Abstract
The inflammatory context of HIV infection has been posited to contribute to the higher comorbidity risk noted in HIV-infected populations. One possible pathway may involve 1,25-dihydroxyvitamin D [1,25(OH)2D], which plays a wide biologic role in many tissues. We sought to investigate whether inflammation was associated with vitamin D metabolites in a cohort of HIV-infected (HIV+) men receiving treatment and HIV-uninfected (HIV−) men. Vitamin D metabolites, including 25-hydroxyvitamin D [25(OH)D] and 1,25(OH)2D, were measured along with 24 inflammatory markers among Multicenter AIDS Cohort Study participants. Exploratory factor analysis reduced inflammatory marker data to a smaller set of inflammatory processes (IPs). Multivariate linear regression was used to evaluate associations between vitamin D metabolites and IPs. There were 466 HIV+ and 100 HIV− men, who contributed 658 stored samples from 1998 to 2008. We found three IPs with IP 1 characterized by sTNF-R2, sIL-2Rα, sCD27, BAFF, sgp130, sCD14, CXCL10 (IP-10), and sIL-6R. While none of the three IPs was associated with 25(OH)D levels in either HIV+ or HIV−, higher levels of IP 1 were significantly associated with the reduced levels of 1,25(OH)2D in HIV+, and a similar although nonsignificant trend was seen in HIV−. The association between 1,25(OH)2D and inflammation found among HIV-infected men suggests a possible mechanism whereby inflammation leads to the increased comorbidity risk noted among HIV-infected individuals.
Keywords: vitamin D, inflammation, biomarker, HIV infected
Introduction
Vitamin D deficiency plays an established role in skeletal disease1 and has also been implicated in the increased risk of other nonskeletal comorbidities, such as cardiovascular disease, metabolic syndrome, neurocognitive decline, and even mortality.2–5 This spectrum of age-related comorbidity is increasingly seen among HIV-infected populations, as treatment-mediated viral suppression has extended life spans and reduced AIDS-related mortality.6 Growing evidence suggests that besides traditional risk factors (smoking, hypertension, diabetes, and hyperlipidemia) and toxicity of cumulative exposure to antiretroviral therapy, HIV-associated inflammation may play a role in the noted excess risk of age-related comorbidity even among well-treated HIV-infected people.6 In the Multicenter AIDS Cohort Study (MACS), seven biomarkers related to innate immune activation remained elevated in HIV virologically suppressed men following treatment, suggesting persistent residual inflammation.7 Furthermore, higher levels of four inflammatory markers after therapy initiation were predictive of later mortality among HIV-suppressed men.8
Given evidence that both poor vitamin D status and residual inflammation may contribute to age-related morbidity risk, the question arises as to whether inflammation and vitamin D metabolism act through a single pathway to increase risk or whether they represent separate mechanisms. Vitamin D has two main forms as follows: 25-hydroxyvitamin D [25(OH)D], the most widely assessed but inactive form, and 1,25-dihydroxyvitamin D [1,25(OH)2D], the active form that regulates calcium-phosphate homeostasis.1 In vitro studies have demonstrated immunomodulating effects of 1,25(OH)2D, as the active form acts on a variety of immune cells.9 For instance, 1,25(OH)2D3 can promote monocyte-to-macrophage differentiation and downregulate the production of pro-inflammatory cytokines and chemokines.9 In some contexts, inflammation also appears to interfere with activation and degradation of 1,25(OH)2D,10 thus impeding the regulatory effects of vitamin D.
Existing data on the relationship between vitamin D and inflammation have come primarily from general elderly populations or from trials of vitamin D supplementation, but the nature of the relationship remains ambiguous.11 HIV-infected populations represent a group with enduring inflammation and, thus, may be an ideal population for looking at this relationship. One recent prospective study among HIV treated individuals reported no change in inflammatory biomarkers following supplementation of vitamin D and restoration of 25(OH)D to normal levels.12 Examining change in inflammatory biomarker levels and corresponding change in both inactive and active vitamin D metabolites within individuals would more definitively establish the potential links between inflammation and vitamin D metabolism.
In this study, we sought to evaluate associations of markers of inflammation and immune activation with vitamin D metabolites in HIV virologically suppressed men following treatment and HIV-uninfected counterparts, to elucidate relationships between general and HIV-specific patterns of inflammation and vitamin D metabolites.
Methods
Study population and design
A detailed description of the MACS has been provided elsewhere.13 Briefly, it is a longstanding prospective cohort study that has followed 7,350 men who have sex with men from four metropolitan locations in the United States with a goal of understanding the clinical course of HIV-1 infection. Data on demographics, behavioral risks, clinical outcomes, and laboratory measurements are collected semiannually. Details on MACS can be found at: http://aidscohortstudy.org The MACS vitamin D ancillary study was nested in an existing MACS substudy of inflammation and immune activation, in which a panel of chemokines, cytokines, and soluble receptors was measured from stored serum samples of 541 HIV seroconverters, 1,279 highly active antiretroviral therapy (HAART) users, and 250 HIV-uninfected men. The MACS vitamin D ancillary study selected available samples of HAART users to measure vitamin D metabolites from pre- and post-HAART visits during which inflammatory biomarkers were measured, aiming to explore the impact of viral suppression and changes in inflammation following HAART initiation on vitamin D metabolites. Multiple samples from HIV-uninfected men were selected for comparison, matching the sampling time points of HIV-infected participants. This vitamin D substudy in MACS was approved by the institutional review boards at all MACS study sites. The study was conducted in accordance with the World Medical Association Declaration of Helsinki. A written informed consent from all participants has been obtained.
The main analysis of present study was restricted to post-HAART samples of HAART users who had experienced viral suppression (defined as <400 copies/mL) following treatment and samples from comparable HIV-uninfected men. Secondary analysis included samples from a subset of above HAART users with both pre- and post-HAART measurements to evaluate relationships of change in inflammatory biomarkers and change in vitamin D metabolites around HAART initiation.
Vitamin D metabolites
Stored serum samples collected between 1999 and 2008 were tested for vitamin D metabolites, including 25(OH)D2, 25(OH)D3, 1,25(OH)2D2, and 1,25(OH)2D3. Testing was completed at University of Washington between October 2014 and February 2015, using immunoaffinity-liquid chromatography-tandem mass spectrometry with yielding limit of quantitation (LOQ) <1 ng/mL and coefficient of variation (CV) <5% for 25(OH)D and LOQ <4 pg/mL and CV <12% for 1,25(OH)2D.14 Total levels of 25(OH)D and 1,25(OH)2D were obtained by summing their corresponding subforms. Consistent with the Institute of Medicine,15 we defined vitamin D deficiency as 25(OH)D <20 ng/mL. To remove influences of race and season on vitamin D metabolites,16 levels of 25(OH)D and 1,25(OH)2D were standardized by race and season by adding residuals of regressing vitamin D on race and season to intercepts.
Inflammatory biomarkers
A total of 24 serologic inflammatory biomarkers were quantified in the MACS inflammation substudy using two multiplex assays and a separate assay for C-reactive protein (CRP).17 Measurement of 15 cytokines, including IL-1β, IL-2, IL-6, CXCL8 (IL-8), IL10, IL-12p70, GM-CSF, INF-γ, TNF-α, CXCL10 (IP-10), CCL11 (eotaxin), CCL2 (MCP-1), CCL13 (MCP-4), CCL4 (MIP-1β), and CCL17 (TARC), was completed using the pro-inflammatory 9-plex and Chemokine 7-plex (Meso-Scale Diagnostics, LLC, Rockville, MD). Another six soluble receptors (sCD14, sgp130, sIL-2Rα, sIL-6R, sTNF-R2, and sCD27), a chemokine CXCL13 (BLA-BCA1), and a cytokine (BAFF) were determined using the fluorescent bead-based multiplex Luminex xMAP system (Fluorokine MAP; R&D Systems, Minneapolis, MN) and analyzed using a Bio-Plex 200 Luminex instrument and Bio-Plex software (Bio-Rad, Hercules, CA). CRP was measured using a high-sensitivity nephelometric assay (Quest Diagnostics, Dade Behring, Inc., Newark, DE). To reduce variations, a single assay lot was chosen to implement testing, and samples for a single person were analyzed on the sample plate. Biomarker values were natural log transformed to achieve approximate normality distribution.
For the present study, 15 of 24 measured biomarkers were included in the main analysis. Excluded biomarkers were those with >10% of undetectable values [IL-1β (46%), GM-CSF (44%), IFN-γ (38%), IL-2 (23%), and IL-12p70 (11%)] and those for which there was no evidence from the literature or MACS data of associations with vitamin D (CXCL13, IL10, CCL17, and CRP).
Remaining missing values due to above or below the limits of detection were imputed using truncated log-normal distributions specific to the distribution of the biomarker.
Covariates
Covariates for inclusion in the analysis were drawn from prior work in the MACS evaluating correlates of vitamin D metabolite levels.16 Covariate values were assessed at the time point of the vitamin D metabolite measurements. Enzyme-linked immunosorbent assays along with confirmatory Western blot determined HIV status (HIV infected vs. HIV uninfected). Race (white, black, and other race) and age (≥50 vs <50 years) were self-reported. Season was determined based on the timing of serum sample collection and categorized as spring (March, April, and May), summer (June, July, and August), fall (September, October, and November), and winter (December, January, and February). Body mass index (BMI) was grouped into three categories: normal (<25.0 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30.0 kg/m2). A few individuals with BMI <18.5 kg/m2 were grouped into the normal BMI category. Hepatitis C virus (HCV) infection was considered present if a plasma RNA test was positive. Plasma HIV RNA was measured using the Roche assays (27% Roche second generation, LOQ <50 copies/mL; 68% Roche COBAS TaqMan, LOQ <20 copies/mL), and viral suppression was defined as <400 copies/mL. CD4+ T lymphocytes were measured using standardized flow cytometry.18 The DHHS/Kaiser Panel guidelines19 were used to define HAART use.
Statistical analyses
Characteristics of the study population were presented by HIV status using frequency and percent and compared using chi-square tests. The geometric mean difference in each biomarker and levels of 25(OH)D and 1,25(OH)2D by HIV status was statistically compared using nonparametric Wilcoxon tests.
We used exploratory factor analysis (EFA) to reduce the number of inflammatory exposure indicators and summarize their contribution to inflammatory processes (IPs). Implementation of EFA methods for inflammatory biomarkers in the MACS has been previously described.20 Briefly, inflammatory biomarker levels were standardized [mean = 0, standard deviation (SD) = 1]. The strength and direction of correlations between biomarkers were then used to infer the presence of underlying latent processes or factors. A weighted linear combination of biomarkers was used to estimate individual levels of each latent process (factor scores). The derived weights (factor loadings) represent the strength and direction of the correlation of each biomarker with the underlying latent IP.
Next, standardized continuous 25(OH)D and 1,25(OH)2D levels were separately regressed on EFA-identified IPs. Generalized estimating equation was used to account for multiple visits contributed by some individuals. We selected covariates into the final model based on literature21 and previous study results.16 Final models were adjusted for age >50 years, BMI, and HCV infection and were run stratified by HIV status.
To evaluate individual biomarker contributions, relationships of individual biomarkers with vitamin D metabolite levels were also explored, accounting for multiple comparisons using the Benjamini–Hochberg method.22 A sensitivity analysis was conducted, treating vitamin D metabolite levels as categorical, with 25(OH)D <20 ng/mL defined as vitamin D deficient and 1,25(OH)2D < 33 pg/mL (lowest 10th percentile) defined as low 1,25(OH)2D. Also a secondary analysis of change in selected individual biomarkers and change in vitamin D metabolites around HAART initiation was conducted. We considered p-value <.05 as indicative of statistical significance. All statistical analyses were completed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Description of study population
A total of 566 men contributed 658 stored serum samples to the analysis: 466 post-HAART samples from HIV virologically suppressed men following treatment and 192 samples from HIV-uninfected men (Table 1). The age distribution was similar between the two groups. There were significant differences in the distribution of race and BMI by HIV status. A higher prevalence of HCV infection was seen among HIV-uninfected men as a result of oversampling in the MACS substudy of inflammation and immune activation.7 Among HIV-infected men, the median time from HAART initiation to the biomarker measurement was 2.2 years [interquartile range (IQR): 1.7–2.3], and the median CD4+ T cell count at the time of the biomarker measurements was 531 cells/mm3 (IQR: 367–717). Among the HIV-infected men, 205 had both pre- and post-HAART samples from which to evaluate the relationship between change in levels of inflammatory biomarkers and change in vitamin D metabolites.
Table 1.
Baseline Characteristics of Study Population by HIV Status
| Variable | HIV+a | HIV−b | p-Value |
|---|---|---|---|
| Number of unique individuals | 466 | 100 | |
| Number of study visits | 466 | 192 | |
| % Age ≥50 years | 132 (28.3) | 66 (34.4) | .124 |
| % Race | |||
| White | 278 (59.7) | 115 (59.9) | <.001 |
| Black | 114 (24.5) | 66 (34.4) | |
| Other | 74 (15.9) | 11 (5.7) | |
| % BMI | |||
| Normal | 239 (51.3) | 89 (46.4) | <.001 |
| Overweight | 173 (37.1) | 52 (27.1) | |
| Obese | 54 (11.6) | 51 (26.6) | |
| % Hepatitis C infection | 38 (8.2) | 28 (14.6) | .013 |
| CD4+ T cell count, median (IQR), cells/mm3 | 531 (367, 717) | ||
| Years since HAART initiation, median (IQR) | 2.2 (1.7, 2.3) | ||
| Standardized 25(OH)D, median (IQR), ng/mL | 18.5 (12.7, 24.7) | 18.0 (12.8, 21.7) | |
| Standardized 1,25(OH)2D, median (IQR), pg/mL | 48.2 (39.2, 58.2) | 47.5 (39.6, 57.4) | |
Refers to post-HAART samples from HIV-infected men with suppressed viral load (≤400 copies/mL).
Refers to samples from HIV-uninfected men.
BMI, body mass index; HAART, highly active antiretroviral therapy; IQR, interquartile range; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Difference in vitamin D metabolites and biomarkers
The median standardized levels of 25(OH)D and 1,25(OH)2D did not statistically differ by HIV status (Table 1). The median change in levels of 25(OH)D and 1,25(OH)2D among 205 HIV virologically suppressed men with two measurements was 0.44 ng/mL (IQR: −5.75, 5.37) and 2.12 pg/mL (IQR: −8.98, 11.99), respectively. As reported previously,7 despite experiencing HAART, more than a half of individual biomarkers measured were higher among HIV-infected men than HIV-uninfected men (Table 2).
Table 2.
Mean Natural Log-transformed Concentration of Fifteen Biomarkers, by HIV Status
| Variable | HIV+ | HIV− | p-Value |
|---|---|---|---|
| Number of unique individuals | 466 | 100 | |
| Number of study visits | 466 | 192 | |
| sTNFR2, mean (SD) | 7.9 (0.4) | 7.7 (0.3) | <.001 |
| sIL-2Rα, mean (SD) | 7.3 (0.4) | 7.2 (0.3) | .005 |
| sCD27, mean (SD) | 9.4 (0.4) | 9.1 (0.3) | <.001 |
| BAFF, mean (SD) | 7.7 (0.4) | 7.6 (0.2) | <.001 |
| sCD14, mean (SD) | 14.7 (0.3) | 14.5 (0.3) | <.001 |
| sIL-6R, mean (SD) | 10.8 (0.4) | 10.8 (0.4) | .400 |
| IP-10, mean (SD) | 5.4 (0.7) | 4.9 (0.7) | <.001 |
| sgp130, mean (SD) | 12.5 (0.2) | 12.4 (0.2) | <.001 |
| TNF-α, mean (SD) | 2.3 (0.6) | 2.3 (0.7) | .024 |
| IL-8, mean (SD) | 3.0 (1.0) | 2.9 (1.0) | .516 |
| IL-6, mean (SD) | 0.1 (0.8) | 0.1 (1.0) | .715 |
| MIP-1, mean (SD) | 4.9 (0.7) | 5.0 (0.7) | .141 |
| MCP-1, mean (SD) | 6.3 (0.4) | 6.2 (0.4) | <.001 |
| Eotaxin, mean (SD) | 7.4 (0.5) | 7.4 (0.5) | .306 |
| MCP-4, mean (SD) | 6.7 (0.4) | 6.7 (0.4) | .571 |
SD, standard deviation.
Exploratory factor analysis
Among 658 samples included, 31 measurements from 6 biomarkers yielded values below or above the limits of detection and were imputed. The correlation between 15 biomarkers and factor loadings indicating the contribution of each biomarker to underlying IPs is consistent with previous reports in the MACS.20 Three IPs were identified. IP 1 was primarily characterized by sTNF-R2, sCD27, sIL-2Rα, BAFF, sgp130, sCD14, CXCL10 (IP-10), and sIL-6R; IP 2 was primarily characterized by TNF-α, IL-6, IL-8, and MIP-1; and IP 3 was mainly characterized by MCP-1, eotaxin, and MCP-4.
Relationship between vitamin D metabolites and IPs
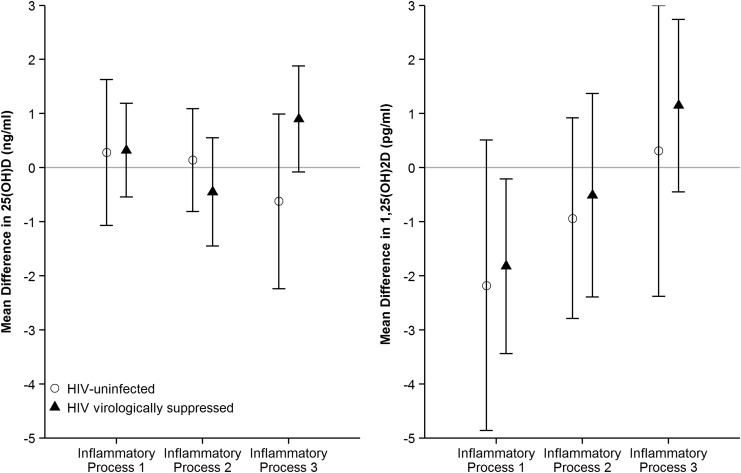
In the multivariate analysis, none of the IPs was associated with levels of 25(OH)D in either HIV group. However, levels of IP 1 were significantly associated with lower levels of 1,25(OH)2D among HIV-infected men, such that every SD increase in IP 1 was associated with a 1.82 pg/mL lower level of 1,25(OH)2D [95% confidence interval (CI): −3.44 to −0.21]. A similar magnitude of effect was seen among HIV-uninfected men, but the point estimate did not reach statistical significance in the smaller HIV-uninfected samples (β = −2.18 pg/mL, 95% CI: −4.86 to 0.51). IP 2 and 3 were not significantly associated with 1,25(OH)2D levels in either HIV group (Fig. 1).
FIG. 1.
Multivariate regression of inflammatory processes on levels of vitamin D metabolites by HIV status. Each inflammatory process was standardized with mean 0 and standard deviation 1.
Sensitivity analyses that categorized 25(OH)2D (<20 pg/mL) and 1,25(OH)2D (<33 pg/mL, the lowest 10th percentile) to evaluate the assumption of linearity of effects provided analogous results: none of IPs was significantly related to deficiency of 25(OH)D in either HIV group, while IP 1 levels were borderline significantly associated with the lowest 10th percentile of 1,25(OH)2D (odds ratio = 1.36, 95% CI: 0.96–1.96) among HIV-infected men.
Relationship between vitamin D metabolites and individual biomarkers from IPs
In the multivariate analysis, we found only a weak association of CXCL10 (IP-10) with levels of 25(OH)D among HIV-uninfected men and that of eotaxin with 25(OH)D levels among HIV-infected men. However, these associations were not statistically significant after adjusting the significance threshold to account for multiple comparisons.
In contrast, two markers, including sTNF-R2 and sCD27, that contributed to IP 1 were significantly associated with lower levels of 1,25(OH)2D levels among HIV-infected men. Among HIV-uninfected men, sTNF-R2 from IP 1 was associated with decreased levels of 1,25(OH)2D, while TNF-α from IP 2 was associated with lower levels of 1,25(OH)2D, and MIP-1 from IP 2 had an opposite effect. After accounting for multiple comparisons, the only significant relationships were with sTNF-R2 among HIV-infected men and TNF-α among HIV-uninfected men. There was no differential effect of biomarkers on either 25(OH)D or 1,25(OH)2D by HIV status (Table 3).
Table 3.
Multivariate Regression of Individual Biomarker on Levels of Vitamin D Metabolites by HIV Status
| HIV+ (N = 466) | HIV− (N = 192) | HIV+ (N = 466) | HIV− (N = 192) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean difference in 25(OH)D, (95% CI) | p-Value | Mean difference in 25(OH)D, (95% CI) | p-Value | Mean difference in 1,25(OH)2D, (95% CI) | p-Value | Mean difference in 1,25(OH)2D, (95% CI) | p-Value | |
| sTNF-R2 | 0.20 (−0.57 to 0.97) | .606 | 0.51 (−0.75 to 1.77) | .428 | −2.26 (−3.69 to −0.82) | .002 | −2.78 (−4.98 to −0.57) | .014 |
| sCD27 | 0.43 (−0.37 to 1.24) | .291 | −0.30 (−1.50 to 0.90) | .620 | −1.56 (−3.02 to −0.09) | .037 | −2.18 (−4.48 to 0.12) | .063 |
| sIL-2Rα | 0.28 (−0.46 to 1.02) | .455 | 0.46 (−0.52 to 1.44) | .360 | −1.05 (−2.54 to 0.44) | .168 | −1.12 (−3.16 to 0.92) | .283 |
| BAFF | −0.63 (−1.37 to 0.11) | .096 | −0.48 (−1.96 to 0.99) | .522 | −0.74 (−2.05 to 0.56) | .265 | −1.47 (−4.12 to 1.17) | .274 |
| sgp130 | 0.17 (−0.58 to 0.93) | .651 | 0.08 (−1.16 to 1.31) | .905 | −0.76 (−2.29 to 0.77) | .332 | −0.72 (−2.60 to 1.17) | .456 |
| sCD14 | 0.45 (−0.37 to 1.27) | .279 | 0.03 (−0.87 to 0.94) | .941 | 0.91 (−0.48 to 2.30) | .198 | 0.53 (−1.55 to 2.60) | .617 |
| IP-10 | 0.20 (−0.66 to 1.06) | .654 | 1.53 (0.34 to 2.72) | .012 | −0.36 (−1.76 to 1.03) | .609 | −0.34 (−3.43 to 2.75) | .830 |
| sIL-6R | 0.37 (−0.36 to 1.11) | .320 | 0.11 (−0.74 to 0.96) | .796 | −0.72 (−1.84 to 0.39) | .202 | 0.77 (−1.93 to 3.47) | .578 |
| TNF-α | 0.09 (−0.69 to 0.87) | .822 | 0.20 (−0.48 to 0.88) | .557 | −1.28 (−2.96 to 0.39) | .134 | −1.76 (−2.74 to −0.78) | <.001 |
| IL-6 | −0.30 (−1.21 to 0.62) | .528 | −0.10 (−0.99 to 0.78) | .819 | −1.13 (−2.78 to 0.53) | .181 | −0.96 (−2.27 to 0.36) | .154 |
| IL-8 | −0.23 (−1.08 to 0.61) | .590 | −0.28 (−1.05 to 0.49) | .482 | 0.18 (−1.38 to 1.74) | .818 | −1.21 (−3.14 to 0.71) | .216 |
| MIP-1 | −0.55 (−1.44 to 0.34) | .229 | 0.23 (−0.62 to 1.08) | .592 | 0.90 (−0.57 to 2.36) | .229 | 2.52 (0.20 to 4.84) | .033 |
| MCP-1 | 0.57 (−0.29 to 1.42) | .194 | −0.70 (−2.03 to 0.63) | .302 | −0.53 (−1.85 to 0.79) | .431 | −0.24 (−2.39 to 1.91) | .827 |
| Eotaxin | 0.82 (0.00 to 1.65) | .049 | −0.78 (−2.02 to 0.46) | .217 | 1.11 (−0.28 to 2.49) | .117 | −0.78 (−3.10 to 1.54) | .508 |
| MCP-4 | 0.28 (−0.47 to 1.04) | .462 | 0.65 (−0.58 to 1.88) | .302 | 0.82 (−0.49 to 2.13) | .222 | 1.22 (−0.97 to 3.41) | .276 |
The concentration of each biomarker had been natural log-transformed and then standardized with mean = 0 and standard deviation = 1.
The model was adjusted for age, BMI, and HCV infection. An example of interpretation is that one SD increase of Eotaxin was significantly associated with 0.82 pg/mL higher levels of 25(OH)D in HIV+.
Bold font refers to P-value <0.05 from the multivariate regression model.
CI, confidence interval; HCV, hepatitis C virus.
Relationship between change in vitamin D metabolite and change in sTNF-R2 or sCD27
Neither change in sTNF-R2 or sCD27 was associated with change in levels of 25(OH)D. However, change in sCD27—a member of the tumor necrosis factor receptor superfamily that is expressed primarily on T cells—was significantly associated with change in 1,25(OH)2D (β = −2.98 pg/mL, 95% CI: −5.38 to −0.58) (Table 4).
Table 4.
Relationship Between Change in sTNF-R2 or sCD27 and Change in Vitamin D Metabolites
| Mean difference of change in 25(OH)D (95% CI) | p-Value | Mean difference of change in 1,25(OH)2D (95% CI) | p-Value | |
|---|---|---|---|---|
| Change in sTNF-R2 | 0.23 (−1.56 to 1.11) | .741 | −1.62 (−4.08 to 0.83) | .195 |
| Change in sCD27 | −0.70 (−2.02 to 0.61) | .297 | −2.98 (−5.38 to −0.58) | .016 |
Change in sTNF-R2 or sCD27 had been standardized with mean = 0 and standard deviation = 1.
The model was adjusted for age, BMI, and HCV infection. The interpretation is similar as above.
Analysis was restricted to 205 HIV+ who had pre- and post-HAART samples and available sTNF-R2 and sCD27.
Bold font refers to P-value <0.05 from the multivariate regression model.
Discussion
In our study, we found an association of markers important for immune responses toward infection and inflammation with levels of 1,25(OH)2D—the active vitamin D metabolite—among HIV-infected men following treatment, but not with levels of 25(OH)D. This relationship appeared to be driven by sTNF-R2 and sCD27, as reflected in the individual biomarker analysis and change analysis around HAART initiation.
In the MACS cohort, HIV infection did not appear to increase the risk of poor 25(OH)D and 1,25(OH)2D status associated with inflammation, at least among those with well-treated HIV. A similar point estimate for the association of IP 1 with lower levels of 1,25(OH)2D was observed among HIV-uninfected to that seen among HIV-infected men; both groups also had comparable standardized levels of 1,25(OH)2D. Thus the inflammatory relationship noted in our study does not appear to be specific to HIV infection, although HIV infection is associated with higher levels of many inflammatory markers.
Some evidence suggested that intracellular bacteria can disrupt vitamin D metabolism leading to increased extrarenal production of 1,25(OH)2D, consequently depleting 25(OH)D while disabling kidney's control of 1,25(OH)2D production.23 Intracellular bacteria may block vitamin D receptors and cause an increase in inflammatory cytokines (e.g., IFN-γ). These cytokines are able to upregulate CYP27B1, a key enzyme converting 25(OH)D to 1,25(OH)2D in the extrarenal tissues. As our data show, the median levels of 25(OH)D, 18.5 ng/mL among HIV-infected men and 18.0 ng/mL among HIV-uninfected men, were relatively lower than the adjusted mean levels of 25(OH)D, 25.2 ng/mL, in the general men population of United States,24 while the median levels of 1,25(OH)2D, 48 pg/mL, in our study samples were at the higher end of the reported normal range, 20–60 pg/mL.25
However, our results were not fully consistent with such a bacterial infection model. Several antibacterial cytokines, including IL-6 and IL-8 (all indicators of IP 2), were not associated with 25(OH)D or 1,25(OH)2D. Of the important chemokine identified in the pathogenesis of bacterial infection,26 only MIP-1α was positively associated with 1,25(OH)2D, but not with 25(OH)D. Likely, the relationship between cytokines and vitamin D metabolites is complex, involving multiple pathways. For example, TNF-α can either upregulate or downregulate the production of 1,25(OH)2D under different conditions.27 Furthermore, 1,25(OH)2D as an immunomodulatory agent can inhibit the production of TNF-α.28 Thus relationships noted in our analysis between an IP related to immune activation (i.e., IP 1) and 1,25(OH)2D but not 25(OH)D levels may represent an alternative mechanism disrupting production of 1,25(OH)2D. Alternatively, the mild-to-moderate inflammatory state of this well-treated and suppressed HIV-infected samples may not reach a threshold sufficient for impacting 25(OH)D levels, as seen in other highly inflammatory conditions (e.g., multiple sclerosis, acute infantile congestive heart failure, inflammatory bowel disease, and cystic fibrosis).29
Examining individual inflammatory markers, it appeared that sTNF-R2 and sCD27 drove the association of IP 1 with levels of 1,25(OH)2D. Looking at changes in marker levels, the association between change in sCD27 and change in levels of 1,25(OH)2D was consistent with a relationship between immune markers and vitamin D metabolites. Both sCD27 and sTNF-R2 belong to members of the TNF-R superfamily,30 and their elevation represents a state of immune activation and chronic inflammation.31 The sTNF-R2, in certain conditions, may act to prolong the half-life of circulated TNF-α or to facilitate the interaction of TNF-α with receptors on the cell surface.32
The strengths of present study include measurements of both vitamin D metabolites and a large number of inflammatory and immune activation biomarkers in a well-characterized HIV cohort. Furthermore, the inclusion of an HIV-uninfected group provides a comparison from which to assess differences due to the context of HIV infection and associated inflammation. However, we did not capture other biomarkers that are important for a full characterization of vitamin D metabolism, including serum calcium and phosphorus, PTH and FGF23, the latter of which play a critical role in regulation of renal production of 1,25(OH)2D.33
Of note, the three IPs were characterized in a data-driven approach instead of a hypothesis-driven approach. The clustering of each IP purely depended on their intercorrelation. Thus identifying biological pathways for these identified processes may be difficult.
In conclusion, we found that an IP mainly driven by sTNF-R2 and sCD27 was associated with lower levels of the active form of vitamin D. These results indicate a potential for immune activation and inflammation to influence vitamin D metabolism and suggest a vitamin D endocrine pathway through which the inflammation could result in the excess risk of comorbidities. Longitudinal studies with repeated measures of inflammatory markers and vitamin D metabolites, as well as comorbidity ascertainment, could establish whether HIV-associated inflammation contributes to comorbidity risk through a pathway involving vitamin D metabolism.
Acknowledgments
The authors appreciate efforts from all collaborators, staffs, and participants of the Multicenter AIDS Cohort Study (MACS). Data in present study were collected by the MACS centers (Principal Investigators, grant number): institution, comprising Baltimore (J.B.M., U01-AI35042): Johns Hopkins Bloomberg School of Public Health; Chicago (Steven M. Wolinsky, U01-AI35039): Northwestern University; Los Angeles (Roger Detels and Otoniel Martinez-Maza, U01-AI35040): University of California, Los Angeles; and Pittsburgh (Charles R. Rinaldo and L.A.K., U01-AI35041): University of Pittsburgh. Data were managed by Data Coordinating Center at Johns Hopkins Bloomberg School of Public Health (L.P.J. and Gypsyamber D’ Souza, UM1-AI35043). The MACS is primarily funded by the National Institute of Allergy and Infectious Diseases, with additional co-funding from the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute of Mental Health. Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute and the National Institute on Deafness and Communication Disorders. MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. The funding for this substudy was supported by the National Institute of Allergy and Infectious Diseases (R21-AI-109817). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The MACS website is located at http://statepi.jhsph.edu/macs/macs.html.
Authors' Contributions
L.Z. and A.G.A. contributed to the analysis and composition of the article. A.G.A., L.P.J., T.T.B., and J.B.M. were responsible for the design and conduct of the study. A.N.H. carried out the measurement of serum samples. A.G.A., L.P.J., T.T.B, M.D.W., F.J.P., L.A.K., and A.T. provided critical intellectual comments on revisions of the article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Holick MF: Vitamin D deficiency. N Engl J Med 2007;357:266–281 [DOI] [PubMed] [Google Scholar]
- 2. Dobnig H, Pilz S, Scharnagl H, et al. : Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med 2008;168:1340–1349 [DOI] [PubMed] [Google Scholar]
- 3. Wang TJ, Pencina MJ, Booth SL, et al. : Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008;117:503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ford ES, Zhao G, Li C, Pearson WS: Serum concentrations of vitamin D and parathyroid hormone and prevalent metabolic syndrome among adults in the United States. J Diabetes 2009;1:296–303 [DOI] [PubMed] [Google Scholar]
- 5. Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC: Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry 2006;14:1032–1040 [DOI] [PubMed] [Google Scholar]
- 6. Deeks SG, Lewin SR, Havlir DV: The end of AIDS: HIV infection as a chronic disease. Lancet 2013;382:1525–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wada NI, Jacobson LP, Margolick JB, et al. : The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015;29:463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wada NI, Bream JH, Martínez-Maza O, et al. : Inflammatory biomarkers and mortality risk among HIV-suppressed men: A multisite prospective cohort study. Clin Infect Dis 2016;63:984–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guillot X, Semerano L, Saidenberg-Kermanac'h N, Falgarone G, Boissier MC: Vitamin D and inflammation. Joint Bone Spine 2010;77:552–557 [DOI] [PubMed] [Google Scholar]
- 10. Hummel DM, Fetahu IS, Gröschel C, Manhardt T, Kállay E: Role of proinflammatory cytokines on expression of vitamin D metabolism and target genes in colon cancer cells. J Steroid Biochem Mol Biol 2014;144 Pt A:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gonçalves de Carvalho CM, Ribeiro SM: Aging, low-grade systemic inflammation and vitamin D: A mini-review. Eur J Clin Nutr 2017;71:434–440 [DOI] [PubMed] [Google Scholar]
- 12. Hoffman RM, Lake JE, Wilhalme HM, Tseng CH, Currier JS: Vitamin D levels and markers of inflammation and metabolism in HIV-infected individuals on suppressive antiretroviral therapy. AIDS Res Hum Retroviruses 2016;32:247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Detels R, Jacobson L, Margolick J, et al. : The multicenter AIDS Cohort Study, 1983 to …. Public Health 2012;126:196–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laha TJ, Strathmann FG, Wang Z, de Boer IH, Thummel KE, Hoofnagle AN: Characterizing antibody cross-reactivity for immunoaffinity purification of analytes prior to multiplexed liquid chromatography-tandem mass spectrometry. Clin Chem 2012;58:1711–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ross AC, Taylor CL, Yaktine AL, et al. : Institute of Medicine (U.S.): Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Press, Washington, DC, 2011, pp. 89–94 [PubMed] [Google Scholar]
- 16. Zhang L, Tin A, Brown T, et al. : Vitamin D deficiency and metabolism in HIV-infected and -uninfected men in the multicenter AIDS cohort study (MACS). AIDS Res Hum Retroviruses 2016;33:261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McKay HS, Margolick JB, Martínez-Maza O, et al. : Multiplex assay reliability and long-term intra-individual variation of serologic inflammatory biomarkers. Cytokine 2017;90:185–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hultin LE, Menendez FA, Hultin PM, et al. : Assessing immunophenotyping performance: Proficiency-validation for adopting improved flow cytometry methods. Cytometry B Clin Cytom 2007;72:249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Available at https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf accessed May1, 2017 [Google Scholar]
- 20. Abraham AG, Darilay A, McKay H, et al. : Kidney dysfunction and markers of inflammation in the multicenter AIDS cohort study. J Infect Dis 2015;212:1100–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vescini F, Cozzi-Lepri A, Borderi M, et al. : Prevalence of hypovitaminosis D and factors associated with vitamin D deficiency and morbidity among HIV-infected patients enrolled in a large Italian cohort. J Acquir Immune Defic Syndr 2011;58:163–172 [DOI] [PubMed] [Google Scholar]
- 22. Benjamini Y, Hochberg Y: Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B Methodol 1995:289–300 [Google Scholar]
- 23. Mangin M, Sinha R, Fincher K: Inflammation and vitamin D: The infection connection. Inflamm Res 2014;63:803–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA: Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr 2008;88:1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lips P: Relative value of 25(OH)D and 1,25(OH)2D measurements. J Bone Miner Res 2007;22:1668–1671 [DOI] [PubMed] [Google Scholar]
- 26. Shanley TP, Schmal H, Friedl HP, Jones ML, Ward PA: Role of macrophage inflammatory protein-1 alpha (MIP-1 alpha) in acute lung injury in rats. J Immunol 1995;154:4793–4802 [PubMed] [Google Scholar]
- 27. Bikle DD, Pillai S, Gee E, Hincenbergs M: Tumor necrosis factor-α regulation of 1, 25-dihydroxy vitamin D production by human keratinocytes. Endocrinology 1991;129:33–38 [DOI] [PubMed] [Google Scholar]
- 28. Zhang Y, Leung DY, Richers BN, et al. : Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 2012;188:2127–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cannell JJ, Grant WB, Holick MF: Vitamin D and inflammation. Dermatoendocrinol 2014;6:e983401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Widney D, Gundapp G, Said JW, et al. : Aberrant expression of CD27 and soluble CD27 (sCD27) in HIV infection and in AIDS-associated lymphoma. Clin Immunol 1999;93:114–123 [DOI] [PubMed] [Google Scholar]
- 31. Regidor DL, Detels R, Breen EC, et al. : Effect of highly active antiretroviral therapy on biomarkers of B-lymphocyte activation and inflammation. AIDS 2011;25:303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carpentier I, Coornaert B, Beyaert R: Function and regulation of tumor necrosis factor receptor type 2. Curr Med Chem 2004;11:2205–2212 [DOI] [PubMed] [Google Scholar]
- 33. Bikle DD: Vitamin D regulation of immune function. Vitam Horm 2011;86:1–21 [DOI] [PubMed] [Google Scholar]