Abstract
Purpose: In Malaysia, an estimated 9.7%–12.4% of transgender women (TW) are HIV positive, with higher estimates among those engaged in sex work. According to the 90–90–90 Joint United Nations Programme on HIV/AIDS strategy, HIV testing is the first crucial step in curbing the HIV epidemic. This study examines correlates of recent HIV testing among TW in Greater Kuala Lumpur, Malaysia.
Methods: TW (N = 199) in Greater Kuala Lumpur completed a survey on healthcare access and utilization, including HIV testing history. Bivariate logistic regression and penalized multivariate logistic regression were used to explore correlates of HIV testing in the last 12 months.
Results: Overall, 41.7% of TW reported having ever been tested for HIV. Among participants who were HIV negative or not sure of their HIV status (n = 187), only 18.7% (n = 35) had been tested for HIV in the last 12 months. The multivariate analysis indicated that having a primary care provider (PCP), being 26–40 years of age, and having higher mental health functioning were positively associated with recent HIV testing. Active amphetamine use and previous depression diagnosis were also associated with recent HIV testing.
Conclusion: HIV testing is the first step in linking individuals to prevention and treatment interventions. Our findings suggest that having a PCP can improve engagement in HIV testing. Moreover, PCPs can serve as a valuable link to HIV treatment and prevention services. Current interventions that target social and behavioral risk factors for HIV, on their own, may be insufficient at engaging all HIV-vulnerable TW.
Keywords: HIV, HIV testing, Malaysia, transgender women, trans woman
Introduction
The Joint United Nations Programme on HIV/AIDS (UNAIDS) has set ambitious 90–90–90 targets to end the AIDS epidemic by 2030.1 Central to this strategy is the importance of identifying 90% of all people living with HIV, for whom HIV testing is the first and most crucial step to either initiate HIV treatment if positive, or if negative, to consider for preexposure prophylaxis (PrEP).2 Among the priorities of this strategy is a focus on key populations.1 Globally, transgender women (TW) have a disproportionate burden of HIV (19.1%; 95% confidence interval [CI] = 17.4–20.7)3 and relative to their cisgender counterparts, they have higher levels of sex work and social stigma, but lower rates of HIV testing and access to gender-affirmative, trans-competent healthcare.4 Barriers to HIV testing among TW include fear of discrimination and legal retribution, limited access to quality HIV care, and mistrust of medical institutions.5,6
In Malaysia, civil and religious law have contributed to the hostile healthcare environment through which transgender individuals must navigate. Religious police conduct raids and arrest TW for so-called “cross-dressing” and same-sex sexual behavior.7 Faced with high levels of stigma, many TW experience significant health disparities compared to their cisgender counterparts, including on HIV-related outcomes. For example, in one study, TW were less likely to receive antiretroviral therapy (ART), had poorer adherence to ART, and were less likely to achieve viral suppression compared to cisgender persons.8 Malaysia has one of the largest HIV epidemics in Southeast Asia.9 Although Malaysia's HIV epidemic has historically been driven by people who inject drugs, the majority of new infections are attributed to sexual transmission.10 Government statistics estimate that HIV prevalence among TW and transgender men collectively is 5.6%, ∼14 times higher compared with the general adult population.10 Unfortunately, the Malaysian government does not provide separate HIV prevalence estimates for TW and transgender men. More recent data, however, from a respondent-driven sampling study of TW sex workers in Greater Kuala Lumpur found an HIV prevalence of 12.4% (95% CI = 7.8–17.1).11
Few studies have looked specifically at correlates of HIV testing among TW. One study in Jamaica demonstrated that variables such as perceived HIV risk, history of sexual assault, and history of a sexually transmitted infection (STI) were correlated with HIV testing.12 Similarly, a study of TW in Vietnam found that STI testing, high level of education, and a history of police harassment were positively associated with HIV testing in the last year.13 In Peru, a study of men who have sex with men and TW identified that previous STI diagnosis, history of transactional sex, and age were positively correlated with recent HIV testing.14 Correlates vary across cultures, suggesting that HIV prevention practices must be created based on data specific to the cultural context within which a transgender woman exists.
Consistent with the UNAIDS focus on the 90–90–90 strategy,1 we sought to examine the correlates of recent HIV testing among TW in Malaysia, a country where HIV incidence and mortality continue to increase.2 Examining the correlates of HIV testing, the first step in the HIV treatment and prevention cascade, will provide important insights necessary to reverse the trend in increasing HIV incidence and mortality in TW, especially in locations where stigma and discrimination remain high.
Methods
Participants and recruitment
In 2014, we recruited 199 TW in Malaysia to complete an in-depth cross-sectional survey on healthcare access and utilization, including HIV testing history. Participants were recruited from the Greater Kuala Lumpur region and surrounding locales by convenience sampling. Inclusion criteria were as follows: (1) identify as a transgender woman, (2) 18 years of age or older, (3) a citizen or resident of Malaysia, and (4) able to read or speak Bahasa Malaysia or English. Participants were recruited using a combination of active and passive recruitment strategies. Specifically, trained outreach workers conducted community-based recruitment at a number of venues used by TW, including community-based organizations, brothels, and outdoor restaurants. The outreach workers and research assistants in this study were all TW. Recruited participants were also able to refer peers. After information sessions were provided, interested participants were referred to a research assistant for further eligibility screening and informed consent procedures.
Procedures
Participants were briefed regarding the purpose of the study, topics covered, and potential risks in participating. Written informed consent was obtained before enrollment and participants were compensated with 20 MYR ($5.70 USD) for their time. After informed consent was obtained, participants completed a computer-based web survey with on-site support from research staff. Due to the sensitive nature of the questions, surveys were conducted in several locations based on participant comfort and safety. Surveys were administered using Qualtrics survey software.15
This study was reviewed and approved by the Yale University Human Investigation Committee and the University of Malaya Medical Ethics Committee. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Measures
Dependent variable
The dependent variable, recent HIV testing, was defined as having received an HIV test in the last 12 months. Although Malaysia does not publish a specific recommended frequency for HIV testing among TW, it does recommend that individuals at risk for HIV are tested annually.
Independent variables
Time since last HIV test was calculated as the number of months since last reported HIV test. Knowledge of HIV was measured using a 9-item questionnaire adapted from the AIDS Indicator Survey.16 A sample question is, “Can using condoms reduce the risk of HIV transmission/infection?” Participants responded using a binary option of “agree” (1) or “disagree” (2) and a mean split procedure was used to stratify HIV knowledge scores as “high” if the score was at or above the mean and “low” if the score was below the mean. Use and knowledge of HIV resources were measured using three binary response (“yes” or “no”) questions, which were “Do you know where you can go to receive an HIV test?,” “In the past 12 months, have you been given sterile needles and syringes by an outreach worker, peer educator, or needle-syringe exchange program?,” and “In the past 12 months, have you been given condoms by an outreach worker, drop-in center, or sexual health clinic?”
Previous diagnosis of HIV was defined as ever having been diagnosed with HIV by a medical professional. Similarly, previous STI diagnosis was defined as ever having been diagnosed with chlamydia, gonorrhea, or syphilis. Participants who reported a previous HIV diagnosis were asked additional questions to measure ART use, including “Are you currently taking antiretroviral therapy to treat your HIV?” with a binary response option of “yes” or “no”. Participants who responded that they were not currently taking ART were then asked, “Have you ever been told by a doctor to start taking ART to treat your HIV?” with a binary response option of “yes” or “no”.
Trust in physician was measured using the 11-item Trust in Physician Scale.17 A sample item is, “I trust my doctor's judgment about my medical care.” Participants responded using a Likert-type scale ranging from “totally disagree” (1) to “totally agree” (5). A mean split procedure was used to classify individuals as having “high trust” in their physician if they scored at or above the mean and “low trust” if they scored below the mean. Three questions measured engagement with healthcare providers, including: “Do you have a primary care provider (PCP)?” and “Have you disclosed your transgender identity to your healthcare provider(s)?” A third question asked “How comfortable are you discussing transgender healthcare with your PCP?,” to which participants responded using a 4-point scale ranging from “not at all comfortable” (1) to “very comfortable” (4), which were later collapsed into a binary measure of “not comfortable” (1) or “comfortable” (2).
Health-related quality of life (HRQoL) was measured using the SF-12, using the physical health composite scores (PCS) and mental health composite scores (MCS), which were stratified as “high” if they were at or above the median score and “low” if they were below the median.18 Utilization of gender-affirming surgery was measured as a binary question (“yes” or “no”), calculated by a “yes” response to having undergone any one of the following procedures as part of gender-affirming care: breast augmentation surgery, facial reconstruction, or the creation of a neovagina. Hormone use was also measured using two questions, “Have you ever taken oral hormones, even just once?” and “Have you ever injected hormones, even just once in your life?”
Experience of childhood physical and sexual violence and adulthood physical violence was measured using the 19-item scale from the U.S. Centers for Disease Control and Prevention's Behavioral Risk Factor Surveillance System (BRFSS) questionnaire for violence and victimization.19 All BRFSS questions were single-item measures with response options of “yes” or “no.” A sample item for experience of childhood physical violence is, “Before the age of 18, were you ever hit, slapped, kicked, or physically hurt by an adult?” An additional binary (“yes” or “no”) question was added to measure lifetime experience of gender-based sexual violence, “Have you ever been forced to engage in unwanted sexual activity because you are transgender?” Previous depression diagnosis was measured as a single-item self-report question, “Have you ever been diagnosed with depression by a medical professional?” with response options of “yes” or “no”.
Transgender-related stigma was measured using an adapted 24-item version of the Berger HIV Stigma Scale.20 An example of an adapted item is, “Being transgender makes me feel like I'm a bad person.” Participants responded using a Likert-type scale ranging from “strongly disagree” (1) to “strongly agree” (4). A mean split procedure was used to stratify each participant as either “high stigma” if they scored at or above the mean and “low stigma” if they scored below the mean. Family support for being transgender was measured by a single-item question, “How supported do you feel your birth family is regarding your transgender identity.” Participants responded using a 4-point scale ranging from “not at all supported” (1) to “very supported” (4), which was later dichotomized as “not supported” (1) or “supported” (2).
Sociobehavioral and demographic measures included age, stratified by three groupings, 18–25, 26–40, and >40 years; education, measured by self-report; ethnicity, measured by self-report as Malay, Indian, or other, which was dichotomized as Malay or non-Malay; and employment status, measured by self-report as full-time employed, part-time employed, or unemployed. Participants were defined as “single” if they reported being widowed or not having a partner. “In a relationship” was defined as being married or having a partner. Housing status was measured as “unstable” if the participant indicated being homeless or currently living in a brothel, shelter, hotel, or motel. Income was stratified as “high” and “low” based on 200% of the Malaysia poverty line (2000 MYR).21
Sex work in the last 12 months was defined as having received money, food, housing, or goods in exchange for sexual activity at least once in the past year. Criminal justice history was measured with two single-item questions: Have you ever been placed in jail by the police and have you ever been sentenced and incarcerated in prison. Drug and alcohol use across the lifetime and last 30 days, defined as active drug use, were also measured. Drugs measured were alcohol, heroin, buprenorphine, morphine, benzodiazepines, cannabis, amphetamines, and lysergic acid diethylamide/3,4-methylenedioxymethamphetamine. Injection drug use, defined as any previous injection of illicit drugs, was measured for both lifetime and last 30 days.
Data analysis
Logistic regression was used to examine bivariate associations between the covariates and the dependent variable (recent HIV testing) using IBM SPSS Statistics version 23 (IBM Corporation, Armonk, NY). We used the Bayesian lasso method to perform variable selection and identify correlates of recent HIV testing in the multivariate analysis.22 This method implements the Bayesian analogue of the penalized regression, offering several advantages, including direct estimation of standard errors.23,24 Penalized regression methods provide a conservative approach to variable selection and estimation of regression coefficients.25 The analysis was implemented using the package “EBglmnet” in R (Foundation for Statistical Computing, Vienna, Austria) with normal—exponential—gamma hierarchical priors.26 The 12 participants who reported being HIV positive were excluded from both the bivariate and multivariate analysis (n = 187). Before the multivariate analysis, collinearity diagnostics were performed between the covariates and the dependent variable using a tolerance threshold of 7. Tolerance was ≤3 for all covariates, except “Comfortable discussing transgender healthcare with your PCP?” and “Disclosed transgender identity to doctor?,” indicating collinearity. As such, the variable “Disclosed transgender identity to doctor?” was removed from the multivariate analysis. All other covariates in Table 3 were included in the multivariate analysis.
Table 3.
Bivariate and Multivariate Penalized Regression for Correlates of Recent HIV Testing (n = 187)
| n (%) | OR | 95% CI | p | AOR | 95% CI | p | |
|---|---|---|---|---|---|---|---|
| Sociodemographic characteristics | |||||||
| Ethnicity | |||||||
| Malay | 112 (59.9) | 1.87 | 0.84–4.16 | 0.126 | |||
| Non-Malay | 75 (40.1) | Ref | — | — | |||
| Employment status | |||||||
| Full time | 153 (81.8) | 0.48 | 0.17–1.36 | 0.166 | |||
| Part time | 14 (7.5) | 0.64 | 0.13–3.14 | 0.579 | |||
| Unemployed | 20 (10.7) | Ref | — | — | |||
| Education | |||||||
| Secondary level or below | 88 (47.1) | Ref | — | — | |||
| Post-secondary or higher | 99 (52.9) | 1.92 | 0.89–4.13 | 0.096 | |||
| Income | |||||||
| MYRa 0–2000 per month | 81 (43.3) | Ref | — | — | |||
| MYR >2000 per month | 106 (56.7) | 0.44 | 0.21–0.92 | 0.030 | |||
| Age, years | |||||||
| 18–25 | 40 (21.4) | Ref | — | — | Ref | — | — |
| 26–40 | 107 (57.2) | 12.52 | 1.64–95.65 | 0.015 | 2.77 | 1.12–6.87 | 0.029 |
| >40 | 40 (21.4) | 9.75 | 1.16–82.11 | 0.036 | — | — | — |
| Relationship status | |||||||
| Single | 138 (73.8) | Ref | — | — | |||
| In relationship | 49 (26.2) | 1.38 | 0.62–3.07 | 0.437 | |||
| Housing status | |||||||
| Unstable | 17 (9.1) | Ref | — | — | Ref | — | — |
| Stable | 170 (90.9) | 0.21 | 0.08–0.60 | 0.003 | 0.41 | 0.15–1.14 | 0.089 |
| Sex work in the last 12 months | |||||||
| No | 35 (18.7) | Ref | — | — | |||
| Yes | 152 (81.3) | 0.73 | 0.30–1.78 | 0.487 | |||
| Drug and alcohol use | |||||||
| Active amphetamine use | |||||||
| No | 141 (75.4) | Ref | — | — | Ref | — | — |
| Yes | 46 (24.6) | 3.43 | 1.58–7.44 | 0.002 | 2.69 | 1.20–6.04 | 0.017 |
| Active alcohol use | |||||||
| No | 113 (60.4) | Ref | — | — | |||
| Yes | 74 (39.6) | 1.02 | 0.48–2.17 | 0.954 | |||
| Active cannabis use | |||||||
| No | 181 (96.8) | Ref | — | — | |||
| Yes | 6 (3.2) | 0.87 | 0.10–7.64 | 0.896 | |||
| Healthcare involvement | |||||||
| Has a primary care provider | |||||||
| No | 176 (94.1) | Ref | — | — | Ref | — | — |
| Yes | 11 (5.9) | 6.08 | 1.74–21.27 | 0.005 | 4.86 | 1.25–18.81 | 0.023 |
| Disclosed transgender identity to doctor? | |||||||
| No | 153 (81.8) | Ref | — | — | |||
| Yes | 34 (18.2) | 2.57 | 1.11–5.96 | 0.028 | |||
| Comfortable discussing transgender healthcare with your PCP? | |||||||
| Not comfortable | 124 (66.3) | Ref | — | — | |||
| Comfortable | 63 (33.7) | 2.18 | 1.03–4.60 | 0.042 | |||
| Trust in physician | |||||||
| Low | 143 (76.5) | Ref | — | — | |||
| High | 44 (23.5) | 1.39 | 0.61–3.17 | 0.437 | |||
| Previous STI diagnosis | |||||||
| No | 175 (93.6) | Ref | — | — | |||
| Yes | 12 (6.4) | 3.45 | 1.03–11.61 | 0.045 | |||
| Previous depression diagnosis | |||||||
| No | 174 (93.0) | Ref | — | — | Ref | — | — |
| Yes | 13 (7.0) | 8.71 | 2.65–28.64 | <0.001 | 6.16 | 1.56–24.24 | 0.010 |
| Any previous gender-affirming surgery | |||||||
| No | 136 (72.7) | Ref | — | — | |||
| Yes | 51 (27.3) | 1.51 | 0.69–3.32 | 0.304 | |||
| HIV knowledge | |||||||
| Low | 60 (32.1) | Ref | — | — | |||
| High | 127 (67.9) | 1.76 | 0.75–4.14 | 0.198 | |||
| Received condoms from outreach worker | |||||||
| No | 41 (21.9) | Ref | — | — | |||
| Yes | 146 (78.1) | 1.15 | 0.46–2.87 | 0.76 | |||
| Criminal justice involvement | |||||||
| Previously in jail | |||||||
| No | 125 (66.8) | Ref | — | — | |||
| Yes | 62 (33.2) | 2.60 | 1.23–5.50 | 0.013 | |||
| Previously in prison | |||||||
| No | 160 (85.6) | Ref | — | — | |||
| Yes | 27 (14.4) | 3.18 | 1.30–7.74 | 0.011 | |||
| Physical and sexual assault | |||||||
| Experienced physical assault in childhood | |||||||
| No | 100 (53.5) | Ref | — | — | |||
| Yes | 87 (46.5) | 1.27 | 0.61–2.66 | 0.519 | |||
| Experienced sexual assault in childhood | |||||||
| No | 151 (80.7) | Ref | — | — | |||
| Yes | 36 (19.3) | 3.94 | 1.75–8.89 | 0.001 | |||
| Experienced physical assault in adulthood | |||||||
| No | 167 (89.3) | Ref | — | — | |||
| Yes | 20 (10.7) | 5.68 | 2.14–15.05 | <0.001 | |||
| Experienced gender-based sexual violence | |||||||
| No | 164 (87.7) | Ref | — | — | Ref | — | — |
| Yes | 23 (12.3) | 6.68 | 2.64–16.94 | <0.001 | 2.18 | 0.88–5.37 | 0.092 |
| Psychosocial well-being | |||||||
| Family supports my transgender identity | |||||||
| No | 126 (67.4) | Ref | — | — | |||
| Yes | 61 (32.6) | 0.67 | 0.29–1.52 | 0.336 | |||
| Transgender-related stigma | |||||||
| Low | 107 (57.2) | Ref | — | — | |||
| High | 80 (42.8) | 1.33 | 0.64–2.79 | 0.443 | |||
| Mental health composite score (SF-12) | |||||||
| Low | 95 (50.8) | Ref | — | — | Ref | — | — |
| High | 92 (49.2) | 3.17 | 1.42–7.06 | 0.005 | 2.27 | 1.04–4.96 | 0.041 |
| Physical health composite score (SF-12) | |||||||
| Low | 81 (43.3) | Ref | — | — | |||
| High | 106 (56.7) | 1.18 | 0.56–2.50 | 0.690 | |||
All variables in Table 3 were included in the multivariate penalized regression procedure with the exception of “Disclosed transgender identity to doctor?,” which was removed due to collinearity.
Malaysian Ringgit.
AOR, adjusted odds ratio; OR, odds ratio; PCP, primary care provider; STI, sexually transmitted infection; TW, transgender woman.
Results
Sample characteristics are summarized in Table 1. Participants' mean age was 34.4 years (standard deviation [SD] = 10.7) and most identified as either ethnic Malay (61.3%) or Indian (34.7%). Participants were mostly single (75.4%) and living in stable housing (89.4%). More than half had completed post-secondary education (53.3%). Just over 1 in 4 TW reported having gender-affirming surgery (28.1%). The majority of participants were employed full time (80.9%), with 61.3% citing sex work as their primary form of employment. Among sex work-involved participants, 83.5% of all income received during the last 6 months was derived from sex work. Overall, transgender-related stigma was low (mean [M] = 1.14, SD = 0.52, range: 1–4), as were both scores for HRQoL on the SF-12, including MCS (M = 25.93, SD = 5.04) and PCS (M = 24.83, SD = 5.42). Trust in Physician scores, however, were moderately high (M = 3.09, SD = 0.28, range: 1–5).
Table 1.
Sample Characteristics (n = 199)
| Variable | n (%) |
|---|---|
| Age (mean, SD) | (34.4, 10.7) |
| Gender-affirming surgery | 56 (28.1) |
| Ethnicity | |
| Malay | 122 (61.3) |
| Indian | 69 (34.7) |
| Other | 8 (4.0) |
| Relationship status | |
| Single | 150 (75.4) |
| In a relationship | 49 (24.6) |
| Education | |
| Secondary level or below | 93 (46.7) |
| Post-secondary or higher | 106 (53.3) |
| Housing | |
| Stable | 178 (89.4) |
| Unstable | 21 (10.6) |
| Employment | |
| Full-time employed | 161 (80.9) |
| Part-time employed | 14 (7.0) |
| Currently unemployed | 24 (12.1) |
| Sex work as primary form of employment | 122 (61.3) |
| Percentage of income from sex work (mean, SD) | (83.5, 30.9) |
SD, standard deviation.
Table 2 shows participants' HIV testing behaviors, as well as use and knowledge of HIV resources. Less than half of participants had ever been tested for HIV (41.7%). Moreover, among participants who reported being HIV negative or not sure of their HIV status, only 18.7% had been tested in the last 12 months, with the mean time since last HIV test being 25.2 months (SD = 39.6). Nearly all (99%) participants reported knowing where they could receive an HIV test and approximately three-quarters (77.4%) had received condoms from an outreach worker in the past 12 months. Twelve (6.0%) participants reported having been previously diagnosed with HIV, of whom, eight were currently on ART. Furthermore, three of those participants not on ART had been told previously by a doctor to begin ART.
Table 2.
HIV Testing Behaviors and Use and Knowledge of HIV Resources (n = 199)
| Variable | n (%) |
|---|---|
| Lifetime HIV testing history | 83 (41.7) |
| Recent HIV testing historya | 35 (18.7) |
| Time since last test in monthsa (mean, SD) | (25.2, 39.6) |
| Ever diagnosed with HIV | 12 (6.0) |
| If HIV+, on ART?b | 8 (66.7) |
| If not on ART, ever been told to start ART?c | 3 (75.0) |
| Use and knowledge of HIV resources | |
| Do you know where you can go to receive an HIV test? | 197 (99.0) |
| In the past 12 months, have you been given sterile needles and syringes by an outreach worker, peer educator, or needle-syringe exchange program? | 3 (1.5) |
| In the past 12 months, have you been given condoms by an outreach worker, drop-in center, or sexual health clinic? | 154 (77.4) |
n = 187; bn = 12; cn = 4.
ART, antiretroviral therapy.
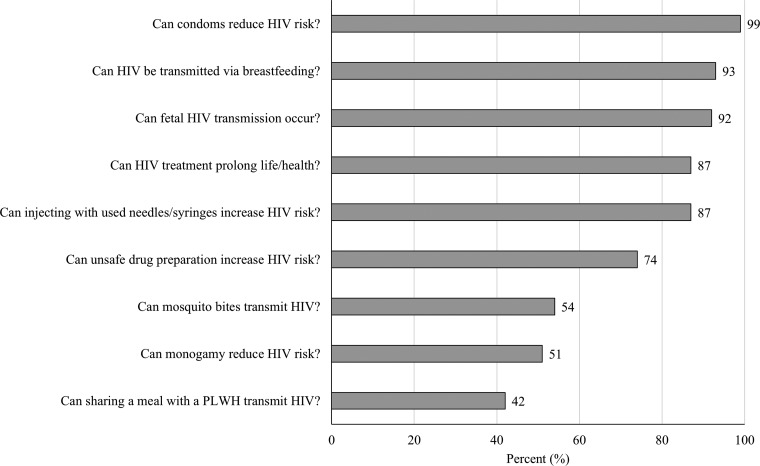
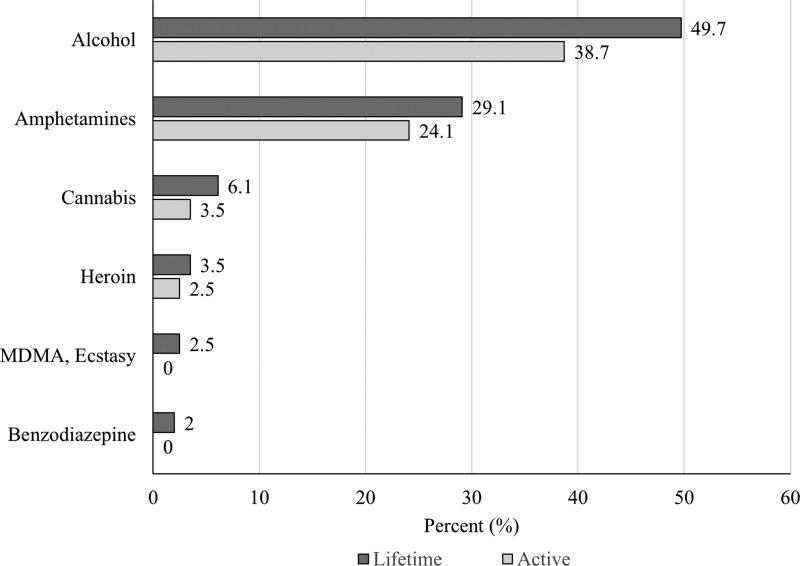
Figure 1 illustrates the percentage of participants who responded correctly to items on the HIV knowledge scale. Overall, participants demonstrated moderate knowledge of HIV, with a mean correct score of 6.9 (SD = 1.4) out of 9. Although most participants correctly identified condom use (99.5%) and use of clean needles and syringes (87.4%) as protective against HIV, many incorrectly identified mosquito bites (46.0%) and sharing a meal with a person living with HIV (58.8%) as a mode of HIV transmission. Figure 2 shows participants' lifetime and active drug use patterns. Alcohol (49.7%), amphetamines (29.1%), and cannabis (6.1%) were the substances most used across the lifetime. The three substances were also the most commonly used in the last 30 days. Only two participants reported ever injecting drugs, one of whom had injected in the last 30 days. Although 71.9% of participants reported having received hormone injections in their lifetime, none reported sharing of hormone injection equipment.
FIG. 1.
Percentage of participants with correct responses to questions on HIV-related knowledge (N = 199). PLWH, person living with HIV.
FIG. 2.
Lifetime and active (last 30 days) drug use (N = 199). MDMA, 3,4-methylenedioxymethamphetamine.
Bivariate associations between covariates and recent HIV testing are presented in Table 3. Covariates were selected based on a review of relevant literature and knowledge of the local Malaysian context. Income over 2000 MYR per month and being stably housed were negatively associated with recent HIV testing. Conversely, older age, active amphetamine use, having a PCP, having disclosed your transgender identity to your PCP, being comfortable discussing transgender healthcare needs with your PCP, previous STI diagnosis, previous depression diagnosis, having been in jail, having been in prison, history of childhood sexual assault, history of physical assault in adulthood, history of gender-based sexual violence, and having a high mental health score on the SF-12 were each positively associated with recent HIV testing.
Results of the multivariate analysis are also presented in Table 3. The multivariate analysis shows a subset of covariates selected by the Bayesian lasso algorithm with normal—exponential—gamma hierarchical priors.26 When adjusted for other covariates in the model, recent HIV testing was associated with being 26–40 years of age (adjusted odds ratio [AOR] = 2.77, 95% CI = 1.12–6.87), active amphetamine use (AOR = 2.69, 95% CI = 1.20–6.04), having a PCP (AOR = 4.86, 95% CI = 1.25–18.81), previous depression diagnosis (AOR = 6.16, 95% CI = 1.56–24.24), and high mental health functioning (AOR = 2.27, 95% CI = 1.04–4.96).
Discussion
The first step of the UNAIDS 90–90–90 HIV control strategy is HIV testing, with attention to key populations.1 To our knowledge, this is the first study to examine correlates of recent HIV testing among TW, an especially high-risk population, in Malaysia. Overall, we found that TW had suboptimal rates of both lifetime (41.7%) and recent HIV testing (18.7%). This is particularly concerning given the high rates of sex work involvement, polysubstance use, and other HIV vulnerabilities, including gender-based sexual violence and poor linkage to healthcare, observed in this study. Results of the multivariate analysis revealed that high mental health functioning was associated with greater likelihood of HIV testing in the last 12 months. One would expect that individuals with a greater capacity to prioritize health testing would be more likely to pursue an HIV test. The association between age and HIV testing has been documented previously14,27,28 and could be explained by young TW perceiving their personal HIV risk incorrectly or feeling uncomfortable about accessing HIV healthcare. Importantly, future research is needed to determine the unique barriers to HIV care engagement among young TW.
Interestingly, previous diagnosis of depression and active amphetamine use were also positively associated with recent HIV testing. Contrary to expectations, these destabilizing factors were associated with an increased likelihood of being tested recently for HIV. History of depression and amphetamine use are both indicators of HIV vulnerability.27–31 Both depressive symptoms and amphetamine use can increase risky health behaviors and inhibit an individual's ability to engage with both preventive HIV care and treatment.32–35 The positive association between these variables and recent HIV testing could be explained by current outreach efforts in Malaysia intended to engage TW in peer counseling and substance use support. It is thus reasonable to posit that TW who experience depression and/or substance use are more likely to access HIV testing because efforts are made to target their coexisting healthcare needs. These instabilities could also lead TW to interface with social services, which in turn may link them into HIV testing. Alternatively, it may be awareness of one's own instability—and in turn, one's HIV vulnerability—that motivates TW to seek HIV testing. As such, interventions to engage TW in HIV testing should ensure they reach those individuals who do not interface with social services and outreach workers, but are still at high risk for HIV.
The data also emphasize the importance of the PCP in linking TW into regular HIV testing. TW who reported having a PCP were six times more likely to have had a recent HIV test, underscoring the impact of having TW engaged in primary care. Unfortunately, few TW had a PCP (6%), although the vast majority had interacted with an HIV outreach worker in the last 12 months and nearly all TW knew where they could receive an HIV test. Despite great awareness of the availability of HIV testing in our sample, TW have suboptimal rates of lifetime and recent HIV testing. As TW in our sample interfaced more often with HIV outreach workers than PCPs, future interventions should include procedures that increase HIV testing uptake by supporting initiatives to train existing groups of TW community healthcare workers in rapid HIV testing. HIV testing as prevention is not a common practice in publicly funded hospitals and clinics where most Malaysian citizens receive healthcare. It is also highly stigmatized to request HIV testing, especially for TW who may avoid contact with healthcare systems altogether for fear of discrimination.36 Several studies have demonstrated that peer-delivered HIV testing is both acceptable and effective at increasing HIV testing uptake.37,38 Providing TW with access to anonymous, free HIV testing within environments where they feel safe discussing their healthcare needs could lead to greater HIV testing uptake and more frequent testing.
Although recent HIV testing in this sample appeared to be the same for sex work-involved and sex work-uninvolved participants, it is nonetheless an important indicator of risk that warrants additional study. In this study, most TW were engaged in sex work, which was also the primary source of their income. The impact of sex work involvement on HIV in TW in Malaysia, however, appears similar to the experience of cisgender women sex workers. For example, a recent study of HIV prevalence among sex workers in Greater Kuala Lumpur, Malaysia, revealed similarly high rates for TW (12.4%, 95% CI = 7.8–17.1) and cisgender women (11.1%, 95% CI = 7.6–14.7).11 Furthermore, innovative interventions to reduce HIV risk among TW sex workers in Malaysia have been explored previously, including the feasibility of microfinance-based interventions.39
Introduction of novel strategies, including peer-based HIV testing, at-home HIV testing, and integration of HIV testing into routine care, may improve overall uptake and frequency of HIV testing for TW in Malaysia. Such strategies are consistent with the Malaysia Ministry of Health's mission to decentralize HIV testing from clinic- to community-based settings.10 Implementation of such HIV screening programs, however, will require sustained support from funders and government agencies that provide funding and other resources for HIV screening services in community settings. Such strategies should be designed to engage patients in the full cascade of care, including linkage to treatment and prevention services, including PrEP. Other models for improving uptake of HIV testing among TW include engagement in primary care settings, especially in settings that provide comprehensive gender-affirming care.36,40–42 To achieve this, medical providers must be trained to provide high-quality and well-informed care to transgender patients.43 Such care necessitates curriculum development and continuing medical education that expand knowledge regarding transgender healthcare. In addition, primary care engagement is essential to initiating and maintaining patients in PrEP treatment. TW would benefit greatly from receiving comprehensive HIV preventive care that includes both frequent testing and access to PrEP.42
Our results also indicate that TW have a mixed level of awareness of modes of HIV transmission. Specifically, most participants correctly identified sharing of drug injection equipment as an HIV risk factor and condom use as protective against HIV. However, a large proportion incorrectly identified mosquito bites and sharing a meal with a person living with HIV as modes of HIV transmission. The incorrect belief in mosquito-mediated HIV transmission is likely due to increased incidence of dengue, chikungunya, and other mosquito-borne illnesses in Southeast Asia.44–46 Accurate knowledge of HIV risk factors can reduce HIV-related stigma and consequently increase utilization of HIV testing services.
TW experience one of the highest rates of HIV globally and, until recently, have not been the beneficiary of targeted evidence-based HIV prevention interventions.3 Testing is the first step in the cascade of HIV care and is a critical component of engaging high-risk populations, specifically TW, in HIV treatment and prevention services. To achieve the UNAIDS 2020 goal of 90–90–90, scale-up of HIV testing among transgender people must be expanded.1 Our data suggest that novel strategies are needed to engage TW who are not linked into HIV preventive care. The findings of this study suggest that although HIV testing services are available, uptake among TW has been low. Thus, it is crucial to understand the determinants of HIV testing to shape current interventions and create new initiatives that are effective in meeting the needs of TW.
Strengths and limitations
Greater Kuala Lumpur, Malaysia, is a predominantly urban and highly populated region. As such, the participants who were sampled likely had greater exposure to HIV resources than individuals in more remote regions. In fact, the low levels of HIV testing reported in this study may underestimate the true healthcare disparity that exists within the transgender community. Given that time since the last HIV test was a self-reported variable, our data may represent an underestimate of the true length of time since the last HIV test. Furthermore, given the moderate knowledge of HIV in this sample, participants may have been aware of the social desirability of reporting more recent HIV testing. This bias could have led to inaccurate reporting and a reduction in the mean time since last test. Finally, as this sample only included TW, it is important not to generalize the findings to all transgender individuals. Transgender men represent a unique demographic with specific healthcare needs that require investigation and analysis separate from TW. Another weakness of our findings is the underrepresentation of persons of Chinese ethnicity who comprise nearly a quarter of Malaysia's population (23.4%).47 Future studies should explore strategies to improve recruitment of Chinese transgender individuals in Malaysia.11
Conclusion
TW are a key population at high risk for HIV in Malaysia and, consistent with the UNAIDS 90–90–90 strategy,1 are a key target for expansion of HIV testing and broader prevention and treatment strategies. Our data demonstrate that TW in Greater Kuala Lumpur face multiple risk factors for HIV, including sex work, substance abuse, and social instability. Current efforts to improve uptake in HIV testing have proven inadequate for the TW population as a whole. Nevertheless, our findings demonstrate that factors that are likely proxies for social instability, such as amphetamine use and previous depression diagnoses, are associated with increased rates of HIV testing. Our data suggest that the most vulnerable TW appear to be engaged in regular HIV testing; however, efforts should be made to ensure that all TW at risk for HIV receive regular HIV testing. HIV testing represents the first step in linking HIV-positive TW to treatment or, if negative, directing TW to effective prevention strategies, including PrEP.
Acknowledgments
This research was supported by grants from the Wilbur G. Downs fellowship at Yale University and the Yale Fund for Lesbian and Gay Studies Grant; National Institute on Drug Abuse Career Development Awards (K01 DA038529, J.A.W. and K24 DA017072, F.L.A.); National Institute on Drug Abuse Research Awards (R36 DA042643, O.M. and R01 DA025943, F.L.A.); and a Malaysia Ministry of Education, University Malaya High Impact Research Grant (E-000001-20001, A.K.). The authors thank their collaborating organizations, including Pertubuhan Advokasi Masyarakat Terpinggir (PAMT), PT Foundation, and SEED Foundation, who aided in recruitment efforts and provided guidance in questionnaire design. A special thanks to Ms. Chella Sri for her assistance with translation, recruitment, and survey administration.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Joint United Nations Programme on HIV/AIDS (UNAIDS): 90-90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva, Switzerland: UNAIDS, 2014. Available at www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf Accessed September9, 2017 [Google Scholar]
- 2. Joint United Nations Programme on HIV/AIDS (UNAIDS): The Gap Report. Geneva, Switzerland: UNAIDS, 2014. Available at www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf Accessed October18, 2017 [Google Scholar]
- 3. Baral SD, Poteat T, Stromdahl S, et al. : Worldwide burden of HIV in transgender women: A systematic review and meta-analysis. Lancet Infect Dis 2013;13:214–222 [DOI] [PubMed] [Google Scholar]
- 4. Poteat T, Reisner SL, Radix A: HIV epidemics among transgender women. Curr Opin HIV AIDS 2014;9:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson JE, Kanters S: Lack of sexual minorities' rights as a barrier to HIV prevention among men who have sex with men and transgender women in Asia: A systematic review. LGBT Health 2015;2:16–26 [DOI] [PubMed] [Google Scholar]
- 6. Silva-Santisteban A, Eng S, de la Iglesia G, et al. : HIV prevention among transgender women in Latin America: Implementation, gaps and challenges. J Int AIDS Soc 2016;19:20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Human Rights Watch. Malaysia: Court ruling sets back transgender rights. 2015. Available at www.hrw.org/news/2015/10/08/malaysia-court-ruling-sets-back-transgender-rights Accessed October24, 2016
- 8. Kalichman SC, Hernandez D, Finneran S, et al. : Transgender women and HIV-related health disparities: Falling off the HIV treatment cascade. Sex Health 2017;14:469–476 [DOI] [PubMed] [Google Scholar]
- 9. HIV/STD Section, Disease Control Division, Ministry of Health, Government of Malaysia: 2010 UNGASS Country Progress Report—Malaysia. Putrajaya, Malaysia: Ministry of Health Malaysia, 2010. Available at http://data.unaids.org/pub/report/2010/malaysia_2010_country_progress_report_en.pdf Accessed October17, 2017 [Google Scholar]
- 10. HIV/STI Section, Disease Control Division, Ministry of Health Malaysia: National Strategic Plan: Ending AIDS: 2016–2030. Putrajaya, Malaysia: Ministry of Health Malaysia, 2015. Available at http://aidsdatahub.org/sites/default/files/publication/Malaysia_National_strategic_plan_2016–2030.pdf Accessed September29, 2017 [Google Scholar]
- 11. Wickersham JA, Gibson BA, Bazazi AR, et al. : Prevalence of human immunodeficiency virus and sexually transmitted infections among cisgender and transgender women sex workers in Greater Kuala Lumpur, Malaysia: Results from a respondent-driven sampling study. Sex Transm Dis 2017;44:663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Logie CH, Lacombe-Duncan A, Wang Y, et al. : Prevalence and correlates of HIV infection and HIV testing among transgender women in Jamaica. AIDS Patient Care STDs 2016;30:416–424 [DOI] [PubMed] [Google Scholar]
- 13. Bao A, Colby DJ, Trang T, et al. : Correlates of HIV testing among transgender women in Ho Chi Minh, Vietnam. AIDS Behav 2016;20:371–378 [DOI] [PubMed] [Google Scholar]
- 14. Lee SW, Deiss RG, Segura ER, et al. : A cross-sectional study of low HIV testing frequency and high-risk behaviour among men who have sex with men and transgender women in Lima, Peru. BMC Public Health 2015;15:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qualtrics [online survey software], 2014. Provo, Utah, USA. Available at https://www.qualtrics.com. Accessed January 1, 2017.
- 16. MEASURE DHS: AIDS Indicator Survey: Model Individual Questionnaire. Rockville, MD: The DHS Program: Demographic and Health Surveys, 2011. Available at https://dhsprogram.com/pubs/pdf/AISQ1/AIS_Individual_QRE_DHS6_8Nov2011.pdf Accessed January1, 2017 [Google Scholar]
- 17. Thom DH, Ribisl KM, Stewart AL, Luke DA: Further validation and reliability testing of the Trust in Physician Scale. The Stanford Trust Study Physicians. Med Care 1999;37:510–517 [DOI] [PubMed] [Google Scholar]
- 18. Ware J, Jr., Kosinski M, Keller SD: A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–233 [DOI] [PubMed] [Google Scholar]
- 19. Centers for Disease Control and Prevention: Behavioral Risk Factor Surveillance System: BRFSS 2006 Survey Data and Documentation—2006 Questionnaire. 2006. Available at www.cdc.gov/brfss/annual_data/annual_2006.htm Accessed July30, 2018
- 20. Berger BE, Ferrans CE, Lashley FR: Measuring stigma in people with HIV: Psychometric assessment of the HIV stigma scale. Res Nurs Health 2001;24:518–529 [DOI] [PubMed] [Google Scholar]
- 21. Nom IE, Sandanasamy SK, Beng KT, Mohamad R: Country Report Malaysia: Poverty alleviation with a focus on vulnerable people. The 8th ASEAN and Japan High Level Officials Meeting on Caring Societies. Tokyo, Japan: Association of Southeast Asian Nations (ASEAN), 2010. Available at www.mhlw.go.jp/bunya/kokusaigyomu/asean/2010/dl/cr05-malaysia.pdf Accessed July25, 2018 [Google Scholar]
- 22. Park T, Casella G: The Bayesian Lasso. J Am Stat Assoc 2008;103:681–686 [Google Scholar]
- 23. Tibshirani R: Regression shrinkage and selection via the Lasso. J Royal Stat Soc Ser B 1996;58:267–288 [Google Scholar]
- 24. Kyung M, Gill J, Ghosh M, Casella G: Penalized regression, standard errors, and Bayesian lassos. Bayesian Anal 2010;5:369–411 [Google Scholar]
- 25. Morozova O, Levina O, Uuskula A, Heimer R: Comparison of subset selection methods in linear regression in the context of health-related quality of life and substance abuse in Russia. BMC Med Res Methodol 2015;15:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang A, Liu D: EBglmnet: A comprehensive R package for sparse generalized linear regression models. Bioinformatics 2016:1–3. DOI: 10.1093/bioinformatics/btw143 [DOI] [PubMed] [Google Scholar]
- 27. Hahm HC, Augsberger A, Feranil M, et al. : The associations between forced sex and severe mental health, substance use, and HIV risk behaviors among Asian American women. Violence Against Women 2017;23:671–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meyer JP, Springer SA, Altice FL: Substance abuse, violence, and HIV in women: A literature review of the syndemic. J Womens Health (Larchmt) 2011;20:991–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Machado Neto AdL, Pereira Rodrigues NL, Tavares de Luna Neto R, et al. : Depression as a risk factor for HIV infection. Int Arch Med 2015;8:1–5 [Google Scholar]
- 30. Treisman G, Angelino A: Interrelation between psychiatric disorders and the prevention and treatment of HIV infection. Clin Infect Dis 2007;45(Suppl. 4):S313–S317 [DOI] [PubMed] [Google Scholar]
- 31. Buchacz K, McFarland W, Kellogg TA, et al. : Amphetamine use is associated with increased HIV incidence among men who have sex with men in San Francisco. AIDS 2005;19:1423–1424 [DOI] [PubMed] [Google Scholar]
- 32. Gonzalez JS, Batchelder AW, Psaros C, Safren SA: Depression and HIV/AIDS treatment nonadherence: A review and meta-analysis. J Acquir Immune Defic Syndr 2011;58:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sikkema KJ, Watt MH, Drabkin AS, et al. : Mental health treatment to reduce HIV transmission risk behavior: A positive prevention model. AIDS Behav 2010;14:252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Colfax G, Shoptaw S: The methamphetamine epidemic: Implications for HIV prevention and treatment. Curr Opin HIV AIDS Rep 2005;2:194–199 [DOI] [PubMed] [Google Scholar]
- 35. Hinkin CH, Barclay TR, Castellon SA, et al. : Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav 2007;11:185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gibson BA, Brown SE, Rutledge R, et al. : Gender identity, healthcare access, and risk reduction among Malaysia's mak nyah community. Glob Public Health 2016;11:1010–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Veronese V, Oo ZM, Thein ZW, et al. : Acceptability of peer-delivered HIV testing and counselling among men who have sex with men (MSM) and transgender women (TW) in Myanmar. AIDS Behav 2018;22:2426–2434 [DOI] [PubMed] [Google Scholar]
- 38. Pawa D, Firestone R, Ratchasi S, et al. : Reducing HIV risk among transgender women in Thailand: A quasi-experimental evaluation of the Sisters program. PLoS One 2013;8:e77113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lall P, Shaw SA, Saifi R, et al. : Acceptability of a microfinance-based empowerment intervention for transgender and cisgender women sex workers in Greater Kuala Lumpur, Malaysia. J Int AIDS Soc 2017;20:21723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wylie K, Knudson G, Khan SI, et al. : Serving transgender people: Clinical care considerations and service delivery models in transgender health. Lancet 2016;388:401–411 [DOI] [PubMed] [Google Scholar]
- 41. Reisner SL, Radix A, Deutsch MB: Integrated and gender-affirming transgender clinical care and research. J Acquir Immune Defic Syndr 2016;72(Suppl. 3):S235–S242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sevelius JM, Deutsch MB, Grant R: The future of PrEP among transgender women: The critical role of gender affirmation in research and clinical practices. J Int AIDS Soc 2016;19:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bauer GR, Zong X, Scheim AI, et al. : Factors impacting transgender patients' discomfort with their family physicians: A respondent-driven sampling survey. PLoS One 2015;10:e0145046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wong LP, Alias H, Aghamohammadi N, et al. : The self-regulation model of illness: Comparison between Zika and dengue and its application to predict mosquito prevention behaviours in Malaysia, a dengue-endemic country. Int J Environ Res Public Health 2016;13:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Packierisamy PR, Ng CW, Dahlui M, et al. : Cost of dengue vector control activities in Malaysia. Am J Trop Med Hyg 2015;93:1020–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liew SM, Khoo EM, Ho BK, et al. : Dengue in Malaysia: Factors associated with dengue mortality from a national registry. PLoS One 2016;11:e0157631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Department of Statistics Malaysia: Current Population Estimates, Malaysia, 2014–2016. Putrajaya, Malaysia: Government of Malaysia, 2016. Available at www.dosm.gov.my/v1/index.php?r=column/pdfPrev&id=OWlxdEVoYlJCS0hUZzJyRUcvZEYxZz09 Accessed October18, 2017 [Google Scholar]