Abstract
BACKGROUND
Bacillus subtilis (B. subtilis) is considered a non-pathogenic microorganism of the genus Bacillus and a common laboratory contaminant. Only scarce reports of B. subtilis central nervous system infection have been reported, mainly in the form of pyogenic meningitis, usually in cases of direct inoculation by trauma or iatrogenically.
CASE SUMMARY
A 51-year-old man, with a free previous medical history, presented to the Emergency Department of our hospital complaining of recurrent episodes of left upper limb weakness, during the last month, which had been worsened the last 48 h. During his presentation in Emergency Department he experienced a generalized tonic-clonic grand mal seizure. Brain magnetic resonance imaging (MRI) scan with intravenous Gadolinium revealed a 3.3 cm × 2.7 cm lesion at the right parietal lobe surrounded by mild vasogenic edema, which included the posterior central gyrus. The core of the lesion showed relatively homogenous restricted diffusion. Post Gadolinium T1W1 image, revealed a ring-shaped enhancement. Due to the imaging findings, brain abscess was our primary consideration. Detailed examination for clinical signs of infectious foci revealed only poor oral hygiene with severe tooth decay and periodontal disease, but without detection of dental abscess. The patient underwent surgical treatment with right parietal craniotomy and total excision of the lesion. Pus and capsule tissue grew B. subtilis and according to antibiogram intravenous ceftriaxone 2 g bids was administered for 4 wk. The patient remained asymptomatic and follow-up MRI scan two months after operation showed complete removal of the abscess.
CONCLUSION
This case highlights the ultimate importance of appropriate oral hygiene and dental care to avoid potentially serious infectious complications and second, B. subtilis should not be considered merely as laboratory contaminant especially when cultivated by appropriate central nervous system specimen.
Keywords: Bacillus subtilis, Brain abscess, Central nervous system infection, Craniotomy, Meningitis, Case report
Core tip: Bacillus subtilis (B. subtilis) is considered a non-pathogenic microorganism of the genus Bacillus and a common laboratory contaminant. We present herein, a rare case of spontaneous cerebral abscess caused by B. subtilis, evolved in a previously healthy immunocompetent male patient. B. subtilis was isolated from both the capsule and pus of the surgically excised brain abscess. Severe tooth decay and periodontitis were the only potential infectious foci. This case highlights the ultimate importance of appropriate oral hygiene and dental care to avoid potentially serious infectious complications and second, B. subtilis should not be considered merely as laboratory contaminant especially when cultivated by appropriate central nervous system specimen.
INTRODUCTION
The bacterial genus bacillus contains predominantly non-pathogenic microorganisms for humans except for the anthrax bacillus. Bacillus subtilis (B. subtilis) is a Gram-positive bacterium, rod-shaped and catalase-positive that is ubiquitous in the environment and are normally found in soil and vegetation[1]. This microorganism is considered non-pathogenic for humans. The eye has been the organ most commonly infected by B. subtilis, majorly by direct inoculation[2]. Only scarce reports of central nervous system infection by B. subtilis have been previously published, all in the form of purulent meningitis[1,3,4]. We present herein, the first case of spontaneous cerebral abscess by B. subtilis, evolved in a previously healthy male patient.
CASE PRESENTATION
Chief complaints
A 51-year-old man presented to the Emergency Department of our hospital complaining of worsening left upper limb weakness. During his presentation in Emergency Department he experienced a generalized tonic-clonic grand mal seizure.
History of present illness
Patient’s symptoms started a month ago with recurrent episodes of left upper limb weakness, which had been worsened the last 48 h.
History of past illness
The patient had a free previous medical history.
Physical examination
After seizure cessation, the patient’s temperature was 36.6 °C, heart rate was 93 bpm, respiratory rate was 16 breaths per minute, blood pressure was 180/90 mmHg and oxygen saturation in room air was 98%. The clinical neurological examination revealed left-sided facial numbness and left upper limb hypoesthesia, a Glasgow Coma scale of 15/15, without any other pathological signs. Our clinical considerations were first a space-occupying brain lesion and second a stroke.
Laboratory examinations
Blood analysis revealed a mild leukocytosis 12 × 109/L, with predominant neutrophils (80%) with normal haematocrit and platelet count. Prothrombin and partial thromboplastin times were normal, and d-dimers were slightly increased at 0.80 μg/mL. Serum C-reactive protein was increased at 4.5 mg/dL (normal range < 0.8 mg/dL) and erythrocyte sedimentation rate at 30 mm/h. The blood biochemistries, as well as urine analysis were normal. Electrocardiogram, chest X-ray and arterial blood gas were also normal.
Imaging examinations
An initial imaging evaluation with brain computed tomography (CT) scan, revealed a 3.2 cm hypodense lesion at right upper parietal lobe. Following contrast media administration, the lesion showed peripheral enhancement. No midline shift was noted.
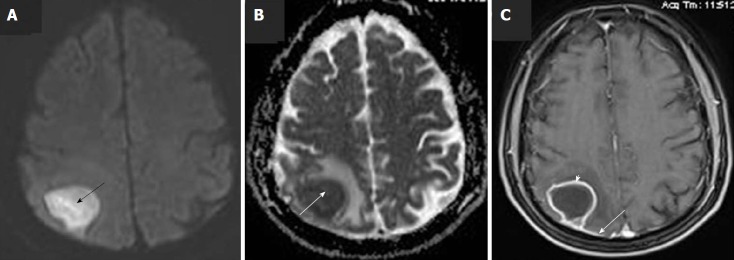
Brain lesion was further evaluated with a brain magnetic resonance imaging (MRI) scan. The latter revealed a 3.3 cm × 2.7 cm lesion at the right parietal lobe surrounded by mild vasogenic edema, which included the posterior central gyrus. The core of the lesion showed relatively homogenous restricted diffusion (Figure 1). Post Gadolinium T1W1 image, revealed a ring-shaped enhancement. Dural enhancement, due to involvement of the adjacent dura was also revealed (Figure 1C). Due to the aforementioned imaging findings, differential diagnosis mainly included the presence of a brain abscess, while the possibility of a restricted diffusion metastatic lesion could not entirely be excluded.
Figure 1.
Pre-operative brain magnetic resonance imaging scan with intravenous Gadolinium administration. A: Axial diffusion-weighted imaging (DWI) image shows high signal of the lesion in right upper parietal lobe (black arrow); B: Axial DWI ADC map reveals low signal at the same area, due to restricted diffusion (white arrow); C: Axial post-Gd image, shows rim enhancement of the lesion (white arrowhead) as well as enhancement of the adjacent dura (white arrow).
Further diagnostic work-up
The patient was further evaluated with blood cultures, urine and stool cultures and examination for ova and parasites which turned out negative. Transthoracic cardiac ultrasound was negative for cardiac vegetations or signs of infection. Considering the facts that blood cultures were negative, our patient had not prosthetic heart valves, congenital heart disease, previous endocarditis, heart murmur or stigmata of endocarditis, transthoracic cardiac ultrasound is sufficient to rule out endocarditis, when the patient presents an optimal echocardiographic window, as in the presented case[5]. Dental examination, including panoramic dental X-ray, revealed poor oral hygiene with severe tooth decay and periodontal disease but without detection of dental abscess. Serology tests for human immunodeficiency virus, human T-lymphotropic virus-1 and virus-2, herpes simplex virus, Epstein-Barr virus, cytomegalovirus and toxoplasma, were negative. Wright, rapid plasma reagin tests and tuberculin skin test were also negative. Serum angiotensin-converting enzyme and protein electrophoresis were normal. Serum complement and levels of immunoglobulins were all normal, as follows: C3: 123 mg%, C4: 27 mg%, IgA: 136 mg/dL, IgG: 1200 mg/dL IgM: 120 mg/dL, IgE: 18 IU/mL, IgD: 15 IU/mL. Total lymphocyte count (1.8 × 109/L) and CD4+ percentage (45%), as well as their absolute count (810 cells/μL), were normal. A full panel of serum tumour markers CEA (1.5 ng/mL), CA 19.9 (12.4 U/mL), CA 125 (8 U/mL), PSA (0.208 ng/mL), α-FP (2.2 IU/mL), β-hCG (0.10 mIU/mL) and β-2-microglobulin (1.35 mg/L) were unrevealing. Abdominal and chest CT scans with intravenous contrast media administration did not reveal any infectious focus or neoplastic lesion. Upper and lower gastrointestinal tract endoscopies were also normal.
Microbiological identification of the causative agent
The etiological factor of patient’s brain abscess, and its susceptibility profile in antibiotics, was determined by using appropriate microbiological analysis. Specifically, pus and capsule tissue of the excised brain lesion were subjected to Gram stain, Acid-Fast Bacilli stain and cultures for aerobes, anaerobes, fungi and mycobacteria. The samples were initially enriched in Thioglycolate broth, incubated overnight and then inoculated onto blood agar plates, McConkey agar plates, Sabouraud agar plates, anaerobe growth media (N-S anaerobe selective supplement, G-N anaerobe selective supplement), and Löwenstein–Jensen medium. Cultures of both pus and capsule tissue grew B. subtilis. Identification of the bacillus was performed by Gram stain, catalase production, motility test and BBL™ Crystal™ Identification Systems for Gram Positive bacteria (BD Diagnostics, Le Pont de Claix, France). Gram stain revealed the presence of Gram-positive rods forming subterminal spors, with a positive motility test, catalase, o-nitrophenyl-β-d-galactopyranoside (ONPG), and citrate (Simmons’) tests. It was identified as B. subtilis, by BBL™ GP Crystal™ Identification Systems (bionumber 2450563773, BD Diagnostics). Antibiotic susceptibility testing was performed by the disk diffusion, and a gradient method (Etest, bioMerieux), according to EUCAST guidelines[6]. Antibiogram demonstrated sensitivity of the microorganism to penicillin, vancomycin, ciprofloxacin, gentamicin and tetracyclines and resistance to clindamycin and rifampicin.
MULTIDISCIPLINARY EXPERT CONSULTATION
Georgios Gatzounis, MD, PhD, Professor and Chief, Department of Neurosurgery, University of Patras Medical School
The patient should undergo surgical treatment with right parietal craniotomy and total excision of the brain lesion.
Stelios F Assimakopoulos, MD, PhD, Assistant Professor of Internal Medicine, Infectious Diseases Specialist, Department of Internal Medicine and Division of Infectious Diseases, University of Patras Medical School; and Markos Marangos, MD, PhD, Professor of Infectious Diseases, Chief of the Division of Infectious Diseases, University of Patras Medical School
Brain abscess should be treated with appropriate empiric antibiotic coverage including Ceftriaxone 2 g bid, Vancomycin 1 g TID and Metronidazole 500 mg QID. After the results of pus and capsule tissue cultures, which grew B. subtilis, and susceptibility testing, Metronidazole and Vancomycin should be discontinued, and the patient continue Ceftriaxone 2 g bid intravenously for 4 wk. Two additional weeks of oral amoxicillin/clavulanic acid 1 g bid is required for treatment completion. Also, the patient should be evaluated for infectious foci and potential underlying immunosuppression, since B. subtilis is considered a non-pathogenic microorganism for humans.
Petros Zampakis, MD, PhD, Assistant Professor of Interventional Neuroradiology, Department of Radiology, University of Patras Medical School
The radiological differential diagnosis mainly includes the presence of a brain abscess, while the possibility of a restricted diffusion metastatic lesion could not entirely be excluded.
Fevronia Kolonitsiou, MD, PhD, Associate Professor of Microbiology, Department of Microbiology, University of Patras Medical School
The microbiological analysis of pus and capsule tissue of the excised brain lesion showed as etiological factor of patient’s brain abscess a considered “non-pathogenic” member of the genus Bacillus, B. subtilis.
FINAL DIAGNOSIS
The final diagnosis of the presented case is spontaneous cerebral abscess due to B. subtilis.
TREATMENT
The patient, following his presenting grand mal seizure in the Emergency Department, was immediately started on dexamethasone 8 mg TID intravenously and phenytoin sodium 300 mg QD orally, without seizure relapse. Considering the brain MRI findings, brain lesion was primarily characterized as brain abscess and empiric intravenous antibiotic therapy with Ceftriaxone 2 g bid, Vancomycin 1 g TID and Metronidazole 500 mg QID was administered. The patient underwent surgical treatment with right parietal craniotomy and total excision of the lesion. After the results of pus and capsule tissue cultures, which grew B. subtilis, and susceptibility testing, Metronidazole and Vancomycin were discontinued, and the patient continued Ceftriaxone 2 g bid intravenously for 4 wk. Upon completion of four weeks of intravenous antibiotic therapy with ceftriaxone, the patient was discharged from the hospital free of symptoms, on oral amoxicillin/clavulanic acid 1 g bid for two additional weeks.
OUTCOME AND FOLLOW-UP
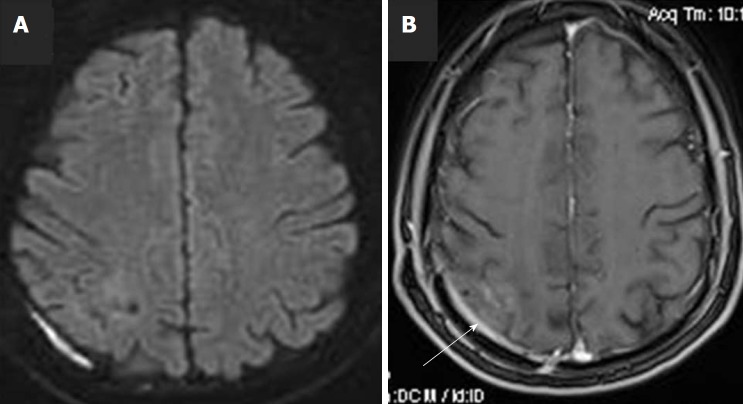
The patient had an uneventful postoperative clinical course, whilst dexamethasone was decreased progressively until its cessation. At follow-up visit, two months after surgical removal of cerebral abscess (one month after hospital discharge), the patient was asymptomatic, and a new MRI scan showed complete removal of the abscess with only minor post-operative findings at the adjacent dura (Figure 2). The patient was advised to treat his dental decay and periodontitis and take care of his oral health and hygiene.
Figure 2.
Follow-up brain magnetic resonance imaging scan with intravenous Gadolinium administration, at two months post operation. A: diffusion-weighted imaging shows complete removal of the lesion, with normal diffusion of the brain parenchyma; B: Axial post-Gd image, shows minimal enhancement of the adjacent dura, due to the previous surgery (white arrow).
DISCUSSION
Brain abscess is a focal infectious collection within the brain parenchyma, which can arise as a complication of a variety of infections, trauma, or surgery. Bacteria can invade the brain either by direct spread, which accounts for 20% to 60% of cases, or through hematogenous seeding, which typically causes multiple lesions[7,8]. A wide range of pathogens can be involved as causative agents in a brain abscess. The microbial profile depends on both how the brain abscess develops and the integrity of patient’s immune system. Mostly a single microbe is isolated, whereas isolation of multiple pathogens has also been described[9]. Aerobic Gram-positive cocci are most commonly encountered and include: viridans streptococci, Streptococcus milleri, microaerophilic streptococci, Streptococcus pneumoniae (rare) and Staphylococcus aureus[9]. Anaerobic bacteria are also common constituents of brain abscesses, originating from odontogenic or otorhinolaryngeal infections[10]. In the immunocompromised host, brain abscess can be caused by opportunistic pathogens, like Toxoplasma gondii, Listeria, Nocardia asteroides and fungi.
The etiologic agent in the presented case was a “non-pathogenic” member of the genus Bacillus, B. subtilis. Only scarce reports implicate this microorganism in human infections. A number of case reports refer to localized ocular infections, by direct inoculation of this organism in the eye, while a fulminating panophthalmitis following penetrating trauma with the vitreous humour has been also described[2]. Also, epidemics of food poisoning attributed to B. subtilis have been previously reported[11]. Regarding implication of B. subtilis or other non-anthrax Bacillus species in disseminated infections, an old review collectively presented 12 cases; eight presenting as meningitis, three bacteraemias and one peritonitis/pericarditis[1]. In meningitis cases, direct portal of entry to the meninges was reported in four patients, while 5 out of 8 patients with meningitis had a fatal outcome. B. subtilis was identified as etiological factor in six of 12 cases; three cases of bacteraemia and three cases of meningitis. We were able to find two additional reports of purulent meningitis by B. subtilis, one complicating a penetrating head injury[3,4]. Collectively, five cases of central nervous system (CNS) infection by B. subtilis have been previously reported, all in the form of purulent meningitis, while direct inoculation of the organism in the CNS seems to be an important pathogenetic factor.
To the best of our knowledge, the presented case is the first in the literature describing the evolution of a cerebral abscess by B. subtilis. The rarity of the presented case is also highlighted by the facts that no direct portal of infection existed, as in most previously described cases of B. subtilis meningitis, nor underlying immunosuppression. Our patient was a healthy man with no previous serious or recurrent or unusual infections, HIV testing was negative, total lymphocyte count and CD4+ percentage, as well as their absolute count, were normal and he was not suffering from underlying malignancy, chronic kidney disease or diabetes mellitus. Regarding the potential mechanism of evolution of cerebral abscess in our patient, the only possible explanation could be the hematogenous spread from the only infectious foci detected, which was the severe tooth decay and periodontitis. Bacillus organisms are often found in the mouth, although they do not appear to be part of the permanent oral flora[12]. Previous studies of odontogenic infections, demonstrated dental caries as the most prevalent predisposing factor, whilst in HIV negative patients, anaerobic bacilli were detected as etiologic agents in 12%[13].
Our patient was successsfully treated with combined total surgical excision of brain abscess, followed by 4 wk of intravenous antibiotic therapy and two weeks of oral therapy, according to antimicrobial susceptibility results. A comprehensive consensus document on controversial issues for the treatment of infections of the central nervous system, published by the Italian Study Group on Severe Infections, recommends that abscesses > 2.5 cm should be surgically removed, followed by 4-6 wk of appropriate antibiotic therapy[14]. Four weeks of intravenous therapy with an antibiotic which exerts favourable pharmacokinetic profile in brain tissue is reasonable. Ceftriaxone at a dose of 2 g bid, which was administered in our patient, is a preferable option if the microorganism has proven susceptible. The total duration of treatment could be completed by sequential oral antibiotics for 2-4 wk, given that susceptible pathogens have been isolated and orally given antibiotics could achieve adequate penetration in brain tissue. In this context, Amoxicillin/clavulanic, which was given to the present patient, trimethoprim-sulfamethoxazole, fluoroquinolone, linezolid and rifampicin are considered effective oral antibiotic options in such cases.
In conclusion, organisms of the genus Bacillus, except for B. anthracis, are not usually considered pathogenic for humans. Occasionally these bacteria can cause serious infections, even in immunocompetent patients, especially if introduced into the vitreous of the eye or subarachnoid space by trauma or iatrogenically. This case highlights the ultimate importance of appropriate oral hygiene and dental care to avoid potentially serious infectious complications and second, B. subtilis should not be considered merely as laboratory contaminant especially when cultivated by appropriate CNS specimen.
CONCLUSION
Only scarce reports of B. subtilis central nervous system infection have been reported, mainly in the form of pyogenic meningitis, usually in cases of direct inoculation by trauma or iatrogenically. B. subtilis is a very rare cause of spontaneous cerebral abscess. B. subtilis should not be considered merely as laboratory contaminant, especially when cultivated by appropriate CNS specimen. Appropriate oral hygiene and dental care is of ultimate importance of to avoid potentially serious infectious complications.
Footnotes
Informed consent statement: Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Invited manuscript
Peer-review started: October 4, 2018
First decision: October 25, 2018
Article in press: November 7, 2018
Specialty type: Medicine, research and experimental
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Shi J, Unger MM S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
Contributor Information
Ioannis Tsonis, Department of Neurosurgery, University of Patras Medical School, Patras 26504, Greece.
Lydia Karamani, Department of Neurosurgery, University of Patras Medical School, Patras 26504, Greece.
Panagiota Xaplanteri, Department of Microbiology, University of Patras Medical School, Patras 26504, Greece.
Fevronia Kolonitsiou, Department of Microbiology, University of Patras Medical School, Patras 26504, Greece.
Petros Zampakis, Department of Radiology, University of Patras Medical School, Patras 26504, Greece.
Georgios Gatzounis, Department of Neurosurgery, University of Patras Medical School, Patras 26504, Greece.
Markos Marangos, Department of Internal Medicine, Division of Infectious Diseases, University of Patras Medical School, Patras 26504, Greece.
Stelios F Assimakopoulos, Department of Internal Medicine, Division of Infectious Diseases, University of Patras Medical School, Patras 26504, Greece. sassim@upatras.gr.
References
- 1.FARRAR WE Jr. Serious infections due to “non-pathogenic” organisms of the genus Bacillus. Review of their status as pathogens. Am J Med. 1963;34:134–141. doi: 10.1016/0002-9343(63)90047-0. [DOI] [PubMed] [Google Scholar]
- 2.Francois J. Le bacille subtilique en pathologie oculaire. Bull et Mem Soc Franc d’Opht. 1934;47:423–424. [Google Scholar]
- 3.Thomas M, Whittet H. Atypical meningitis complicating a penetrating head injury. J Neurol Neurosurg Psychiatry. 1991;54:92–93. doi: 10.1136/jnnp.54.1.92-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Kalbermatten JP. [Considerations on the pathogenic role of Bacillus subtilis. Apropos of a case of purulent meningitis] Praxis. 1969;58:615–618. [PubMed] [Google Scholar]
- 5.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O’Gara P, Taubert KA; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015;132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 6.European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 8.1 ed. 2018. Available from: http://www.eucast.org/clinical_breakpoints/
- 7.Chun CH, Johnson JD, Hofstetter M, Raff MJ. Brain abscess. A study of 45 consecutive cases. Medicine (Baltimore) 1986;65:415–431. [PubMed] [Google Scholar]
- 8.Muzumdar D, Jhawar S, Goel A. Brain abscess: an overview. Int J Surg. 2011;9:136–144. doi: 10.1016/j.ijsu.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Xiao F, Tseng MY, Teng LJ, Tseng HM, Tsai JC. Brain abscess: clinical experience and analysis of prognostic factors. Surg Neurol. 2005;63:442–449; discussion 449-450. doi: 10.1016/j.surneu.2004.08.093. [DOI] [PubMed] [Google Scholar]
- 10.Le Moal G, Landron C, Grollier G, Bataille B, Roblot F, Nassans P, Becq-Giraudon B. Characteristics of brain abscess with isolation of anaerobic bacteria. Scand J Infect Dis. 2003;35:318–321. doi: 10.1080/00365540310000265. [DOI] [PubMed] [Google Scholar]
- 11.Dack G. Food Poisoning. 3rd ed. Chicago: University of Chicago Press; 1956. pp. 220–221. [Google Scholar]
- 12.Burnett GW, Scherp HW, Shuster GS. Oral Microbiology and Infectious Disease. Baltimore: Williams Wilkins Co; 1957. p. 254. [Google Scholar]
- 13.Kityamuwesi R, Muwaz L, Kasangaki A, Kajumbula H, Rwenyonyi CM. Characteristics of pyogenic odontogenic infection in patients attending Mulago Hospital, Uganda: a cross-sectional study. BMC Microbiol. 2015;15:46. doi: 10.1186/s12866-015-0382-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arlotti M, Grossi P, Pea F, Tomei G, Vullo V, De Rosa FG, Di Perri G, Nicastri E, Lauria FN, Carosi G, Moroni M, Ippolito G; GISIG (Gruppo Italiano di Studio sulle Infezioni Gravi) Working Group on Brain Abscesses. Consensus document on controversial issues for the treatment of infections of the central nervous system: bacterial brain abscesses. Int J Infect Dis. 2010;14 Suppl 4:S79–S92. doi: 10.1016/j.ijid.2010.05.010. [DOI] [PubMed] [Google Scholar]