Abstract
Objective: Wound management recommendations usually group dressings by base substrate material or reimbursement codes, even when functional differences are vast (e.g., honey-containing alginates, super-absorbent hydrogels). Polymeric membrane dressings (PMDs) diverge dramatically from conventional foam dressings in functional attributes, indications, and patient results, providing an opportunity to demonstrate the evidence for categorizing dressings based upon functional differences.
Approach: A search of ALL published literature describing the use of PMDs, with no date or language limits, was conducted. Documents simply listing a PMD brand name (e.g., PolyMem) as one of many “foam” dressings were eliminated. The subset of evidence evaluating PMDs for tissue damage resulting from pressure (pressure ulcers, pressure injuries, henceforth: PUs) was summarized.
Results: Studies of PMDs, primarily from independent clinician-researchers, have accumulated into a significant evidence base over the past 30 years. PMDs actively cleanse and debride wounds, balance moisture, relieve pain, and limit inflammation: all functions not shared by conventional foams.
Innovation: This article supports a paradigm shift for wound management guidance materials to embrace a more evidence-based, patient-centered method of classifying products. The results presented here, using PMDs for PUs as an example, show that functional attributes, indications, and patient results are not always dictated by dressing substrates. Rather than being comparable with conventional foam dressings, PMDs have substantially enhanced functions and results.
Conclusion: These results strongly support the author's assertion that evidence-based wound management requires guidelines and recommendations that categorize advanced dressings based upon how they function in real-life settings, rather than upon their base substrate.
Keywords: wounds and injuries, pressure ulcer, wound healing, occlusive dressings/standards, debridement/methods, pain management
Linda L. Benskin, PhD, RN, SRN (Ghana), CWCN, CWS, DAPWCA.

Introduction
Traditionally, wound dressings are categorized by substrate (e.g., alginate, foam, hydrocolloid).1 However, dressing substrates do not always predict function (e.g., honey dressings with alginate substrates, polymeric membrane dressings [PMDs], super-absorbent hydrogels).1,2 Because PMDs diverge dramatically from conventional foam dressings in functional attributes, indications, and patient results, they provide an opportunity to demonstrate the benefits of using evidence-based functional attributes to categorize dressings.3,4 Recent clinical evidence reviews for PMDs support claims of multiple unique functional attributes (Table 1).2,3,5 The distinctive functions of PMDs (see the Materials and Methods section) were described in detail previously (Benskin, 2016), with citations to support all biological plausibility claims.3 A total of 144 independent authors described ∼4000 PMD patients, consistently representing PMDs as a unique dressing category.3
Table 1.
Functional attributes of PMDs and conventional foams compared with the ideal dressing
| Defining polymeric membrane dressings (PMDs) by their functional attributes | |||
|---|---|---|---|
| Functional attributes desired of dressings by wound experts (PMDs, but not conventional foams, have bolded attributes) | Conventional foams | PMDs | Ideal dressings wish list authors |
| Absorbs excess exudate | All | All | Winter,7 Turner,37 Witowski & Parish36 |
| Retains fluid under pressure | Some | All | |
| Requires infrequent dressing changes | Some | All (after PMDs clean wound) | Jones, Gray, & Harding14 |
| Maintains a moist wound environment | Some | All | Winter, Turner, Witowski & Parish |
| Hydrates dry wounds (components in PMDs pull fluid from the body and redistribute it to hydrate dry areas and structures) | None | All | Witowski & Parish |
| “Intelligent” backing adjusts evaporation rate to maintain ideal wound moisture and concentrate exudate | Some | All (except cavity fillers) | Winter |
| Prevents maceration (PMD's components coat periwound) | Some | All | Witowski & Parish, Thomas14 |
| Outer backing protects from microbes and is gas and water vapor permeable | Some | All (except cavity fillers) | Winter, Turner, Witowski & Parish, Scales,14 Baranoski & Ayello,14 others |
| Soft, flexible, conformable, stretchy (even over joints) | Some | All | Scales; Jones, Gray, & Harding, Thomas |
| Stays in place while being easy to apply and remove | Some | All | Fowler;36 Thomas |
| Fills voids to prevent fluid pooling | Some | All | Winter |
| Cushions the wound area, protects from further trauma | All | All | Winter; Jones, Gray, & Harding |
| Provides thermal insulation | All | All | Turner, Witowski & Parish, others |
| Recommended for infected wounds | Some | All | |
| Supports autolytic debridement | All | All | Baranoski & Ayello, Thomas |
| Exhibits effective cleansing activity (PMDs gradually release a surfactant to break bonds adhering slough and contaminants) | None | All | Thomas |
| Actively debrides wounds; pulls loosened contaminants onto dressing | Some | All | Turner, Witowski & Parish, Thomas |
| Minimizes bacterial growth | Some | All | Witowski & Parish |
| Minimizes disruption of wound bed (routine rinsing is not recommended; also preserves beneficial wound nutrients) | None | All | Fowler, Thomas |
| Controls wound odor (PMDs gradually release odor-absorbent) | None | All | Fowler, Thomas |
| Comfortable, even when used in tunneling, undermining, and dead space | Some | All | Jones, Gray, & Harding; Baranoski & Ayello |
| Occlusion decreases pain | All | All | |
| Reduce or eliminate persistent wound pain (PMDs alter the nociceptor response at the spinal cord level to relieve pain) | None | All | Baranoski & Ayello, Witowski & Parish |
| Subdues inflammation, limiting it to the specific site of injury | None | All | |
| Decreases bruising and edema | None | All | |
| Be nontoxic and nonallergenic (even when providing antimicrobial protection) | Some | All, including antimicrobial silver | Turner, Scales, Thomas |
| Does not leave residue in wound bed | Some | All | Turner; Jones, Gray, & Harding; Thomas |
| Does not allow tissue ingrowth into dressings | Some | All | Scales |
| Atraumatic removal, slippery, nonadherent | Some | All | Turner, Baranoski & Ayello, others |
| Indicates when dressings require changing | Some | All | |
| Promotes brisk granulation tissue formation, facilitates reepithelialization | Some | All | Witowski & Parish, Thomas |
| Improves ischemic wound nutrition (PMDs supply glycerol) | None | All | |
| Pulls enzyme- and nutrient-rich fluid from the body into the wound bed to speed debridement and healing | None | All | |
| Strengthens scar (PMDs provide continued nociceptor inhibition through the intact skin during remodeling) | None | All | |
| Saves time, is cost-effective, is quick and easy to use | Some | All | Fowler, Thomas |
Scales proposed attributes of ideal dressings in 1954. Since then, wound experts have proposed many ideal dressings lists, and continue to do so. These attributes are to be compared with clinical study results to determine how closely a dressing meets the standard of an ideal dressing. For additional information see reference.41
PMD, polymeric membrane dressing
Clinical Problem Addressed
PMDs are usually listed with conventional foam dressings in guidance materials.6 This exemplifies the problem: substrate-based categorization misleads clinicians and is not evidence based.1 Conventional foams and PMDs have completely different functional characteristics and indications (Table 1).2,3 PMDs incorporate features of foams, thin films, and hydrocolloids, and have some unique features as well.7 The AHRQ's (Agency for Healthcare Research and Quality) Comparative Effectiveness Review of dressings for PUs (pressure ulcers, pressure injuries, henceforth: PUs) listed PMDs separately from foams.8 Several textbooks recommend PMDs specifically for pain relief, continuous cleansing, and promotion of brisk healing.9–11 A 2016 dressing selection article in Advances in Wound Care specifically recommended PMDs for five of the seven wound characteristics described.2 The authors noted that PMDs “are revolutionizing the way dressings are made, as these dressings can be used on any type of wound.”2 When one classification committee used functional categories, PMDs fit in five: primary contact layers, debriding agents, topical antiseptics (nonreleasing), moisture absorption, and pain reduction.12 Functional categories can, and should, be used in guidelines.
Materials and Methods
Materials
All occlusive, nonadherent dressings soothe wounds. However, PMDs are the only drug-free dressings demonstrated to “relieve pain”4,10,13–15 by subduing and focusing the nociceptor response, even over intact skin.16,17 This influences tissue healing and allows PMDs to help resolve stage I PUs and deep tissue injuries (DTIs).18,19 Clinical studies verify PMD use leads to significantly decreased pain, bruising, and inflammation, and speeds healing of both open and closed tissue injuries.20–24 Recent articles summarize data and explain how PMDs interact with the body to achieve these results.3,4,25
PMDs also augment autolytic debridement with a powerful multicomponent continuous wound cleansing system that atraumatically hastens separation of adherent contaminants from the wound bed.3,4,25,26 Clinical studies found PMDs cleanse and keep both chronic and infected acute wounds clean without routine rinsing at dressing changes.7,17,21,22 Denyer recommends PMDs for infants with epidermolysis bullosa (EB) who cannot be bathed because of birth damage, and for older children with EB who refuse bathing.27 Independent clinicians using PMDs alone achieved effective debridement, cleansing, odor management, and closure of wounds that were initially severely infected, fungating, or covered with thick slough or dry eschar.27–31
Conventional foam dressings are appropriate only for moderately to heavily exudating wounds.2,6 PMDs are indicated for all depths of wounds with all levels of exudation, even when structures such as tendons or bone are exposed.2,3,14,25,32 Components in PMDs balance moisture by recruiting and redistributing fluid from the body to dry areas while simultaneously absorbing excess fluid from highly exudative areas, locking it into the dressing.2,3 Because PMDs draw fluid from the body, clinicians should anticipate a dramatic increase in exudate during initial PMD use.3,25
Some other wound products provide individual aspects of PMD's functions, but none furnish the integrated solutions PMDs provide.4,5,14,25 The combination of continuously cleansing, decreasing pain, focusing inflammation, and balancing moisture have made PMDs, apart from foams, the dressing type recommended in some guidelines for particularly challenging wounds.2,12,33,34 For example, the NPUAP and EPUAP recommended PMDs for controlling exudate, decreasing pain, and wound cleansing in palliative care.34 And, although “foam” dressings are contraindicated, a PMD is the “first choice dressing,” for most situations in children with EB.33
Methods
PMDs diverge dramatically from conventional foam dressings in functional attributes, indications, and patient results, providing an opportunity to demonstrate the benefits of categorizing dressings based upon evidence-based performance.3,4 This brief review focuses on PMD evidence related to tissue damage resulting from pressure (PUs).
Following the Joanna Briggs Institute's (2014) recommendations for reviews of effectiveness35 and guided by the question, “Does the currently available evidence support recommending PMDs, distinct from conventional foam dressings, to manage PUs?,” the author searched PubMed and Google Scholar for ALL articles, chapters, and major conference posters (electronically searching abstracts and walking poster halls) that included PMDs, including those sponsored by competitors, with no date or language limits. A colleague searched CINAHL (Cumulative Index to Nursing and Allied Health Literature) and SCOPUS®. The manufacturer's records were reviewed for references. The searches are current through May 2018. Documents simply listing a PMD brand name without distinguishing between it and conventional foam dressings were eliminated, as were studies in which PUs could not be isolated from other wound types. All other located studies reporting PMD use for PUs are summarized in the Results section in chronological order, concluding with a statistical analysis summarizing the case study/series findings.
Results
The first (1990) published study of PMDs on PUs compared results on 18 PUs in 13 long-term care patients with published data (a historical control group) in a 70-day trial.36 All patients with noninfected stage I–III PUs from two facilities were included.36 Most had severe comorbidities.36 The patients' mean age was 78.8 years; mean weight was only 95.2 pounds.36 Half of the PUs had been present for 75 days or longer (mean duration: 144 days).36 None had responded to treatment with then-popular conventional dressings (hydrocolloids, thin film dressings, and betadine or acetic acid wet-to-dry dressings).36 The only aspect of patient care changed during the study was the dressing protocol.36 Using a bordered 5 cm2 PMD according to the Instructions for Use (IFUs), 94% of these recalcitrant wounds improved and most closed within the 70-day study period, with fewer average days to resolution (40.9) than historical controls (52.6).36 Nurses were less apt to disturb the dressing prematurely because the dressing backing indicated when PMDs should be changed.36 PMDs absorbed well and kept wound beds moist.36 The patients did not experience secondary infections, maceration, or skin irritation.36 Nurses reported time savings because of ease of application and removal of PMDs, the infrequent need for dressing changes, and the fact that little or no wound preparation was required at dressing changes.36 Other dressings fragmented or melted into the wound; PMDs promoted eschar and necrotic tissue dissolution and removal.36 “Wound odor and pain were essentially nonexistent.”36 The authors concluded that PMDs meet all the criteria for an ideal dressing.36
Fowler and Papen (1991) used PMDs on 12 neurological inpatients from two skilled nursing facilities with 19 stage II–IV PUs.7 Eight were bedridden and the remaining four were chairfast, nine were aphasic, and only one was continent.7 Nine of the patients had been transferred to the facility with PUs of unknown duration.7 Prior to the study, the PUs had been managed with various wet/dry dressings, lubricating sprays and creams, and hydrocolloid dressings, most for >6 weeks.7 Following the IFUs, island PMDs were changed when saturated or dislodged, with intervals ranging from 12 h to 3 days.7 Each patient's progress was compared with their own lack of improvement with previous wound management (historical trial with patients serving as their own controls).7 The 19 PUs all resolved or improved (defined by a cleaner wound base, decreased size and depth, and improved color).7 Periwound edema and erythema also improved quickly and maceration did not occur.7 Fourteen of the PUs closed within the 91-day study period, three improved significantly, and the remaining two improved minimally.7 Two of the PUs that did not fully close had at least 4 cm of undermining initially, and another was 4 cm deep (PMD cavity fillers were not yet available).7 The researchers found that the dressing membrane is soft, flexible, nonadherent, and became like a smooth gel in contact with the wound.7 There was minimal need for cleansing and minimal pain at dressing changes.7
Yastrub (2004) used an NPUAP grant to conduct a randomized controlled trial (RCT) comparing PMDs with usual practice on 44 institutionalized geriatric post–cerebral vascular accident stage II PU patients.37 Yastrub used the Pressure Ulcer Scale for Healing (PUSH) to evaluate wound improvement.37 Usual practice was antibiotic ointment covered with a dry clean dressing.37 All participants received standard of care: pressure relief mattresses, turning every 2 h, and nutritional supplements and monitoring.37 Over the 4-week study, 87% (18 of 21) of the PMD group, compared with only 65.2% (15 of 23) of the antibiotic ointment group, showed improvement.37 The difference in PUSH scores (mean of 3.32381 for PMD vs. 1.6087 for usual practice) was significant at the 0.001 level.37
Harrison (2009), a wound specialist whose practice includes all care settings, conducted an independent evaluation of a mesh-reinforced PMD silver rope on 10 consecutive patients with cavity wounds.30 The first two patients had stage III PUs.30 After 2 days of PMD rope use, the first patient, whose wound included two tracts, 4 and 2 cm, with 80% black eschar, achieved a clean pink wound bed: all slough and eschar was floating and was easily removed with a hemostat.30 This MRSA+ (methicillin-resistant Staphylococcus aureus positive) wound closed completely in just 1 month.30 The second patient, managed for at least 3 months with a conventional foam dressing, had a chronic coccyx PU with a 50% yellow-slough–covered cavity.30 PMD rope use quickly resulted in a clean granulating wound bed.30 All 10 patients in the evaluation had extremely positive experiences with the PMD rope. Harrison noted that pain was dramatically reduced and the rope was easy for lay people (often the patients themselves) to insert and remove.30 Managing wounds with the new rope, rather than with negative pressure therapy, increased effectiveness and patient comfort while decreasing the complication rate.30 Moisture was balanced and PMD rope promoted quick closure of cavities and tracts.30
Evidence that PMDs reduce edema, pain, and inflammation led Wilson (2010) to compare results using PMDs on 10 consecutive skilled nursing facility and hospice patients with newly discovered stage I PUs with historical results from the facility and the literature.18 Previously, stage I PUs were managed with ointments.18 Both groups were appropriately offloaded.18 With appropriate care, historically, 80% of stage I PUs will resolve in 10–14 days, whereas 20% will reveal an open (stages II–IV) PU.18 With PMDs, 100% of the PUs resolved: 80% by the first dressing change (day 4) and the remainder by the next inspection (day 7 or 8).18 One hundred percent of the patients reported a great reduction in pain, itching, and burning, with most reporting a complete elimination of PU discomfort within 2 h of PMD application.18 Staff time to manage stage I PUs was dramatically reduced (staff simply removed the PMD after 4 days and replaced it if needed).18
Henson (2017) conducted a quasi-experimental study on managing DTIs at two skilled nursing facilities.19 In the control group, a skin barrier wipe was applied twice a day to 8 DTIs on 6 patients (facility standard of care).19 In the intervention group, PMDs were applied twice a week or as needed over 13 DTIs on 10 patients.19 If any DTIs opened, they were managed with various other advanced wound care products in the control group, and with PMDs in the intervention group.19 PMDs were very easy to use.19 Nursing wound care time was only 20 min/week/patient for PMDs, compared with 70 min/week/patient with skin barrier wipes (71.4% time saved).19 The time saved reduced overtime and allowed nurses more time for documentation, skin checks, and application of lotions or creams.19 Patients used the time previously spent waiting in their rooms for wound management for rehabilitation and activities.19 The nurse practitioner spent less time with wound rounding, freeing her time for primary rounds.19 And, DTIs resolved more quickly with PMDs.19 The control group experienced a 50% (4 of 8) DTI conversion to open PUs, whereas only 23% (3 of 13) of the PMD group's DTIs opened: a 54% reduction in DTIs opening into stage II–IV PUs with PMDs.19 PMDs padded the area for patients with spasms, and in the three cases when the DTIs opened up, excess wound exudate was immediately absorbed by the PMD.19 DTIs that opened resolved faster with PMDs, further reducing costs.19
Case and Bolhuis (2017) conducted a cost comparison study in which eight patients with “slow healing wounds” using the skilled nursing facility's best practice served as their own controls.38 The authors noted, “The most cost-effective wound is a closed wound.”38 Best practice (silver sulfadiazine, collagenase, adhesive hydrocellular foam dressings, maltodextrin hydrophilic dressings, nonadherent dressings, triple antibiotic ointment, petrolatum and bismuth tribromophate dressings, hydrocolloids, and silicone dressings) maintained the wounds in a chronic state, but they were not progressing toward closure.38 The wounds were initially cleansed, then dressed with standard PMDs secured with a gauze wrap.38 PMDs were changed every 3–5 days as needed, based upon exudate amount, without additional cleansing.38 Fewer dressing changes and no routine cleansing reduced both labor time and ancillary supply use.38 The facility's investment in daily wound care decreased by 74% compared with previous approaches.38 And, nurse satisfaction increased: providing effective wound care was empowering.38 One patient passed away because of unrelated factors with the wound 40% closed.38 The remaining seven patients' wounds closed completely.38 Of these, five had PUs.38 Previous best practice, used for an average of 42.6 days on these PUs, produced no appreciable improvement and totaled $2047.75.38 In contrast, PMDs used for an average of 25.4 days closed all five PUs, totaling only $321.45.38 The average cost for ineffective wound management was $9.05/PU/day; effective wound management with PMDs cost only $2.53/PU/day.38
Medical gerontologist Agathangelou (2017) conducted an observational study of 65 patients with PUs at the site of heel cracks.39 The patients' average age was 83 years; 40 had type 1 diabetes and 25 had type 2 diabetes; many were smokers.39 Previous treatment was iodine solution and ointment covered with paraffin gauze.39 When indicated, the posterior tibial artery was revascularized (15 patients; one refused) and systemic antibiotics were provided (23 patients).39 One Pseudomonas-infected patient with renal insufficiency was successfully managed using only silver PMDs, rather than systemic antibiotics.39 Education; pressure relief with orthotic devices, crutches, or wheelchairs; and PMDs changed every 48 h brought PU closure for 58 of the 65 patients (89%) within 4–7 months.39 One patient died of a heart attack, the remaining six patients refused to appropriately offload.39 Initial visual analogue scale pain scores (when applicable) were 7–9, dropping to 4 after a week; by day 12, all patients were pain free.39 PMDs were continued to prevent recurrence.39
A facility tracheostomy site PU rate of 12.5% led clinician researchers O'Toole et al. to develop changes.40 Outcomes from 183 consecutive adults were recorded (control group).40 Then, a prevention program was implemented and the outcomes and compliance levels were recorded for the next 155 adults (experimental group).40 The initial characteristics of the two groups were statistically similar.40 The new protocol included neutral head positioning, Velcro ties, suture removal within 7 days, and a hydrocolloid or PMD placed in the operating room.40 In either case, PMDs were put in place when sutures were removed (by day 7 at the latest).40 PMDs were changed when 75% saturated and as needed, or every 7 days.40 Twenty patients in the preprotocol group developed trach site PUs (10.93%), compared with only two in the protocol group (1.29%), a statistically significant difference (p = 0.0003).40 The PU rate with the new protocol with PMDs was reduced by 90%.40 The researchers had calculated the trach PU rate in the year before the formal study, and staff had heightened awareness of the problem during the study's initial (control) year.40 The lack of improvement between these 2 years suggests that the results were not the result of a natural decline.40
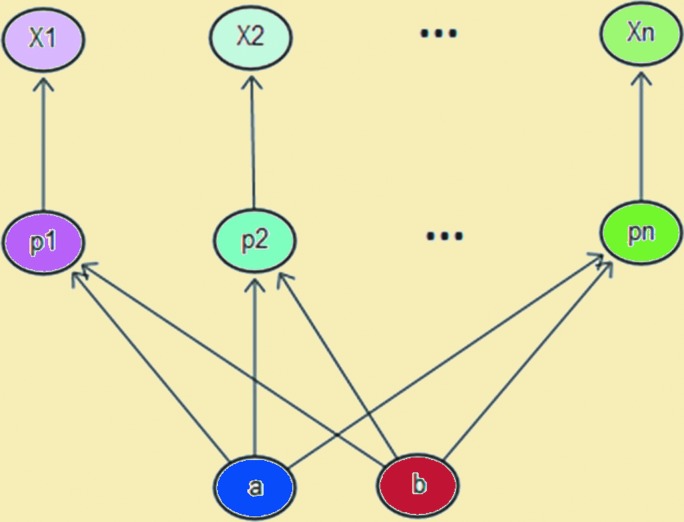
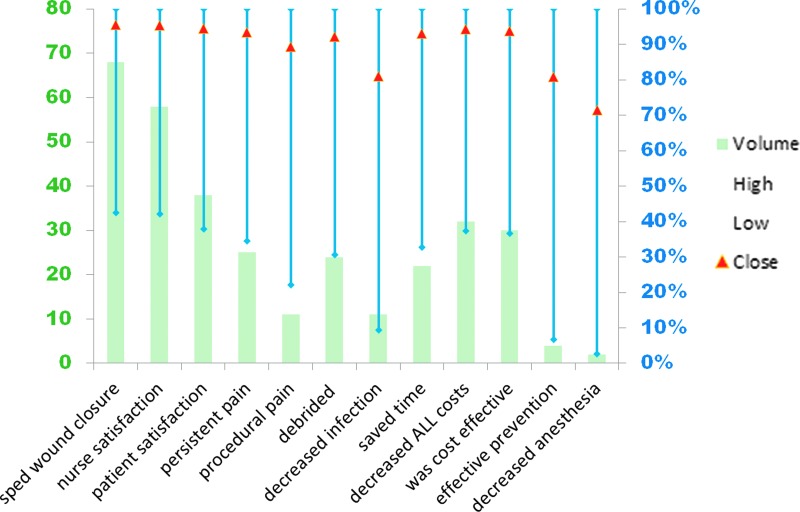
The remaining studies located in the search were case study/series (2006–2017) describing 79 PUs managed with PMDs. These results were summarized and statistically analyzed (details of statistical analysis available upon request). Because this study design is inherently weak, heterogeneity was assumed and explicitly accounted for by treating the probability of success as a random variable embedded in a hierarchical structure (Fig. 1), leading to wide credibility intervals because of the uncertainty in probability of success engendered by heterogeneity. Up to 12 outcomes were reported by the case study/series authors (Fig. 2), none of whom received corporate funding to conduct their studies. Despite the wide 95% credibility intervals, p0.975 is consistently 99% or higher, and p0.50 ranges from 71.42% to 95.44%, indicating a high level of credibility that, in similar patients, PMDs will likely provide the benefits the case study/series authors describe.
Figure 1.
Structural account of heterogeneity. Each wound (X) has its own success parameter (p) arising from a common distribution with random parameters (a, b).
Figure 2.
Polymeric membrane pressure ulcer case study and case series wound data analysis. Up to 12 aspects of wound management (x axis) were coded as dichotomous variables for each wound; wound counts for each aspect (left, y axis) are indicated by the columns. For each aspect of wound management, the 95% credibility intervals (p0.275–p0.975) are indicated by the blue bars (right, y axis), with red triangles marking p0.50. PU, pressure ulcer.
Discussion
All these authors represented PMDs as a unique dressing type, reporting benefits not achieved with conventional foam dressings. Although the PU patients in many of these studies were so debilitated that they would usually be excluded from funded RCTs, they represent the real-world population who most needs an effective dressing solution. Most of the researchers were independent clinicians, enhancing the credibility of their results. The results of studies using PMDs on PUs clearly show that categorizing PMDs by their foam substrate is inappropriate.
Innovation
This article supports a paradigm shift for wound management guidance materials to embrace a more evidence-based, patient-centered method of classifying products. The study results presented here, using PMDs for PUs as an example, clearly show that functional attributes, indications, and patient results are not always dictated by dressing substrates. Unlike conventional foam dressings, PMDs meet all the criteria of an ideal dressing (Table 1).7,14,36,37 These results strongly support the author's assertion that evidence-based wound management requires guidelines that categorize advanced dressings based upon how they function in real-life settings, rather than upon their substrate.
Key Findings.
Although PMDs are classified as foams, their indications and functional attributes are unique
PMDs cleanse; balance moisture; decrease pain, edema, and bruising; and speed healing
PMDs outperform conventional dressings for PUs
Evidence for dressing categorization based upon functional attributes and indications, rather than on substrate or reimbursement code, is compelling
Acknowledgments and Funding Sources
The author thanks John Newton for his assistance in the initial literature search and Jeff Weintraub for providing the appropriately customized statistical analysis of the case study/case series data. No external funding was received for this study.
Abbreviations and Acronyms
- DTI
deep tissue injury
- EB
epidermolysis bullosa
- IFUs
instructions for use
- PMD
polymeric membrane dressing
- PU
tissue damage resulting from pressure, also called pressure ulcer or pressure injury
- PUSH
pressure ulcer scale for healing
Author Disclosure and Ghostwriting
As a result of her extensive experience using PolyMem on her wound patients in a wide variety of settings, the sole author, Linda L. Benskin, became so passionate about the benefits of these unique dressings that she is currently an employee of Ferris Mfg. Corp., the makers of PolyMem. No ghostwriters were involved in the preparation of this article.
About the Author
Linda L. Benskin, PhD, RN, SRN (Ghana), CWCN, CWS, DAPWCA, as a result of her extensive experience managing wound patients while working for 5 years in a remote clinic in northern Ghana, West Africa, became so passionate about the benefits of PMDs that she is currently an employee of Ferris Mfg. Corp., the makers of PolyMem. Dr. Benskin also works independently developing village health worker training programs for Christians in rural areas of tropical developing countries. She is currently gathering data for sustainable wound management options for lay health providers in rural areas of tropical developing countries using improvised dressings.
References
- 1. Bolton L. Operational definition of moist wound healing. J Wound Ostomy Cont Nurs 2007;34:23–29 [DOI] [PubMed] [Google Scholar]
- 2. Dabiri G, Damstetter E, Phillips T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv Wound Care 2016;5:32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benskin LL. Polymeric Membrane Dressings for topical wound management of patients with infected wounds in a challenging environment: a protocol with 3 case examples. Ostomy Wound Manag 2016;62:42–62 [PubMed] [Google Scholar]
- 4. Cutting KF, Vowden P, Wiegand C. Wound inflammation and the role of a multifunctional polymeric dressing. Wounds Int J 2015;6:41–46 [Google Scholar]
- 5. Davies SL, White RJ. Defining a holistic pain-relieving approach to wound care via a drug free polymeric membrane dressing. J Wound Care 2011;20:250, 252, 254 passim [DOI] [PubMed] [Google Scholar]
- 6. Cunningham J, Henry M, Milne C. Wound Source 20th Year [Internet]. Hinesburg VT: Kestrel Health Information, 2017. www.woundsource.com (last accessed July3, 2018) [Google Scholar]
- 7. Fowler E, Papen JC. Clinical evaluation of a polymeric membrane dressing in the treatment of dermal ulcers. Ostomy Wound Manage 1991;35:35–38, 40–44 [PubMed] [Google Scholar]
- 8. Saha S, Smith MB, Totten A, et al. . Pressure Ulcer Treatment Strategies: Comparative Effectiveness [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013. [cited 2014. February 27]. (AHRQ Comparative Effectiveness Reviews). www.ncbi.nlm.nih.gov/books/NBK143657 (last accessed October5, 2018) [PubMed] [Google Scholar]
- 9. Mona Baharestani, Elena Pope. Chronic Wounds in Neonates and Children. In: Krasner D, Rodeheaver GT, Sibbald RG, eds. Chronic Wound Care: A Clinical Source Book for Healthcare Professionals. 4th ed. Malvern, PA: HMP Communications; 2007:685 [Google Scholar]
- 10. Lee BY, editor. The Wound Management Manual. New York: McGraw-Hill, Medical Pub. Division, 2005:382 [Google Scholar]
- 11. Thomas S. Surgical Dressings and Wound Management. 2nd ed. Hinesburg VT: Kestrel Health Information; 2012:708 [Google Scholar]
- 12. Mulder M. The Selection of Wound Care Products for Wound Bed Preparation. Wound Heal South Afr 2009;2:76–78 [Google Scholar]
- 13. Cunningham J, Henry M. WoundSource The Kestrel Wound Product Sourcebook 10th Anniversary Edition. Hinesburg, VT: Kestrel Health Information; 2007. p.9 [Google Scholar]
- 14. Benskin LL. PolyMem The Ideal Dressing [Internet]. Ferris Mfg. Corp.; 2015. www.researchgate.net/publication/325930476_MKL662_PolyMem_Ideal_Dressing_Chart_Booklet_Complete_0815 (last accessed October5, 2018)
- 15. Sessions RC. Can a drug‐free dressing decrease inflammation and wound pain? what does the evidence say? J Wound Ostomy Continence Nurs 2009;36(Suppl):S63 [Google Scholar]
- 16. Beitz AJ, Newman A, Kahn AR, Ruggles T, Eikmeier L. A polymeric membrane dressing with antinociceptive properties: analysis with a rodent model of stab wound secondary hyperalgesia. J Pain 2004;5:38–47 [DOI] [PubMed] [Google Scholar]
- 17. Weissman O, Hundeshagen G, Harats M, et al. . Custom-fit polymeric membrane dressing masks in the treatment of second degree facial burns. Burns 2013;39:1316–1320 [DOI] [PubMed] [Google Scholar]
- 18. Wilson D. Application of Polymeric Membrane Dressings to Stage I Pressure Ulcers Speeds Resolution, Reduces Ulcer Site Discomfort and Reduces Staff Time Devoted to Management of Ulcers. Poster # CS-128 presented at: Symposium on Advanced Wound Care (SAWC)SPRING; 2010April17; Orlando, FL, USA [Google Scholar]
- 19. Henson A. Positive Outcomes Managing Deep Tissue Pressure Injuries (DTPIs) with Polymeric Membrane Dressings. Poster #4 presented at: Wild on Wounds (WOW) Conference; 2017October4; Las Vegas, NV, USA [Google Scholar]
- 20. Hayden JK, Cole BJ. The effectiveness of a pain wrap compared to a standard dressing on the reduction of postoperative morbidity following routine knee arthroscopy: a prospective randomized single-blind study. Orthopedics 2003;26:59–63; discussion 63. [DOI] [PubMed] [Google Scholar]
- 21. Dawson, Lewis C, Boch R. Total Joint Replacement Surgical Site Infections Eliminated by Using Multifunctional Dressing. 900 Cases Report over 4 years. Poster presented at: Australian College of Operating Room Nurses (ACORN); 2010May19; Perth, Australia [Google Scholar]
- 22. Rahman S, Shokri A. Total Knee Arthroplasty (TKA) Infections Eliminated and Rehabilitation Improved Using Polymeric Membrane Dressing Circumferential Wrap Technique: 120 Patients at 12-month Follow-up. Poster #398 presented at: European Wound Management Association (EWMA); 2013May15; Copenhagen, Denmark [Google Scholar]
- 23. Kim SH, Lee JH, Lee DE. Dressing Materials in the STSG Donor Site Management: A Comparative Study. J Korean Soc Plast Reconstr Surg 2004;31:71–75 [Google Scholar]
- 24. Edwards J, Mason S. An evaluation of the use of PolyMem Silver in burn management. J Community Nurs 2010;24:16–19 [Google Scholar]
- 25. Benskin LLL. PolyMem Wic Silver Rope: A Multifunctional Dressing for Decreasing Pain, Swelling, and Inflammation. Adv Wound Care 2012;1:44–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agathangelou C. Painless Enhanced Autolytic Debridement on 252 Chronic Wounds by Using Polymeric Membrane Dressings. Poster #CS-006 presented at: Symposium on Advnaced Wound Care (SAWC); 2016April13; Atlanta, GA, USA [Google Scholar]
- 27. Denyer JE. Wound Management for Children with Epidermolysis Bullosa. Dermatol Clin 2010;28:257–264 [DOI] [PubMed] [Google Scholar]
- 28. Agathangelou C. Huge sacral pressure ulcer closed in four months using silver polymeric membrane cavity filler and dressings. Poster #40 presented at: NPUAP 11th Biennial Conference; 2009February27; Arlington, VA, USA [Google Scholar]
- 29. Agathangelou C. Treating Fungating Breast Cancer Wounds with Polymeric Membrane Dressings, an Innovation in Fungating Wound Management. Poster #CS-008 presented at: Symposium on Advanced Wound Care (SAWC); 2016April13; Atlanta, GA, USA [Google Scholar]
- 30. Harrison JE. An Independent Evaluation of a New Mesh‐ Reinforced Silver Rope Dressing. J Wound Ostomy Continence Nurs 2009;36(Suppl):S35 [Google Scholar]
- 31. Agathangelou C. Large Necrotic Malodorous Pressure Ulcer Closed Using Unique Silver Dressings. Poster #35 presented at: NPUAP 11th Biennial Conference; 2009February27; Arlington, VA, USA [Google Scholar]
- 32. Cahn A, Kleinman Y. A novel approach to the treatment of diabetic foot abscesses - a case series. J Wound Care 2014;23:394., 396–399. [DOI] [PubMed] [Google Scholar]
- 33. Denyer JE, Pillay E. Best practice guidelines for skin and wound care in epidermolysis bullosa - International Consensus [Internet]. DEBRA; 2012. [cited 2015. March 30]. https://www.woundsinternational.com/resources/details/best-practice-guidelines-for-skin-and-wound-care-in-epidermolysis-bullosa (last accessed October5, 2018) [Google Scholar]
- 34. Langemo DK, Black J, National Pressure Ulcer Advisory Panel. Pressure ulcers in individuals receiving palliative care: a National Pressure Ulcer Advisory Panel white paper. Adv Skin Wound Care 2010;23:59–72 [DOI] [PubMed] [Google Scholar]
- 35. Aromataris E, Munn Z. Joanna Briggs Institute Reviewer's Manual 3.2.5 Search Strategy [Internet]. 2017th ed. The Joanna Briggs Institute; [cited 2018. February 1]. https://wiki.joannabriggs.org/display/MANUAL/3.2.5+Search+strategy (last accessed October5, 2018) [Google Scholar]
- 36. Carr RD, Lalagos DE. Clinical evaluation of a polymeric membrane dressing in the treatment of pressure ulcers. Decubitus 1990;3:38–42 [PubMed] [Google Scholar]
- 37. Yastrub DJ. Relationship between type of treatment and degree of wound healing among institutionalized geriatric patients with stage II pressure ulcers. Care Manag J 2004;5:213–218 [DOI] [PubMed] [Google Scholar]
- 38. Case E, Bolhuis J. Providing Affordable Care in a Skilled Nursing Facility to Promote Best Practice. Poster #CS091 presented at: 30th Annual Symposium on Advanced Wound Care (SAWC); 2017April5; San Diego, CA [Google Scholar]
- 39. Agathangelou C. Treatment of Diabetic Heel Ulcerations with the Help of Polymeric Membrane Dressings. Poster #EP0009 presented at: European Wound Management Association (EWMA); 2017May3; Amsterdam, The Netherlands [Google Scholar]
- 40. O'Toole TR, Jacobs N, Hondorp B, et al. . Prevention of Tracheostomy-Related Hospital-Acquired Pressure Ulcers. Otolaryngol-Head Neck Surg 2017;156:642–651 [DOI] [PubMed] [Google Scholar]
- 41. Benskin L. Ideal dressing booklet. Fort Worth, TX: Ferris Mrg. Corp, 2015. DOI: 10.13140/RG.2.2.25858.79048 [DOI] [Google Scholar]