Abstract
Outcomes can be challenging to predict in children with mild traumatic brain injury (TBI). Transcranial Doppler (TCD) ultrasound has become an increasingly useful modality in adult and pediatric TBI by measuring blood flow velocities within the circle of Willis. In children with moderate-to-severe TBI, multiple studies have correlated abnormal TCD measurements and poor outcomes. Additionally, TCD has shown value in assessing adults with mild brain injury. To date, there are no studies that correlate TCD findings and outcomes in children with mild TBI. We hypothesize that altered cerebral blood flow after mild TBI is associated with poor functional outcome using the Glasgow Outcome Scale-Extended, Pediatrics (GOS-E Peds). TCD was performed within 24 h of admission on 60 patients at a tertiary Level 1 children's hospital. A secondary analysis was performed on the subgroup of 28 mild TBI patients. GOS-E Peds was measured at the time of hospital discharge and 4–6 weeks post-discharge. Cerebral blood flow velocities did not show correlation with outcome. At discharge, the right-sided Spearman's correlation coefficient was 0.19 (p value = 0.33) and the left-sided was 0.36 (p = 0.06). At follow up the right-sided coefficient was −0.04 (p = 0.84), the left-sided was −0.25 (p = 0.24). Pulsatility index likewise showed no correlation. Right and left-sided correlation at discharge were −0.25 (p = 0.19) and 0.01 (p = 0.96), respectively. At follow up the right side showed 0.004 (p = 0.99), and the left showed 0.18 (p = 0.41). Although our data did not show correlation, it showed that the investigation could feasibly be done in pediatric patients with mild TBI. The study was limited by small sample size and infrequent outcome of interest. Future studies may help define the role of TCD in the large population of mild pediatric TBI patients.
Keywords: CBF velocity, outcome measures, pediatric brain injury, transcranial Doppler ultrasound
Introduction
Traumatic brain injury (TBI) has been classified as a serious public health concern by the Centers for Disease Control and Prevention. Annually in the United States, there are 475,000 children seen in emergency departments for a traumatic brain injury, with 35,000 subsequent inpatient admissions.1 A more recent study estimated that in 2014, approximately 630,000 children (< 20 years old) were treated in emergency departments for TBI. Of those, 60,000 were hospitalized and 7,500 died as a result of their TBI.2
Predicting outcomes after TBI remains a challenge, especially early in the care of a brain injured child. This is especially true in those that present with mild TBI (initial Glasgow Coma Scale [GCS] score of 13–15).3 Important decisions regarding the extent of diagnostic work-up and the child's disposition after TBI are frequently made in the early post-injury phase. Much work has been done to standardize ways to predict which of the apparently well-appearing children will go on to have further sequelae in both the short-term and long-term.4–6 Clinical history remains the most important tool, but imaging modalities have proven to be useful and predictive in many cases. The mainstay of emergent neuroimaging, the computer tomography (CT) scan, comes with limitations associated with radiation exposure and the high cost of the study. Conversely, transcranial Doppler (TCD) ultrasound is a non-invasive and low-cost diagnostic study that has been established as a modality for assessment of traumatic brain injury.7–8
TCD ultrasound as a clinical diagnostic tool was initially developed to assess the cerebral blood flow velocity in adult patients with aneurysmal subarachnoid hemorrhage.9–12 With time, further uses of TCD have been pursued including evaluation of traumatic brain injury.13–15 A study by Oertel and his team prospectively performed daily TCD on 299 post-traumatic adults. They found that the incidence of abnormal cerebral blood flow velocities after trauma was similar to those found after aneurysmal subarachnoid hemorrhage.16 Additionally, Zeigler and colleagues showed that after severe TBI in adults normal cerebral blood flow velocities correlated with favorable neurocognitive outcome, while abnormal flow velocities predicted poor neurocognitive outcome.17
With Bode's description of age stratified cerebral blood flow velocity values, investigators saw a potential for TCD use for pediatric patients with moderate and severe TBI.18–21 Trabold and colleagues22 and Ojha and colleagues23 found that abnormal TCD findings correlated with poor outcomes both at discharge and at 12 month follow up, respectively. More recently, the largest study of TCD use in children with moderate to severe TBI found a strong correlation between neurocognitive outcomes and abnormal cerebral blood flow velocities as measured by TCD.24
The evidence that supports correlation of outcome and cerebral blood flow velocities in mild TBI is less robust.25 Bouzat and his team performed TCDs in the emergency department on 356 mild to moderately injured patients ages 15 years or older across 17 sites. Mild TBI was defined as s GCS score of 13–15 and moderate TBI as a GCS score of 9–12. In patients with mild TBI, the sensitivity of predicting secondary neurologic decline was 91% (95% confidence interval [CI], 59 −100%), while the specificity was 80% (95% CI, 75–85%). Further, his team found that patients who demonstrated an abnormal TCD on admission were more likely to have an abnormal disability rating score on Day 28 compared with those with normal TCD findings. Based on these results, TCD evaluation in the first 24 h post-injury was recommended to assist with clinical decision making related to length of observation periods, further neuroimaging, and follow up.26
To date, there are no studies that describe cerebral blood flow velocities in children (< 15 years of age) who sustain a mild TBI. We hypothesize that altered cerebral blood flow velocities after mild pediatric TBI is associated with poor functional outcome at time of discharge and 6 weeks post-hospital discharge. Secondary objectives are to describe cerebral blood flow velocity characteristics in children with mild TBI and identify potential risk factors for abnormal cerebral blood flow velocities in this population.
Methods
This study examines 35 patients with mild TBI (GCS 13-15) enrolled in a larger (n = 60) prospective, observational parent study (National Institutes of Health/National Institute of Nursing Research, 1P30-NR014139; Robert Wood Johnson Foundation, 71244). It was performed in a single, large academic medical center in the U.S. It was reviewed and approved by the Institutional Review Board, and patients were enrolled after obtaining parental consent. Patients enrolled (2012–2015) in the parent study included children ages 10 days to 15 years admitted to the hospital for a TBI (mild, moderate, and severe) as a result of accidental or abusive head trauma. All children had to both be previously healthy and have no history of developmental delay. All injuries were isolated head trauma, and had no previous history of TBI. Both English- and Spanish-speaking families were enrolled.
Inclusion criteria for the 35 children in the subgroup analysis were GCS score 13–15, availability of bilateral middle cerebral artery flow velocity measurements, and a neurocognitive outcome score using the Glasgow Outcome Scale-Extended, Pediatrics (GOS-E Peds). The GOS-E Peds score was captured at hospital discharge and at 4–6 weeks post-hospital discharge. Patients determined to have an abusive head injury were excluded because of the challenge to determine time of injury, potential for multiple episodes of injury prior to presentation, and other potential historical confounders.
Transcranial Doppler ultrasound was performed within 24 h of admission to the hospital. All patients received standard care, and all patient providers were blinded to the TCD findings. Head CT scans were performed to determine location and type of injury on all patients, although this was not requisite for study inclusion.
Transcranial Doppler ultrasound
TCD is a non-invasive portable ultrasound modality. The advantages of TCD include ease of use, reproducibility, absence of radiation exposure, availability at bedside, real-time results and relatively low cost. Transcranial Doppler ultrasound utilizes a low-frequency (2-MHz) transducer on the surface of the scalp to measure blood flow velocities within the circle of Willis using four common transcranial acoustic windows (temporal, transforaminal, transorbital, and submandibular). We report data from the M1/M2 sections of bilateral middle cerebral arteries obtained through the temporal acoustic window. Data obtained is reported as mean flow velocity (MV), peak flow velocity (PV), and end diastolic flow velocity (EDV). In addition, a pulsatility index (PI) was calculated on both left and right sides. PI is derived from the difference in the PV and EDV divided by MV (PV - EDV/MV).27 The pulsatility index represents a measure of cerebral vascular reactivity; values greater than 1.3 have been independently correlated with poor neurofunctional outcome.28,29
GOS-Extended, Peds
Functional outcomes were measured using the GOS-E Peds score at the time of hospital discharge and at a 6 weeks post-discharge follow-up visit. The GOS-E Peds instrument is designed to be developmentally appropriate for children younger than 17 years of age, and was validated in a trial of 159 patients. The Glasgow Outcome Scale scores from a range of 1 to 8, with lower scores indicating a better functional outcome. The eight categories are as follows:1 = Upper Good Recovery (performs all age-appropriate activities as before); 2 = Lower Good Recovery (normal activities with mild impairment in social activities or family relationships); 3 = Upper Moderate Disability (reduced capacity for school work and moderate impairment in social activities and family relationships); 4 = Lower Moderate Disability (assistance of another person at home is essential for most, but not all, activities of everyday living or child's ability to interact outside the home is impaired as expected for age); 5 = Upper Severe Disability (requires sheltered work or school environment, or is unable to attend school and unable to participate in social activities or has daily severe disruptions in family relationships); 6 = Lower Severe Disability (assistance of another person at home is essential for all activities of everyday living as expected for age); 7 = Vegetative State (unable to follow simple commands or interact beyond reflexes) and; 8 = Death. Scores from 1 to 3 are most often described as a “good outcome” whereas scores 4 to 8 as a “poor outcome”.30
Statistical analysis
Analyses were conducted using SAS 9.4 (Cary, NC). Descriptive statistics summarized participant characteristics. Correlations of the standardized cerebral flow velocities and pulsatility index with GOS-E Peds score were evaluated using Spearman correlation coefficients as GOS-E Peds is measured on an ordinal scale. Because our sample's cerebral flow velocities in each level of gender and location of injury are not normally distributed with a small sample size, Wilcoxon rank-sum tests were carried out to assess the relationship of gender and location of injury with cerebral flow velocities. Paired t-tests were used to assess the difference between left- and right-side cerebral flow velocities. Power analysis was conducted using the SAS power procedure for estimating power of a Pearson's correlation based on a two-sided hypothesis test with a significance level of 0.05.
Analysis was performed using the highest mean flow velocity in the middle cerebral artery. Left and right sides were analyzed separately. Values were normalized for age and gender based on published means and standard deviations.24 The highest measured pulsatility index value was used for analysis.
Results
Of the initial 60 patients from the parent study, 35 (58%) were found to have mild TBI. Four children were excluded due to lack of bilateral MCA flow data and 3 were excluded because their mechanism of injury was determined to be as a result of an abusive head trauma. Therefore, 28 children were included in the analysis. Of note, 25 of 28 (89%) children in the analysis had follow-up GOS-E Peds scores.
Study characteristics included children ages 10 days old to 15 years old. The age group with the largest number of children was school age (10 to 15 years). Genders were divided equally and the majority (71%) of study participants presented with a GCS score of 15, with fewer presenting with values of 13 (7%) and 14 (21%). Forty-three percent of patients were determined to need intensive care unit monitoring. No children required invasive monitoring or surgical intervention during their admission. Right-sided injury represented a small majority over left-sided injury (43% vs 32%, respectively). Epidural hemorrhages were the most common injury found (23%), and 40% of the children had a cranial fracture. (Table 1)
Table 1.
Demographics Characteristics
| Characteristics | n (%) |
|---|---|
| Gender | |
| Female | 14 (50) |
| Male | 14 (50) |
| Age | |
| 0–10 days | 1 (4) |
| 11–90 days | 2 (7) |
| 3 – 11.9 months | 2 (7) |
| 12 months – 2.9 years | 1 (4) |
| 3 – 5.9 years | 5 (18) |
| 6 – 9.9 years | 5 (18) |
| 10 – 15 years | 12 (43) |
| Ethnicity | |
| Non-Hispanic or Latino | 24 (86) |
| Hispanic or Latino | 4 (14) |
| Race | |
| Black or African-American | 3 (11) |
| White/Caucasian | 25 (89) |
| Initial GCS | |
| 13 | 2 (7) |
| 14 | 6 (21) |
| 15 | 20 (71) |
| Admit location | |
| ICU | 12 (43) |
| Non-ICU | 16 (57) |
| Mechanism of injury | |
| MVC | 4 (14) |
| Fall | 18 (64) |
| Other (recreational) | 6 (21) |
| Location of injury | |
| Right | 12 (43) |
| Left | 9 (32) |
| Bilateral | 3 (11) |
| Global | 4 (14) |
| Frontal | 6 (28) |
| Temporal | 7 (25) |
| Parietal | 7 (28) |
| Occipital | 1 (4) |
| Type of injury | |
| Subdural | 6(21) |
| Epidural | 10 (36) |
| Subarachnoid | 7 (25) |
| Fracture | 17 (61) |
| CSF leak | 1 (4) |
| Contusion | 2 (7) |
GCS, Glasgow Coma Scale; ICU, intensive care unit; MVC, motor vehicle collision; CSF, cerebrospinal fluid.
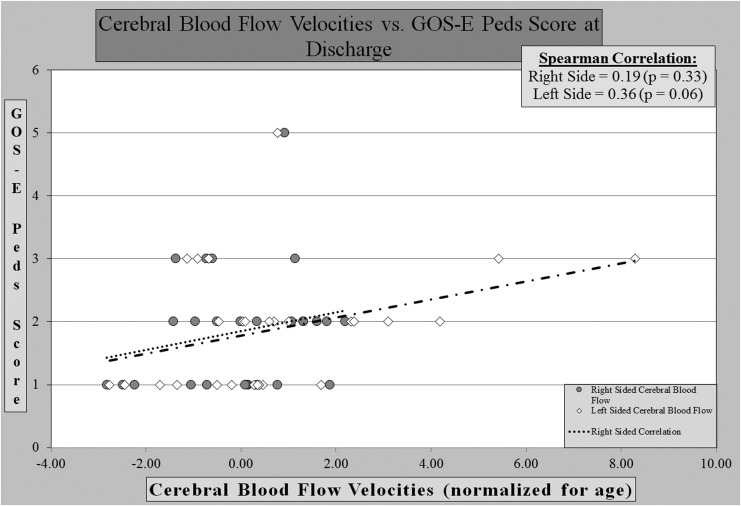
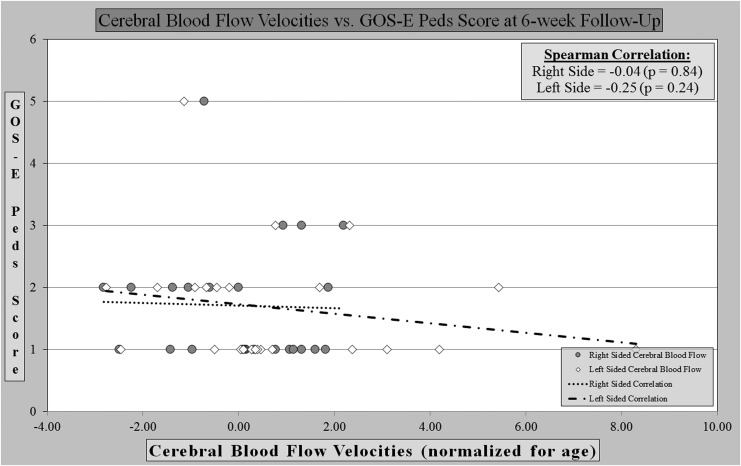
We found no significant correlation between cerebral blood flow velocities and GOS-E Peds score at the time of hospital discharge or at a 4–6 weeks post-discharge follow-up visit. At the time of discharge, cerebral blood flow velocities showed no significant positive or negative correlation with the left or right sides. (Fig. 1) At follow-up, the right- and left-sided blood flow velocities showed no correlation. (Fig. 2)
FIG. 1.
Cerebral blood flows vs. outcome score at discharge.
FIG. 2.
Cerebral blood flows vs. outcome score at 6-week follow-up.
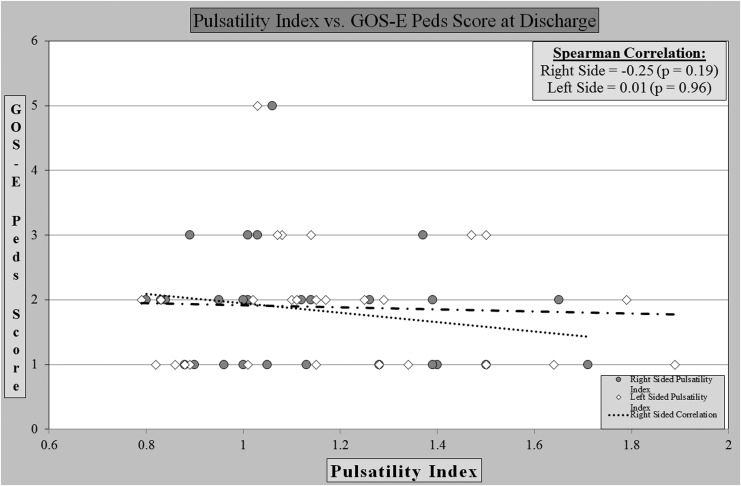
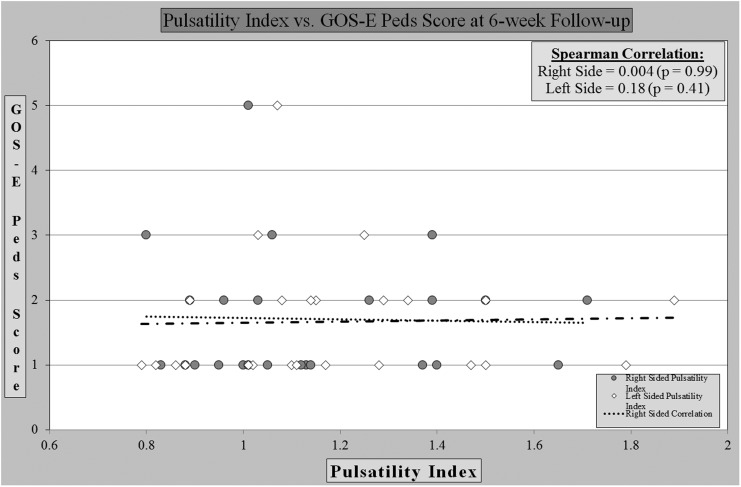
Further, the analysis of the PI revealed no correlation between PI and outcome at discharge (Fig. 3) or at post-discharge follow-up (Fig. 4). We found that eight children had a PI greater than 1.3, which in prior studies has correlated with poor outcome, but was not seen in this sample.
FIG. 3.
Pulsatility index vs. outcome score at discharge.
FIG. 4.
Pulsatility index vs. outcome score at 6-week follow-up.
When further analyzed we found that seven (28%) children had the same GOS-E Peds score between the two discharge measurements; seven (28%) had worse outcomes and 11 (44%) had improved outcomes. We had three (11%) children who were lost to follow up for the 6-week post-hospital discharge visit. Of our 28 children, only two had GOS-E Peds scores of 4 or greater (Lower Moderate Disability). As mentioned, one patient had a 5 (Upper Severe Disability) that improved to a 3 (Upper Moderate Disability) at follow-up, and another patient initially had a 3 (Upper Moderate Disability) and worsened to a 5 (Upper Severe Disability). Both children had normal cerebral blood flow on both sides. When a univariate analysis was performed for age, injury mechanism, type, and location of injury, no correlation was seen in relationship to changes in functional outcomes.
Discussion
This is the first study to report bilateral MCA cerebral blood flow velocities in children with mild TBI. Consistent with most pediatric TBI studies, the children included in the study represent a heterogeneous group with a wide range of mechanism of injuries and anatomical lesions. In our study, examples of mechanisms of injury included motor vehicle injuries, falls, ATV, and golf cart injuries. Injuries noted by neuroimaging ranged from no acute intracranial lesion to traumatic subarachnoid hemorrhage. This wide variability of mechanism and injury has the potential to dilute any signal in the data.
As expected with mildly injured children, few children had cerebral blood flow velocities outside of the normal range for age and gender. Six children had cerebral blood flow velocities greater than 2 standard deviations above normal. We did not appreciate a correlation with poor outcome among these patients. The mechanism of injury in this group included four falls, one motor vehicle collision, and one baseball to the head. Injury patterns included traumatic subarachnoid hemorrhage (3 of 6), subdural hemorrhage (2), cerebral contusion (1), and one patient had no intracranial injury at all. Of the six children, three were found to have a traumatic subarachnoid hemorrhage.
Three children (all females) had cerebral flow velocities 2 standard deviations below normal. All had GOS-E Peds scores consistent with good outcomes at both discharge and at the 4- to 6-week follow-up. In the data set, we unexpectedly observed that females had overall lower flow velocities than males. (Table 2) This is in contrast to prior reported data showing that after 5 years of age, females have higher baseline cerebral flow velocities compared with age-matched males. Even with lower cerebral blood flow velocities for age, the females demonstrated good functional outcomes. Although there is no clear mechanism for this finding, it does provide new information regarding potential variation in cerebral flow velocities of female children who experience a mild TBI.
Table 2.
Comparison of Gender and Location of Injury with Cerebral Blood Flow Velocities
| Gender | Location of injury | |||||
|---|---|---|---|---|---|---|
| Female (n = 14) mean (SD) | Male (n = 14) mean (SD) | p value* | Left (n = 9) mean (SD) | Right (n = 11) mean (SD) | p value* | |
| Normalized flow rate (left) | 0.46 (3.07) | 0.99 (1.63) | 0.1061 | 1.32 (1.06) | −0.19 (2.23) | 0.0401 |
| Normalized flow rate (right) | −0.52 (1.48) | 0.56 (1.01) | 0.0601 | 0.93 (0.98) | −0.64 (1.40) | 0.0294 |
| Mean flows for left and right sides | −0.10 (2.16) | 0.61 (0.98) | 0.1030 | 1.13 (0.98) | −0.79 (1.27) | 0.0162 |
Wilcoxon two-sample test.
SD, standard deviation.
We also unexpectedly found a difference in mean blood flow velocities between the left and right side. Left-sided cerebral blood flow velocities were significantly higher than the right-sided flow velocities on a patient-by-patient basis. (Table 3) This finding has yet to be reported and we could not find any existing studies differentiating left- and right-sided MCA flow velocities. O'Brien and colleagues did show that left-sided cerebral vasospasm started slightly later than the right side, and had a wider range of duration of days.24 The mechanism for this difference is not clear in our data. In our study, the proportion of right- and left-sided hemispheric injuries was not significantly different. Additionally, there was no difference between the incidence of right- and left-sided skull fractures or intracranial bleeding.
Table 3.
Comparison of Left- and Right-Sided Cerebral Blood Flow Velocities
| Mean difference between left and right side (n = 26) μ (SD) | p value | |
|---|---|---|
| Cerebral blood flow | 0.60 (1.59) | 0.0633 |
SD, standard deviation.
Although an insignificant correlation was found between cerebral blood flow velocity and functional outcome, some interesting insights were revealed in our population of study. Our findings support the premise that most children have a good functional outcome after experiencing a mild TBI. We also observed that abnormal cerebral blood flow velocities occur at a rate similar to children with moderate-to-severe injury, and is most likely under-recognized in pediatric patients of all levels of injury severity. 24
All normative values we used to analyze cerebral blood flow velocity in this patient population are parameters described by LaRovere and O'Brien27 and Reuter-Rice,28 and will serve to set the stage for future Common Data Elements for TCD use in children with TBI. Perhaps our findings may prompt the question of a need for larger studies of normative data based on age and gender findings.
Based on the strength of prior studies, the use of only one time-point for cerebral blood flow velocity reporting in this study was intentional. We aimed to demonstrate that early one-time TCD could potentially provide prognostic information. Appreciating that abnormal cerebral blood flow velocities have been seen as early as the day of injury, the early capture with portable TCD could be beneficial to support initial decision making related to level of care and disposition. The ability of early capture could also allow for earlier intervention.
Limitations
The small sample may limit the generalizability of findings and result in insufficient power to detect actual correlation between cerebral blood flow velocities and clinical outcome. Though limited by sample size, this study is the first published data of cerebral blood flow velocities in children with mild TBI, and adds information to our understanding of this large and variable population. Additional limitations include loss of three children to post-discharge follow-up at 4–6 weeks. It is possible that these children showed a loss of function that made them less likely to follow up resulting in confounding of our results. The lack of extracranial internal carotid data limits our ability to characterize the higher cerebral flow velocities seen in some of our children. Generalizability would be enhanced by including more centers in the data collection.
Conclusion
This study adds to the growing body of literature using TCD to measure cerebral blood flow velocities in children who present with a TBI. The importance of understanding the potential role of TCD as a non-invasive diagnostic ultrasound to better appreciate cerebral blood flow velocity in children with mild TBI could lead to better outcomes. Although we could not establish that altered cerebral blood flow velocities are a prime factor in outcome for mild pediatric brain injury, larger studies in adult patients have demonstrated a correlation. Further study in pediatric TBI is warranted.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Faul M., Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA [Google Scholar]
- 2. Thurman D.J.The epidemiology of traumatic brain injury in children and youths: a review of research since 1990. (2016). J. Child Neurol. 31, 20–27 [DOI] [PubMed] [Google Scholar]
- 3. Barlow K.M., Crawford S., Stevenson A., Sandhu S.S., Belanger F., and Dewey D. (2010). Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics 126, e374–e381 [DOI] [PubMed] [Google Scholar]
- 4. Schunk J.E., Rodgerson J.D., and Woodward G.A. (1996). The utility of head computed tomographic scanning in pediatric patients with normal neurologic examination in the emergency department. Pediatr. Emerg. Care 12, 160. [DOI] [PubMed] [Google Scholar]
- 5. Kuppermann N., Holmes J.F., and Dayan P.S. (2009). Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet 374, 1160–1170 [DOI] [PubMed] [Google Scholar]
- 6. Hahn Y.S. and McLone D.G. (1993). Risk factors in the outcome of children with minor head injury. Pediatr. Neurosurg. 19, 135. [DOI] [PubMed] [Google Scholar]
- 7. Ract,C., Le Moigno S., Bruder N., and Vigue B. (2007). Transcranial Doppler ultrasound goal-directed therapy for the early management of severe traumatic brain injury. Intensive Care Med. 33, 645–651 [DOI] [PubMed] [Google Scholar]
- 8. Sloan M.A., Alexandrov A.V., and Tegeler C.H. (2004). Assessment: transcranial doppler ultrasonography: report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology 62, 1468. [DOI] [PubMed] [Google Scholar]
- 9. Lindegaard K.H.F., Nornes H., Bakke S.J., Sorteberg W., and Nakstad P. (1989). Cerebral vasospasm diagnosis by means of angiography and blood velocity measurements. Acta Neurochir. (Wien.) 100, 12–24 [DOI] [PubMed] [Google Scholar]
- 10. Kassell N.F., Sasaki T., Colohan A.R., and Nazar G. (1985). Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke 16, 562–572 [DOI] [PubMed] [Google Scholar]
- 11. Aaslid R., Markwalder T.M., and Nornes H. (1982). Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J. Neurosurg. 57, 769–774 [DOI] [PubMed] [Google Scholar]
- 12. Connolly E.S., Jr., Rabinstein A.A., and Carhuapoma J.R. (2012). Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 43, 1711. [DOI] [PubMed] [Google Scholar]
- 13. Grolimund P., Weber M., Seiler R.W., and Reulen H.J. (1988) Time course of cerebral vasospasm after severe head injury. Lancet 21, 1173. [DOI] [PubMed] [Google Scholar]
- 14. Chan K.H., Dearden N. M., Miller J.D., and Batjer H.H. (1992) The significance of posttraumatic increase in cerebral blood flow velocity: a transcranial Doppler ultrasound study. Neurosurgery 30, 697–700 [PubMed] [Google Scholar]
- 15. Amyot F., Arciniegas D. B., Brazaitis M. P., Curley K. C., Diaz-Arrastia R., and Gandjbakhche A. (2015). A review of the effectiveness of neuroimaging modalities for the detection of traumatic brain injury. J. Neurotrauma 32, 1693–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oertel M., Boscardin W.J., Obrist W.D., Glenn T.C., McArthur D.L., Gravori T., Lee J.H., and Martin N.A. (2005). Posttraumatic vasospasm: the epidemiology, severity, and time course of an underestimated phenomenon: a prospective study performed in 299 patients. J. Neurosurg. 103, 812–824 [DOI] [PubMed] [Google Scholar]
- 17. Ziegler D.W., Cravens G., Poche G., Gandhi R., and Tellez M. (2017). Use of transcranial doppler in patients with severe traumatic brain injuries. J. Neurotrauma, 34, 121–127 [DOI] [PubMed] [Google Scholar]
- 18. Bode H. and Wais U. (1998). Age dependence of flow velocities in basal cerebral arteries. Arch. Dis. Child. 63, 606–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bode H. and Eden A. (1989). Transcranial doppler sonography in children. J. Child. Neurol. 4(Suppl), S68–S76 [DOI] [PubMed] [Google Scholar]
- 20. Schöning M., Staab M., and Walter J. (1993). Transcranial color duplex sonography in childhood and adolescence. Age dependence of flow velocities and waveform parameters. Stroke 24, 1305–1309 [DOI] [PubMed] [Google Scholar]
- 21. Mandera. M., Larysz. D., and Wojtacha M. (2002). Changes in cerebral hemodynamics assessed by transcranial Doppler ultrasonography in children after head injury. Childs Nerv. Syst. 18, 124–128 [DOI] [PubMed] [Google Scholar]
- 22. Trabold F., Meyer P.G., Blanot S., Carli P.A., and Orliaguet G.A. (2004). The prognostic value of transcranial Doppler studies in children with moderate and severe head injury. Intensive Care Med. 30, 108–112 [DOI] [PubMed] [Google Scholar]
- 23. Ojha B.K., Jha D.K., Kale S.S., and Mehta V.S. (2005). Transcranial Doppler in severe head injury: evaluation of pattern of changes in cerebral blood flow velocity and its impact on outcome. Surg. Neurol. 2, 174–179 [DOI] [PubMed] [Google Scholar]
- 24. O'Brien N.F., Maa T., and Yeates K.O. (2015). The epidemiology of vasospasm in children with moderate-to-severe traumatic brain injury. Crit. Care Med. 43, 674–685 [DOI] [PubMed] [Google Scholar]
- 25. Jaffres P., Brun J., Declety P., Bosson J.L., and Fauvage B. (2005). Transcranial doppler to detect on admission patients at risk for neurological deterioration following mild and moderate brain trauma. Intensive Care Med. 31, 785–790 [DOI] [PubMed] [Google Scholar]
- 26. Bouzat P., Almeras L., and Manhes P. (2016). Transcranial Doppler to predict neurologic outcome after mild to moderate traumatic brain injury. Anesthesiology 125, 346–354 [DOI] [PubMed] [Google Scholar]
- 27. LaRovere K. and O'Brien N. (2015). Transcranial doppler sonography in pediatric neurocritical care: A review of clinical applications and case illustrations in the pediatric intensive care unit. J. Ultrasound Med. 34, 2121–2132 [DOI] [PubMed] [Google Scholar]
- 28. Reuter-Rice K. (2017). Transcranial Doppler ultrasound use in pediatric traumatic brain injury. J. Radiol. Nurs. 36, 3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beers S.R., Wisniewski S.R., and Garcia-Filion P. (2012) Validity of a pediatric version of the Glasgow Outcome Scale-Extended. J. Neurotrauma 29, 1126–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]