hence, the complications. Early precutting decreases the risk of PEP while maintaining a similar cannulation success rate. The overall complication rate is the same in both techniques[20]. Therefore, precut sphincterotomy should only be performed by experienced endoscopists who have a selective biliary cannulation success rate of more than 80%[18]. There is no consensus on the timing of early precutting. Studies reported diverse timings, varying between immediately after a single failed standard cannulation attempt and 15 min of attempts[19,21,22]. Experience of the endoscopist is a determining factor in the timing of precutting. ESGE recommends precutting when bile duct cannulation cannot be achieved after 5 attempts or 5 min[16].
The choice between precut sphincterotomy techniques mostly depends on the experience of the endoscopist. However, it may be prudent to perform fistulotomy in patients who have a dilated, bulging intraduodenal portion of the common bile duct and prefer needle knife papillotomy in those with a small papilla. Transpancreatic biliary sphincterotomy can be performed when the main pancreatic duct is unintentionally cannulated during biliary cannulation attempts and the patient has some features making precutting difficult with other techniques, such as a small papilla or an unfavorable anatomy. The depth and direction of incision can be more easily controlled with transpancreatic bilary sphincterotomy compared to needle-knife papillotomy. In patients with unintentional cannulation of the main pancreatic duct, another alternative is to perform needle knife papillotomy or fistulotomy over a plastic stent. In this technique, a plastic stent is inserted into the main pancreatic duct and precut sphincterotomy is performed with either needle knife papillotomy which is preferred in patients with a small papilla; or fistulotomy in those with a bulging intraduodenal bile duct. The insertion of a pancreatic stent has 2 advantages: it acts as a guide to sphincterotomy and decreases the risk of PEP[20,23]. A 5Fr plastic stent is more appropriate for this procedure. The stent should be checked 1 week after its insertion and removed if still in place. Some authors use plastic stents without an internal flange, which increases the rate of spontaneous migration and hence, decreases the need for endoscopy to remove it. On the other hand, early migration is not desired, as the stent should remain in the pancreatic duct for at least 24 h to decrease the risk of PEP[24].
ALTERNATIVES TO ENDOSCOPIC SPHINCTEROTOMY
Balloon dilation of the papilla can be considered as an alternative to sphincterotomy in patients with coagulopathy and altered (e.g., Billroth II gastrectomy) or unfavorable anatomy. It is mostly used to facilitate the extraction of common bile duct stones. A 8 mm diameter dilation balloon is recommended regardless of the common bile duct diameter[16]. There is not a standard protocol for duration and number of dilations. The efficacy of balloon dilation is similar with EST in the extraction of small to moderate sized stones. However, it frequently requires additional procedures, such as mechanical lithotripsy, especially in the extraction of large stones[25,26]. Large-balloon dilation (≥ 12 mm) combined with a variable size of EST is a safe and effective method for the extraction of large stones[27-29].
Balloon dilation of the papilla is significantly associated with more PEP and less bleeding compared to EST in the short term[26,30]. Choosing a balloon diameter smaller than the diameter of the overlying common bile duct, increasing the duration of balloon inflation and performing a small EST before dilation, may decrease the risk of PEP[16,26,31]. Balloon dilation is associated with a lower frequency of complications in the long term, such as stone recurrence, since preserving the sphincter function prevents duodenobiliary reflux and bacterial colonization of the bile duct[32,33].
BILIARY EST IN PATIENTS WITH AN ALTERED OR DIFFICULT ANATOMY
Billroth II gastrectomy
In Billroth II gastrectomy, papilla is approached through the afferent loop in a retrograde fashion. Therefore the papilla has an upside-down appearance and the bile duct is located at 5-6 o’clock. Sphincterotomy is more difficult and can be done by either a rotatable sphincterotome or a needle knife papillotomy over a plastic stent inserted into the common bile duct[34-36]. In the second technique, stent facilitates the identification of the roof of the papilla and acts as a guide in determining the direction and depth of the incision. If there are safety concerns during EST due to difficulty in obtaining an appropriate position or identifying the anatomy, balloon dilation of the papilla can be performed instead of insisting on sphincterotomy[37]. Finally, in patients with Billroth II gastrectomy, EST should be performed by experienced endoscopists, since the techniques are more difficult and associated with a higher risk of complications compared to standart sphincteromy techniques[16,37].
Sometimes, forward viewing endoscopes can be used if afferent loop cannot be intubated with a duodenoscope. However, it is not possible to obtain an en-face position to the papilla with forward viewing endoscopes and they do not have an elevator. Therefore, cannulation and sphincterotomy may be more difficult with a forward viewing endoscope but can be accomplished if the endoscope can take a position below the papilla[38]. The use of a forward viewing endoscope fitted with a cap attachment may increase the therapeutic success.
Periampullary diverticula
Selective cannulation may be difficult in patients with a periampullary diverticula. If the pancreatic duct is unintentionally cannulated during biliary cannulation attempts, double guidewire technique (DGWT) can be used to facilitate the biliary cannulation. In cases when DGWT also fails, a stent is inserted into the pancreatic duct and precutting can be performed with either needle knife papillotomy or fistulotomy over the stent. After selective cannulation is achieved, incision is extended by using a standard sphincterotome. During sphincterotomy, only a few milimeters of the cutting wire should be inside the papilla and sphincterotome should not be directed towards the base of the diverticulum. Sometimes, e.g., in patients with a papilla located in the margin of the diverticulum, it may be difficult to determine the direction and length of the sphincterotomy or properly position the cutting wire. In such cases, a plastic stent can be inserted into the common bile duct and needle knife papillotomy over the stent can be done. Another alternative is to perform balloon dilation of the papilla with or without a preceeding EST, which is at least as effective and safe as EST alone[39,40].
Some retrospective studies reported an increase in EST bleeding rates in patients with periampullary diverticula. However, prospective studies did not confirm these findings and found no significant difference in the overall and specific type of complications[41,42].
COMPLICATIONS OF BILIARY ENDOSCOPIC SPHINCTEROTOMY
Biliary EST is associated with several complications both in the short term and long term. These complications are inevitable due to the invasive nature of ERCP and observed in a variable ratio of patients, depending on some patient and procedure related factors. Early identification and appropriate management of complications is essential to reduce mortality and morbidity. The short term complications of EST are bleeding, perforation, pancreatitis and cholangitis. They have an incidence between 2.5% and 11.8%[43]. When talking about complications, clinicians should keep in mind that EST cannot be evaluated as an independent risk factor in most of the time, in other words, the incidence of complications more or less depends on other procedures of ERCP, such as cannulation and therapeutic success of the whole procedure, etc. Herein, we will review the incidence, risk factors and management of these complications.
Bleeding
Incidence and severity: EST bleeding is classified as immediate or delayed depending on the timing of presentation. Immediate bleeding occurs during or immediately after EST. It is seen in upto 30% of patients and self-limiting in most of the time[44]. Delayed bleeding occurs from a few hours upto 2 wk after the procedure[45]. It is more significant and frequently requires therapeutic interventions[46]. Clinically significant bleeding is defined as the presence of hematemesis and/or melena, hemoglobin drop > 2 g/dL or requirement for interventions, such as transfusion or endoscopy. It has an estimated incidence between 0.1% and 2%[47,48]. Cotton et al[49], categorized the severity of clinically significant bleeding into 4 groups based on the number of transfusions and requirement for interventions: mild (hemoglobin drop < 3 g/dL and no need for blood transfusions), moderate (blood transfusion ≤ 4 units), severe (requiring angiographic or surgical treatment or blood transfusion ≥ 5 units) and fatal[49]. The incidence of severe bleeding varies between 0.1% and 0.5%[50].
Risk factors: In a cornerstone study, multivariable analysis identified 5 independent risk factors for bleeding: the presence of any coagulopathy or thrombocytopenia, active cholangitis, anticoagulant therapy within 3 d after ERCP, endoscopist’s low case volume and occurrence of any observed bleeding during ERCP[51]. Other possible risk factors are cirrhosis, dilated common bile duct, periampullary diverticulum, precut sphincterotomy, uncontrolled cutting (zipper cut) and ampullary stone impaction[45,51]. Longer sphincterotomy incision and use of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) do not seem to increase risk of bleeding[51].
Prevention and management: Correcting the coagulopathies before the procedure and adjusting the antiaggregant and anticoagulant drugs according to the recommendations of the current guidelines may prevent EST bleeding. Avoiding zipper-cut and using a microprocessor-controlled generator may also decrease the risk of bleeding[13]. An anatomy study evaluating the distribution of papillary arteries around the circumference of the papillary orifice revealed that arterial vascularity of the postero-superior quadrant, especially in the combined 10 and 11 o’clock position, was lower than the antero-superior (1-3 o’clock) quadrant; the most densely arterialized region of the papilla[52]. Therefore, when an unexpected bleeding develops during sphincterotomy in a patient with no risk factor, such as coagulopathy and zipper-cut, endoscopist should discontinue the procedure and check the direction of the incision. If a proper direction cannot be attained, EST must be terminated and endoscopic balloon dilation of the papilla should be considered. Finally, in a critically ill patient with coagulopathy, a biliary stent can be inserted and EST can be performed after the patient’s clinical condition has improved.
Initial management of EST bleeding includes fluid resuscitation, correction of coagulopathy and blood transfusion, when necessary. Endoscopic treatment is indicated for immediate bleeding, either obscuring the endoscopic field or persisting at the end of ERCP, or for clinically significant delayed bleeding. Epinephrine injection is the most commonly used method of endoscopic treatment. Diluted epinephrine (1:10000) is injected directly into and around the bleeding point in highly variable amounts (0.5-30 mL). When bleeding point cannot be identified, injections are targeted to the apex of the incision. Care should be taken to avoid injection around the pancreatic orifice, since the resulting edema may lead to pancreatitis. Epinephrine injection is cheap and easy to perform. It has a high success rate between 96% and 100%, with a recurrence rate of 4%-16%[53,54]. Balloon tamponade using a stone extraction or dilation balloon, may allow temporary control of bleeding and visualization of the bleeding site[45]. Thermal therapies using a multipolar or heater probe may be used to coagulate the bleeding vessel[55]. The settings are similar to those used for peptic ulcer bleeding. Larger diameter probes (10Fr) may be more effective because they coagulate a wider area and provide more effective tamponade[55]. During coagulation, caution should be taken to avoid thermal injury to the pancreatic orifice. There are no randomized trials, which proved that thermal therapies in combination with epinephrine injection are superior to epinephrine injection, alone. Application of hemoclips to the bleeding site through the working channel of a duodenoscope is technically challenging because the sheath of the hemoclips may kink while passing over the elevator of the duodenoscope, leading to failure of deployment. Cap-assisted hemoclip application with a forward-viewing endoscope may overcome this problem[56]. A fully covered self-expandable metal stent (FC-SEMS) may be inserted into the biliary system to tamponade the bleeding site. It has the advantage to tamponade the bleeding originating from deeper sites of the biliary system, but has a considerable risk of migration. Studies revealed excellent success rates in the treatment of refractory bleeding cases[57,58]. Finally, angiographic embolization and surgery may be used for the treatment of bleeding refractory to endoscopic treatments.
Perforation
Incidence and severity: Biliary Endoscopic Sphincterotomy is the most common cause of ERCP related perforation[59]. The incidence of sphincterotomy related perforation, also named Type 2 duodenal perforation, is between 0% and 1.8%[43]. Extensions of sphincterotomy beyond the intraduodenal segment of the bile duct and towards the wrong direction are the most frequent mechanisms. Perforation risk increases after needle knife precutting and in patients with sphincter of Oddi dysfunction[44]. Cotton et al[49] categorized the severity of perforations into 3 groups: mild (possible or only slight leak of fluid or contrast, treated by fluids and suction for 3 d or less), moderate (definite perforation treated medically for 4-10 d) and severe (medical treatment for more than 10 d or need for percutaneous or surgical intervention)[49]. Limiting the length of cutting wire inside the papilla during sphincterotomy, performing stepwise incision and using a microprocessor controlled electrosurgical generator may decrease the incidence of perforation.
Diagnosis: Early diagnosis of perforation is crucial and determines the rate of morbidity and mortality. Endoscopists should carefully check for the signs of perforation, during and at the end of ERCP procedure, especially if the patient had an altered anatomy or underwent risky procedures, such as precutting. Severe epigastric pain and tenderness after the procedure should alert the clinician to the possibility of perforation. Subsequently, patients may develop peritonitis with fever, tachycardia and generalized abdominal wall rigidity. The clinical scenario is not always the same. Epigastric pain may be mild at the onset of perforation or peritonitis may not develop in patients with a small leak.
Perforation can be detected by several ways. Free air in the retroperitoneum makes retroperitoneal structures, such as the psoas muscles and the kidneys, visible under fluoroscopy. Injection of a small amount of contrast while the sphincterotome is pulled through the incision can demonstrate extravasation. If perforation cannot be confidently excluded with these methods, abdominal computed tomography (CT) with oral contrast should be performed. CT is the most sensitive modality that demonstrates retrroperitoneal free air and the leak. The amount of free air detected during CT only reflects the amount of air insufflated during the procedure. It does not correlate with the size of perforation or its probability of requiring surgery.
Management: Most of the sphincterotomy related perforations recover completely with conservative treatment. Pooled data from 11 studies revealed a surgery rate of 21%[59]. The main principle of conservative treatment is to prevent retroperitoneal leakage and contamination. For this purpose, there are various endoscopic methods used to close or seal the defect and divert bile from the perforation site. One of them is to close the defect with endoclips and divert bile with nasobiliary tube or plastic stent insertion[60]. Another one is to deploy FC-SEMS, which both seals the defect and diverts the bile[61,62]. FC-SEMS has made a revolution in the treatment of perforation. Case series reported excellent results with no need for surgery after FC-SEMS insertion in patients who received an early diagnosis[63,64]. Endoscopic methods can be performed if biliary system was already cannulated at the time of perforation. Otherwise, bile can be diverted from the perforation site with a percutaneously placed external drainage catheter.
The patient is hospitalized after the endoscopic treatment and put on fasting, intravenous fluids, nasogastric suction, proton pump inhibitors and broad-spectrum intravenous antibiotics. During the follow-up, serial physical examinations and laboratory tests should be done to search for development of peritoneal irritation signs and systemic inflammatory response. Routine imaging is not necessary. Surgery is indicated when patients have a significant leak with ongoing contrast extravasation, deterioration in clinical condition due to peritonitis and/or sepsis, fluid collections not amenable for percutaneous drainage and unresolved problems, such as retained stones. However, during surgery, it may not be possible to detect the site of perforation in some of the patients (16%-80%) or the tissues may be too edematous for primary repair. Therefore, surgery carries a high mortality rate[59].
Pancreatitis
Risk factors: Pancreatitis is the most common complication of ERCP. The incidence depends on some patient and procedure-related factors. Biliary EST is not an independent risk factor for PEP[65]. Patient-related risk factors that are independently associated with PEP include young age, female sex, normal bilirubin, suspected sphincter of Oddi dysfunction, absence of chronic pancreatitis, previous recurrent pancreatitis and prior post-ERCP pancreatitis. Procedure-related independent risk factors include difficult or failed cannulation, precut sphincterotomy, pancreatic sphincterotomy, pancreatic tissue sampling, repetitive pancreatic guidewire cannulation, pancreatic duct injection, balloon dilation of the intact biliary sphincter and endoscopic papillectomy[24,44]. Increased risk of PEP after precut sphincterotomy may be due to repeated cannulation attempts with standard techniques, leading to papillary trauma.
Prevention and management: Atraumatic cannulation with a guidewire cannulation technique, early precut sphincterotomy in patients with difficult biliary access, combining papillary balloon dilation with endoscopic sphincterotomy and pancreatic stenting, particularly in patients with a high risk of PEP, may reduce the risk of pancreatitis[66,67]. Pharmacologic prophylaxis includes rectal NSAID administration either before or immediately after ERCP and periprocedural aggressive intravenous hydration[68-70].
Management of PEP is similar to other causes of pancreatitis. Fasting, goal directed fluid therapy, pain control, enteral nutrition and early diagnosis and management of complications are the cornerstones of treatment.
Cholangitis
Risk factors: The incidence of cholangitis after biliary EST is between 1% and 3%[51]. Risk factors include incomplete biliary drainage, performance of a combined percutaneous and endoscopic procedure, jaundice especially caused by malignancy and inexperience of the endoscopist.
Prevention and management: Complete biliary drainage is the most important factor in reducing the risk of cholangitis. A stent or nasobiliary tube may be inserted for this purpose. If complete biliary drainage is not feasible with endoscopic methods, percutaneous or surgical interventions should be done without delay. Routine antibiotic prophylaxis is not recommended in patients with complete biliary drainage, other than those with primary sclerosing cholangitis or biliary strictures after liver transplantation. The risk of cholangitis may also be reduced by minimizing the volume of contrast injection in patients with obstruction, aspirating infected bile before contrast injection to avoid an increase in biliary pressure and limiting contrast injection to segments already cannulated with a guidewire. Management of cholangitis include supportive care, systemic antibiotic therapy and biliary decompression procedures in a timely manner.
Long-term complications
Long-term complications of EST include recurrent common bile duct stone, cholecystitis, cholangitis, hepatic abscess, papillary stenosis and biliary stricture. Recurrent common bile duct stone is the most common complication. It can be seen in up to 17% of patients. Large diameter of the common bile duct, presence of a periampullary diverticulum, gallstones and the use of mechanical lithotripsy during previous stone extraction increase the risk of recurrence[43]. Stones can be extracted after extension of the previous sphincterotomy and/or balloon dilation of the papilla. Papillary stenosis develops in 1%-3.9% of sphincterotomies done for choledocholithiasis and in 16.8% done for Oddi stenosis[71,72]. SOD and ischemia of the ampulla due to previous complicated endoscopic procedures, such as injection and heater probe for the treatment of bleeding, are the risk factors for papillary stenosis. In sphincterotomy associated biliary stricture, the stricture extends over the intraduodenal segment of the bile duct to the distal part of extraduodenal common bile duct. It develops in 1% of patients[73]. Papillary stenosis can be managed by extension of the previous sphincterotomy. Sphincterotomy associated biliary stricture requires sequential stenting.
CONCLUSION
Biliary endoscopic sphincterotomy is an essential procedure of the ERCP for the treatment and palliation of a wide spectrum of biliary and papillary diseases. Every practitioner who performs ERCP needs to know different techniques and the clinical and anatomic parameters related to the efficacy and safety of the procedure. Although it has some complications, management of those complications is often not difficult.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests fort his review.
Manuscript source: Invited manuscript
Peer-review started: September 28, 2018
First decision: October 18, 2018
Article in press: November 26, 2018
Specialty type: Medicine, research and experimental
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Armellini E, Neri V, Skok P S- Editor: Wang JL L- Editor: A E- Editor: Wu YXJ
Contributor Information
Aydın Şeref Köksal, Department of Gastroenterology, Sakarya University, School of Medicine, Sakarya 54290, Turkey. akoksal@sakarya.edu.tr.
Ahmet Tarik Eminler, Department of Gastroenterology, Sakarya University, School of Medicine, Sakarya 54290, Turkey.
Erkan Parlak, Department of Gastroenterology, Hacettepe University, School of Medicine, Ankara 41000, Turkey.
References
- 1.Kawai K, Akasaka Y, Murakami K, Tada M, Koli Y. Endoscopic sphincterotomy of the ampulla of Vater. Gastrointest Endosc. 1974;20:148–151. doi: 10.1016/s0016-5107(74)73914-1. [DOI] [PubMed] [Google Scholar]
- 2.Classen M, Demling L. [Endoscopic sphincterotomy of the papilla of vater and extraction of stones from the choledochal duct (author’s transl)] Dtsch Med Wochenschr. 1974;99:496–497. doi: 10.1055/s-0028-1107790. [DOI] [PubMed] [Google Scholar]
- 3.Nelson DB, Freeman ML. Major hemorrhage from endoscopic sphincterotomy: risk factor analysis. J Clin Gastroenterol. 1994;19:283–287. doi: 10.1097/00004836-199412000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Veitch AM, Vanbiervliet G, Gershlick AH, Boustiere C, Baglin TP, Smith LA, Radaelli F, Knight E, Gralnek IM, Hassan C, et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Gut. 2016;65:374–389. doi: 10.1136/gutjnl-2015-311110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prat F, Tennenbaum R, Ponsot P, Altman C, Pelletier G, Fritsch J, Choury AD, Bernades P, Etienne JP. Endoscopic sphincterotomy in patients with liver cirrhosis. Gastrointest Endosc. 1996;43:127–131. doi: 10.1016/s0016-5107(06)80114-8. [DOI] [PubMed] [Google Scholar]
- 6.Adler DG, Haseeb A, Francis G, Kistler CA, Kaplan J, Ghumman SS, Laique SN, Munigala S, Taylor LJ, Cox K, et al. Efficacy and safety of therapeutic ERCP in patients with cirrhosis: a large multicenter study. Gastrointest Endosc. 2016;83:353–359. doi: 10.1016/j.gie.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Navaneethan U, Njei B, Zhu X, Kommaraju K, Parsi MA, Varadarajulu S. Safety of ERCP in patients with liver cirrhosis: a national database study. Endosc Int Open. 2017;5:E303–E314. doi: 10.1055/s-0043-102492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikarashi S, Katanuma A, Kin T, Takahashi K, Yane K, Sano I, Yamazaki H, Maguchi H. Factors associated with delayed hemorrhage after endoscopic sphincterotomy: Japanese large single-center experience. J Gastroenterol. 2017;52:1258–1265. doi: 10.1007/s00535-017-1347-9. [DOI] [PubMed] [Google Scholar]
- 9.Nakaji S, Hirata N, Matsui H, Shiratori T, Kobayashi M, Yoshimura S, Kanda K, Kawamitsu N, Harasawa H. Hemodialysis is a strong risk factor for post-endoscopic sphincterotomy bleeding in patients with choledocholithiasis. Endosc Int Open. 2018;6:E568–E574. doi: 10.1055/a-0587-4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ASGE Technology Committee. Kethu SR, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, Kaul V, Kwon RS, Mamula P, Pedrosa MC, Rodriguez SA, Tierney WM. ERCP cannulation and sphincterotomy devices. Gastrointest Endosc. 2010;71:435–445. doi: 10.1016/j.gie.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 11.ASGE Technology Committee, Shah RJ, Somogyi L, Petersen BT, Tierney WM, Adler DG, Chand B, Conway JD, Croffie JM, Disario JA, Mishkin DS, Wong Kee Song LM. Short-wire ERCP systems. Gastrointest Endosc. 2007;66:650–657. doi: 10.1016/j.gie.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 12.Verma D, Kapadia A, Adler DG. Pure versus mixed electrosurgical current for endoscopic biliary sphincterotomy: a meta-analysis of adverse outcomes. Gastrointest Endosc. 2007;66:283–290. doi: 10.1016/j.gie.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Perini RF, Sadurski R, Cotton PB, Patel RS, Hawes RH, Cunningham JT. Post-sphincterotomy bleeding after the introduction of microprocessor-controlled electrosurgery: does the new technology make the difference? Gastrointest Endosc. 2005;61:53–57. doi: 10.1016/s0016-5107(04)02454-x. [DOI] [PubMed] [Google Scholar]
- 14.Parlak E, Köksal AŞ, Öztaş E, Dişibeyaz S, Ödemiş B, Yüksel M, Yıldız H, Şaşmaz N, Şahin B. Is there a safer electrosurgical current for endoscopic sphincterotomy in patients with liver cirrhosis? Wien Klin Wochenschr. 2016;128:573–578. doi: 10.1007/s00508-014-0677-3. [DOI] [PubMed] [Google Scholar]
- 15.Elta GH, Barnett JL, Wille RT, Brown KA, Chey WD, Scheiman JM. Pure cut electrocautery current for sphincterotomy causes less post-procedure pancreatitis than blended current. Gastrointest Endosc. 1998;47:149–153. doi: 10.1016/s0016-5107(98)70348-7. [DOI] [PubMed] [Google Scholar]
- 16.Testoni PA, Mariani A, Aabakken L, Arvanitakis M, Bories E, Costamagna G, Devière J, Dinis-Ribeiro M, Dumonceau JM, Giovannini M, et al. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016;48:657–683. doi: 10.1055/s-0042-108641. [DOI] [PubMed] [Google Scholar]
- 17.Mavrogiannis C, Liatsos C, Papanikolaou IS, Psilopoulos DI, Goulas SS, Romanos A, Karvountzis G. Safety of extension of a previous endoscopic sphincterotomy: a prospective study. Am J Gastroenterol. 2003;98:72–76. doi: 10.1111/j.1572-0241.2003.07166.x. [DOI] [PubMed] [Google Scholar]
- 18.Goff JS. Common bile duct pre-cut sphincterotomy: transpancreatic sphincter approach. Gastrointest Endosc. 1995;41:502–505. doi: 10.1016/s0016-5107(05)80011-2. [DOI] [PubMed] [Google Scholar]
- 19.Choudhary A, Winn J, Siddique S, Arif M, Arif Z, Hammoud GM, Puli SR, Ibdah JA, Bechtold ML. Effect of precut sphincterotomy on post-endoscopic retrograde cholangiopancreatography pancreatitis: a systematic review and meta-analysis. World J Gastroenterol. 2014;20:4093–4101. doi: 10.3748/wjg.v20.i14.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubota K, Sato T, Kato S, Watanabe S, Hosono K, Kobayashi N, Hisatomi K, Matsuhashi N, Nakajima A. Needle-knife precut papillotomy with a small incision over a pancreatic stent improves the success rate and reduces the complication rate in difficult biliary cannulations. J Hepatobiliary Pancreat Sci. 2013;20:382–388. doi: 10.1007/s00534-012-0552-4. [DOI] [PubMed] [Google Scholar]
- 21.Navaneethan U, Konjeti R, Venkatesh PG, Sanaka MR, Parsi MA. Early precut sphincterotomy and the risk of endoscopic retrograde cholangiopancreatography related complications: An updated meta-analysis. World J Gastrointest Endosc. 2014;6:200–208. doi: 10.4253/wjge.v6.i5.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cennamo V, Fuccio L, Zagari RM, Eusebi LH, Ceroni L, Laterza L, Fabbri C, Bazzoli F. Can early precut implementation reduce endoscopic retrograde cholangiopancreatography-related complication risk? Meta-analysis of randomized controlled trials. Endoscopy. 2010;42:381–388. doi: 10.1055/s-0029-1243992. [DOI] [PubMed] [Google Scholar]
- 23.Bourke MJ, Costamagna G, Freeman ML. Biliary cannulation during endoscopic retrograde cholangiopancreatography: core technique and recent innovations. Endoscopy. 2009;41:612–617. doi: 10.1055/s-0029-1214859. [DOI] [PubMed] [Google Scholar]
- 24.Dumonceau JM, Andriulli A, Elmunzer BJ, Mariani A, Meister T, Deviere J, Marek T, Baron TH, Hassan C, Testoni PA, et al. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - updated June 2014. Endoscopy. 2014;46:799–815. doi: 10.1055/s-0034-1377875. [DOI] [PubMed] [Google Scholar]
- 25.Baron TH, Harewood GC. Endoscopic balloon dilation of the biliary sphincter compared to endoscopic biliary sphincterotomy for removal of common bile duct stones during ERCP: a metaanalysis of randomized, controlled trials. Am J Gastroenterol. 2004;99:1455–1460. doi: 10.1111/j.1572-0241.2004.30151.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Su P, Lin S, Xiao K, Chen P, An S, Zhi F, Bai Y. Endoscopic papillary balloon dilatation versus endoscopic sphincterotomy in the treatment for choledocholithiasis: a meta-analysis. J Gastroenterol Hepatol. 2012;27:464–471. doi: 10.1111/j.1440-1746.2011.06912.x. [DOI] [PubMed] [Google Scholar]
- 27.Ersoz G, Tekesin O, Ozutemiz AO, Gunsar F. Biliary sphincterotomy plus dilation with a large balloon for bile duct stones that are difficult to extract. Gastrointest Endosc. 2003;57:156–159. doi: 10.1067/mge.2003.52. [DOI] [PubMed] [Google Scholar]
- 28.Heo JH, Kang DH, Jung HJ, Kwon DS, An JK, Kim BS, Suh KD, Lee SY, Lee JH, Kim GH, et al. Endoscopic sphincterotomy plus large-balloon dilation versus endoscopic sphincterotomy for removal of bile-duct stones. Gastrointest Endosc. 2007;66:720–726; quiz 768, 771. doi: 10.1016/j.gie.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 29.Itoi T, Itokawa F, Sofuni A, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Moriyasu F. Endoscopic sphincterotomy combined with large balloon dilation can reduce the procedure time and fluoroscopy time for removal of large bile duct stones. Am J Gastroenterol. 2009;104:560–565. doi: 10.1038/ajg.2008.67. [DOI] [PubMed] [Google Scholar]
- 30.Liao WC, Tu YK, Wu MS, Wang HP, Lin JT, Leung JW, Chien KL. Balloon dilation with adequate duration is safer than sphincterotomy for extracting bile duct stones: a systematic review and meta-analyses. Clin Gastroenterol Hepatol. 2012;10:1101–1109. doi: 10.1016/j.cgh.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Liao WC, Lee CT, Chang CY, Leung JW, Chen JH, Tsai MC, Lin JT, Wu MS, Wang HP. Randomized trial of 1-minute versus 5-minute endoscopic balloon dilation for extraction of bile duct stones. Gastrointest Endosc. 2010;72:1154–1162. doi: 10.1016/j.gie.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka S, Sawayama T, Yoshioka T. Endoscopic papillary balloon dilation and endoscopic sphincterotomy for bile duct stones: long-term outcomes in a prospective randomized controlled trial. Gastrointest Endosc. 2004;59:614–618. doi: 10.1016/s0016-5107(04)00157-9. [DOI] [PubMed] [Google Scholar]
- 33.Zhao HC, He L, Zhou DC, Geng XP, Pan FM. Meta-analysis comparison of endoscopic papillary balloon dilatation and endoscopic sphincteropapillotomy. World J Gastroenterol. 2013;19:3883–3891. doi: 10.3748/wjg.v19.i24.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hintze RE, Veltzke W, Adler A, Abou-Rebyeh H. Endoscopic sphincterotomy using an S-shaped sphincterotome in patients with a Billroth II or Roux-en-Y gastrojejunostomy. Endoscopy. 1997;29:74–78. doi: 10.1055/s-2007-1004078. [DOI] [PubMed] [Google Scholar]
- 35.Lin LF, Siauw CP, Ho KS, Tung JC. ERCP in post-Billroth II gastrectomy patients: emphasis on technique. Am J Gastroenterol. 1999;94:144–148. doi: 10.1111/j.1572-0241.1999.00785.x. [DOI] [PubMed] [Google Scholar]
- 36.Ciçek B, Parlak E, Dişibeyaz S, Koksal AS, Sahin B. Endoscopic retrograde cholangiopancreatography in patients with Billroth II gastroenterostomy. J Gastroenterol Hepatol. 2007;22:1210–1213. doi: 10.1111/j.1440-1746.2006.04765.x. [DOI] [PubMed] [Google Scholar]
- 37.Bergman JJ, van Berkel AM, Bruno MJ, Fockens P, Rauws EA, Tijssen JG, Tytgat GN, Huibregtse K. A randomized trial of endoscopic balloon dilation and endoscopic sphincterotomy for removal of bile duct stones in patients with a prior Billroth II gastrectomy. Gastrointest Endosc. 2001;53:19–26. doi: 10.1067/mge.2001.110454. [DOI] [PubMed] [Google Scholar]
- 38.Bove V, Tringali A, Familiari P, Gigante G, Boškoski I, Perri V, Mutignani M, Costamagna G. ERCP in patients with prior Billroth II gastrectomy: report of 30 years’ experience. Endoscopy. 2015;47:611–616. doi: 10.1055/s-0034-1391567. [DOI] [PubMed] [Google Scholar]
- 39.Liao WC, Huang SP, Wu MS, Lin JT, Wang HP. Comparison of endoscopic papillary balloon dilatation and sphincterotomy for lithotripsy in difficult sphincterotomy. J Clin Gastroenterol. 2008;42:295–299. doi: 10.1097/MCG.0b013e31802c3458. [DOI] [PubMed] [Google Scholar]
- 40.Kim HW, Kang DH, Choi CW, Park JH, Lee JH, Kim MD, Kim ID, Yoon KT, Cho M, Jeon UB, et al. Limited endoscopic sphincterotomy plus large balloon dilation for choledocholithiasis with periampullary diverticula. World J Gastroenterol. 2010;16:4335–4340. doi: 10.3748/wjg.v16.i34.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zoepf T, Zoepf DS, Arnold JC, Benz C, Riemann JF. The relationship between juxtapapillary duodenal diverticula and disorders of the biliopancreatic system: analysis of 350 patients. Gastrointest Endosc. 2001;54:56–61. doi: 10.1067/mge.2001.115334. [DOI] [PubMed] [Google Scholar]
- 42.Panteris V, Vezakis A, Filippou G, Filippou D, Karamanolis D, Rizos S. Influence of juxtapapillary diverticula on the success or difficulty of cannulation and complication rate. Gastrointest Endosc. 2008;68:903–910. doi: 10.1016/j.gie.2008.03.1092. [DOI] [PubMed] [Google Scholar]
- 43.Ryozawa S, Itoi T, Katanuma A, Okabe Y, Kato H, Horaguchi J, Fujita N, Yasuda K, Tsuyuguchi T, Fujimoto K. Japan Gastroenterological Endoscopy Society guidelines for endoscopic sphincterotomy. Dig Endosc. 2018;30:149–173. doi: 10.1111/den.13001. [DOI] [PubMed] [Google Scholar]
- 44.Freeman ML. Complications of endoscopic retrograde cholangiopancreatography: avoidance and management. Gastrointest Endosc Clin N Am. 2012;22:567–586. doi: 10.1016/j.giec.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Rustagi T, Jamidar PA. Endoscopic retrograde cholangiopancreatography-related adverse events: general overview. Gastrointest Endosc Clin N Am. 2015;25:97–106. doi: 10.1016/j.giec.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Cotton PB, Garrow DA, Gallagher J, Romagnuolo J. Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years. Gastrointest Endosc. 2009;70:80–88. doi: 10.1016/j.gie.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 47.Wilcox CM, Canakis J, Mönkemüller KE, Bondora AW, Geels W. Patterns of bleeding after endoscopic sphincterotomy, the subsequent risk of bleeding, and the role of epinephrine injection. Am J Gastroenterol. 2004;99:244–248. doi: 10.1111/j.1572-0241.2004.04058.x. [DOI] [PubMed] [Google Scholar]
- 48.Ferreira LE, Fatima J, Baron TH. Clinically significant delayed postsphincterotomy bleeding: a twelve year single center experience. Minerva Gastroenterol Dietol. 2007;53:215–223. [PubMed] [Google Scholar]
- 49.Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 50.Barthet M, Lesavre N, Desjeux A, Gasmi M, Berthezene P, Berdah S, Viviand X, Grimaud JC. Complications of endoscopic sphincterotomy: results from a single tertiary referral center. Endoscopy. 2002;34:991–997. doi: 10.1055/s-2002-35834. [DOI] [PubMed] [Google Scholar]
- 51.Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–918. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 52.Mirjalili SA, Stringer MD. The arterial supply of the major duodenal papilla and its relevance to endoscopic sphincterotomy. Endoscopy. 2011;43:307–311. doi: 10.1055/s-0030-1256229. [DOI] [PubMed] [Google Scholar]
- 53.Leung JW, Chan FK, Sung JJ, Chung SC. Endoscopic sphincterotomy-induced hemorrhage: A study of risk factors and the role of epinephrine injection. Gastrointest Endosc. 1995;42:550–554. doi: 10.1016/S0016-5107(95)70009-9. [DOI] [PubMed] [Google Scholar]
- 54.Tsou YK, Lin CH, Liu NJ, Tang JH, Sung KF, Cheng CL, Lee CS. Treating delayed endoscopic sphincterotomy-induced bleeding: epinephrine injection with or without thermotherapy. World J Gastroenterol. 2009;15:4823–4828. doi: 10.3748/wjg.15.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuran S, Parlak E, Oguz D, Cicek B, Disibeyaz S, Sahin B. Endoscopic sphincterotomy-induced hemorrhage: treatment with heat probe. Gastrointest Endosc. 2006;63:506–511. doi: 10.1016/j.gie.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 56.Liu F, Wang GY, Li ZS. Cap-assisted hemoclip application with forward-viewing endoscope for hemorrhage induced by endoscopic sphincterotomy: a prospective case series study. BMC Gastroenterol. 2015;15:135. doi: 10.1186/s12876-015-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itoi T, Yasuda I, Doi S, Mukai T, Kurihara T, Sofuni A. Endoscopic hemostasis using covered metallic stent placement for uncontrolled post-endoscopic sphincterotomy bleeding. Endoscopy. 2011;43:369–372. doi: 10.1055/s-0030-1256126. [DOI] [PubMed] [Google Scholar]
- 58.Shah JN, Marson F, Binmoeller KF. Temporary self-expandable metal stent placement for treatment of post-sphincterotomy bleeding. Gastrointest Endosc. 2010;72:1274–1278. doi: 10.1016/j.gie.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Vezakis A, Fragulidis G, Polydorou A. Endoscopic retrograde cholangiopancreatography-related perforations: Diagnosis and management. World J Gastrointest Endosc. 2015;7:1135–1141. doi: 10.4253/wjge.v7.i14.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin YJ, Jeong S, Kim JH, Hwang JC, Yoo BM, Moon JH, Park SH, Kim HG, Lee DK, Jeon YS, et al. Clinical course and proposed treatment strategy for ERCP-related duodenal perforation: a multicenter analysis. Endoscopy. 2013;45:806–812. doi: 10.1055/s-0033-1344230. [DOI] [PubMed] [Google Scholar]
- 61.Jeon HJ, Han JH, Park S, Youn S, Chae H, Yoon S. Endoscopic sphincterotomy-related perforation in the common bile duct successfully treated by placement of a covered metal stent. Endoscopy. 2011;43 Suppl 2 UCTN:E295–E296. doi: 10.1055/s-0030-1256464. [DOI] [PubMed] [Google Scholar]
- 62.Vezakis A, Fragulidis G, Nastos C, Yiallourou A, Polydorou A, Voros D. Closure of a persistent sphincterotomy-related duodenal perforation by placement of a covered self-expandable metallic biliary stent. World J Gastroenterol. 2011;17:4539–4541. doi: 10.3748/wjg.v17.i40.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tringali A, Pizzicannella M, Andrisani G, Cintolo M, Hassan C, Adler D, Dioscoridi L, Pandolfi M, Mutignani M, Di Matteo F. Temporary FC-SEMS for type II ERCP-related perforations: a case series from two referral centers and review of the literature. Scand J Gastroenterol. 2018;53:760–767. doi: 10.1080/00365521.2018.1458894. [DOI] [PubMed] [Google Scholar]
- 64.Odemis B, Oztas E, Kuzu UB, Parlak E, Disibeyaz S, Torun S, Kayacetin E. Can a Fully Covered Self-Expandable Metallic Stent be Used Temporarily for the Management of Duodenal Retroperitoneal Perforation During ERCP as a Part of Conservative Therapy? Surg Laparosc Endosc Percutan Tech. 2016;26:e9–e17. doi: 10.1097/SLE.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 65.Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, Aas J, Ryan ME, Bochna GS, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425–434. doi: 10.1067/mge.2001.117550. [DOI] [PubMed] [Google Scholar]
- 66.Freeman ML, Guda NM. ERCP cannulation: a review of reported techniques. Gastrointest Endosc. 2005;61:112–125. doi: 10.1016/s0016-5107(04)02463-0. [DOI] [PubMed] [Google Scholar]
- 67.Sundaralingam P, Masson P, Bourke MJ. Early Precut Sphincterotomy Does Not Increase Risk During Endoscopic Retrograde Cholangiopancreatography in Patients With Difficult Biliary Access: A Meta-analysis of Randomized Controlled Trials. Clin Gastroenterol Hepatol. 2015;13:1722–1729.e2. doi: 10.1016/j.cgh.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 68.Ding X, Chen M, Huang S, Zhang S, Zou X. Nonsteroidal anti-inflammatory drugs for prevention of post-ERCP pancreatitis: a meta-analysis. Gastrointest Endosc. 2012;76:1152–1159. doi: 10.1016/j.gie.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 69.Buxbaum J, Yan A, Yeh K, Lane C, Nguyen N, Laine L. Aggressive hydration with lactated Ringer’s solution reduces pancreatitis after endoscopic retrograde cholangiopancreatography. Clin Gastroenterol Hepatol. 2014;12:303–7.e1. doi: 10.1016/j.cgh.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi JH, Kim HJ, Lee BU, Kim TH, Song IH. Vigorous Periprocedural Hydration With Lactated Ringer’s Solution Reduces the Risk of Pancreatitis After Retrograde Cholangiopancreatography in Hospitalized Patients. Clin Gastroenterol Hepatol. 2017;15:86–92.e1. doi: 10.1016/j.cgh.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 71.Prat F, Malak NA, Pelletier G, Buffet C, Fritsch J, Choury AD, Altman C, Liguory C, Etienne JP. Biliary symptoms and complications more than 8 years after endoscopic sphincterotomy for choledocholithiasis. Gastroenterology. 1996;110:894–899. doi: 10.1053/gast.1996.v110.pm8608900. [DOI] [PubMed] [Google Scholar]
- 72.Seifert E. Long-term follow-up after endoscopic sphincterotomy (EST) Endoscopy. 1988;20 Suppl 1:232–235. doi: 10.1055/s-2007-1018182. [DOI] [PubMed] [Google Scholar]
- 73.Pozsár J, Sahin P, László F, Topa L. Endoscopic treatment of sphincterotomy-associated distal common bile duct strictures by using sequential insertion of multiple plastic stents. Gastrointest Endosc. 2005;62:85–91. doi: 10.1016/s0016-5107(05)00547-x. [DOI] [PubMed] [Google Scholar]


