Abstract
Background: Lymphedema is a chronic, incurable condition that occurs most commonly in lower limbs (legs and feet). Increased morbidity is seen with this form of lymphedema, but there are few studies and even fewer tools intended to assess symptom burden in patients impacted by this condition. A questionnaire, the Lymphedema Symptom Intensity and Distress Survey-Lower Limb (LSIDS-L), was developed to fill this gap. The measure is composed of several clusters of symptoms thought to characterize lower limb lymphedema. The initial work was conducted to propose and assess the face validity of the clusters. Subsequently, work was undertaken to empirically evaluate the presence of the symptom clusters, assess the reliability of the cluster scores, and evaluate the validity of the scores by studying associations with other valid measures.
Methods and Results: During the initial work, the LSIDS-L was tested with lower limb lymphedema patients only, and in the subsequent work the LSIDS-L and valid measures were administered to patients with no lymphedema and with lower limb lymphedema. A total of 388 volunteers participated, 111 of whom indicated no diagnosis of lymphedema, and 277 indicated a diagnosis of lower limb lymphedema. Cluster analysis resulted in the exclusion of 5 items, yielding 8 clusters with a total of 31 items. Cluster scores demonstrated acceptable internal consistency, distinguished nonlymphedema patients from lower lymphedema patients, and demonstrated expected convergent and divergent validity with other valid measures.
Conclusion: The LSIDS-L is a valid tool for detecting and quantifying symptom burden in patients with lower limb lymphedema.
Keywords: lymphedema, lower limb lymphedema, instrument development, symptoms, quality of life, questionnaire
Introduction
Lymphedema is a chronic swelling condition affecting ∼140–250 million people worldwide.1 Lymphedema can be primary, related to congenital lymphatic abnormalities and/or abnormal structural growth; or secondary, triggered by harm to the lymphatic system from surgery, radiation, parasitic infection, inflammation, malignancy, or trauma. Regardless of the cause, lymphedema is a progressive, debilitating, and potentially painful condition that can be treated but generally not cured.2
Lymphedema is traditionally viewed as a non-life-threatening diagnosis with potential for serious complications.1,2 However, studies that focus on symptoms and functional deficits in patients with lymphedema illuminate a broad constellation of symptoms common to lymphedema patients, some of which are very serious and even life-threatening.3–6 Viehoff et al.'s6,7 preliminary work toward developing a functional assessment of lymphedema patients based on the International Classification of Functioning, Disability and Health (ICF) suggest that some symptoms may be universal for lymphedema patients, while some may be unique to the area of the body affected by lymphedema. For example, symptoms related to swelling, emotional turmoil, pain, disruption to temperature regulation, and abnormal sensation are commonly experienced by most patients with lymphedema, while ingestion problems seem to be unique to patients with head and neck lymphedema. Problems moving around and participating in recreational activities seem to be far more prevalent in patients with lower limb lymphedema than in patients with other areas of lymphedema.6
Lymphedema that is not related to cancer or cancer treatment particularly impacts the lower limb region.8 Noncancer-related lymphedema more often affects both limbs and leads to more cellulitis diagnoses than lymphedema, resulting from cancer or cancer treatment. In regions where lymphedema diagnosis and treatment generally takes place only in the context of cancer supportive care, noncancer-related lymphedema populations could be underdiagnosed and underserved. Considering the increased morbidity of this group, a lack of proper treatment and monitoring could adversely affect morbidity, mobility, and quality of life.8
Despite being the most common form of lymphedema, studies assessing symptoms associated with lower limb lymphedema are lacking. There are few symptom assessment tools for use in patients with lower limb lymphedema.9 The lower-extremity Lymphedema Screening Questionnaire serves as a screening tool for lower limb lymphedema,10 and the Gynecological Cancer Lymphedema Questionnaire (GCLQ) uses a yes/no response option to detect the presence of 20 symptoms in gynecological cancer patients.11 Developed in Europe, the Lymphoedema Functioning, Disability, and Health Questionnaire (Lymph-ICF-LL) incorporates a scale of 0 (not at all) to 10 (a lot) in the evaluation of 28 lower limb symptoms.12 Two of these scales, the GCLQ and the Lymph-ICF-LL, provide symptom information; however, they do not address the intensity and distress associated with symptoms of lower limb lymphedema. Assessing the intensity and distress associated with lymphedema-related symptoms in a quantifiable manner is important not only to help illustrate the disease burden on patients but also to measure the effect of treatment on patient symptom burden and quality of life.13 In the United States, Medicare now requires verification of functional change by some objective measure,14 intensifying the need for a comprehensive assessment of lymphedema-related symptoms and their impact on patients. To this end, our team is developing a comprehensive battery of lymphedema symptom assessment tools.
The initial component of our battery of lymphedema symptom assessment tools was the Lymphedema Symptom Intensity and Distress Survey-Arm (LSIDS-A). The reliability and validity of the seven distinct symptom clusters of symptoms identified in breast cancer survivors during the development of the tool have been published.15 A second component of this battery focuses on lymphedema of the head and neck, the LSIDS-Head and Neck. Preliminary assessment of the measurement quality of that measure has been published3 and continued testing is in-progress.
The next component of the battery under development assesses intensity and distress of lymphedema symptoms of the lower limb, the LSIDS-Lower Limb (LSIDS-L). The face validity of the preliminary 36-item measure and the resulting symptom prevalence profile have been reported.16,17 The purpose of this article is to report on the further refinement and testing of this instrument.16,17
Materials and Methods
Instrument development
As noted in the introduction, the LSIDS-A, for which validity has been established for symptoms of lymphedema in the arms, informed the development of the LSIDS-L.15 An expert panel revised items to reflect similar symptoms likely to be manifested for patients with lymphedema in the leg(s)/lower limb, as well as to add symptoms thought to apply specifically to lower limb locations such as “Difficulty Standing.” The initial expert-established face-valid version of the LSDS-L consisted of 36 yes/no questions with follow-up 5-point intensity and distress ratings for “yes” responses. Intensity and distress questions displayed an anchor label of “slight” for a rating of 1 and “severe” for a rating of 5.16,17
Sample
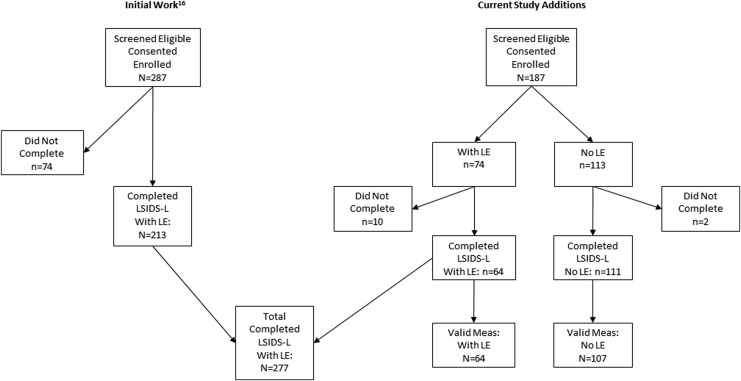
The initial study of the face validity and composition of the LSIDS-L included only individuals with lower limb lymphedema. The extension of that work reported on here included not only those individuals but also additional, newly recruited individuals with and without lymphedema. Details of how the final samples were arrived at for this work are shown in Figure 1. Ultimately, there were a total of 277 participants with lower limb lymphedema and 111 without completed the LSIDS-L measure. Within that sample, 64 participants with lymphedema and 107 without also completed the set of established valid measures. All components of this work were approved as exempt by the Vanderbilt Institutional Review Board (IRB), and all data were collected in accordance with standards of the responsible conduct of human research and the Declaration of Helsinki 1975, as revised in 2008. Individuals were recruited from several sources that included a registry of individuals that consented to be contacted for future research, an internal research distribution list, ResearchMatch (a national health research volunteer registry), and flyers posted in local lymphedema clinics, on the National Lymphedema Network and Lymphatic Education and Research Network websites, and social media.
FIG. 1.
Compilation of participants from the previous work16 with the additional participants recruited to arrive at the total number individuals with data for the LSIDS-L (N = 388) and the subset with data for the valid measures (n = 171). LE, lymphedema; LSIDS-L, Lymphedema Symptom Intensity and Distress Survey-Lower Limb.
Patients in the initial face-validity component of this work were screened for eligibility by phone. Once eligibility was established, a survey link was sent for completion of data collection (see Stolldorf et al.16 for further details). Individuals recruited subsequent to that work were screened via an online process. They completed a brief, anonymous screening survey to report that they either did not have any lymphedema (for the nonlymphedema cohort) or had lower limb lymphedema. All participants were required to read and write in English and provide informed consent before participating in the study.
Data collection process and instruments
All data were collected via REDCap (Research Electronic Data Capture), a secure, web-based application. REDCap servers are housed in a local data center at Vanderbilt, and all web-based information transmission is encrypted. REDCap was developed specifically around HIPAA-Security guidelines and is recommended to Vanderbilt researchers by both the Privacy Office and IRB.18 The survey used for collecting the study data contained all of the study measures in multiple sections. Once initiated, participants were able to save their responses and return at a later time to work further on the survey if they preferred. Most participants completed the survey in one sitting.
Instruments
The following measures were completed by participants in the initial face validity study and in the current study incorporating established valid measures.
Demographics: A self-report form included questions about gender, race/ethnicity, marital and employment status, insurance, and income.
Health and Lymphedema Form: A self-report form that included questions about medications, health conditions and surgeries, lymphedema diagnosis, type, cause, location, and treatment.
LSIDS-L: The version of this measure used was the initial 36-item self-report survey coming out of the expert panel face-validity work. It assessed the presence of each listed symptom and, if present, a rating of the intensity and distress of the respective symptom on a 5-point scale. Individual LSIDS-L item scores were calculated by assigning a value of 0 for all “No” responses, and summing the Intensity and Distress ratings for all “Yes” responses.
In addition to the above listed instruments, participants in the current validation study also completed the following instruments.
Marlowe–Crowne Social Desirability Scale Short Form C (MCSDS-SFC): This 13-item survey has demonstrated reliable and valid assessment of social desirability bias in adults using true/false questions.19–21 The measure is scored by reversing items 1, 2, 3, 4, 6, 8, 11, and 12 before calculating a total score. Scores from this measure have been found to have an internal consistency of 0.76 in prior research21 (Kuder–Richardson formula 20) and correlate strongly with the 33-item full Marlowe–Crowne scale (r = 0.93).19–21 The scores generated in this study had an internal consistency of 0.71 (Kuder–Richardson index).
Functional Assessment Screening Questionnaire (FAS-Q): The FAS-Q is a 15-item survey shown to be reliable and valid in assessing functional impairment in adults using 5-point Likert responses22,23 that are summed to arrive at a total score. The total scores generated in our study had an internal consistency of 0.92 (Cronbach's α).
Profile of Mood States-Short Form (POMS-SF): The POMS-SF is a 37-item survey that has been shown to be reliable and valid in quantifying mood in adults using 5-point Likert responses.24,25 Six “mood state” subscale scores are obtained by summing the constituent items (number of items per subscale ranged from 5 to 8). A total score is obtained by reversing the Vigor subscale score and then summing all subscale scores. The subscale scores generated in this study had Cronbach's α coefficients ranging from 0.82 to 0.96; the respective Cronbach's α for the total score was 0.95.
Statistical analysis
Data from both phases of this study were combined and analyzed using IBM SPSS Statistics 24 (Armonk, NY) and SAS 9.4 (Cary, NC). Frequency distributions were used to summarize the nominal and ordinal study data distributions, including LSIDS-L symptom prevalence. Median and interquartile range (IQR) were used to summarize the continuous variables due to skewness of the distributions. Empirical generation of the clusters of LSIDS-L symptom responses was conducted using SAS PROC VARCLUS. This approach is statistically derived from hierarchical clustering methods and loosens many of the assumptions inherent in traditional factor analysis (e.g., normality of item response distributions), thus making it most suitable for symptom clustering. The internal consistency of the study measure scores and the derived LSIDS-L cluster scores were assessed using Kuder–Richardson index (Marlowe–Crowne) and Cronbach's α (all other scores).
Group comparisons were conducted using chi-square tests of independence for nominal and ordinal data. Mann–Whitney tests were used for continuous data. Patterns of associations of the derived LSIDS-L scores with the other study measure scores were assessed using Spearman correlations. Effect sizes were more important in this study than a minimal statistical significance level (p < 0.05). Effect sizes used included both Cohen's d statistic and Spearman correlation.
Results
Sample characteristics
The analysis sample for evaluation of the LSIDS-L instrument comprised 388 individuals: 277 with lower limb lymphedema and 111 without lymphedema. The sample was largely female (87%), white (85%, non-Hispanic (96%), and married or living with a partner (65%).
Demographic characteristics of the groups with lower limb lymphedema and without lymphedema are summarized in Table 1. The median age of those with lymphedema was 53 years (IQR = 41–60) with median of 16 years of education (IQR = 14–18). A majority of the participants were employed (59%), 24% lived in a relatively rural area, most had some type of health insurance (97%), and 67.5% reported an annual household income of >$30,000. Compared to those individuals with lymphedema, those who did not have lymphedema were younger, slightly more educated, had higher income levels, were more likely to live in suburbs rather than rural areas, and have private insurance (see Table 1, p < 0.01).
Table 1.
Demographics by Lymphedema Status (N = 388)
| Overall (N = 388) | No lymphedema (n = 111) Median [IQR] | Lymphedema (n = 277) | p | |
|---|---|---|---|---|
| Age (N = 386, n lymph = 275) | 50.0 [36–59] | 36.0 [27–54] | 53.0 [41–60] | <0.001 |
| Years of education | 16.0 [14–18] | 17.0 [16–18] | 16.0 [14–18] | <0.001 |
| n (%) | ||||
|---|---|---|---|---|
| Gender | 0.711 | |||
| Female | 336 (86.6) | 95 (85.6) | 241 (87.0) | |
| Male | 52 (13.4) | 16 (14.4) | 36 (13.0) | |
| Race (N = 387, n no lymph = 110) | 0.003 | |||
| Multiracial | 8 (2.1) | 7 (6.4) | 1 (0.4) | |
| Black or African American | 33 (8.5) | 8 (7.3) | 25 (9.0) | |
| White | 328 (84.8) | 90 (81.8) | 238 (85.9) | |
| Othera | 18 (4.7) | 5 (4.5) | 13 (4.7) | |
| Ethnicity (N = 382, n lymph = 271) | 0.171 | |||
| Hispanic or Latino | 15 (3.9) | 2 (1.8) | 13 (4.8) | |
| Not Hispanic or Latino | 367 (96.1) | 109 (98.2) | 258 (95.2) | |
| Relationship status (N = 387, n lymph = 276) | 0.158 | |||
| Single/other | 136 (35.1) | 45 (40.5) | 91 (33.0) | |
| Married/living w/partner | 251 (64.9) | 66 (59.5) | 185 (67.0) | |
| Employment | <0.001 | |||
| Not employed | 100 (25.8) | 11 (9.9) | 89 (32.1) | |
| Employed | 262 (67.5) | 99 (89.2) | 163 (58.8) | |
| Other | 26 (6.7) | 1 (0.9) | 25 (9.0) | |
| Residence | <0.001 | |||
| City | 211 (54.4) | 54 (48.6) | 157 (56.7) | |
| Country | 78 (20.1) | 13 (11.7) | 65 (23.5) | |
| Suburb/other | 99 (25.5) | 44 (39.6) | 55 (19.9) | |
| Insurance (N = 385, n lymph = 274) | <0.001 | |||
| None | 10 (2.6) | 2 (1.8) | 8 (2.9) | |
| Government | 78 (20.3) | 14 (12.6) | 64 (23.4) | |
| Private | 239 (62.1) | 89 (80.2) | 150 (54.7) | |
| Other | 52 (13.5) | 5 (4.5) | 47 (17.2) | |
| Multiple types | 6 (1.6) | 1 (0.9) | 5 (1.8) | |
| Annual household income (N = 380, n lymph = 269) | 0.007 | |||
| $30,000 or less | 75 (19.8) | 17 (15.3) | 58 (21.6) | |
| Over $30,000 | 272 (71.8) | 91 (82.0) | 181 (67.5) | |
| Do not care to respond | 32 (8.4) | 3 (2.7) | 29 (10.8) | |
Includes American Indian/Alaskan Natives, Asians, Native Hawaiian or Other Pacific Islanders, and other.
IQR, interquartile range.
Bolding indicates a statistically significant difference, p < .01.
Clinical characteristics of the individuals with lower limb lymphedema (n = 277) are summarized in Table 2. Approximately 20% could not identify the cause of their lymphedema (n = 55). Of those reporting a known cause (n = 222), 51.4% indicated primary lymphedema, while 48.6% indicated that their lymphedema was secondary to cancer (47.2%), noncancer (47.2), or other/unknown events (5.6%). Of the individuals providing treatment information (n = 271), 44 (16.2%) were not currently in treatment at all, 20.7% (n = 56) were using a compression garment as the only treatment, and only 5.2% reported currently using complex decongestive therapy (Table 2). Median duration of lower limb lymphedema was 5.4 years (IQR = 1.9–13.2), with a range of 0.0–54.8 years.
Table 2.
Clinical Characteristics of Individuals with Lymphedema (N = 277)
| Characteristic | n (%) |
|---|---|
| Lymphedema cause | |
| Unknown | 55 (19.9) |
| Known | 222 (80.1) |
| Primary | 114 (51.4) |
| Secondary | 108 (48.6) |
| Cancer | 51 (47.2) |
| Noncancer | 51 (47.2) |
| Other/unknown | 6 (5.6) |
| Current lymphedema treatment (N = 271) | |
| None | 44 (16.2) |
| Complex decongestive therapy by therapist | 14 (5.2) |
| Compression garment only | 56 (20.7) |
| Pump only | 2 (0.7) |
| Elevation only | 14 (5.2) |
| Medication only | 14 (5.2) |
| Complex decongestive therapy and pump | 4 (1.5) |
| Pump and compression garment | 30 (11.1) |
| Elevation and medication | 6 (2.2) |
| Night bandaging | 8 (3.0) |
| Exercises, skin care, compression sleeve, and bandaging | 35 (12.9) |
| Laser | 2 (0.7) |
| Othera | 54 (19.9) |
Other treatments listed included various alternative combinations of the above treatments (n = 36), as well as self-massage (n = 4), compression wraps (n = 4), compression pantyhose or stockings (n = 5), ice (n = 1), massage therapy (n = 5), Reid sleeves (n = 3), yoga (n = 1), and low sodium diet (n = 1).
Scores from the valid measures for 64 individuals with lower limb lymphedema and 107 without lymphedema are summarized in Table 3. Statistically significant differences between the two groups were observed for all of the measures with the exception of the POMS confusion subscale score and the Marlowe–Crowne measure of social desirability. Individuals without lymphedema tended to have higher FAS-Q and POMS-SF Vigor scores, and lower POMS-SF Tension, Depression, Anger, Fatigue, and Overall scores (effect sizes: 0.44–1.5, all p < 0.01, Table 3).
Table 3.
Valid Measures Scores by Lymphedema Status (N = 171)
| No lymphedema (n = 107) | Lymphedema (n = 64) | |||
|---|---|---|---|---|
| Median [IQR] | p | Cohen's d | ||
| FAS-Q (0–60)a | 58.0 [55–60] | 50.0 [40–54] | <0.001 | 1.50 |
| POMS-SF total (0–148)b | 33.0 [28–41] | 40.0 [31–61] | 0.001 | 0.53 |
| POMS-SF subscalesc | ||||
| Tension (0–24) | 3.0 [1–6] | 5.0 [2–10] | <0.001 | 0.44 |
| Depression (0–32) | 2.0 [0–4] | 4.0 [2–17] | 0.005 | 0.64 |
| Anger (0–28) | 2.0 [0–4] | 4.0 [1–10] | <0.001 | 0.44 |
| Vigor (0–24) | 11.0 [7–15] | 6.5 [4–10] | <0.001 | 0.78 |
| Fatigue (0–20) | 4.0 [2–7] | 6.0 [3–14] | 0.001 | 0.53 |
| Confusion (0–20) | 2.0 [1–3] | 2.0 [1–4] | 0.235 | 0.18 |
| MCSDS-SFC (0–13)d | 8.0 [6–10] | 9.0 [7–10] | 0.249 | 0.18 |
FAS-Q; higher scores indicate higher function.
POMS-SF; higher scores indicate more negative mood state.
Each subscale score indicates higher levels of that state; note that vigor is reversed for POMS-SF total scoring because it is the only subscale for which higher scores indicate a more positive mood state.
MCSDS-SFC; higher scores indicate an increased tendency to socially desirable responses over accurate responses.
FAS-Q, Functional Assessment Screening Questionnaire; IQR, interquartile range; MCSDS-SFC, Marlowe–Crowne Social Desirability Scale-Short Form C; POMS-SF, Profile of Mood States-Short Form.
Symptoms
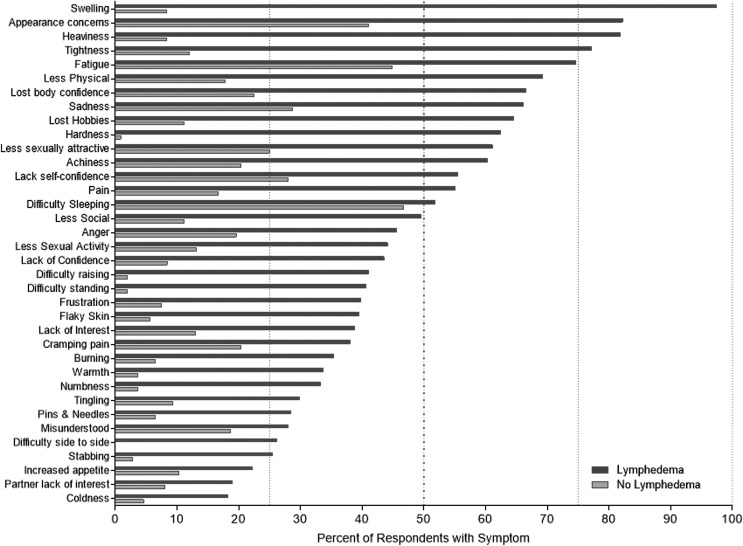
The symptoms assessed by the LSIDS-L were consistently more prevalent in individuals with lower limb lymphedema compared with those without lymphedema (Fig. 2). Differences were statistically significant (p < 0.02) for every symptom with the exception of difficulty sleeping (51.8% vs. 46.7%, p = 0.372) and being misunderstood by a significant other (28.0% vs. 18.7%, p = 0.061). None of the symptoms was reported by >50% of nonlymphedema individuals. In contrast, 15 symptoms were experienced by >50% of individuals with lower limb lymphedema: swelling (97.4%), appearance concerns (82.2%), heaviness (81.8%), tightness (77.2%), fatigue (74.6%), less physical activity (69.2%), loss of body confidence (66.5%), sadness (66.1%), loss of hobbies (64.6%), hardness (62.4%), less sexually attractive (61.1%), achiness (60.3%), lack of self-confidence (55.5%), pain (55.1%), and difficulty sleeping (51.8%) (see Fig. 2). Among individuals with lower limb lymphedema, only two symptoms were reported by <20%: partner lack of interest in sex (18.9%) and coldness in the leg(s) (18.2%).
FIG. 2.
Symptom prevalence for individuals with lower limb lymphedema (n = 277) and without lymphedema (n = 111).
Clustering of symptoms (content and structural validity)
Consistent with the previously validated LSIDS-A measure,16 ∼20% of the individuals chose not to respond to one or more of the 3 items about sexuality. Therefore, those items were grouped (clustered) together based on face validity only and not included in the statistical clustering analysis. Based on results from the initial cluster analysis, 5 of the remaining 33 original symptoms were excluded due to poor cluster loading and/or poor consistency of response with the other symptoms in their respective cluster: cramping pain in legs, warmth in legs, coldness in legs, flaky skin on legs, and increased appetite. The remaining 28 items resulted in 7 clusters of symptoms labeled: activity, soft tissue sensation, pain, resources, biobehavioral, neurological sensation, and function. The composition of those clusters as well as the items comprising the sexuality cluster is displayed in Table 4. Scores for each of the clusters were generated by averaging the constituent item scores for the individuals who provided responses to all of the items comprising the respective cluster. Reliability coefficients in the form of the internal consistency of the responses used to generate those scores ranged from 0.77 to 0.89 (Cronbach's α). An overall score for the LSIDS-L was computed by averaging all 31 items (28 non-sexuality, 3 sexuality), allowing a total of 3 missing item responses. The Cronbach's α reliability coefficient of that overall score was 0.94 (see Table 4).
Table 4.
Lymphedema Symptom Intensity and Distress Survey-Lower Limb Clustering Face and Content Validity, Internal Consistency Among Individuals with Lymphedema (N = 277)
| Cluster | Na | Cronbach's α |
|---|---|---|
| Overall (all items) | 168 | 0.940 |
| Activity | 271 | 0.858 |
| Fatigue | ||
| Difficulty sleeping | ||
| Inability to do hobbies | ||
| Decreased social activity | ||
| Decreased physical activity | ||
| Soft tissue sensation | 268 | 0.851 |
| Heaviness in lower limb(s) | ||
| Tightness in lower limb(s) | ||
| Swelling in lower limb(s) | ||
| Hardness in lower limb(s) | ||
| Pain | 265 | 0.801 |
| Burning pain in lower limb(s) | ||
| Stabbing pain in lower limb(s) | ||
| Pain in lower limb(s) | ||
| Achiness in lower limb(s) | ||
| Resources | 270 | 0.892 |
| Lack of confidence in insurance | ||
| Frustration with insurance | ||
| Biobehavioral | 234 | 0.884 |
| Sadness | ||
| Anger | ||
| Lack of confidence in self | ||
| Concerns about appearance | ||
| Feeling misunderstood | ||
| Felling less sexually attractive | ||
| Loss of confidence in body | ||
| Neurological sensation | 265 | 0.768 |
| Numbness in lower limb(s) | ||
| Tingling in lower limb(s) | ||
| Pins and needles in lower limb(s) | ||
| Function | 273 | 0.825 |
| Difficulty moving side to side | ||
| Difficulty raising lower limb(s) | ||
| Difficulty standing | ||
| Sexuality | 204 | 0.783 |
| Lack of interest in sex | ||
| Partner lack of interest in sex | ||
| Decreased sexual activity |
N refers to the number of cases providing scores for all of the items in the respective cluster or in the case of the “Overall” score, all items in the measure.
Concurrent criterion and construct validity
An initial evaluation of the criterion validity of the LSIDS-L symptom cluster and overall scores included comparing the scores generated from the individuals with lower limb lymphedema to those without lymphedema. As expected, statistically significantly higher scores were observed for the group with lymphedema than for those without (p < 0.001, see Table 5). All of these differences remained after controlling for the observed differences in the demographic characteristics of the two samples (p < 0.002). The lowest effect size was observed for sexuality cluster scores (Cohen's d = 0.60), while the highest was observed for soft tissue sensation (Cohen's d = 2.28). Furthermore, as illustrated by the summaries of the percentages of lowest and highest scores in each of the groups, there were very clear differences in the reports that the measure captured considerable variability within the groups (see Table 5).
Table 5.
Lymphedema Symptom Intensity and Distress Survey-Lower Limb Scores by Lymphedema Status (N = 388)
| No lymphedema (n = 111) | Lymphedema (n = 277) | |||
|---|---|---|---|---|
| LSIDS-L | Median [IQR] n (% lowest, % highest) | p | Cohen's d | |
| Overall | 0.3 [0–1] 107 (57, 0) | 3.4 [1–6] 245 (5, <1) | <0.001 | 1.69 |
| Clusters | ||||
| Activity | 0.6 [0–2] 107 (46, 0) | 4.4 [1–8] 271 (11, 7) | <0.001 | 1.11 |
| Soft tissue sensation | 0.0 [0–0] 108 (81, 0) | 5.4 [3–8] 268 (2, 7) | <0.001 | 2.28 |
| Pain | 0.0 [0–1] 108 (73, 0) | 2.0 [0–5] 265 (25, 2) | <0.001 | 1.12 |
| Resources | 0.0 [0–0] 107 (89, 0) | 0.0 [0–8] 270 (52, 17) | <0.001 | 0.78 |
| Biobehavioral | 0.3 [0–2] 104 (53, 0) | 4.3 [1–7] 234 (15, 4) | <0.001 | 1.07 |
| Neurological sensation | 0.0 [0–0] 108 (85, 0) | 0.7 [0–3] 265 (49, 1) | <0.001 | 0.76 |
| Function | 0.0 [0–0] 108 (97, 0) | 1.3 [0–5] 273 (48, 3) | <0.001 | 1.01 |
| Sexuality | 0.0 [0–0] 99 (78, 0) | 0.0 [0–5] 204 (51, 4) | <0.001 | 0.60 |
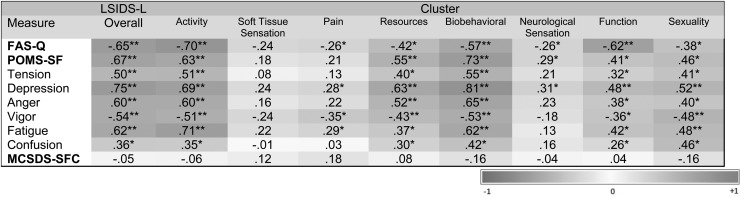
Patterns of association of the LSIDS-L scores with the scores of the valid measures within the subset of individuals with lower limb lymphedema who completed all of the measures (n = 64) was used to investigate the construct validity of the cluster scores. Based on previous work with the LSIDS-A,15 it was hypothesized that the overall LSIDS-L score and more specifically the soft tissue sensation, biobehavioral, sexuality, and activity scores would correlate more strongly with the FAS-Q scores than with the other measures. Relative to associations with the other subscale scores on the POMS-SF, the LSIDS-L neurological sensation scores were expected to correlate most strongly with the fatigue subscale score while LSIDS-L function scores could correlate more strongly with the tension and confusion subscales. The LSIDS-L resource score was hypothesized to correlate with POMS-SF tension subscale score. All of the LSIDS-L scores were hypothesized to demonstrate weak associations with the POMS-SF anger and vigor scores, as well as the Marlowe–Crowne score.
The patterns of association are illustrated in Figure 3. As expected the correlations of the LSIDS-L cluster scores with the Marlowe–Crowne were very weak, demonstrating that social desirability did not account for a meaningful percentage of the variability in the LSIDS-L scores (absolute rs = ±0.04 to 0.18, p > 0.05). In terms of expected convergence of the scores, some of the hypotheses were supported; however, in general the delineations between the FAS-Q and POMS-SF correlations were not as distinct as expected. The LSIDS-L overall, activity, and biobehavioral scores demonstrated strong correlations with not only FAS-Q but also with all the scales in the POMS-SF, with the exception of confusion (absolute rs = 0.50 to 0.75, p < 0.001). Contrary to expectations, the LSIDS-L soft tissue sensation, neurological sensation, and pain scores demonstrated relatively weak associations with all the FAS-Q and POMS-SF scores (absolute rs = 0.01 to 0.35). Associations of the LSIDS-L resources, function, and sexuality scores with the FAS-Q and POMS-SF were generally moderate and demonstrated some delineations in construct.
FIG. 3.
Correlations of lymphedema symptom intensity and distress survey cluster scores with valid measures scores (N = 64). *p < 0.05, **p < 0.001. FAS-Q, Functional Assessment Screening Questionnaire; MCSDS-SFC, Marlowe–Crowne Social Desirability Scale-Short Form C; POMS-SF, Profile of Mood States-Short Form.
Discussion of Results
The purpose for the development of the LSIDS-L was to build on the foundational work of the LSIDS-A measure. The ultimate goal is to develop a battery of tools that will assess both systemic symptoms believed to be common to any manifestation of lymphedema (arm, lower limb, etc.) and symptoms specific to each area. Unlike other available tools, the LSIDS-L not only captures the presence of symptoms but also the intensity and distress (psychological component) of each reported symptom. Thus, the tool provides actionable clinical information from which decisions can not only be made regarding the need for additional symptom management interventions (e.g., pain management) but also the need for supportive care (e.g., social services or psychological counseling). In this study, 15 symptoms were experienced by >50% of the individuals with lower limb lymphedema. In previous research, 8 of those same 15 symptoms were found to occur in >50% of individuals with arm lymphedema.16 Those symptoms include swelling, appearance concerns, heaviness, tightness, fatigue, less physical activity, achiness, pain, and difficulty sleeping, suggesting that there may indeed be a group of systemic symptoms associated with lymphedema regardless of body location. Furthermore, findings that >50% of individuals with lower limb lymphedema experienced loss of body confidence, sadness, loss of hobbies, hardness, feeling less sexually attractive, and lack of self-confidence (findings absent in individuals with arm lymphedema) suggests that location of lymphedema may result in unique symptoms. Findings such as these provide further support for Viehoff et al.'s7 suggestion that some symptoms may be universally experienced by individuals with lymphedema and some may be specific to the lymphedema location. Thus, an instrument that captures systemic, universal lymphedema and location-specific lymphedema symptoms has optimal research and clinical value.
Findings from the clustering of the symptoms reported in this study were also illuminating. Seven clusters with a total of 30 symptoms were previously identified in patients with arm lymphedema.15 Those same symptom clusters were mirrored in the lower limb sample in this study: activity, soft tissue sensation, resources, biobehavioral, neurological sensation, sexuality, and functional. Yet in this study, a distinct cluster of pain-related symptoms emerged. Those symptoms clustered with neurological sensation in the previous research in the population of individuals with arm lymphedema.
Overall, results from this study indicated that the LSIDS-L scores were internally consistent and consistent with the reliability coefficients for cluster scores generated from the LSIDS-A measure.15 Thus, this measure shows promise for further use as a reliable measure of symptoms in individuals with lower limb lymphedema. Test-retest reliability will still need to be assessed. Patterns of associations of LSIDS-L cluster scores with the existing valid measures demonstrated expected findings. Furthermore, higher score values for the group with lower limb lymphedema compared to the group without lymphedema provides evidence of LSIDS-L sensitivity in the intended population. Individuals were able to complete the instrument online without difficulty and voiced no complaints regarding completion time. Thus, the instrument appears to be both valid and feasible for use with individuals with lower limb lymphedema.
Limitations
Findings from this study should be considered in light of its inherent limitations. First, the nonlymphedema individuals were younger, more affluent, more educated, and less reflective of rural-dwelling persons. While analyses were conducted that provided statistical control for those differences, it is possible that a more closely matched comparator group could have resulted in an alternative symptom profile for that group. Second, we did not objectively measure limb volume. Although another study found that perceived volume difference impacted reported symptoms more than actual volume difference,26 the influence of actual volume upon symptom reporting in lower limb lymphedema is unclear. Further work is also needed to assess the measure's test–retest reliability and thus usefulness as an indicator of change. Finally, no valid measures for pain or sensation were administered for comparison to the LSIDS-L, and the LSIDS-L clusters for soft tissue sensation, pain, and neurological sensation demonstrated weak associations with the included valid mood and function measures. Further testing is indicated to assess sensation-related validity.
Conclusion
The LSIDS-L extends the development of a battery of tools that will assess both systemic and area-specific symptoms to include individuals with lower limb lymphedema. The instrument appears to be a viable option for symptom assessment in this population. The development of symptom assessment tools for individuals with truncal and head and neck lymphedema is ongoing. Such development will provide additional information regarding universal and area-specific symptoms in varied populations of individuals with lymphedema.
Acknowledgments
The authors thank Nancy Kidd, Melissa Adair, and Rebecca Palmer for their work on this study. The project described was supported by resources funded by the Martha Rivers Ingram Chair of Nursing and CTSA award no. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Földi M, Földi E, Strößenreuther C, Kubik S. Földi's Textbook of Lymphology: For Physicians and Lymphedema Therapists, 3rd ed. Munich: Elsevier Health Sciences; 2012 [Google Scholar]
- 2. Lee B-B, Bergan J, Rockson SG. Lymphedema: A Concise Compendium of Theory and Practice. London, England: Springer Science + Business Media; 2011 [Google Scholar]
- 3. Deng J, Ridner SH, Murphy BA, Dietrich MS. Preliminary development of a lymphedema symptom assessment scale for patients with head and neck cancer. Support Care Cancer 2012; 20:1911–1918 [DOI] [PubMed] [Google Scholar]
- 4. Klernäs P, Johnsson A, Horstmann V, Kristjanson LJ, Johansson K. Lymphedema quality of life inventory (LyQLI)-development and investigation of validity and reliability. Qual Life Res 2014; 24:427–439 [DOI] [PubMed] [Google Scholar]
- 5. Ridner SH, McMahon E, Dietrich MS, Hoy S. Home-based lymphedema treatment in patients with cancer-related lymphedema or noncancer-related lymphedema. Oncol Nurs Forum 2008; 35:671–680 [DOI] [PubMed] [Google Scholar]
- 6. Viehoff PB, Gielink PDC, Damstra RJ, Heerkens YF, van Ravensberg DC, Neumann MHA. Functioning in lymphedema from the patients' perspective using the International Classification of Functioning, Disability and health (ICF) as a reference. Acta Oncol 2015; 54:411–421 [DOI] [PubMed] [Google Scholar]
- 7. Viehoff P, Hidding J, Heerkens Y, van Ravensberg C, Neumann H. Coding of meaningful concepts in lymphedema-specific questionnaires with the ICF. Disabil Rehabil 2013; 35:2105–2112 [DOI] [PubMed] [Google Scholar]
- 8. Shallwani SM, Hodgson P, Towers A. Comparisons between cancer-related and noncancer-related lymphedema: An overview of new patients referred to a specialized hospital-based center in Canada. Lymphat Res Biol 2017; 15:64–69 [DOI] [PubMed] [Google Scholar]
- 9. Cemal Y, Jewell S, Albornoz CR, Pusic A, Mehrara BJ. Systematic review of quality of life and patient reported outcomes in patients with oncologic related lower extremity lymphedema. Lymphat Res Biol 2013; 11:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yost KJ, Cheville AL, Weaver AL, Al Hilli M, Dowdy SC. Development and validation of a self-report lower-extremity lymphedema screening questionnaire in women. Phys Ther 2013; 93:694–703 [DOI] [PubMed] [Google Scholar]
- 11. Carter J, Raviv L, Appollo K, Baser RE, Iasonos A, Barakat RR. A pilot study using the Gynecologic Cancer Lymphedema Questionnaire (GCLQ) as a clinical care tool to identify lower extremity lymphedema in gynecologic cancer survivors. Gynecol Oncol 2010; 117:317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Devoogdt N, De Groef A, Hendrickx A, et al. . Lymphoedema Functioning, Disability and Health Questionnaire for Lower Limb Lymphoedema (Lymph-ICF-LL): Reliability and validity. Phys Ther 2014; 94:705–721 [DOI] [PubMed] [Google Scholar]
- 13. Saito T, Unno N, Yamamoto N, et al. . Low lymphatic pumping pressure in the legs is associated with leg edema and lower quality of life in healthy volunteers. Lymphat Res Biol 2015; 13:154–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. US Department of Health and Human Services. Federal Register Final Rule: 77 FR 68891. 2012; Vol 77 (No. 222). Available at: www.gpo.gov/fdsys/pkg/FR-2012-11-16/html/2012-26900.htm Accessed September22, 2017
- 15. Ridner SH, Dietrich MS. Development and validation of the Lymphedema Symptom and Intensity Survey-Arm. Support Care Cancer 2015; 23:3103–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stolldorf DP, Dietrich MS, Ridner SH. Symptom frequency, intensity, and distress in patients with lower limb lymphedema. Lymphat Res Biol 2016; 14:78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stolldorf DP, Dietrich MS, Ridner SH. A comparison of the quality of life in patients with primary and secondary lower limb lymphedema: A mixed-methods study. West J Nurs Res 2016; 38:1313–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crowne DP, Marlowe D. A new scale of social desirability independent of psychopathology. J Consult Psychol 1960; 24:349–354 [DOI] [PubMed] [Google Scholar]
- 20. Goldfried MR. A cross-validation of the Marlowe–Crowne social desirability scale items. J Soc Psychol 1964; 64:137–145 [DOI] [PubMed] [Google Scholar]
- 21. Robinette RL. The relationship between the Marlowe–Crowne form C and the validity scales of the MMPI. J Clin Psychol 1991; 47:396–399 [DOI] [PubMed] [Google Scholar]
- 22. Millard RW. The functional assessment screening questionnaire: Application for evaluating pain-related disability. Arch Phys Med Rehabil 1989; 70:303–307 [PubMed] [Google Scholar]
- 23. Seltzer GB, Granger CV, Wineberg DE. Functional assessment—Bridge between family and rehabilitation-medicine within an ambulatory practice. Arch Phys Med Rehabil 1982; 63:453–457 [PubMed] [Google Scholar]
- 24. Curran SL, Andrykowski MA, Studts JL. Short-form of the profile of mood states (POMS-SF)—Psychometric information. Psychol Assess 1995; 7:80–83 [Google Scholar]
- 25. Shacham S. A shortened version of the profile of mood states. J Pers Assess 1983; 47:305–306 [DOI] [PubMed] [Google Scholar]
- 26. Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer 2005; 13:904–911 [DOI] [PubMed] [Google Scholar]