Abstract
Significance: Diabetic cardiomyopathy (DCM) is a frequent complication occurring even in well-controlled asymptomatic diabetic patients, and it may advance to heart failure (HF).
Recent Advances: The diabetic heart is characterized by a state of “metabolic rigidity” involving enhanced rates of fatty acid uptake and mitochondrial oxidation as the predominant energy source, and it exhibits mitochondrial electron transport chain defects. These alterations promote redox state changes evidenced by a decreased NAD+/NADH ratio associated with an increase in acetyl-CoA/CoA ratio. NAD+ is a co-substrate for deacetylases, sirtuins, and a critical molecule in metabolism and redox signaling; whereas acetyl-CoA promotes protein lysine acetylation, affecting mitochondrial integrity and causing epigenetic changes.
Critical Issues: DCM lacks specific therapies with treatment only in later disease stages using standard, palliative HF interventions. Traditional therapy targeting neurohormonal signaling and hemodynamics failed to improve mortality rates. Though mitochondrial redox state changes occur in the heart with obesity and diabetes, how the mitochondrial NAD+/NADH redox couple connects the remodeled energy metabolism with mitochondrial and cytosolic antioxidant defense and nuclear epigenetic changes remains to be determined. Mitochondrial therapies targeting the mitochondrial NAD+/NADH redox ratio may alleviate cardiac dysfunction.
Future Directions: Specific therapies must be supported by an optimal understanding of changes in mitochondrial redox state and how it influences other cellular compartments; this field has begun to surface as a therapeutic target for the diabetic heart. We propose an approach based on an alternate mitochondrial electron transport that normalizes the mitochondrial redox state and improves cardiac function in diabetes.
Keywords: NAD, NADH, redox balance, mitochondria, diabetes, cardiomyopathy
Diabetes and the Heart
Clinical overview of diabetes mellitus incidence and etiology
Diabetes mellitus (DM) has been a rapidly growing health epidemic worldwide (47, 53). As a chronic metabolic disease resulting from either autoimmune destruction of the insulin-secreting pancreatic β cells (type 1 DM, T1D) or impaired insulin secretion secondary to systemic insulin resistance (type 2 DM, T2D), DM culminates in chronic hyperglycemia and dyslipidemia (8). Although historically considered separate conditions, contemporary evaluation of T1D and T2D suggests a significant overlap in presentation, complications, and health outcomes, particularly with respect to the heart (8).
The heart as a primary target of DM pathology
Cardiovascular disease is the leading cause of morbidity and mortality in DM patients. There is an appreciable contribution to DM cardiovascular pathology from atherosclerotic disease; however, this review will emphasize the effect of the diabetic milieu directly on the myocardium through processes that promote myocardial dysfunction. Disease pathology in the myocardium is indicated by the presence of a unique clinical entity, diabetic cardiomyopathy (DCM), which was first documented in the 1970 s in a small DM patient cohort for which no attributable origin could be defined (159). This pathological condition garnered support as clinically important by epidemiological data in the Framingham study establishing a link between DM and heart failure (HF), which demonstrated DM as an independent risk factor for cardiac events when correcting for comorbidities such as hypertension, coronary artery disease, and dyslipidemia (99). Indeed, DCM develops in a large proportion of well-controlled DM patients, emphasizing the need for investigation into the underlying molecular events driving disease and development of targeted therapies.
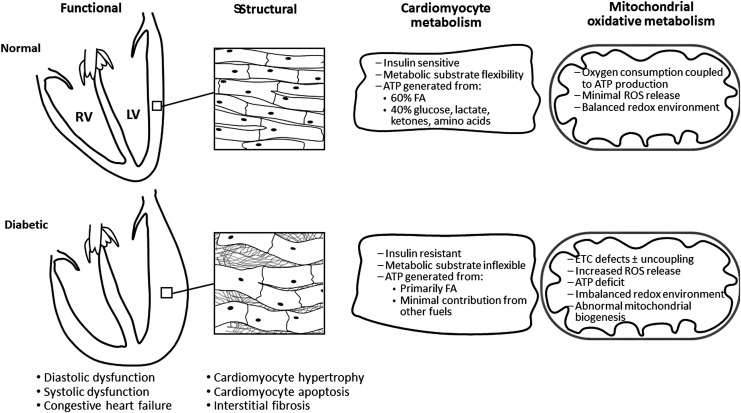
Since its initial observation, DCM has been further characterized, and it is typically indicated by a number of morphological and molecular changes (summarized in Fig. 1). Gross structural aspects of DCM include an increase in left ventricle (LV) mass and wall thickness. Histological examination reveals interstitial fibrosis, and cardiomyocyte rarefaction and hypertrophy (66). Current clinical diagnosis of DCM relies on noninvasive imaging, that is, echocardiography or MRI. An early decline in diastolic function (ventricular filling/relaxation) often occurs without detectable changes in ejection fraction (diastolic HF with preserved ejection fraction) in both human subjects (95) and animal models (55). This subclinical diastolic dysfunction in DM patients is also evident by analysis of blood flow velocities at the LV valves using Doppler echocardiography, demonstrating prolonged filling times and rates (7, 166). As diastolic function persists, systolic dysfunction may develop and evolve into ultimately HF with reduced ejection fraction, an end-stage condition without specific therapeutic approaches.
FIG. 1.
Morphological and metabolic features of diabetic cardiomyopathy. Diabetic cardiomyopathy is defined by the presence of an initial decreased myocardial diastolic dysfunction [heart failure with preserved ejection fraction (55)] in the absence of diabetes-induced standard cardiac risk factors, and may evolve to systolic dysfunction (heart failure with reduced ejection fraction) and congestive heart failure. These functional abnormalities are progressively induced by thickness and stiffness of the ventricular wall (defect in cardiomyocyte relaxation, interstitial fibrosis, and cardiomyocyte hypertrophy), cardiomyocyte death, and eventually contractile dysfunction. The heart relies on a large and constant energy supplied by oxidative metabolism. The normal heart is free to switch fuel substrates for oxidation and ATP synthesis to respond to energy demands, whereas the diabetic heart is insulin resistant and metabolically inflexible experiencing a decrease in glucose oxidation, and an increase in FA oxidation. Diabetic cardiomyopathy is considered a disease of the myocardial energetic metabolism characterized by a decreased efficiency of oxygen utilization induced by mitochondrial dysfunction. Healthy mitochondria release minimal ROS levels that are compatible with the physiological signaling, whereas the ROS flux released by diabetic cardiac mitochondria is reported to be increased by most studies. Other critical processes that are interrelated with mitochondrial failure are changes in the redox environment (described in this review) and Ca2+ handling (not shown in the picture). FA, fatty acids; LV, left ventricle; ROS, reactive oxygen species.
The underlying mechanism that initiates the pathology and leads to compromised cardiac function has yet to be fully understood. In addition to the derangement of circulating substrates, metabolic changes in the myocardium are apparent very early in DM, preceding compromised pump function and thus pointing toward the crucial pathogenic role of energy metabolism.
Energy Metabolism in the Diabetic Heart
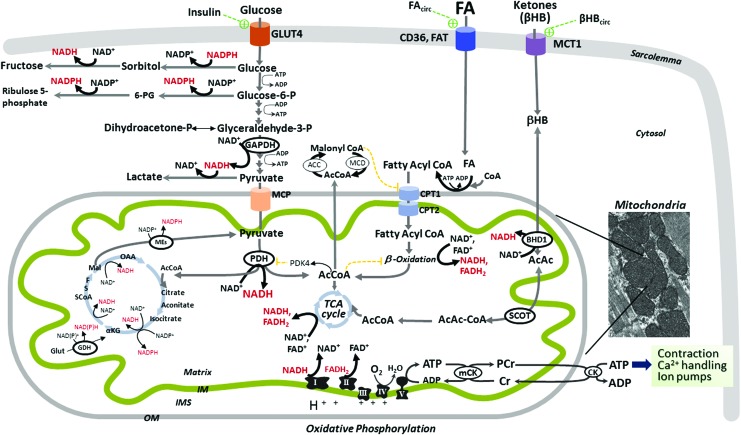
Optimal contractile function depends on continuous mitochondrial oxidative metabolism to form the ATP needed daily to sustain the cardiac output. To accomplish this energetic mission, cardiac mitochondria transform the chemical energy stored in fuel substrates (fatty acids [FA], glucose, lactate, ketone bodies, and amino acids) into ATP through oxidative phosphorylation (Fig. 2). The normal adult heart obtains 60% of energy from FA oxidation with the remaining 40% originating from the oxidation of other fuel substrates, and it has the flexibility to switch substrates as energy sources depending on physiological conditions (55). This characteristic is vital for the ability of the normal heart to respond to the energy demand as energetic substrates have different energy efficiency, which is defined as the amount of ATP produced for the amount of oxygen consumed, and assessed by the P/O ratio. FA oxidation generates the greatest ATP yield by using the highest amount of oxygen (P/O ∼2.3). Glucose is the most efficient energy substrate with a P/O ratio of 2.58. (55). The oxidation of β-hydroxybutyrate (βHB) has an intermediate efficiency with a P/O∼2.5. βHB is oxidized by the normal heart in proportion to its availability at the expense of FA and glucose (147); the diabetic heart acquires the ability to shift the acetyl-CoA toward ketone body synthesis, a characteristic of the fetal heart (48). Glucose oxidation is required for normal cardiac metabolism as a decrease induces diastolic dysfunction (1, 181). A reversal back to a fetal metabolic state with overreliance on glucose oxidation and decreased FA oxidation occurs in the failing heart, and is associated with a state of “energy starvation” as glucose, although a low oxygen-consuming substrate, is also a low ATP-yield fuel (per mole). Conversely, an excessive dependence on FA oxidation occurs in the diabetic heart, which supports the central postulate to explain the negative impact of metabolic inflexibility on cardiac function in diabetes.
FIG. 2.
Metabolism of fuel substrates drives the levels of reduced equivalents (NADH and NADPH) in normal cardiomyocytes. Normal adult heart obtains 40% of energy from metabolism of glucose, lactate, and ketones (mainly βHB), with the remaining 60% delivered from FA oxidation (55). Glucose uptake into cardiomyocytes is insulin dependent, whereas FA and βHB uptake is not hormonally regulated, and is driven by their bloodstream availability (FAcirc, βHBcirc) (17, 147). Glucose enters cardiomyocytes predominantly via the insulin-dependent glucose transporter 4 (GLUT4), and it follows multiple metabolic pathways including glycolysis, glycogen synthesis, and polyol pathway (with sorbitol and fructose formation), or is shuttled into the hexosamine biosynthetic or pentose phosphate pathways (with 6-Phosphogluconate, 6-PG, and ribulose 5-phosphate formation). Pyruvate is either converted to lactate or transported into mitochondria via the MPC, and converted by PDH to acetyl-CoA (AcCoA) for the TCA cycle. PDH is inhibited by pyruvate kinase (PDK4) that is activated by an excess of AcCoA and NADH. For simplicity, fluxes through glycogen synthesis and hexosamine pathways are not shown. After entry into cardiomyocytes (via CD36 and FA translocase, FAT), long chain FA are activated to FA-CoA that is either esterified as triacylglycerol (stored in the cytosol, not shown) or enters the mitochondria via carnitine palmitoyltransferases (CPT1 and 2), and they are oxidized via FA β-oxidation. The end products of each FA β-oxidation cycle are NADH, FADH2, and acetyl-CoA, which are further oxidized by ETC complexes or TCA, respectively, ultimately leading to ATP synthesis via mitochondrial oxidative phosphorylation. FA β-oxidation is controlled at different steps, including the inhibitory effect of malonylCoA (formed from AcCoA via AcCoA carboxylase, ACC), FADH2/FAD+ and NADH/NAD+ redox ratios, and acetyl-CoA/CoA ratio, all of which are unfavorable to FA oxidation. MalonylCoA is degraded by MCD, thus releasing its inhibitory effect on CPT1. The control of FA β-oxidation provided by post-translational modifications of β-oxidation enzymes and their transcriptional regulation is not depicted in the figure. βHB is the main ketone body utilized by the heart as an energy fuel. Produced by the liver at rates that are proportional to FA oxidation and NADH/NAD+ ratio, βHB's cardiomyocyte uptake is facilitated by the monocarboxylate transporter 1 (MCT1). Within mitochondria, βHB is oxidized by mitochondrial βHB dehydrogenase (BDH1) to acetoacetate (AcAc) that is converted to acetoacetyl-CoA (AcAc-CoA) by the enzyme succinyl-CoA:3 oxoacid-CoA transferase (SCOT). AcAc-CoA is then converted to acetyl-CoA for TCA cycle. Mitochondrial NADH/NAD+ redox is unfavorable to the βHB oxidative flux (147). Cardiac mitochondria can also fully metabolize branched chain amino acids (leucine, isoleucine, and valine), providing acetyl-CoA for the TCA cycle and succinyl-CoA for anaplerosis (not depicted in the figure). TCA cycle is a source of reducing equivalents in the form of NADH and NADPH. The figure depicts other sources of reducing equivalents. GDH converts glutamate to α-ketoglutarate that is coupled to either NAD+ or NADP+ reduction. Mitochondrial isoforms of MEs also can reduce NADP+ to NADPH. Mitochondrial oxidative phosphorylation provides more than 95% of the cardiac ATP (55), with the remainder derived from glycolysis. Electrons are transferred from the reducing equivalents, NADH and FADH2, to oxygen by the ETC complexes, whereas an electrochemical gradient is built across the mitochondrial IM, which is used by the ATP synthase (complex V) to form ATP. Mitochondrial-generated ATP is transferred to the cytosol by the mitochondrial and cytosolic creatine kinases (CK) for contractile apparatus, sarcoplasmic reticulum Ca2+-ATPase, and other ion pumps. Components of the contractile apparatus and calcium handling are affected by oxidative damage (9, 10), supporting a close link between mitochondrial bioenergetics, redox state, and myocardial contraction. The inset represents an electron micrograph of cardiac muscle showing mouse interfibrillar mitochondria. For simplicity, the nicotinamide nucleotide transhydrogenase, a mitochondrial IM enzyme that reduces NADPH+ by oxidizing NADH and using the mitochondrial proton motive force, is not shown in this figure. The reduced NADH and NADPH are shown in red. βHB, β-hydroxybutyrate; CPT1, carnitine palmitoyltransferase 1; ETC, electron transport chain; GDH, glutamate dehydrogenase; IM, inner membrane; MCD, malonylCoA decarboxylase; ME, malic enzyme; MPC, mitochondrial pyruvate carrier; PDH, pyruvate dehydrogenase; TCA, tricarboxylic acid cycle. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Alterations in the cardiac metabolic profile: changes in substrate utilization
There is an increased FA utilization in the hearts of asymptomatic diabetic human subjects, suggesting that changes in mitochondrial metabolism precede cardiac pathology and are detrimental to the heart (82). Insulin resistance is not the primary mechanism for this metabolic switch in models of insulin resistance and obesity (32). Cardiomyocyte-specific deletion of the insulin receptor leads to decreased insulin-stimulated glucose uptake/oxidation and increased FA oxidation but only modest cardiac dysfunction in the absence of systemic insulin resistance (19). This work suggests that although cardiac insulin resistance is an important event over the course of obesity-T2D, increased FA availability to the myocardium induced by systemic insulin resistance is required to trigger cardiac dysfunction in DM.
The allosteric activation of FA uptake and mitochondrial oxidation precedes the transcriptional regulation of metabolic remodeling. Hyperlipidemia, common in obesity and DM, arises from both diet and adipose lipolysis that increases circulating FA. Excessive FA cardiac uptake is observed in rodent models of T1D (122), obesity (138), and T2D (40) indicating that cardiac FA utilization is largely driven by circulating lipid availability. Once inside cardiomyocytes, FA are esterified to fatty acyl-CoA esters and transported into mitochondria to be oxidized as energetic fuel (Fig. 2), translocate to the nucleus, or undergo esterification to triglycerides (TG) for cytosolic storage (105). Within the nucleus, FA activate transcription factors such as the nuclear peroxisome proliferator-activated receptor-alpha (PPARα) to further promote mitochondrial FA oxidation, and PPARγ coactivator 1α (PGC-1α) to improve mitochondrial oxidative capacity through a coordinated transcriptional program that drives metabolic remodeling (59, 112). PPARα increases the expression of genes that promote FA utilization and inhibit glucose oxidation (59, 73); its expression is increased in several rodent models of DCM (65), and its cardiac-specific overexpression phenocopies the diabetic heart (65). Despite increased FA utilization, there remains an excessive intramyocardial TG accumulation leading to incompletely oxidized and potentially detrimental lipid metabolites (29, 110) that contributes to cardiac damage. Which one of these two mechanisms of “lipotoxicity” is prevalent is not known. Strong evidence in favor of the detrimental effect of overreliance on mitochondrial FA oxidation for ATP originates from studies showing that increased FA oxidation comes with a higher oxygen cost, thus reducing cardiac efficiency (cardiac contractile force as a function of oxygen consumed) in obese (28), insulin-resistant (32), T1D (90), and T2D diabetic rodents (90).
Despite the detrimental role of excessive FA in cardiac function in DM, there is intriguing evidence of an acute beneficial effect of palmitate on contractile function in hearts from diabetic rodents exposed to high glucose (24, 63) and adrenergic stimulation to mimic the diabetic heart during stress (188). Diabetic hearts efficiently oxidized the excess of FA and developed a higher contractile force whereas palmitate was detrimental on normal hearts, consistent with the notion that increased FA availability to the normal heart may trigger cardiac pathology. The short-term benefit of FA on the diabetic heart is further addressed in subsequent sections.
Mitochondrial electron transport chain defects in the diabetic heart
Measuring the cardiac high-energy phosphate metabolites such as phosphocreatine/ATP ratio (PCr/ATP) as an indirect assessment of oxidative phosphorylation shows that DM patients with normal cardiac function have a decreased PCr/ATP that is negatively correlated with circulating FA (168). Oxidative phosphorylation rates of mitochondria from atrial tissue of T2D patients are decreased (9). More recently, an impaired mitochondrial function with cardiac contractile dysfunction was reported in diabetic patients but not in “metabolically healthy” obese patients (125), suggesting that mitochondrial dysfunction is central to developing cardiac dysfunction in the transition from obesity to DM.
Despite the lack of consensus regarding the specific mitochondrial electron transport chain (ETC) site defective in the DM heart in both human subjects (Table 1) and animal models (Table 2), heart mitochondria are very susceptible to DM-induced ETC dysfunction in comparison with kidney and liver mitochondria (34). The impact of ETC defect on limiting FA oxidation in the DM heart is also unclear. Mitochondrial palmitoylcarnitine oxidation was decreased in cardiac samples from T2D subjects (9), suggesting that the ETC defects limit FA oxidation. In contrast, in rodent models of DM, increased FA oxidation is not limited by defects in complexes I (192) and III (27). Intriguingly, complex I inhibition with rotenone led to a metabolic shift to increase FA oxidation and glutamate utilization to compensate for the impaired energy production (199).
Table 1.
Bioenergetic Impairment in the Hearts of Human Subjects with Obesity and T2D
| Tissue/sample analyzed | Type of bioenergetic impairment | Ref. |
|---|---|---|
| Atrial fibers | ↓ Respiratory state 3 rate (with glutamate and palmitoylcarnitine, but not with pyruvate and succinate) in permeabilized atrial fibers from T2D patients with coronary artery bypass graft surgery | (9) |
| ↓ Respiratory state 3 rate (with glutamate/malate and succinate) in permeabilized atrial fibers from T2D patients with coronary artery bypass graft surgery | (57) | |
| ↓ Respiratory state 3 rate and RCR (with palmitoylcarnitine and pyruvate/malate) in permeabilized fibers from obese and T2D patients | (125) | |
| ↓ Complex I activity in obese patients | ||
| ↓ Complex II and III activities in obese T2D patients | ||
| Atrial mitochondria | ↓ Respiratory state 3 rate (with glutamate/malate and palmitoylcarnitine) | (52) |
| ↓ Complex I and IV activities in subsarcolemmal mitochondria from atrial appendages in T2D patients |
↓, decreased; RCR, respiratory control ratio (state 3, ADP-induced/state 4, ADP limited); T2D, type 2 diabetes mellitus.
Table 2.
Bioenergetic Impairment in the Hearts of T1D and T2D Animal Models
| Type of diabetes | Type of bioenergetic impairment | Ref. |
|---|---|---|
| Heart tissue, LV fibers, and isolated mitochondria | ||
| T1D | ↓ Respiratory state 3 rate (with succinate and TMPD +ascorbate, but not with glutamate/malate) in cardiac mitochondria isolated from guinea pigs with STZ-induced T1D | (189) |
| ↓ Respiratory state 3 rate (with pyruvate/malate) and RCR in cardiac mitochondria isolated from OVE26 mice with T1D | (173) | |
| ↓ Respiratory state 3 rate (with pyruvate/malate) in cardiac mitochondria isolated from rats with STZ-induced T1D; rates were improved with insulin treatment | (67) | |
| ↓ Respiratory state 3 (with glutamate and succinate) | (192) | |
| ↓ Complex I and II activities | ||
| FA oxidation is not limited | ||
| ↓ Respiratory state 4 rate (with succinate), ↔ ATP in cardiac mitochondria isolated from rats with STZ-induced T1D | (81) | |
| ↓ Respiratory state 3 rate (with glutamate/malate and succinate), improved after insulin treatment | (111) | |
| ↓ Complex I and II activities in cardiac mitochondria isolated from rats with STZ-induced T1DM | ||
| ↓ Respiratory state 3 rate (with glutamate/malate and pyruvate/malate, but not with palmitoylcarnitine) in LV fibers from Akita mice with T1D | (33) | |
| ↓ Respiratory state 3 rate (with glutamate/malate and succinate) that was improved after insulin treatment | (111) | |
| ↓ Complex I and II activities in cardiac mitochondria isolated from rats with STZ-induced T1D | ||
| T2D | ↓ Respiratory state 3 rate (with glutamate/malate and palmitoylcarnitine) in SSM (but not in IFM) | (54) |
| ↓ Complexes I, III, IV, and V in SSM (but not in IFM) in cardiac mitochondria isolated from db/db mice | ||
| ↓ Respiratory state 3 rate (with glutamate/malate, pyruvate/malate, palmitoylcarnitine) | (28) | |
| ↑ Respiratory state 4 rate (with palmitoylcarnitine), ↓ ATP due to FA-induced uncoupling in LV fibers from db/db mice | ||
| ↓ RCR (with glutamate/malate, succinate, TMPD) in cardiac mitochondria isolated from db/db mice with T2D | (188) | |
| ↓ Respiratory state 3 rate (with glutamate/malate, pyruvate/malate, and palmitoylcarnitine), ↓ ATP in LV fibers from ob/ob mice | (27) | |
| ↔ Respiratory state 3 rate, ↑ respiratory state 4 rate (with palmitoylcarnitine), ↓ ATP due to mitochondrial uncoupling in LV fibers from db/db mice | (93) | |
| ↓ Complex I and IV activities in LV tissue from ZDF rats | (151) | |
↓, decreased; ↔, unchanged; LV, left ventricle; IFM, interfobrillar mitochondria (between the myofibrilles); TMPD, N,N,N′,N′-tetramethyl-p-phenylenediamine; RCR, respiratory control ratio (state 3, ADP induced/state 4 ADP limited); STZ, streptozotocin; SSM, subsarcolemmal mitochondria (beneath the sarcolemma); T1D, type 1 diabetes mellitus.
Will the increase in mitochondrial FA oxidation efficiently maintain a positive cardiac energy balance? Part of the electrochemical proton gradient generated by FA oxidation, usually used to generate ATP, is reported to be consumed in T2D (27) and some (111, 192) but not all (33, 81) T1D models to generate heat as a result of mitochondrial uncoupling, and did not match a parallel increase in cardiac contractile force leading to cardiac inefficiency. The data suggest that in some models of diabetes the heart may become energy starved.
Consequences of Mitochondrial Metabolic Changes on Critical Pathogenic Mechanisms of DCM
Oxidative stress
Two major features of DCM are particularly sensitive to antioxidant therapy, namely cardiac hypertrophy (130) and fibrosis (205), highlighting the role of oxidative stress as a critical pathogenic mechanism for DCM (174, 201). Multiple sources contribute to reactive oxygen species (ROS) in the obese and DM heart, including NADPH oxidases (92, 171), uncoupled nitric oxide synthase (62), monoamine oxidase in both humans (57) and animal models (180), and ETC. The classical concept is that the univalent reduction of oxygen to form superoxide occurs with increased electron pressure at specific ETC sites caused by interruption of the electron flow in ETC defects. A novel mechanism of increased oxidative stress in the diabetic heart was reported by Nakamura et al. (129), demonstrating oxidative stress-induced activation of p53 signaling leading to increased cytochrome c oxidase assembly protein and subsequent increases in mitochondrial respiration, FA oxidation, and ROS generation in T1D and T2D mouse models. These data support a novel concept about the nuclear-mitochondrial interaction, and they indicate that early genomic instability induced by the diabetic milieu activates p53 pathway in the heart to induce mitochondrial oxidative stress (183). In addition, they bring forth an interesting hypothesis that an increase, rather than a decrease, in mitochondrial electron flow may increase oxidative stress in the diabetic heart.
Is the oxidation of excessive energetic sources responsible for the increased oxidative stress? Mitochondrial oxidation of excessive glucose and subsequent ROS generation has been put forth as the unifying pathogenic mechanism for chronic diabetic complications based on work in endothelial cells that are readily permeable to glucose in the absence of insulin (136). During the development of overfeeding-induced obesity and DM, the heart oxidizes an excessive amount of FA, increasing the reducing equivalent pool (NADH and FADH2, Fig. 2), leading to increased ETC electron flow, and generating a greater mitochondrial inner membrane electrochemical gradient that is usually used to drive ATP synthase. In the normal heart, mitochondrial oxygen consumption increases when the proton gradient is collapsed by an uncoupler (a compound that dissociates substrate oxidation from ADP phosphorylation), indicating a physiologic limitation of oxidative phosphorylation by the phosphorylation apparatus (ATP synthase, adenine nucleotide translocase, and mitochondrial phosphate carrier) (157). Therefore, the larger electrochemical gradient produced during substrate oversupply that is not consumed for ADP phosphorylation may increase electron pressure at ETC sites that are known to leak electrons and form superoxide such as the Qo in complex III (42). In addition, sites in the mitochondrial FA oxidation pathway also leak electrons during increased electron flux in normal heart mitochondria, independent of the proton gradient magnitude (177). We reported similar findings from a T1D rodent model examining mitochondria from kidney proximal tubules, a structure that also over-relies on mitochondrial FA oxidation in DM (158). ETC defects that interrupt electron flow create additional sites for increased electron pressure and leak as recently reported for complex II (192). Interestingly, mice bearing a genetic complex I defect in the heart do not exhibit increased oxidative stress (100), implying that mitochondrial sites other than ETC are also responsible for superoxide formation in the diabetic heart. Alternatively, complex III may be the principal site of ROS generation during excessive FA oxidation by diabetic cardiac mitochondria, and a defect in complex I is protective by limiting electron flow into complex III.
Oxidative stress triggers a vicious cycle whereupon mitochondria are both the site and targets of oxidative damage. The induction of the mitochondrial transition pore opening is sensitive to oxidative stress, adding to mitochondrial dysfunction (175) and inducing apoptosis (184). In addition, ROS lead to cardiac fibrosis and damage the contractile apparatus (174). Mitochondrial uncoupling proteins dissipate the inner membrane potential and are inducible by oxidative stress, suggesting that they protect mitochondria by reducing the electron pressure created by excessive substrate oxidation. Indeed, human gene polymorphisms that decrease uncoupling protein 2 expression lead to enhanced oxidative stress (162).
Mitochondrial biogenesis
Mitochondrial biogenesis depends on a coordinated program to regulate the formation of all mitochondrial components. This process is orchestrated by PGC-1α that co-activates the nuclear respiratory factors 1 and 2 (NRF-1 and NRF-2) and enhances expression of the nuclear-encoded mitochondrial transcription factor A (TFAM) (200). TFAM binds to mtDNA, and it initiates mitochondrial transcription and genome replication (36). PGC-1α is considered the master regulator of mitochondrial biogenesis (102), and is beneficial for cardiac function during development and adaptation, but leads to cardiomyopathy with overexpression (160). Although PGC-1 coactivator is required for high-capacity mitochondrial respiratory function (13, 113), it is dispensable for maintenance of mitochondrial density and cardiac function under basal conditions in the adult heart.
Decreased cardiac PGC-1α is noted early in genetically induced DM (58); whereas during high fat diet (HFD)-induced insulin resistance, PGC-1α is initially increased, but then progressively declines (124). Cardiac cells cultured with various FA concentrations exhibit an increase in PGC-1α-mediated mitochondrial biogenic response with high FA levels (124). Increased PGC-1α, mtDNA content, and mitochondrial mass have been reported in some models of T1D and T2D (27, 173) though this biogenic response was associated with decreased mitochondrial function, suggesting an accumulation of defective mitochondria. As in animal studies, cardiac mitochondrial biogenesis is reduced in patients with advanced stages of DM (152, 154).
Calcium handling
Cardiomyocyte contraction depends on intracellular calcium concentration and mitochondrial-generated ATP. Ca2+ influx via the L-type Ca2+ channels triggers sarcoplasmic reticulum Ca2+ release, which dramatically increases the cytosolic-free Ca2+ to trigger contraction. Ventricular relaxation and filling is induced by Ca2+ removal from cytosol primarily by the ATP-dependent sarcoplasmic reticulum Ca-ATPase 2a (SERCA2a). A decreased excitation-contraction coupling induced by decreased SERCA2a was reported in the hearts of both T1D (133) and T2D (20) rodents. Recent research has focused on mitochondrial Ca2+ handling in DCM. Normal mitochondria act not only as Ca2+ buffers to prevent cytosolic Ca2+ overload but also as sensors with Ca2+-sensitive mitochondrial dehydrogenases that are activated to improve mitochondrial oxidative capacity (74). Decreased mitochondrial Ca2+ uptake precedes the contractile decline in a model of T1D (67), suggesting that Ca2+ handling is compromised in the DM heart.
Overall, alterations in mitochondrial biogenesis and metabolism are linked to contractile dysfunction via oxidative stress and Ca2+ mishandling, likely contributing to reduced diastolic function. However, earlier events driving the metabolic inflexibility that precede these critical pathogenic loops have yet to be uncovered.
Mitochondria as Mediators of Redox Status and Signaling in DCM
The concept of redox status and signaling
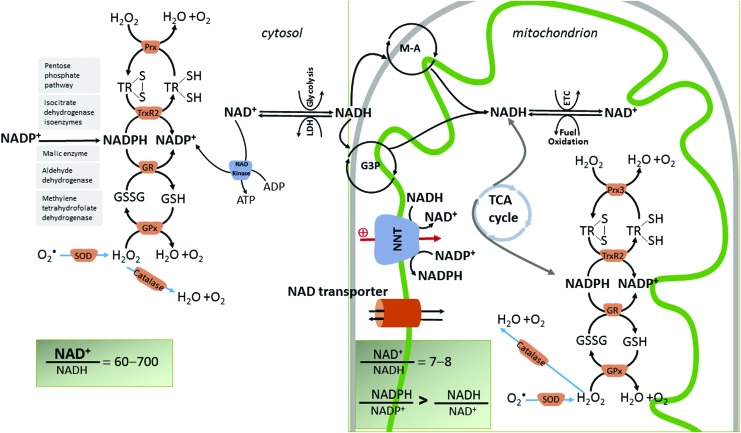
Redox reactions involve electron transfer between reduced and oxidized compounds. Classic examples are the transfer of electrons in the mitochondrial ETC from reduced to oxidized subunits according to their redox potential until the ultimate tetravalent addition of electrons to oxygen. There are four major redox couples (redox players) (97) that reflect the cellular redox status, and they are involved in redox signaling: NAD+ (oxidized)/NADH (reduced), NADP+/NADPH, GSSG (glutathione disulfide)/GSH (glutathione), as well as TrxSS (oxidized disulfide thioredoxin)/TrxSH2 (reduced thioredoxin) (Fig. 3).
FIG. 3.
The four main redox couples governing the redox balance in cardiac mitochondria (NAD+/NADH, NADP+/NADPH, GSH/GSSG, and TrxSH2/TrxSS) are hinged by NNT. Normal cardiomyocytes maintain a constant NAD pool mostly by converting biosynthetic precursors via the salvage pathway rather than de novo synthesis [not shown in the figure, revised in (83)]. Mitochondrial NAD content is increased by the import of cytosolic NAD through hypothetical transport mechanisms (35) that were identified in many species but not in mammals. However, it is believed that a bi-directional mitochondrial-cytosol transport system exists for both NAD and its precursors because exogenously added NAD leads to a greater accumulation in mitochondria than the cytosol (145). NAD+ is both a coenzyme for redox enzymes and an enzyme substrate for nonredox reactions in which the adenine diphosphate ribose moiety of NAD+ is cleaved, leading to the depletion of the NAD pool (not shown). Nicotinamide adenine nucleotide (NAD+) is a phosphate acceptor being converted to the phosphorylated form, NADP, via the enzyme NAD kinase (120). Only the cytosolic isoform of the NAD kinase is depicted in the figure. Therefore, NAD+ is a precursor for NADP. Both NAD+ and NADP+ are hybrid acceptors, and are converted to the reduced forms, NADH and NADPH. NADH transfers reducing equivalents that are derived from fuel oxidation, and, therefore, the NAD+/NADH couple is critical for energy generation. The NADPH/NADP+ redox couple is central to anabolism and antioxidant defense by donating electrons to glutathione (GSH/GSSG) and thioredoxin [Trx(SH)2/TrxSS)] systems, both of which are critical in scavenging H2O2 (generated from superoxide, O•2, by dismutation) via the enzymes GR, GPx, and thioredoxin reductase-Prx, respectively. Mitochondrial antioxidant system is mirrored by a similar scavenging mechanism in the cytosol. In these reactions, the reduced and oxidized members of the redox couples interconvert but are not consumed. Unlike these antioxidant mechanisms, catalase, which also scavenges H2O2, does not require reducing equivalents from NADPH for its function. Mitochondrial NADH/NAD+ and NADPH/NADP+ redox couples are linked by the enzyme NNT that reduces NADP+ at the expense of NADH oxidation and utilizing the mitochondrial IM proton motive force to drive this process. NNT maintains mitochondrial matrix NADPH/NADP+ pool in a reduced form, and it is a physiologically relevant source of NADPH to drive the enzymatic degradation of H2O2. The figure shows that the mitochondrial redox state of the NADH/NAD+ and NADPH/NADP+ redox couples are maintained different as the nucleotides have different metabolic roles. The NADH/NAD+ pool supports the divergent transfer of electrons from fuel substrates to both the ETC and antioxidant system via NNT, and thus is only partially reduced in comparison to NADPH/NADP+. NNT maintains the NADPH/NADP+ ratio several fold higher than the NADH/NAD+ (161). Cytosolic NADPH is regenerated via the pentose phosphate pathway, and redox reactions are catalyzed by isocitrate dehydrogenase, malic enzyme, aldehyde dehydrogenase, and methylene tetrahydropholate dehydrogenase (115). The cytosolic NADH is imported in mitochondria by redox shuttles, most commonly the M-A and glycerol 3 phosphate shuttles (G3P). GPx, glutathione peroxidase; GR, glutathione reductase; GSH, glutathione; GSSG, glutathione disulfide; H2O2, hydrogen peroxide; NNT, nicotinamide nucleotide transhydrogenase; M-A, malate-aspartate; Prx, peroxiredoxin. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Redox state is compartmentalized within the cell. Energized mitochondria have a high NADH concentration to provide electrons for oxidative phosphorylation (100). In the cytosol, NAD+ exceeds NADH, reflecting a relatively oxidized state with NAD+ available as a cofactor for oxidative reactions (i.e., glyceraldehyde-3-phosphate dehydrogenase reaction in glycolysis). In contrast, the cytosolic NADPH/NADP+ ratio is maintained in a reduced state via several enzymatic reactions (Fig. 3) to drive reductive biosynthesis and maintain antioxidant defense. The cytosolic GSH/GSSG couple is also maintained in a reduced state for ROS detoxification. These redox couples are interconnected (Fig. 3). In mitochondria, the nicotinamide nucleotide transhydrogenase (NNT) reduces NADP+ at the expense of NADH oxidation, utilizing the mitochondrial inner membrane protonmotive force to drive this process. Although there are additional enzymatic oxidative reactions that generate NADPH (isocitrate dehydrogenase, malic enzyme, and glutamate dehydrogenase, depicted in Fig. 2), NNT is a physiologically relevant source of mitochondrial NADPH (155). The NADPH/NADP+ redox couple is central to the antioxidant defense by donating electrons to glutathione and thioredoxin systems, both of which are critical in scavenging hydrogen peroxide (H2O2) via the enzymes glutathione peroxidase (GPx) and thioredoxin reductase-peroxiredoxin (Prx), respectively. The mitochondrial antioxidant system is mirrored by a similar scavenging mechanism in the cytosol. Mitochondrial redox state of the NADH/NAD+ and NADPH/NADP+ redox couples is maintained independently as the nucleotides have different metabolic roles. The NADH/NAD+ couple supports the divergent transfer of electrons from fuel substrates to both the ETC (via complex I) and the antioxidant system (via NNT). The NADPH/NADP+ couple is far more reduced as it supplies electrons to GSH reductase and thioredoxin reductase 2 to keep mitochondrial GSH and thioredoxin pools reduced. NNT maintains the NADPH/NADP+ ratio several fold higher than the NADH/NAD+ in mitochondria (161).
In conclusion, redox signaling regulates metabolism whereas metabolic state influences redox signaling. The NAD+/NADH redox couple is a critical node integrating metabolic and signaling events. Our discussion will emphasize changes in the NAD+/NADH redox couple in the heart over the course of obesity, insulin resistance, and DM.
Total NAD pool
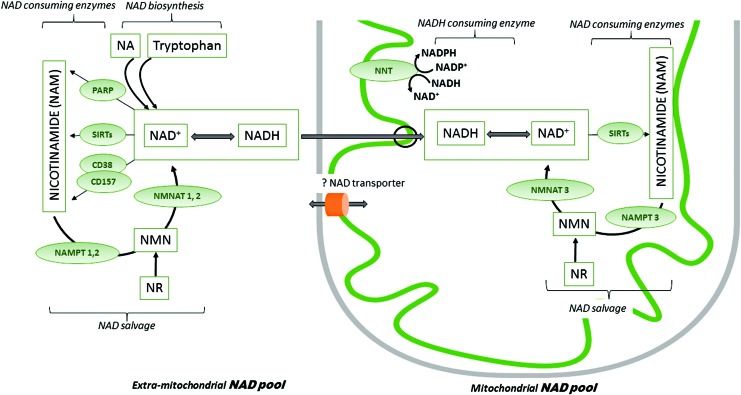
The redox signaling network linked to the NAD+/NADH couple depends on the total extramitochondrial and mitochondrial NAD pools (Fig. 4). NAD is a substrate for enzymes, including the family of sirtuins (SIRTs) and poly ADP ribose polymerases (PARPs), which continuously converts NAD+ to nicotinamide. As NAD is continuously degraded, cardiomyocytes must maintain a constant pool by de novo synthesis from diet-derived tryptophan and conversion of nicotinamide mononucleotide (NMN), nicotinamide (NAM), or nicotinamide riboside (NR) to NAD. However, mammalian cells, including cardiomyocytes, rely mainly on the NAD salvage pathways that recycle NAM generated by NAD-consuming enzymes to replenish NAD. Transformation of NAM to NMN is catalyzed by nicotinamide phosphoribosyltransferases (NAMPTs), rate-limiting enzymes in the salvage pathway. Conversion of NMN to NAD is then catalyzed by NMN adenylyltransferases (NMNATs) with different isoforms (NMNAT1 is nuclear, NMNAT2 is in the Golgi apparatus, and NMNAT3 is mitochondrial) supporting organelle cellular NAD pools. In cardiomyocytes, the mitochondrial NAD pool is relatively high, matching its critical role in mitochondrial function (4). It is unclear whether NAD pools are completely segregated or exchanged between subcellular compartments. Recently, it was shown that nuclear and mitochondrial NAD concentrations match the Michaelis constants of the respective SIRTs that are also compartmentalized (Fig. 4). The rate of NAD depletion is similar in the nucleus and cytosol on PARP activation, whereas mitochondrial NAD is minimally affected by the cytosolic NAD-consuming enzymes, further indicating that the mitochondrial NAD pool is finely balanced. Mitochondrial NAD pool is dependent on the conversion of NMN via NMNAT3 and the potential import of cytosolic NAD through transport mechanisms that are not fully elucidated in mammals (35), although its existence is supported by the fact that exogenously added NAD leads to greater accumulation in mitochondria than the cytosol (145).
FIG. 4.
Maintenance of mitochondrial and extramitochondrial NAD pools. NAD occurs as either oxidized (NAD+) or reduced (NADH) forms. In the extramitochondrial compartment, NAD is a co-substrate for enzymes, including PARPs, sirtuins (SIRT 1, 2, 5, 6, 7), and cyclic ADP-ribose (cADPR) synthases (CD38, CD157). These enzymes decrease the extramitochondrial NAD pool by continuously degrading NAD to NAM. The major mitochondrial NAD consumers are sirtuins 3, 4, and 5. The NAD biosynthetic pathway maintains a stable NAD subcellular concentration, and it relies on precursors such as dietary NA, tryptophan, NAM, and NR. The latter two may be also administered exogenously. NAD salvage pathway recycles the NAM generated as a by-product of the NAD-consuming enzyme activities. Transformation of NAM to NMN is catalyzed by nicotinamide phosphoribosyltransferases (NAMPT 1, 2, and 3), which are rate limiting in the salvage pathway. Conversion of NMN to NAD is then catalyzed by NMN adenylyltransferases (NMNAT 1, 2, and 3). As the mitochondrial membrane is impermeable for NADH, the reducing equivalents generated in the cytosol are transferred to the mitochondria via redox shuttles. Although the NMNAT3 isoform is present in mitochondria, the mitochondrial NAD salvage pathway has not been fully elucidated. Exogenous NAD increases mitochondrial function, but a mammalian NAD transporter has yet to be found. NNT reduces NADP+ to NADPH at the expense of NADH oxidation. NA, nicotinic acid; NAM, nicotinamide; NMN, nicotinamide mononucleotide; NR, nicotinamide riboside; PARP, poly(ADP-ribose) polymerase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Compared with exercise-induced cardiac hypertrophy, pathological cardiac hypertrophy is associated with a decrease in the cardiomyocyte NAD pool whereas NAD repletion inhibits the cardiac hypertrophic response (144). Why is the cardiomyocyte NAD pool decreased in cardiac pathology? One explanation is that chronic oxidative stress with secondary DNA alterations stimulates PARP to consume NAD for DNA repair (143). Oxidative stress may also cause NAD loss via connexins that are sensitive to oxidative stress (144). A reduced NAMPT and NAD synthetic pathways may be also responsible for NAD depletion (144). NAMPT inhibition with either an NAD analog (139) or a genetic approach (91) leads to a decreased NAD pool in cardiomyocytes associated with mitochondrial dysfunction, insulin resistance, and decreased cardiomyocyte ability to respond to stress. Is the diabetic or obese heart NAD deficient? The myocardial NAD pool was decreased in a model of DM, and NAD replenishment was protective against streptozotocin (STZ)-induced destruction of pancreatic β-cells (104). A more specific question then emerges: Are subcellular NAD pools altered in the obese and DM heart? This is yet to be determined.
Whole body increases in NAD availability appear to benefit metabolic health, especially with conditions of nutrient excess such as obesity and metabolic syndrome. Boosting NAD with NR increases the NAD+/NADH ratio (41). However, skeletal muscle-specific overexpression of NAMPT increased the NAD pool in parallel with NADH without altering the NAD+/NADH ratio in any cellular compartment (69). Apparently, the increase in the total skeletal NADH reflects an equilibrium between the oxidized and reduced forms of NAD to maintain the NAD+/NADH ratio within a range of absolute concentrations. These mice were not protected against obesity and metabolic syndrome, and this begs the question as to whether changing the NAD+/NADH redox ratio rather than the absolute NAD concentration mediates the effects of NAD-boosting interventions. The increase in NADH without changing the redox status did not prevent metabolic disease, suggesting that excessively increasing the reducing equivalents (NADH) is not beneficial (69).
Mitochondrial NAD+/NADH redox state and its regulation
NAD+ serves as a cofactor in fuel breakdown, whereas NADH is a substrate for complex I in the ETC and NNT. Therefore, the NAD+/NADH ratio reflects the overall status of mitochondrial metabolism. Although the NAD+/NADH redox ratio has been extensively studied in other organs, a description of the NAD+/NADH redox ratio in the obese or diabetic heart, and its involvement in diabetic cardiac pathology remains to be elucidated.
NADH is produced from NAD+ during energy substrate oxidation, serving as a key energy-transfer intermediate. In comparison with glucose oxidation that consumes 10 NAD+, palmitate (long-chain FA) consumes 31 NAD+ during mitochondrial oxidation. In addition to greater NAD+ consumption, the increased NADH from FA β-oxidation activates pyruvate dehydrogenase kinase to phosphorylate and inhibits pyruvate dehydrogenase, thus limiting glucose oxidation by the glucose-FA cycle (Randle cycle) (137, 150). It has not been determined whether increased circulating ketone bodies are associated with an increased cardiac uptake and oxidation. Therefore, mitochondrial FA oxidation is the major NADH producer in the diabetic heart.
NAD+ adjusts cell metabolism to meet energetic challenges. During energy crises (caloric restriction, fasting, exercise), NAD+ level rises (38); whereas during energy overload (i.e., increased fuel substrates in the form of FA), the NAD+ decreases and its reduced form, NADH, increases. The amount of NADH production and NAD+ consumption is tightly linked to energetic fuel oxidation, whereas mitochondrial NAD recycling (NADH consumption and NAD+ production) is linked to the ETC and decreases with ETC defects. For example, Complex I (100) and IV (183) defects lead to increased mitochondrial NADH content. The deficiency of frataxin, a mitochondrial protein integral to the assembly and function of iron-sulfur proteins in ETC complexes I, II, and III and aconitase (tricarboxylic acid cycle, TCA cycle), is associated with an 85-fold decrease in cardiomyocyte NAD+/NADH ratio and pathologic cardiac hypertrophy (194). Approaches to correct mitochondrial ETC defects increased NAD+ content (3). In conclusion, the disruption of the electron flow to oxygen by ETC defects increases NADH, causing a highly reduced redox environment within mitochondria.
Cardiac NAD+ is reduced in HF (89). Is there a change in the NAD+/NADH redox couple or its components in the obese and diabetic heart? Though a plethora of reasons suggest it is, direct measures of NAD+ or NADH in the obese and diabetic heart are limited. In OLETF rats, a model of T2D, diastolic dysfunction, and stiffness correlated with ATP depletion; whereas the NAD+/NADH redox ratio was unchanged (109). In the ZDF rat T2D model, NADH was unchanged in cardiac tissue in the absence of dysfunction (24). Although NADH/NAD+ redox ratio determines the production of mitochondrial ROS, the NADPH/NADP ratio is key to antioxidant defense. They are linked by the enzyme NNT that transfers electrons from NADH to NADP+ (Fig. 3). Although they are engaged in distinct metabolic pathways, their reduced forms, NADH and NADPH are spectrally identical, making specific quantification technically challenging, though fluorescence lifetime imaging (25) has been promising in distinguishing the two reduced pyridine nucleotide cofactors in the heart (135). Most studies measure a mixture of NADH and NADPH (24, 188, 189). Using a protocol that does not allow NADPH interference (Cell Technology), we found an increase in NADH in isolated cardiac mitochondria energized with FA substrates (21); whereas Bhatt et al. report a decrease in the steady-state NAD(P)H in diabetic cardiomyocytes incubated with high glucose and palmitate (24). These results suggest that the reducing power of NADH excess [induced by either excessive FA oxidation (21) or defective complex I (100, 192)] may be split between ATP production and antioxidant defense (to NADPH, via NNT). The increase in the NADH/NAD+ ratio and NAD+ decrease is supported by our work showing decreased activity of mitochondrial NAD+-dependent SIRT3 with secondary increases in protein lysine acetylation (21).
Two critical observations suggest that the NAD+/NADH ratio is decreased in the diabetic heart mitochondria: (i) The activity of NAD+-dependent sirtuins is decreased, leading to increased lysine acetylation of mitochondrial proteins; (ii) NAD replenishment or interventions (i.e., caloric restriction) to normalize the NAD+/NADH are beneficial for DCM. Therefore, manipulating the NAD+/NADH redox couple with novel therapeutic strategies is a promising, yet unexplored target to improve cardiac mitochondrial metabolism and to alleviate DCM. The next sections will discuss these topics.
The NAD+/NADH Couple in Redox Signaling
The role of the NAD+ as a cellular signaling regulator has only begun to be understood. NAD+ modulates key cellular processes, including energetic metabolism, mitochondrial integrity, gene expression, cell death, and degeneration; all of these are altered in DCM.
NAD+ is an electron acceptor and functions as a cofactor for enzymes that catalyze reduction-oxidation (redox) reactions. Redox reactions are readily reversible, and they do not contribute directly to changes in the total NAD pool in a specific subcellular compartment. NAD+ and NADH interconvert but are not irreversibly consumed. NAD+ participates in all major energetic pathways, including glycolysis, TCA, FA oxidation, ketone body metabolism, and ETC (Fig. 2). NAD+ is a potent activator of the TCA enzymes, whereas NADH is a TCA allosteric inhibitor and increases in ETC defects (3, 100, 194). Approaches to correct mitochondrial ETC defects increased NAD+ content (3). Therefore, the NAD+/NADH redox couple regulates energy production. NAD is also used as a co-substrate by enzymes, including sirtuins and PARPs.
Sirtuins
SIRTs remove an acetyl group from lysine residues in an NAD+-dependent manner by cleaving NAD+ to nicotinamide. SIRT2 was initially reported to mediate longevity in response to caloric restriction in Saccharomyces cerevisiae (94). Sirtuin orthologs also enhance lifespan in mammals, including mice (165). There are seven mammalian sirtuins that differ in their cellular localization, suggesting that sirtuin activities are compartmentalized in parallel with NAD+ pools. Although all sirtuins are NAD+ dependent, SIRT1 and 3 are well-known players in the heart, and they are the focus of this review.
Sirtuin1 has been observed in both the nucleus and the cytosol (185), where it serves several roles, including (i) epigenetic regulation by targeting specific lysine residues on histones to alter gene transcription, (ii) metabolic control by acting on transcription factors (p53, NF-kB, PGC1α, forkhead box O [FOXO]3a), and components of insulin signaling, and (iii) mediating the cardiomyocyte circadian clock. SIRT1 protein abundance is relatively stable, whereas its deacetylation of key lysine residues of histone 3 follows circadian NAD+ variations (128). Fasting activates SIRT1 through adrenergic-dependent phosphorylation by cAMP/PKA to sensitize the enzyme to NAD+ fluctuations and maintain energy homeostasis during stress (72). SIRT1 protects against pathologic cardiac hypertrophy as knockout mice exhibit developmental cardiac defects (43). Sustained SIRT1 overexpression causes cardiomyopathy whereas moderate SIRT1 expression ameliorates age-induced cardiac hypertrophy and dysfunction (5), suggesting that its effect is dose dependent. SIRT1 also protects mitochondrial function by activating PGC-1α (132) to increase mitochondrial FA oxidation (71). Deacetylation by SIRT1 facilitates insulin sensitivity. The effect of SIRT1 on mitochondrial FA oxidation is complex and varies according to the setting, a topic addressed later in this review. In a model of T1D, SIRT1 suppresses cardiomyocyte apoptosis (78). Overall, SIRT1 is critical to the heart in obesity and DM by regulating pathological hypertrophy, insulin sensitivity, and mitochondrial metabolism.
SIRT3 is the major mitochondrial NAD+-dependent deacetylase (118). Although the SIRT3-deficient mice do not exhibit baseline metabolic changes (118), an HFD regimen recapitulates the human metabolic syndrome characterized by obesity, glucose intolerance, dyslipidemia, liver steatosis, protein hyperacetylation, and chronic inflammation (86). A single nucleotide polymorphism in the human SIRT3 gene was identified that reduces enzymatic activity, suggesting a genetic component to the metabolic syndrome, and further underscoring the role of sirtuins as master regulators of metabolism (86). Genetic manipulation of SIRT3 gene has revealed its role in deacetylating and increasing hepatic mitochondrial enzyme activity involved in oxidative phosphorylation, FA oxidation (84), antioxidant defense, and mediating the effect of caloric restriction on age-induced hearing loss (176). SIRT3 also mediates the circadian control of mitochondrial oxidative phosphorylation (142).
Sirtuin3 regulates cardiac changes in the setting of obesity and DM such as hypertrophy, insulin sensitivity, and fuel metabolism. SIRT3 knockout causes age-related cardiac hypertrophy and failure with pathologic challenges (106), whereas overexpression protects against pathological hypertrophy by activating FOXO3a and its downstream antioxidant genes, mitochondrial manganese Superoxide dismutase (MnSOD), and catalase (182). SIRT3 also targets cyclophilin D, a modulator of the mitochondrial permeability transition pore (mPTP), to suppress pore opening, apoptosis, and cardiac hypertrophy (79). Modulation of the mPTP and mitochondrial Ca+ by cyclophilin D maintains the mitochondrial metabolism and NAD+/NADH redox state, and it controls the mitochondrial acetylome (134).
SIRT1 and SIRT3 act in concert to regulate energy metabolism during energetic crises. SIRT3 deacetylation activates enzymes involved in glycolysis (96, 140), FA oxidation (22, 84, 86), TCA cycle (176), and the ETC (2). During fasting, SIRT1 mediates a switch from glucose to FA oxidation in response to increased circulating FA (75) and increased NAD+ (37) in the muscle. By upregulating metabolic machinery during states of decreased fuel availability, SIRT3 appears to be a critical metabolic regulator of coupling substrate oxidation with the formation of reducing equivalents to ATP production, thus maximizing efficiency. However, most studies in this area have been performed in extracardiac tissues, comparing the fasting versus fed conditions and/or genetic manipulation of sirtuins. The effect of fasting on NAD+-dependent deacetylase has not been investigated in the heart.
SIRT1 and SIRT3 are classically defined as NAD+-dependent deacetylases, but more recent data suggest that SIRT3 depends on the NAD+/NADH ratio rather than the absolute [NAD+] (100). NADH exhibited a dose-dependent inhibition of SIRT3 activity despite constant NAD+ concentrations, suggesting that NADH competes with NAD at the binding site. In vitro experiments show that NADH inhibits SIRT3 at concentrations that are nonphysiologic, thus unlikely to regulate SIRT3 activity in vivo. This conundrum is complicated by the observation that mice bearing a genetic defect in complex I, the major NADH oxidation site, do not exhibit changes in mitochondrial NAD+ concentrations but rather a significant increase in NADH and a dramatic increase in lysine acetylation of cardiac proteins (100). We also showed that decreasing NADH consumption at complex I facilitates mitochondrial lysine acetylation in the diabetic heart (192), and that [NADH] rather than NAD+ correlates with increased SIRT3 activity (21).
NAD+-dependent sirtuins have been investigated as therapeutic targets in DCM. For example, resveratrol, a polyphenol and well-known SIRT1 activator, alleviated DCM in models of both T1D and T2D via activating sirt1, 2, 3, and 5 (14, 15), improved glucose metabolism in human subjects (23, 117), and decreased oxidative stress in cultured cardiomyocytes (186). In a rodent model of genetic obesity, resveratrol mitigated cardiac fibrosis and improved FA metabolism (18)
SIRT1 compete with PARPs for the NAD pool. PARP activity increases in chronic diseases, including obesity-induced DM due to increased DNA damage, and is reported to be a more rapid and efficient NAD+ consumer than SIRT1, suggesting that its activation may limit SIRT1 activity in these conditions. Competition for the NAD+ pool was validated in a mouse model of human accelerated aging where either PARP inhibition or NAD pool replenishment was protective (167). Increasing NAD availability by inhibiting PARP-1 increased SIRT1 activity, mitochondrial content, energy expenditure, oxidative metabolism, and protected against metabolic disease (16). PARP-1 inhibition in a mouse model of T1D alleviated DCM (148). However, therapeutic manipulation of PARP-1 activity with the purpose to correct the NAD+/NADH redox state, increase sirtuin activity, and protect against DCM should be considered with care due to the critical role of PARPs in the cellular response to stress and DNA repair.
NAD+/NADH ratio, sirtuins, and mitochondrial health in DCM
NAD+ is a vital cofactor in the cardiac metabolic program and mitochondrial fitness (39) through regulating mitochondrial biogenesis, dynamics (fusion/fission processes), and mitochondrial quality control.
Mitochondrial mass depends on the formation of mitochondria (biogenesis) and degradation of defective mitochondria (mitophagy). Both SIRT1 and SIRT3 regulate PGC-1α, the master regulator of mitochondrial biogenesis. SIRT1 deacetylates and increases the transcriptional activity of PGC-1α (132), whereas SIRT3 is a PGC-1α target as its activation is required for PGC-1α-induced mitochondrial biogenesis (107). The decrease in nuclear NAD+ and SIRT1 activity caused a downregulation of TFAM, the major factor responsible for the replication and transcription of the mitochondrial genome, with decreased mitochondrial respiration and increased glycolysis, a pseudohypoxic phenomenon that is mitigated by NAD supplementation (76). SIRT1 and PGC-1α were recently found to be associated with mitochondrial DNA nucleoids and TFAM (26), suggesting that both SIRT1 and PGC-1α can directly affect mitochondrial transcription.
ETC protein subunits are encoded by both nuclear and mitochondrial genomes. Unbalanced gene expression results in accumulation of unassembled subunits and induces proteotoxic stress with activation of the mitochondrial unfolded protein response (UPRmt) (98) as part of mitochondrial quality control. The UPRmt induces a nuclear gene expression program, including FOXO3a, MnSOD, and catalase, along with stimulating autophagy. SIRT3 is a critical coordinator of the UPRmt by enhancing the antioxidant defense and mitophagy (141). SIRT3 exerts cardioprotection by activating the FOXO3a-Parkin-mediated mitophagy, thus preserving mitochondrial homeostasis and alleviating cardiomyopathy in a model of T1D (202). SIRT3 also regulates mitochondrial morphology by influencing factors that are responsible for fusion/fission processes (163).
Complex I is the major hybrid acceptor from mitochondrial NADH; its inhibition traps this redox couple in its reduced form with a decrease in NAD+, SIRT3 inhibition, and increased mitochondrial protein lysine acetylation with accelerated HF in the presence of stress (100). NR supplementation in mouse models of mitochondrial defects increased cellular NAD with an increase in mitochondrial biogenesis (41, 103). Known targets of the NAD-dependent deacetylase SIRT1 are the tumor suppressor p53, the myocyte-specific enhancer factor 2, the FOXO factor, and PGC-1α, all of which activate transcriptional programs and promote mitochondrial function (11). Gomes et al. (76) reported that the control of mitochondrial metabolism exerted by the nuclear NAD+-dependent SIRT1 is regulated by energy supply in the heart. With normal or increased energy supply, the acetyl-CoA-dependent acetyltransferase GCN5 acetylates and inhibits PGC-1α (64) to depress mitochondrial biogenesis and function. In contrast, caloric restriction increases nuclear NAD+ and SIRT1 activity to improve the nuclear-mitochondrial communication and metabolism by alleviating the state of “pseudohypoxia” induced by the decreased NAD+, and it increases TFAM promoter activity to enhance transcription and mtDNA replication. This signaling pathway is believed to operate in insulin-resistant tissues, including the heart (146). In conclusion, NAD+-dependent sirtuins may be a key therapeutic target to maintain mitochondrial integrity in DCM.
How Mitochondrial NAD+/NADH Redox Dysregulation Potentially Drives DCM
The interplay between bioenergetics and NAD+/NADH redox state is a critical point of regulation in the heart. Understanding the nature of how this relationship fails in DM may reveal novel targets for treating DCM. We propose that an excess energy supply with a secondary shift toward a highly reduced NAD+/NADH redox couple is an early event in cardiac pathology of overfeeding-induced obesity and DM. In the setting of increased fuel availability, their full oxidation provides excessive NADH and acetyl-CoA.
The role of high glucose
Because hyperglycemia is the hallmark of diabetes, and carbohydrates are major parts of the Western diet, a reasonable question arises: Does glucose contribute to the increased mitochondrial Acetyl-CoA/CoA and NADH/NAD+ ratios in the heart in these conditions? Optimal glycemic control in diabetes reduces cardiovascular morbidity (153), suggesting that hyperglycemia is an important pathogenic factor for cardiovascular complications. Glucose is taken up by cardiomyocytes via GLUT1 and GLUT4, with the latter being responsible for insulin-dependent glucose uptake. Absence of GLUT4 significantly decreased cardiac glucose uptake under insulin-stimulated conditions but did not fully abolish (61) the observed increase of basal cardiac glucose uptake and glycogen content (1). Because GLUT1 is responsible for the bulk of basal glucose uptake, these data suggest that circulating hyperglycemia increases GLUT1-mediated insulin-independent glucose uptake. However, glycolysis and pyruvate oxidation are decreased in the diabetic heart (164). This shift away from glucose oxidative metabolism is not restricted to diabetes as it precedes the diastolic dysfunction on angiotensin II infusion (126) and pressure-overload hypertrophy models (203). In addition, transgenic mice with a mutation that decreases pyruvate oxidation develop both diastolic (1) and systolic (181) dysfunction, supporting the concept that glucose oxidation is required for normal cardiac metabolism. Retaining the capacity to freely switch between energy substrates to optimally respond to energy demands is a characteristic of normal cardiomyocytes, skeletal and liver cells (172). The question that arises pertains to the role of hyperglycemia in DCM.
If cardiomyocyte glucose uptake is only moderately affected whereas glycolysis and pyruvate oxidation are inhibited, it is unlikely that hyperglycemia contributes to the increase in mitochondrial acetyl-CoA/CoA and decreased NAD+/NADH in the diabetic heart. Rather, the increased flux through alternative non-ATP-producing glucose pathways such as polyol pathway, activation of protein kinase C, formation of advanced glycation end products, and hexosamine pathways, recognized as central to diabetic chronic microvascular complications, are more likely to mediate cytosolic redox changes (Fig. 5). Do they affect the myocardium? Glucose conversion to sorbitol (via aldose reductase with NADPH oxidation) and then to fructose (via sorbitol dehydrogenase with NAD+ reduction) has been extensively studied in microvascular complications, but its effect on myocardium in diabetes has not been established. High cytosolic glucose increases diacylglycerol, causing chronic activation of protein kinase C isoforms and cardiomyopathy (193). Excessive glucose and glucose-derived dicarbonyls (i.e., methylglyoxal) react with lysine and arginine amino groups of proteins, forming advanced glycation end-products that damage cardiomyocytes. Intracellular targets of methylglyoxal have been reported, including mitochondrial proteins (156) and components of calcium cycling (187), indicating a direct effect on energetic metabolism and cardiac contraction (131). The hexosamine pathway provides the end product UDP-N-acetyl-glucosamine (UDP-GlcNAc) used by the enzyme O-GlcNAc transferase to modify proteins by O-GlcNAcylation, thus causing contractile dysfunction (149), epigenetic modifications, and changes in gene expression in the diabetic heart [reviewed in (101)]. Diversion of the glycolytic intermediates to pathways others than full oxidation and ATP production is favored by the inhibition of the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by oxidation. In addition, chronic oxidative stress and ROS-mediated DNA damage activate DNA repair mechanisms, including PARP1 that polyADP-ribosylates and inhibits GAPDH, thus creating an amplification loop by depleting cytosolic NAD+ and inhibiting SIRT1 (Fig. 5).
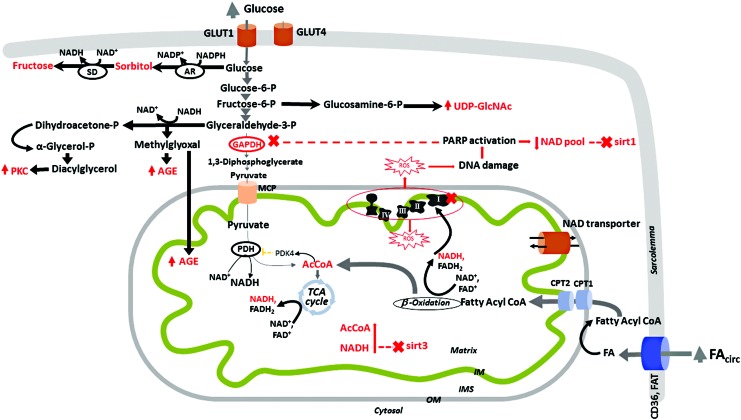
FIG. 5.
Excess of glucose and fatty acids changes the NAD+/NADH ratio in cardiomyocytes in different compartments. Glucose is taken up by cardiomyocytes via GLUT1 and GLUT4, with the latter being responsible for the insulin-dependent glucose uptake. As insulin activity is decreased and GLUT-1 is responsible for the bulk of basal glucose uptake, circulating hyperglycemia leads to an increase in the GLUT1-mediated insulin-independent glucose uptake by the diabetic heart. There is a metabolic shift away from glycolysis and pyruvate oxidation (164), and, therefore, these pathways are not responsible for changes in the mitochondrial acetyl-CoA/CoA and NAD+/NADH ratios in diabetes. In the cytosol, glucose follows alternative non-ATP-producing pathways such as polyol and advanced glycation end products formation (131, 156, 187), and activation of protein kinase C (193) and hexosamine pathways (101, 149). Glucose conversion to sorbitol (via aldose reductase, AR, with NADPH oxidation) and then to fructose (via sorbitol dehydrogenase, SD, with NAD+ reduction), although extensively studied in microvascular complications, has no established role in the diabetic myocardium. Diversion of the glycolytic intermediates to pathways others than full oxidation and ATP production is favored by the inhibition of the glycolytic enzyme GAPDH by oxidation (60), NADH (31), and polyADP-ribosylation (172), thus creating an amplification loop, depleting the cytosolic NAD pool, and inhibiting cytosolic NAD+-dependent SIRT1. In mitochondria, increased FA oxidation and ETC defects (i.e., complex I defect) cause an increase in NADH and acetyl-CoA, which favor the decreased activity of mitochondrial SIRT3. The cytosolic and mitochondrial NAD pools communicate via a potentially bi-directional NAD transporter on the mitochondrial IM as the OM is freely permeable for small molecules. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; OM, outer membrane. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The role of FA
In comparison with glucose, chronically excessive FA reach the mitochondria, and pose a great challenge to mitochondrial metabolism by increasing NADH, thus favoring ETC electron flow and mitochondrial inner membrane hyperpolarization that favors superoxide formation (108). In addition, the various ETC defects in the diabetic heart will further increase NADH. Therefore, in contrast to the general consensus that the diabetic heart functions under more oxidizing conditions that drive pathology (12), we propose that a reductive redox stress may precede and induce the observed oxidative stress.
A mainstream hypothesis in the field of DCM supports the dependency of the net mitochondrial ROS efflux on the mitochondrial redox environment, and it states that the ROS levels are minimal at intermediate redox environment values [redox optimized ROS balance hypothesis; for more details, readers are referred to an excellent review on this topic (12)]. The mitochondrial redox environment is provided by the mitochondrial redox couples [NADH/NAD+, NADPH/NADP+, GSH/GSSG, and Trx(SH)2/TrxSS], ranging from extremely reduced to oxidized values depending on mitochondria energetic state. Although the mechanisms differ, both extremes are associated with increased mitochondrial ROS release. At a highly oxidized redox state, the mitochondrial antioxidant defense is overwhelmed. A highly reduced mitochondrial redox state is achieved when the levels of the reducing equivalents in the redox couples are increased. As seen in Figure 3, primary changes in NADH may induce secondary alterations in other redox couples via the forward NNT reaction, and they may occur when NADH is either excessively produced or not oxidized. An excessive oxidation of energy substrates may cause increased mitochondrial NADH, whereas either mitochondrial ETC defects (i.e., complex I defect) or NNT deficiency may decrease NADH oxidation. However, NNT deficiency has been reported to protect the heart against overload-induced HF (135), and, therefore, this is unlikely to contribute to an overly reduced redox environment. Due to increased oxidation of energy substrates with high reducing power and mitochondrial ETC defects, diabetic heart mitochondria may achieve an extremely reduced redox environment. For example, we reported that the NADH/NAD+ ratio is increased in cardiac mitochondria oxidizing palmitoyl-CoA, and normalizing the NADH/NAD+ ratio is beneficial to the diabetic heart (21).
Our hypothesis does not exclude the redox-optimized ROS balance hypothesis, and it proposes that the effect of excessive FA oxidation by the metabolically inflexible diabetic heart is more complex, and exceeds the participation in oxidative stress. The early increase in the reactive metabolites, NADH and acetyl-CoA, may initiate cardiac damage through pathogenic mechanisms such as lysine acetylation of cardiac proteins. Increased circulating FA seems to be the primary event that changes the cardiac NAD+/NADH redox state during nutrient challenges. For example, long-term caloric restriction reduces circulating FA (68) and activates NAD+-dependent mitochondrial sirtuins to deacetylate ETC proteins and alleviate diastolic dysfunction and DCM in T2D (46). In contrast, a short-term (overnight) fasting triggers adipose lipolysis to increase circulating nonesterified FA (75, 80) and decrease cardiac NAD+.
In addition to NAD+/NADH redox couple regulating protein lysine acetylation and energetic metabolism (described in previous sections), the role of increased acetyl-CoA derived from oxidation of excessive fuel substrates is gaining recognition. The importance of acetyl-CoA as a sensor of caloric excess is supported by experiments in mice bearing a deficiency of carnitine acetyltransferase (transports mitochondrial acetyl-CoA to the cytosol), which exhibit increased mitochondrial lysine acetylation induced by excessive mitochondrial acetyl-CoA (127). In addition, an increase in nuclear acetyl-CoA caused histone lysine acetylation and induction of genes involved in utilizing the nutrient excess (197). Acetyl-CoA is used by acetyltransferases as a substrate to acetylate protein lysines. Histone acetyltransferases (p300, CBP, GCN5) regulate chromatin dynamics to activate gene transcription. General control of amino acid synthesis 5 (GCN5) is involved in lysine acetylation and inhibition of PGC-1α (114). A mitochondrial acetyltransferase, GCN5-1, was recently described to counter the effect of SIRT3 on ETC proteins (170). With low binding affinity for acetyl-CoA, acetyltransferases are controlled by acetyl-CoA availability within cellular compartments (196), and are inhibited by CoA, suggesting that the acetyl-CoA/CoA is equally important in regulating their activity (44). Acetyl-CoA can also promote nonenzymatic lysine acetylation (195), and is increased in skeletal muscle (110) and liver mitochondria (88) with high fat feeding.
Both acetyl-CoA excess and NAD+-dependent sirtuin deficiency favor lysine acetylation of cardiomyocyte proteins with a purportedly inhibitory effect on metabolic enzymes to limit the further generation of acetyl-CoA and NADH. This is illustrated in the context of SIRT3 deficiency where known targets, including mitochondrial FA oxidation enzymes (85, 169) and protein subunits of ETC complexes I and II (2, 45), are hyperacetylated and inhibited. However, this potentially sets in motion a vicious cycle that favors increased NADH and a mitochondrial reduced state, further facilitating protein lysine acetylation. In the setting of over-nutrition, a paradoxical association arises: increased cardiac fat utilization not limited by lysine acetylation and mitochondrial ETC defects. In a T1D rodent model, we found increased lysine acetylation of cardiac FA oxidation enzymes despite unchanged SIRT3 protein abundance, which was associated with increased mitochondrial FA oxidation (192). Similarly, HFD led to increased lysine acetylation of FA oxidation enzymes and long chain acylCoA dehydrogenase, LCAD, activation, thus enhancing cardiac FA oxidation (6). We also reported that improving the NAD+/NADH redox state and decreasing lysine acetylation of FA oxidation enzymes limited mitochondrial FA oxidation and improved heart function in T1D (21) (Fig. 6). In conclusion, in the setting of high FA availability, mitochondrial FA oxidation enzymes are activated when hyperacetylated.
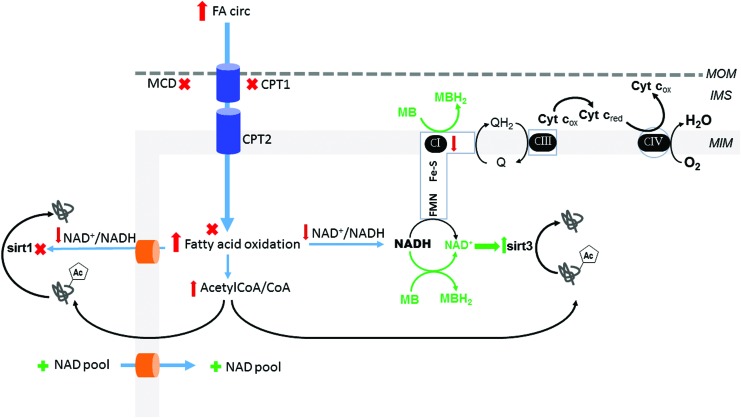
FIG. 6.
Potential therapeutic targets to normalize the NAD+/NADH ratio in the heart during excess energetic supply. The irreversible inhibition (X) of CPT1 with etomoxir proved efficacious in DM patients but was stopped due to side effects (87). MalonylCoA inhibits CPT1, but it is degraded by MCD. MCD inhibitors reduce FA oxidation, increase glucose oxidation, and improve insulin sensitivity (179). Further, MCD-deficient mice were protected against insulin resistance on HFD (190), suggesting that MCD is an important therapeutic target to balance cardiac metabolism in DM, but its benefit has not been evaluated clinically. Trimetazidine, an inhibitor of 3-ketoacyl-CoA thiolase, the last enzymatic step in FA oxidation, increases glucose oxidation (119) and is cardioprotective in diet-induced obese (191) and DM (116) mice, in addition to improving cardiac function in diabetic patients with cardiomyopathy (204). A novel therapeutic approach in diabetes is the use of mitochondrial alternative electron carriers to normalize the NAD+/NADH redox ratio, decrease lysine acetylation, and alleviate the metabolic rigidity and cardiac dysfunction of the diabetic heart (21). The figure shows a proposed mechanism for the lysine deacetylating effect of methylene blue (MB). Diabetic-induced complex I defect causes a decrease in NADH oxidation. In experimental models of rotenone-induced complex I defect, MB accepts electrons from catalytic subunits of complex I and becomes reduced (MBH2) whereas cytochrome c reoxidizes MBH2 to MB [20]. Therefore, MB provides an alternative electron route within the diabetic complex I-deficient cardiac mitochondria and favors NADH oxidation, thus increasing NAD+ and SIRT3 activity. The administration of exogenous NAD or precursors (+) improved the mitochondrial NAD pool and cardiac function (discussed in the main text). DM, diabetes mellitus; HFD, high fat diet. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
This paradox suggests that the effect of sirtuins and lysine acetylation on mitochondrial FA oxidation depends on both the pre-existing biochemical environment and tissue type, and that additional regulatory mechanisms may operate during nutrient excess in the heart. The details underlying this unique mechanism have begun to unfold. One aspect of additional regulation occurs during HFD where SIRT3 is downregulated and the NAD+/NADH ratio is decreased; heat shock protein 10 (SIRT3 substrate) becomes acetylated and induces optimal folding of FA oxidation enzymes to enhance activity and, subsequently, mitochondrial FA oxidation (121). In addition, both enzymatic and nonenzymatic acetylation is operative during nutrient overload, leading to acetylation of lysine sites beyond those recognized as sirtuin targets. Future research will establish whether nonspecific lysine acetylation activates FA oxidation, overriding the inhibitory effect of SIRT3-targeted lysines. An interesting hypothesis was raised by Griffin et al. regarding the functional effect of the site-specific lysine acetylation; the authors noted that several proteomic studies have identified more than 2000 acetylated lysine residues in 400 mitochondrial proteins that function within metabolic pathways. In comparison to phosphorylation, the functional impact of the large number of lysine acetylation sites remains unknown (77). The authors suggest a similarity of acetylation with the oxidative changes of proteins that commonly occur with yet-to-be-known functional changes, but are likely determined by the intensity to which the protein is “decorated” with these post-translational modifications (77). This hypothesis emphasizes the need to understand the critical and specific role of the NADH/NAD+ and acetyl-CoA/CoA as energy sensors, between many others, and pathogenic factors in DCM.
Despite the mainstream postulate supporting the detrimental role of the chronic exposure to excessive FA on cardiac function in DM, there is evidence of an acute beneficial effect of palmitate on cardiac contractile function in models of T1D (189) and T2D (24, 188) during both basal and adrenergic stimulation. In the presence of high glucose, the diabetic hearts efficiently oxidized FA and developed a higher contractile force whereas palmitate was harmful to the normal hearts.
Mitochondrial oxidation of high levels of glucose has been established as having a central mechanistic role in the development of chronic complications in tissues with predominantly insulin-independent glucose uptake, such as the endothelium during microvascular complications (31). In contrast, as pyruvate oxidation decreases in the DM heart, chronic hyperglycemia induces an excessive oxidative redox environment and exhausts mitochondrial antioxidant scavenging systems (24, 188, 189) via extra-mitochondrial mechanisms. As the glutathione and thioredoxin redox couples (major mitochondrial and cytosolic scavengers for the highly diffusible H2O2) become more oxidized during hyperglycemia, short-term mitochondrial palmitate oxidation provides reducing equivalents (NADH) that normalize the mitochondrial redox status, restore the antioxidant potential, and provide a higher energetic budget that rescues the diabetic heart. This emphasizes the short-term benefit of optimizing the high glucose-induced oxidative redox environment by using palmitate, a powerful reducing equivalent generator. A similar benefit was obtained with GSH, emphasizing the acute benefit of correcting the oxidized redox environment to where the net mitochondrial ROS flux is minimal. Chronic administration of palmitate has not been investigated; thus, the results remain in an unresolved contradiction to support the detrimental role of increased mitochondrial FA oxidation in cardiac function.
In light of metabolic changes, the existence of a difference in FA handling between the normal and diabetic heart is not surprising. Chronic exposure of the diabetic hearts to both hyperglycemia and excessive FA has multiple long-term metabolic effects. In addition to decreased insulin signaling, acetyl-CoA and NADH from excessive FA oxidation activate PDK4 (pyruvate dehydrogenase [PDH] inhibitor), thus inhibiting pyruvate oxidation and requiring enhanced FA oxidation to meet cellular demand. Moreover, metabolic gene expression reprograming via PPARα facilitates FA oxidation and suppresses glucose oxidation. Therefore, in contrast to the normal heart, the diabetic heart is conditioned to oxidize the FA excess over glucose. Noteworthy is that ATP is not always measured in DM myocardial samples, raising the possibility that diabetic samples exposed to experimental conditions of high glucose as the only energetic fuel may be energetically deficient and this accounts for the observed decline in cardiac function and palmitate benefit (188).
Acute palmitate has also been reported to rescue the diabetic heart exposed to a β-adrenergic agonist mimicking a physiological increase in workload (188). On physiologic stress, the β-adrenergic-induced increase in cardiomyocyte Ca2+ levels stimulates TCA cycle enzymes (30) to produce NADH to respond to increased NADH demand resulting from increased consumption by the ETC to produce ATP for contraction. FA oxidation fits this “parallel activation” (49), whereas glucose oxidation by the diabetic heart does not [a very informative review is provided in (198)]. On a nonphysiologic workload, such as an increase in preload or postload, the primary mechanism to increase inotropism is governed by the Frank Starling mechanism without an appropriate adrenergic signaling and increase in Ca2+ cardiomyocyte level. The beneficial effect of palmitate has not been studied when the diabetic heart is under these pathological stresses.
Nevertheless, these experiments are in agreement with our hypothesis that FA oxidation is detrimental to the normal heart, and may trigger the cardiac pathology on substrate oversupply. In the oxidized cytosolic redox environment induced by hyperglycemia, mitochondrial FA oxidation offers maximal reducing power and optimizes the redox environment to a level where the mitochondrial ROS release is minimal. However, at higher concentrations, FA oxidation induces an increase in ROS mitochondrial flux (50). We propose that chronic, excessive FA oxidation by the metabolically inflexible diabetic heart is more complex than changing the level of oxidative stress, and includes lysine acetylation and epigenetic changes as key mechanistic factors in DCM.
Future Directions
Cardiac dysfunction induced by overfeeding-induced obesity and diabetes has a multifactorial pathogenesis, and the initiating mechanism is yet to be understood. These conditions cause an increased energetic supply. The increase in mitochondrial FA oxidation at the expense of glucose oxidation indicates the onset of metabolic inflexibility. Caloric restriction alleviates obesity and diabetes-induced diastolic dysfunction in humans and mice; its health benefits are partly mediated by sirtuins, enzymes that remove the acetyl group from lysine residues on proteins, and regulate their activity in an NAD+-dependent manner. NADH, the reduced form of NAD, is produced by fuel oxidation and consumed by mitochondria to produce the oxidized form, NAD+, in the process of oxidative phosphorylation while ATP is formed. In addition, NADH serves as an electron donor used by the enzyme NNT in the forward reaction to form NADPH to maintain optimal antioxidant response. Therefore, the NAD+/NADH redox ratio reflects the mitochondrial function, and it is a cellular regulation node. Although the mitochondrial NAD+/NADH redox ratio has been studied in other organs, its relationship with other cellular compartments and involvement in obesity and diabetes-induced cardiac pathology remains to be elucidated. Exogenous administration of NAD or its precursors increases mitochondrial NAD pool and is beneficial to the metabolic health by maintaining mitochondrial integrity. Their use in the setting of the obese and diabetic heart needs further investigation. Normalizing the mitochondrial redox by shifting electrons away from NADH without changing the NAD pool proved a beneficial therapeutic approach for the diabetic heart (Fig. 6). The role of the NNT to connect fuel oxidation with the antioxidant potential also needs to be unfolded.
Acknowledgments
The authors want to acknowledge the contribution of all research colleagues contributing to this field, and they apologize to those who were not cited in this review due to space limitations. This work was supported by an internal research grant of the University of Medicine and Pharmacy of Timisoara, Romania (PIII-C5-PCFI-2017/2018-01 to D.M.M.), grants from the Juvenile Diabetes Research Foundation International, United Mitochondrial Disease Foundation, and start-up funds from Central Michigan University College of Medicine (to M.G.R.).
Abbreviations Used
- βHB
β-hydroxybutyrate
- CPT1
carnitine palmitoyltransferase 1
- DCM
diabetic cardiomyopathy
- DM
diabetes mellitus
- ETC
electron transport chain (mitochondrial)
- FA
fatty acids
- FOXO
forkhead box O
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GCN5
general control of amino acid synthesis 5
- GSH
glutathione
- GSSG
glutathione disulfide
- GPx
glutathione peroxidase
- H2O2
hydrogen peroxide
- HF
heart failure
- HFD
high fat diet
- LCAD
long chain acylCoA dehydrogenase
- LV
left ventricle
- MCD
malonylCoA decarboxylase
- MnSOD
manganese Superoxide dismutase
- mPTP
mitochondrial permeability transition pore
- NAM
nicotinamide
- NAMPT
nicotinamide phosphoribosyltransferase
- NMN
nicotinamide mononucleotide
- NMNAT
NMN adenylyltransferase
- NNT
nicotinamide nucleotide transhydrogenase
- NR
nicotinamide riboside
- NRF
nuclear respiratory factor
- PARP
poly(ADP ribose) polymerase
- PDH
pyruvate dehydrogenase
- PGC-1α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PPARα
Peroxisome proliferator-activated receptor α
- Prx
peroxiredoxin
- ROS
reactive oxygen species
- SIRT
Sirtuin
- STZ
streptozotocin
- T1D
type 1 DM
- T2D
type 2 DM
- TCA
tricarboxylic Acid Cycle
- TFAM
mitochondrial transcription factor A
- TG
triglycerides
- UPRmt
mitochondrial unfolded protein response
Author Disclosure Statement
The authors confirm that no competing financial interests exist. The authors apologize for not referring other studies due to the length constraints.
References
- 1. Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, Quist W, Lowell BB, Ingwall JS, and Kahn BB. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest 104: 1703–1714, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, and Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A 105: 14447–14452, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akie TE, Liu L, Nam M, Lei S, and Cooper MP. OXPHOS-mediated induction of NAD+ promotes complete oxidation of fatty acids and interdicts non-alcoholic fatty liver disease. PLoS One 10: e0125617, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alano CC, Tran A, Tao R, Ying W, Karliner JS, and Swanson RA. Differences among cell types in NAD(+) compartmentalization: a comparison of neurons, astrocytes, and cardiac myocytes. J Neurosci Res 85: 3378–3385, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, and Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 100: 1512–1521, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Alrob OA, Sankaralingam S, Ma C, Wagg CS, Fillmore N, Jaswal JS, Sack MN, Lehner R, Gupta MP, Michelakis ED, Padwal RS, Johnstone DE, Sharma AM, and Lopaschuk GD. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc Res 103: 485–497, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altun G, Babaoglu K, Binnetoglu K, Ozsu E, Yesiltepe Mutlu RG, and Hatun S. Subclinical left ventricular longitudinal and radial systolic dysfunction in children and adolescents with type 1 diabetes mellitus. Echocardiography 33: 1032–1039, 2016 [DOI] [PubMed] [Google Scholar]
- 8. American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 40: S11–S24, 2017 [DOI] [PubMed] [Google Scholar]
- 9. Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, and Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol 54: 1891–1898, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, and Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andreux PA, Houtkooper RH, and Auwerx J. Pharmacological approaches to restore mitochondrial function. Nat Rev Drug Discov 12: 465–483, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aon MA, Tocchetti CG, Bhatt N, Paolocci N, and Cortassa S. Protective mechanisms of mitochondria and heart function in diabetes. Antioxid Redox Signal 22: 1563–1586, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, and Spiegelman BM. Transcriptional coactivator PGC-1α controls the energy state and contractile function of cardiac muscle. Cell Metab 1: 259–271, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Bagul PK, Deepthi N, Sultana R, and Banerjee SK. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J Nutr Biochem 26: 1298–1307, 2015 [DOI] [PubMed] [Google Scholar]
- 15. Bagul PK, Dinda AK, and Banerjee SK. Effect of resveratrol on sirtuins expression and cardiac complications in diabetes. Biochem Biophys Res Commun 468: 221–227, 2015 [DOI] [PubMed] [Google Scholar]
- 16. Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, and Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 13: 461–468, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bayeva M, Sawicki KT, and Ardehali H. Taking diabetes to heart—deregulation of myocardial lipid metabolism in diabetic cardiomyopathy. J Am Heart Assoc 2: e000433, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beaudoin MS, Perry CG, Arkell AM, Chabowski A, Simpson JA, Wright DC, and Holloway GP. Impairments in mitochondrial palmitoyl-CoA respiratory kinetics that precede development of diabetic cardiomyopathy are prevented by resveratrol in ZDF rats. J Physiol 592: 2519–2533, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, Zhang D, Cooksey RC, McClain DA, Litwin SE, Taegtmeyer H, Severson D, Kahn CR, and Abel ED. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest 109: 629–639, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Belke DD, Swanson EA, and Dillmann WH. Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes 53: 3201–3208, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Berthiaume JM, Hsiung CH, Austin AB, McBrayer SP, Depuydt MM, Chandler MP, Miyagi M, and Rosca MG. Methylene blue decreases mitochondrial lysine acetylation in the diabetic heart. Mol Cell Biochem 432: 7–24, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bharathi SS, Zhang Y, Mohsen AW, Uppala R, Balasubramani M, Schreiber E, Uechi G, Beck ME, Rardin MJ, Vockley J, Verdin E, Gibson BW, Hirschey MD, and Goetzman ES. Sirtuin 3 (SIRT3) protein regulates long-chain acyl-CoA dehydrogenase by deacetylating conserved lysines near the active site. J Biol Chem 288: 33837–33847, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhatt JK, Thomas S, and Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res 32: 537–541, 2012 [DOI] [PubMed] [Google Scholar]
- 24. Bhatt NM, Aon MA, Tocchetti CG, Shen X, Dey S, Ramirez-Correa G, O'Rourke B, Gao WD, and Cortassa S. Restoring redox balance enhances contractility in heart trabeculae from type 2 diabetic rats exposed to high glucose. Am J Physiol Heart Circ Physiol 308: H291–H302, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blacker TS, Mann ZF, Gale JE, Ziegler M, Bain AJ, Szabadkai G, and Duchen MR. Separating NADH and NADPH fluorescence in live cells and tissues using FLIM. Nat Commun 5: 3936, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bogenhagen DF. Mitochondrial DNA nucleoid structure. Biochim Biophys Acta 1819: 914–920, 2012 [DOI] [PubMed] [Google Scholar]
- 27. Boudina S, Sena S, O'Neill BT, Tathireddy P, Young ME, and Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation 112: 2686–2695, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, and Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 56: 2457–2466, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Brindley DN, Kok BP, Kienesberger PC, Lehner R, and Dyck JR. Shedding light on the enigma of myocardial lipotoxicity: the involvement of known and putative regulators of fatty acid storage and mobilization. Am J Physiol Endocrinol Metab 298: E897–E908, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Bround MJ, Wambolt R, Cen H, Asghari P, Albu RF, Han J, McAfee D, Pourrier M, Scott NE, Bohunek L, Kulpa JE, Chen SR, Fedida D, Brownsey RW, Borchers CH, Foster LJ, Mayor T, Moore ED, Allard MF, and Johnson JD. Cardiac Ryanodine Receptor (Ryr2)-mediated Calcium Signals Specifically Promote Glucose Oxidation via Pyruvate Dehydrogenase. J Biol Chem 291: 23490–23505, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54: 1615–1625, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, and Abel ED. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 146: 5341–5349, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Bugger H, Boudina S, Hu XX, Tuinei J, Zaha VG, Theobald HA, Yun UJ, McQueen AP, Wayment B, Litwin SE, and Abel ED. Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes 57: 2924–2932, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bugger H, Chen D, Riehle C, Soto J, Theobald HA, Hu XX, Ganesan B, Weimer BC, and Abel ED. Tissue-specific remodeling of the mitochondrial proteome in type 1 diabetic akita mice. Diabetes 58: 1986–1997, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cambronne XA, Stewart ML, Kim D, Jones-Brunette AM, Morgan RK, Farrens DL, Cohen MS, and Goodman RH. Biosensor reveals multiple sources for mitochondrial NAD(+). Science 352: 1474–1477, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Campbell CT, Kolesar JE, and Kaufman BA. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim Biophys Acta 1819: 921–929, 2012 [DOI] [PubMed] [Google Scholar]
- 37. Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, and Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458: 1056–1060, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, and Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab 11: 213–219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Canto C, Menzies KJ, and Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: a Balancing Act between Mitochondria and the Nucleus. Cell Metab 22: 31–53, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carley AN, Atkinson LL, Bonen A, Harper ME, Kunnathu S, Lopaschuk GD, and Severson DL. Mechanisms responsible for enhanced fatty acid utilization by perfused hearts from type 2 diabetic db/db mice. Arch Physiol Biochem 113: 65–75, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Cerutti R, Pirinen E, Lamperti C, Marchet S, Sauve AA, Li W, Leoni V, Schon EA, Dantzer F, Auwerx J, Viscomi C, and Zeviani M. NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab 19: 1042–1049, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, and Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278: 36027–36031, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, and Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A 100: 10794–10799, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Choudhary C, Weinert BT, Nishida Y, Verdin E, and Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat Rev Mol Cell Biol 15: 536–550, 2014 [DOI] [PubMed] [Google Scholar]
- 45. Cimen H, Han MJ, Yang Y, Tong Q, Koc H, and Koc EC. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry 49: 304–311, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cohen K, Waldman M, Abraham NG, Laniado-Schwartzman M, Gurfield D, Aravot D, Arad M, and Hochhauser E. Caloric restriction ameliorates cardiomyopathy in animal model of diabetes. Exp Cell Res 350: 147–153, 2017 [DOI] [PubMed] [Google Scholar]
- 47. Collaboration NCDRF. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387: 1513–1530, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cook GA, Lavrentyev EN, Pham K, and Park EA. Streptozotocin diabetes increases mRNA expression of ketogenic enzymes in the rat heart. Biochim Biophys Acta 1861: 307–312, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cortassa S, Aon MA, O'Rourke B, Jacques R, Tseng HJ, Marban E, and Winslow RL. A computational model integrating electrophysiology, contraction, and mitochondrial bioenergetics in the ventricular myocyte. Biophys J 91: 1564–1589, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cortassa S, Sollott SJ, and Aon MA. Mitochondrial respiration and ROS emission during beta-oxidation in the heart: an experimental-computational study. PLoS Comput Biol 13: e1005588, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. This reference has been deleted.
- 52. Croston TL, Thapa D, Holden AA, Tveter KJ, Lewis SE, Shepherd DL, Nichols CE, Long DM, Olfert IM, Jagannathan R, and Hollander JM. Functional deficiencies of subsarcolemmal mitochondria in the type 2 diabetic human heart. Am J Physiol Heart Circ Physiol 307: H54–H65, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dabelea D, Rewers A, Stafford JM, Standiford DA, Lawrence JM, Saydah S, Imperatore G, D'Agostino RB, Jr., Mayer-Davis EJ, Pihoker C, and Group SfDiYS. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics 133: e938–e945, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dabkowski ER, Baseler WA, Williamson CL, Powell M, Razunguzwa TT, Frisbee JC, and Hollander JM. Mitochondrial dysfunction in the type 2 diabetic heart is associated with alterations in spatially distinct mitochondrial proteomes. Am J Physiol Heart Circ Physiol 299: H529–H540, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. De Jong KA. and Lopaschuk GD. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can J Cardiol 33: 860–871, 2017 [DOI] [PubMed] [Google Scholar]
- 56. This reference has been deleted.
- 57. Duicu OM, Lighezan R, Sturza A, Balica R, Vaduva A, Feier H, Gaspar M, Ionac A, Noveanu L, Borza C, Muntean DM, and Mornos C. Assessment of mitochondrial dysfunction and monoamine oxidase contribution to oxidative stress in human diabetic hearts. Oxid Med Cell Longev 2016: 8470394, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Duncan JG, Fong JL, Medeiros DM, Finck BN, and Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation 115: 909–917, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Duncan JG. and Finck BN. The PPARalpha-PGC-1alpha axis controls cardiac energy metabolism in healthy and diseased myocardium. PPAR Res 2008: 253817, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Eaton P, Wright N, Hearse DJ, and Shattock MJ. Glyceraldehyde phosphate dehydrogenase oxidation during cardiac ischemia and reperfusion. J Mol Cell Cardiol 34: 1549–1560, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Fam BC Rose LJ, Sgambellone R, Ruan Z, Proietto J, and Andrikopoulos S. Normal muscle glucose uptake in mice deficient in muscle GLUT4. J Endocrinol 214: 313–327, 2012 [DOI] [PubMed] [Google Scholar]
- 62. Faria AM, Papadimitriou A, Silva KC, Lopes de Faria JM, and Lopes de Faria JB. Uncoupling endothelial nitric oxide synthase is ameliorated by green tea in experimental diabetes by re-establishing tetrahydrobiopterin levels. Diabetes 61: 1838–1847, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fauconnier J, Andersson DC, Zhang SJ, Lanner JT, Wibom R, Katz A, Bruton JD, and Westerblad H. Effects of palmitate on Ca(2+) handling in adult control and ob/ob cardiomyocytes: impact of mitochondrial reactive oxygen species. Diabetes 56: 1136–1142, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Fernandez-Marcos PJ. and Auwerx J. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 93: 884S–890S, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, and Kelly DP. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest 109: 121–130, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fischer VW, Barner HB, and Larose LS. Pathomorphologic aspects of muscular tissue in diabetes mellitus. Hum Pathol 15: 1127–1136, 1984 [DOI] [PubMed] [Google Scholar]
- 67. Flarsheim CE, Grupp IL, and Matlib MA. Mitochondrial dysfunction accompanies diastolic dysfunction in diabetic rat heart. Am J Physiol 271: H192–H202, 1996 [DOI] [PubMed] [Google Scholar]
- 68. Fontana L, Meyer TE, Klein S, and Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A 101: 6659–6663, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Frederick DW, Davis JG, Davila A, Jr., Agarwal B, Michan S, Puchowicz MA, Nakamaru-Ogiso E, and Baur JA. Increasing NAD synthesis in muscle via nicotinamide phosphoribosyltransferase is not sufficient to promote oxidative metabolism. J Biol Chem 290: 1546–1558, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. This reference has been deleted.
- 71. Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, and Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 26: 1913–1923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gerhart-Hines Z, Dominy JE, Jr., Blattler SM, Jedrychowski MP, Banks AS, Lim JH, Chim H, Gygi SP, and Puigserver P. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+). Mol Cell 44: 851–863, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gilde AJ, van der Lee KA, Willemsen PH, Chinetti G, van der Leij FR, van der Vusse GJ, Staels B, and van Bilsen M. Peroxisome proliferator-activated receptor (PPAR) alpha and PPARbeta/delta, but not PPARgamma, modulate the expression of genes involved in cardiac lipid metabolism. Circ Res 92: 518–524, 2003 [DOI] [PubMed] [Google Scholar]
- 74. Glancy B. and Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 51: 2959–2973, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Goldrick RB. and Hirsch J. Serial Studies on the Metabolism of Human Adipose Tissue. Ii. Effects of caloric restriction and refeeding on lipogenesis, and the uptake and release of free fatty acids in obese and nonobese individuals. J Clin Invest 43: 1793–1804, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, and Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155: 1624–1638, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Griffin TM, Humphries KM, Kinter M, Lim HY, and Szweda LI. Nutrient sensing and utilization: getting to the heart of metabolic flexibility. Biochimie 124: 74–83, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Guo R, Liu W, Liu B, Zhang B, Li W, and Xu Y. SIRT1 suppresses cardiomyocyte apoptosis in diabetic cardiomyopathy: an insight into endoplasmic reticulum stress response mechanism. Int J Cardiol 191: 36–45, 2015 [DOI] [PubMed] [Google Scholar]
- 79. Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, and Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2: 914–923, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hammer S, van der Meer RW, Lamb HJ, Schar M, de Roos A, Smit JW, and Romijn JA. Progressive caloric restriction induces dose-dependent changes in myocardial triglyceride content and diastolic function in healthy men. J Clin Endocrinol Metab 93: 497–503, 2008 [DOI] [PubMed] [Google Scholar]
- 81. Herlein JA, Fink BD, O'Malley Y, and Sivitz WI. Superoxide and respiratory coupling in mitochondria of insulin-deficient diabetic rats. Endocrinology 150: 46–55, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Herrero P, Peterson LR, McGill JB, Matthew S, Lesniak D, Dence C, and Gropler RJ. Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. J Am Coll Cardiol 47: 598–604, 2006 [DOI] [PubMed] [Google Scholar]
- 83. Hershberger KA, Martin AS, and Hirschey MD. Role of NAD+ and mitochondrial sirtuins in cardiac and renal diseases. Nat Rev Nephrol 13: 213–225, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr., Alt FW, Kahn CR, and Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464: 121–125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hirschey MD, Shimazu T, Huang JY, Schwer B, and Verdin E. SIRT3 regulates mitochondrial protein acetylation and intermediary metabolism. Cold Spring Harb Symp Quant Biol 76: 267–277, 2011 [DOI] [PubMed] [Google Scholar]
- 86. Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stancakova A, Goetzman E, Lam MM, Schwer B, Stevens RD, Muehlbauer MJ, Kakar S, Bass NM, Kuusisto J, Laakso M, Alt FW, Newgard CB, Farese RV, Jr., Kahn CR, and Verdin E. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell 44: 177–190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Holubarsch CJ, Rohrbach M, Karrasch M, Boehm E, Polonski L, Ponikowski P, and Rhein S. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO (etomoxir for the recovery of glucose oxidation) study. Clin Sci (Lond) 113: 205–212, 2007 [DOI] [PubMed] [Google Scholar]
- 88. Horie S, Isobe M, and Suga T. Changes in CoA pools in hepatic peroxisomes of the rat under various conditions. J Biochem 99: 1345–1352, 1986 [DOI] [PubMed] [Google Scholar]
- 89. Horton JL, Martin OJ, Lai L, Riley NM, Richards AL, Vega RB, Leone TC, Pagliarini DJ, Muoio DM, Bedi KC, Jr., Margulies KB, Coon JJ, and Kelly DP. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight 2: pii:, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. How OJ, Aasum E, Severson DL, Chan WY, Essop MF, and Larsen TS. Increased myocardial oxygen consumption reduces cardiac efficiency in diabetic mice. Diabetes 55: 466–473, 2006 [DOI] [PubMed] [Google Scholar]
- 91. Hsu CP, Oka S, Shao D, Hariharan N, and Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res 105: 481–491, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Huynh K, Kiriazis H, Du XJ, Love JE, Gray SP, Jandeleit-Dahm KA, McMullen JR, and Ritchie RH. Targeting the upregulation of reactive oxygen species subsequent to hyperglycemia prevents type 1 diabetic cardiomyopathy in mice. Free Radic Biol Med 60: 307–317, 2013 [DOI] [PubMed] [Google Scholar]
- 93. Ilkun O, Wilde N, Tuinei J, Pires KM, Zhu Y, Bugger H, Soto J, Wayment B, Olsen C, Litwin SE, and Abel ED. Antioxidant treatment normalizes mitochondrial energetics and myocardial insulin sensitivity independently of changes in systemic metabolic homeostasis in a mouse model of the metabolic syndrome. J Mol Cell Cardiol 85: 104–116, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Imai S, Armstrong CM, Kaeberlein M, and Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403: 795–800, 2000 [DOI] [PubMed] [Google Scholar]
- 95. Jensen MT, Sogaard P, Andersen HU, Bech J, Hansen TF, Galatius S, Jorgensen PG, Biering-Sorensen T, Mogelvang R, Rossing P, and Jensen JS. Prevalence of systolic and diastolic dysfunction in patients with type 1 diabetes without known heart disease: the Thousand & 1 Study. Diabetologia 57: 672–680, 2014 [DOI] [PubMed] [Google Scholar]
- 96. Jing E, O'Neill BT, Rardin MJ, Kleinridders A, Ilkeyeva OR, Ussar S, Bain JR, Lee KY, Verdin EM, Newgard CB, Gibson BW, and Kahn CR. Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes 62: 3404–3417, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jones DP. and Sies H. The Redox Code. Antioxid Redox Signal 23: 734–746, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jovaisaite V. and Auwerx J. The mitochondrial unfolded protein response-synchronizing genomes. Curr Opin Cell Biol 33: 74–81, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kannel WB, Hjortland M, and Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 34: 29–34, 1974 [DOI] [PubMed] [Google Scholar]
- 100. Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC, Jr., Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, and Tian R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab 18: 239–250, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Keating ST, Plutzky J, and El-Osta A. Epigenetic changes in diabetes and cardiovascular risk. Circ Res 118: 1706–1722, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kelly DP. and Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev 18: 357–368, 2004 [DOI] [PubMed] [Google Scholar]
- 103. Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsstrom S, Pasila L, Velagapudi V, Carroll CJ, Auwerx J, and Suomalainen A. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol Med 6: 721–731, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Khanra R, Dewanjee S, Dua TK, Sahu R, Gangopadhyay M, De Feo V, and Zia-Ul-Haq M. Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J Transl Med 13: 6, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kienesberger PC, Pulinilkunnil T, Nagendran J, and Dyck JR. Myocardial triacylglycerol metabolism. J Mol Cell Cardiol 55: 101–110, 2013 [DOI] [PubMed] [Google Scholar]
- 106. Koentges C, Pfeil K, Schnick T, Wiese S, Dahlbock R, Cimolai MC, Meyer-Steenbuck M, Cenkerova K, Hoffmann MM, Jaeger C, Odening KE, Kammerer B, Hein L, Bode C, and Bugger H. SIRT3 deficiency impairs mitochondrial and contractile function in the heart. Basic Res Cardiol 110: 36, 2015 [DOI] [PubMed] [Google Scholar]
- 107. Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, and Chang Y. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One 5: e11707, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Korshunov SS, Skulachev VP, and Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416: 15–18, 1997 [DOI] [PubMed] [Google Scholar]
- 109. Kouzu H, Miki T, Tanno M, Kuno A, Yano T, Itoh T, Sato T, Sunaga D, Murase H, Tobisawa T, Ogasawara M, Ishikawa S, and Miura T. Excessive degradation of adenine nucleotides by up-regulated AMP deaminase underlies afterload-induced diastolic dysfunction in the type 2 diabetic heart. J Mol Cell Cardiol 80: 136–145, 2015 [DOI] [PubMed] [Google Scholar]
- 110. Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, and Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008 [DOI] [PubMed] [Google Scholar]
- 111. Lashin OM, Szweda PA, Szweda LI, and Romani AM. Decreased complex II respiration and HNE-modified SDH subunit in diabetic heart. Free Radic Biol Med 40: 886–896, 2006 [DOI] [PubMed] [Google Scholar]
- 112. Lehman JJ, Boudina S, Banke NH, Sambandam N, Han X, Young DM, Leone TC, Gross RW, Lewandowski ED, Abel ED, and Kelly DP. The transcriptional coactivator PGC-1alpha is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am J Physiol Heart Circ Physiol 295: H185–H196, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, and Kelly DP. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3: e101, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, and Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab 3: 429–438, 2006 [DOI] [PubMed] [Google Scholar]
- 115. Lewis CA, Parker SJ, Fiske BP, McCloskey D, Gui DY, Green CR, Vokes NI, Feist AM, Vander Heiden MG, and Metallo CM. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol Cell 55: 253–263, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Li YJ, Wang PH, Chen C, Zou MH, and Wang DW. Improvement of mechanical heart function by trimetazidine in db/db mice. Acta Pharmacol Sin 31: 560–569, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Liu K, Zhou R, Wang B, and Mi MT. Effect of resveratrol on glucose control and insulin sensitivity: a meta-analysis of 11 randomized controlled trials. Am J Clin Nutr 99: 1510–1519, 2014 [DOI] [PubMed] [Google Scholar]
- 118. Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Jr., Weissman S, Verdin E, and Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27: 8807–8814, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lopaschuk GD, Barr R, Thomas PD, and Dyck JR. Beneficial effects of trimetazidine in ex vivo working ischemic hearts are due to a stimulation of glucose oxidation secondary to inhibition of long-chain 3-ketoacyl coenzyme a thiolase. Circ Res 93: e33–e37, 2003 [DOI] [PubMed] [Google Scholar]
- 120. Love NR, Pollak N, Dolle C, Niere M, Chen Y, Oliveri P, Amaya E, Patel S, and Ziegler M. NAD kinase controls animal NADP biosynthesis and is modulated via evolutionarily divergent calmodulin-dependent mechanisms. Proc Natl Acad Sci U S A 112: 1386–1391, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lu Z, Chen Y, Aponte AM, Battaglia V, Gucek M, and Sack MN. Prolonged fasting identifies heat shock protein 10 as a Sirtuin 3 substrate: elucidating a new mechanism linking mitochondrial protein acetylation to fatty acid oxidation enzyme folding and function. J Biol Chem 290: 2466–2476, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Luiken JJ, Arumugam Y, Bell RC, Calles-Escandon J, Tandon NN, Glatz JF, and Bonen A. Changes in fatty acid transport and transporters are related to the severity of insulin deficiency. Am J Physiol Endocrinol Metab 283: E612–E621, 2002 [DOI] [PubMed] [Google Scholar]
- 123. This reference has been deleted
- 124. Mitra R, Nogee DP, Zechner JF, Yea K, Gierasch CM, Kovacs A, Medeiros DM, Kelly DP, and Duncan JG. The transcriptional coactivators, PGC-1alpha and beta, cooperate to maintain cardiac mitochondrial function during the early stages of insulin resistance. J Mol Cell Cardiol 52: 701–710, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Montaigne D, Marechal X, Coisne A, Debry N, Modine T, Fayad G, Potelle C, El Arid JM, Mouton S, Sebti Y, Duez H, Preau S, Remy-Jouet I, Zerimech F, Koussa M, Richard V, Neviere R, Edme JL, Lefebvre P, and Staels B. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation 130: 554–564, 2014 [DOI] [PubMed] [Google Scholar]
- 126. Mori J, Alrob OA, Wagg CS, Harris RA, Lopaschuk GD, and Oudit GY. ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: a critical role of PDK4. Am J Physiol Heart Circ Physiol 304: H1103–H1113, 2013 [DOI] [PubMed] [Google Scholar]
- 127. Muoio DM, Noland RC, Kovalik JP, Seiler SE, Davies MN, DeBalsi KL, Ilkayeva OR, Stevens RD, Kheterpal I, Zhang J, Covington JD, Bajpeyi S, Ravussin E, Kraus W, Koves TR, and Mynatt RL. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab 15: 764–777, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nakahata Y, Sahar S, Astarita G, Kaluzova M, and Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324: 654–657, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Nakamura H, Matoba S, Iwai-Kanai E, Kimata M, Hoshino A, Nakaoka M, Katamura M, Okawa Y, Ariyoshi M, Mita Y, Ikeda K, Okigaki M, Adachi S, Tanaka H, Takamatsu T, and Matsubara H. p53 promotes cardiac dysfunction in diabetic mellitus caused by excessive mitochondrial respiration-mediated reactive oxygen species generation and lipid accumulation. Circ Heart Fail 5: 106–115, 2012 [DOI] [PubMed] [Google Scholar]
- 130. Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, and Namba M. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation 98: 794–799, 1998 [DOI] [PubMed] [Google Scholar]
- 131. Nam DH, Han JH, Lee TJ, Shishido T, Lim JH, Kim GY, and Woo CH. CHOP deficiency prevents methylglyoxal-induced myocyte apoptosis and cardiac dysfunction. J Mol Cell Cardiol 85: 168–177, 2015 [DOI] [PubMed] [Google Scholar]
- 132. Nemoto S, Fergusson MM, and Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. J Biol Chem 280: 16456–16460, 2005 [DOI] [PubMed] [Google Scholar]
- 133. Netticadan T, Temsah RM, Kent A, Elimban V, and Dhalla NS. Depressed levels of Ca2+-cycling proteins may underlie sarcoplasmic reticulum dysfunction in the diabetic heart. Diabetes 50: 2133–2138, 2001 [DOI] [PubMed] [Google Scholar]
- 134. Nguyen TT, Wong R, Menazza S, Sun J, Chen Y, Wang G, Gucek M, Steenbergen C, Sack MN, and Murphy E. Cyclophilin D modulates mitochondrial acetylome. Circ Res 113: 1308–1319, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Nickel AG, von Hardenberg A, Hohl M, Loffler JR, Kohlhaas M, Becker J, Reil JC, Kazakov A, Bonnekoh J, Stadelmaier M, Puhl SL, Wagner M, Bogeski I, Cortassa S, Kappl R, Pasieka B, Lafontaine M, Lancaster CR, Blacker TS, Hall AR, Duchen MR, Kastner L, Lipp P, Zeller T, Muller C, Knopp A, Laufs U, Bohm M, Hoth M, and Maack C. Reversal of Mitochondrial Transhydrogenase Causes Oxidative Stress in Heart Failure. Cell Metab 22: 472–484, 2015 [DOI] [PubMed] [Google Scholar]
- 136. Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, and Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000 [DOI] [PubMed] [Google Scholar]
- 137. Nuutila P, Koivisto VA, Knuuti J, Ruotsalainen U, Teras M, Haaparanta M, Bergman J, Solin O, Voipio-Pulkki LM, Wegelius U, and Yki-Järvinen H. Glucose-free fatty acid cycle operates in human heart and skeletal muscle in vivo. J Clin Invest 89: 1767–1774, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ouwens DM, Diamant M, Fodor M, Habets DD, Pelsers MM, El Hasnaoui M, Dang ZC, van den Brom CE, Vlasblom R, Rietdijk A, Boer C, Coort SL, Glatz JF, and Luiken JJ. Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia 50: 1938–1948, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Oyarzun AP, Westermeier F, Pennanen C, Lopez-Crisosto C, Parra V, Sotomayor-Flores C, Sanchez G, Pedrozo Z, Troncoso R, and Lavandero S. FK866 compromises mitochondrial metabolism and adaptive stress responses in cultured cardiomyocytes. Biochem Pharmacol 98: 92–101, 2015 [DOI] [PubMed] [Google Scholar]
- 140. Ozden O, Park SH, Wagner BA, Yong Song H, Zhu Y, Vassilopoulos A, Jung B, Buettner GR, and Gius D. SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells. Free Radic Biol Med 76: 163–172, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Papa L. and Germain D. SirT3 regulates the mitochondrial unfolded protein response. Mol Cell Biol 34: 699–710, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, Levine DC, Bacsik DJ, Gius D, Newgard CB, Goetzman E, Chandel NS, Denu JM, Mrksich M, and Bass J. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342: 1243417, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pillai JB, Isbatan A, Imai S, and Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem 280: 43121–43130, 2005 [DOI] [PubMed] [Google Scholar]
- 144. Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, Samant S, Ravindra PV, Isbatan A, and Gupta MP. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem 285: 3133–3144, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pittelli M, Felici R, Pitozzi V, Giovannelli L, Bigagli E, Cialdai F, Romano G, Moroni F, and Chiarugi A. Pharmacological effects of exogenous NAD on mitochondrial bioenergetics, DNA repair, and apoptosis. Mol Pharmacol 80: 1136–1146, 2011 [DOI] [PubMed] [Google Scholar]
- 146. Ptitsyn A, Hulver M, Cefalu W, York D, and Smith SR. Unsupervised clustering of gene expression data points at hypoxia as possible trigger for metabolic syndrome. BMC Genomics 7: 318, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Puchalska P. and Crawford PA. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab 25: 262–284, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Qin WD, Liu GL, Wang J, Wang H, Zhang JN, Zhang F, Ma Y, Ji XY, Li C, and Zhang MX. Poly(ADP-ribose) polymerase 1 inhibition protects cardiomyocytes from inflammation and apoptosis in diabetic cardiomyopathy. Oncotarget 7: 35618–35631, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ramirez-Correa GA, Ma J, Slawson C, Zeidan Q, Lugo-Fagundo NS, Xu M, Shen X, Gao WD, Caceres V, Chakir K, DeVine L, Cole RN, Marchionni L, Paolocci N, Hart GW, and Murphy AM. Removal of abnormal myofilament O-GlcNAcylation restores Ca2+ sensitivity in diabetic cardiac muscle. Diabetes 64: 3573–3587, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Randle PJ, Garland PB, Hales CN, and Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1: 785–789, 1963 [DOI] [PubMed] [Google Scholar]
- 151. Raza H, John A, and Howarth FC. Alterations in glutathione redox metabolism, oxidative stress, and mitochondrial function in the left ventricle of elderly Zucker diabetic fatty rat heart. Int J Mol Sci 13: 16241–16254, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ren J, Pulakat L, Whaley-Connell A, and Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med (Berl) 88: 993–1001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Riddle MC. Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation 122: 844–846, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, and Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54: 8–14, 2005 [DOI] [PubMed] [Google Scholar]
- 155. Ronchi JA, Francisco A, Passos LA, Figueira TR, and Castilho RF. The contribution of nicotinamide nucleotide transhydrogenase to peroxide detoxification is dependent on the respiratory state and counterbalanced by other sources of NADPH in liver mitochondria. J Biol Chem 291: 20173–20187, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Rosca MG, Mustata TG, Kinter MT, Ozdemir AM, Kern TS, Szweda LI, Brownlee M, Monnier VM, and Weiss MF. Glycation of mitochondrial proteins from diabetic rat kidney is associated with excess superoxide formation. Am J Physiol Renal Physiol 289: F420–F430, 2005 [DOI] [PubMed] [Google Scholar]
- 157. Rosca MG. and Hoppel CL. Mitochondria in heart failure. Cardiovasc Res 88: 40–50, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Rosca MG, Vazquez EJ, Chen Q, Kerner J, Kern TS, and Hoppel CL. Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes 61: 2074–2083, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, and Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30: 595–602, 1972 [DOI] [PubMed] [Google Scholar]
- 160. Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, Medeiros DM, Valencik ML, McDonald JA, and Kelly DP. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res 94: 525–533, 2004 [DOI] [PubMed] [Google Scholar]
- 161. Rydstrom J. Mitochondrial NADPH, transhydrogenase and disease. Biochim Biophys Acta 1757: 721–726, 2006 [DOI] [PubMed] [Google Scholar]
- 162. Salpea KD, Talmud PJ, Cooper JA, Maubaret CG, Stephens JW, Abelak K, and Humphries SE. Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis 209: 42–50, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Samant SA, Zhang HJ, Hong Z, Pillai VB, Sundaresan NR, Wolfgeher D, Archer SL, Chan DC, and Gupta MP. SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress. Mol Cell Biol 34: 807–819, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Sankaralingam S, Alrob OA, Zhang L, Jaswal JS, Wagg CS, Fukushima A, Padwal RS, Johnstone DE, Sharma AM, and Lopaschuk GD. Lowering body weight in obese mice with diastolic heart failure improves cardiac insulin sensitivity and function: implications for the Obesity Paradox. Diabetes 64: 1643–1657, 2015 [DOI] [PubMed] [Google Scholar]
- 165. Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, and Imai S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab 18: 416–430, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Schannwell CM, Schneppenheim M, Perings S, Plehn G, and Strauer BE. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology 98: 33–39, 2002 [DOI] [PubMed] [Google Scholar]
- 167. Scheibye-Knudsen M, Mitchell SJ, Fang EF, Iyama T, Ward T, Wang J, Dunn CA, Singh N, Veith S, Hasan-Olive MM, Mangerich A, Wilson MA, Mattson MP, Bergersen LH, Cogger VC, Warren A, Le Couteur DG, Moaddel R, Wilson DM, 3rd, Croteau DL, de Cabo R, and Bohr VA. A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in cockayne syndrome. Cell Metab 20: 840–855, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, and Clarke K. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 107: 3040–3046, 2003 [DOI] [PubMed] [Google Scholar]
- 169. Schwer B, Bunkenborg J, Verdin RO, Andersen JS, and Verdin E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc Natl Acad Sci U S A 103: 10224–10229, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Scott I, Webster BR, Li JH, and Sack MN. Identification of a molecular component of the mitochondrial acetyltransferase programme: a novel role for GCN5 L1. Biochem J 443: 655–661, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Serpillon S, Floyd BC, Gupte RS, George S, Kozicky M, Neito V, Recchia F, Stanley W, Wolin MS, and Gupte SA. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am J Physiol Heart Circ Physiol 297: H153–H162, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Shah MS. and Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 118: 1808–1829, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Shen X, Zheng S, Thongboonkerd V, Xu M, Pierce WM, Jr., Klein JB, and Epstein PN. Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am J Physiol Endocrinol Metab 287: E896–E905, 2004 [DOI] [PubMed] [Google Scholar]
- 174. Shen X, Zheng S, Metreveli NS, and Epstein PN. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes 55: 798–805, 2006 [DOI] [PubMed] [Google Scholar]
- 175. Sivitz WI. and Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal 12: 537–577, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, and Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143: 802–812, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. St-Pierre J, Buckingham JA, Roebuck SJ, and Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277: 44784–44790, 2002 [DOI] [PubMed] [Google Scholar]
- 178. This reference has been deleted
- 179. Stanley WC, Morgan EE, Huang H, McElfresh TA, Sterk JP, Okere IC, Chandler MP, Cheng J, Dyck JR, and Lopaschuk GD. Malonyl-CoA decarboxylase inhibition suppresses fatty acid oxidation and reduces lactate production during demand-induced ischemia. Am J Physiol Heart Circ Physiol 289: H2304–H2309, 2005 [DOI] [PubMed] [Google Scholar]
- 180. Sturza A, Duicu OM, Vaduva A, Danila MD, Noveanu L, Varro A, and Muntean DM. Monoamine oxidases are novel sources of cardiovascular oxidative stress in experimental diabetes. Can J Physiol Pharmacol 93: 555–561, 2015 [DOI] [PubMed] [Google Scholar]
- 181. Sun W, Quan N, Wang L, Yang H, Chu D, Liu Q, Zhao X, Leng J, and Li J. Cardiac-Specific Deletion of the Pdha1 Gene Sensitizes Heart to Toxicological Actions of Ischemic Stress. Toxicol Sci 153: 411, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, and Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 119: 2758–2771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Sung HJ, Ma W, Wang PY, Hynes J, O'Riordan TC, Combs CA, McCoy JP, Jr., Bunz F, Kang JG, and Hwang PM. Mitochondrial respiration protects against oxygen-associated DNA damage. Nat Commun 1: 5, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Sung MM, Hamza SM, and Dyck JR. Myocardial metabolism in diabetic cardiomyopathy: potential therapeutic targets. Antioxid Redox Signal 22: 1606–1630, 2015 [DOI] [PubMed] [Google Scholar]
- 185. Tanno M, Sakamoto J, Miura T, Shimamoto K, and Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem 282: 6823–6832, 2007 [DOI] [PubMed] [Google Scholar]
- 186. Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, Shimamoto K, and Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem 285: 8375–8382, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Tian C, Alomar F, Moore CJ, Shao CH, Kutty S, Singh J, and Bidasee KR. Reactive carbonyl species and their roles in sarcoplasmic reticulum Ca2+ cycling defect in the diabetic heart. Heart Fail Rev 19: 101–112, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Tocchetti CG, Caceres V, Stanley BA, Xie C, Shi S, Watson WH, O'Rourke B, Spadari-Bratfisch RC, Cortassa S, Akar FG, Paolocci N, and Aon MA. GSH or palmitate preserves mitochondrial energetic/redox balance, preventing mechanical dysfunction in metabolically challenged myocytes/hearts from type 2 diabetic mice. Diabetes 61: 3094–3105, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Tocchetti CG, Stanley BA, Sivakumaran V, Bedja D, O'Rourke B, Paolocci N, Cortassa S, and Aon MA. Impaired mitochondrial energy supply coupled to increased H2O2 emission under energy/redox stress leads to myocardial dysfunction during Type I diabetes. Clin Sci (Lond) 129: 561–574, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Ussher JR, Koves TR, Jaswal JS, Zhang L, Ilkayeva O, Dyck JR, Muoio DM, and Lopaschuk GD. Insulin-stimulated cardiac glucose oxidation is increased in high-fat diet-induced obese mice lacking malonyl CoA decarboxylase. Diabetes 58: 1766–1775, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Ussher JR, Fillmore N, Keung W, Mori J, Beker DL, Wagg CS, Jaswal JS, and Lopaschuk GD. Trimetazidine therapy prevents obesity-induced cardiomyopathy in mice. Can J Cardiol 30: 940–944, 2014 [DOI] [PubMed] [Google Scholar]
- 192. Vazquez EJ, Berthiaume JM, Kamath V, Achike O, Buchanan E, Montano MM, Chandler MP, Miyagi M, and Rosca MG. Mitochondrial complex I defect and increased fatty acid oxidation enhance protein lysine acetylation in the diabetic heart. Cardiovasc Res 107: 453–465, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Verma SK, Deshmukh V, Liu P, Nutter CA, Espejo R, Hung ML, Wang GS, Yeo GW, and Kuyumcu-Martinez MN. Reactivation of fetal splicing programs in diabetic hearts is mediated by protein kinase C signaling. J Biol Chem 288: 35372–35386, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Wagner GR, Pride PM, Babbey CM, and Payne RM. Friedreich's ataxia reveals a mechanism for coordinate regulation of oxidative metabolism via feedback inhibition of the SIRT3 deacetylase. Hum Mol Genet 21: 2688–2697, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Wagner GR. and Hirschey MD. Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Mol Cell 54: 5–16, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Weinert BT, Iesmantavicius V, Moustafa T, Scholz C, Wagner SA, Magnes C, Zechner R, and Choudhary C. Acetylation dynamics and stoichiometry in Saccharomyces cerevisiae. Mol Syst Biol 10: 716, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, and Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324: 1076–1080, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Williams GS, Boyman L, and Lederer WJ. Mitochondrial calcium and the regulation of metabolism in the heart. J Mol Cell Cardiol 78: 35–45, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Worth AJ, Basu SS, Snyder NW, Mesaros C, and Blair IA. Inhibition of neuronal cell mitochondrial complex I with rotenone increases lipid beta-oxidation, supporting acetyl-coenzyme A levels. J Biol Chem 289: 26895–26903, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, and Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999 [DOI] [PubMed] [Google Scholar]
- 201. Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, and Epstein PN. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes 53: 1336–1343, 2004 [DOI] [PubMed] [Google Scholar]
- 202. Yu W, Gao B, Li N, Wang J, Qiu C, Zhang G, Liu M, Zhang R, Li C, Ji G, and Zhang Y. Sirt3 deficiency exacerbates diabetic cardiac dysfunction: role of Foxo3A-Parkin-mediated mitophagy. Biochim Biophys Acta 1863: 1973–1983, 2016 [DOI] [PubMed] [Google Scholar]
- 203. Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M, and Lopaschuk GD. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ Heart Fail 6: 1039–1048, 2013 [DOI] [PubMed] [Google Scholar]
- 204. Zhao P, Zhang J, Yin XG, Maharaj P, Narraindoo S, Cui LQ, and Tang YS. The effect of trimetazidine on cardiac function in diabetic patients with idiopathic dilated cardiomyopathy. Life Sci 92: 633–638, 2013 [DOI] [PubMed] [Google Scholar]
- 205. Zhao W, Zhao T, Chen Y, Ahokas RA, and Sun Y. Oxidative stress mediates cardiac fibrosis by enhancing transforming growth factor-beta1 in hypertensive rats. Mol Cell Biochem 317: 43–50, 2008 [DOI] [PubMed] [Google Scholar]