Abstract
Spinal cord injury (SCI) disrupts autonomic regulation of visceral organs. As a result, a leading cause of mortality in the SCI population is metabolic dysfunction, and an organ central to metabolic control is the liver. Our recent work showed that rodent SCI promotes Kupffer cell (hepatic macrophage) activation, pro-inflammatory cytokine expression, and liver steatosis. These are symptoms of nonalcoholic steatohepatitis (NASH), the hepatic manifestation of metabolic syndrome, and these pre-clinical data replicate aspects of post-SCI human metabolic dysfunction. Because metabolic profile is highly dependent on lifestyle, including diet, it is likely that lifestyle choices prior to injury influence metabolic and hepatic outcomes after SCI. Therefore, in this study we tested if a diet rich in green tea extract (GTE), a known hepatoprotective agent, that began 3 weeks before SCI and was maintained after injury, reduced indices of liver pathology or metabolic dysfunction. GTE treatment significantly reduced post-SCI hepatic iron accumulation and blunted circulating glucose elevation compared with control-diet rats. However, GTE pre-treatment did not prevent Kupffer cell activation, hepatic lipid accumulation, increased serum alanine transaminase, or circulating non-esterified fatty acids, which were all significantly increased 6 weeks post-injury. Spinal cord pathology also was unchanged by GTE. Thus, dietary GTE prior to and after SCI had only a minor hepatoprotective effect. In general, for optimal health of SCI individuals, it will be important for future studies to evaluate how other lifestyle choices made before or after SCI positively or negatively impact systemic and intraspinal outcomes and the overall metabolic health of SCI individuals.
Keywords: catechin, fatty liver, inflammation, interleukin-1, tumor necrosis factor
Introduction
Spinal cord injury (SCI) triggers rapid and widespread systemic inflammation, including elevated chemokines and leukocytes in the circulation, liver, lungs, and kidneys.1–5 This systemic inflammation impairs peripheral organ function.6–9 We previously showed that within 3 weeks of injury in rats, SCI induced hepatic lipid deposition and inflammation, symptoms of nonalcoholic steatohepatitis (NASH), which is the hepatic manifestation of metabolic syndrome.10 Metabolic syndrome, including high blood pressure, hyperglycemia, dyslipidemia, and central adiposity, is more common in individuals with SCI than the general population.6,11–17 Consequently, SCI patients have increased risk of heart disease, stroke and diabetes,13,18–23 and increased morbidity and mortality compared with the general population.6,12,13,19,20
Understanding why metabolic syndrome is more prevalent in the SCI population is an important clinical goal that requires a better understanding of what drives post-SCI organ responses. This is especially true for the liver, as it plays a central role in overall metabolism. Liver health and function are strongly influenced by dietary factors, making it feasible that dietary choices prior to SCI influence hepatic outcomes.24–27 Therefore, in this study, we tested if administering a diet rich in green tea extract (GTE) for 3 weeks before SCI was hepatoprotective. GTE is rich in bioactive polyphenolic catechins including epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), and epigallocatechin gallate (EGCG).28–31 Green tea and its predominant catechin EGCG reduce the obesity-related hallmarks of non-alcoholic fatty liver disease (NAFLD) and NASH,30,32–39 likely by limiting the hepatic nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) activation and downstream inflammatory responses that contribute to liver steatosis and oxidative stress.32–34,40,41 These mechanistic pre-clinical findings are supported by epidemiological evidence suggesting that drinking green tea lowers circulating liver injury biomarkers, lipids, and ferritin; reduces cardiovascular-related mortality; decreases liver steatosis; and improves liver function.29,42–46
Here, we tested the hypothesis that pre-feeding rats a clinically feasible dose of GTE before and throughout recovery from SCI would reduce hepatic post-injury NASH symptoms. Because EGCG in green tea also has neuroprotective effects in models of stroke, transient ischemia, hydrocephalus, and traumatic brain and spinal cord injury when given peripherally or directly into the damaged tissue, we also examined if having a GTE-enriched diet at the time of injury reduced spinal cord pathology and improved locomotor recovery after spinal contusion in rats. Although obviously not designed as a therapeutic study that begins treatment after SCI, studies such as these may begin to provide clues as to how patient lifestyle choices prior to SCI positively or negatively influence metabolic profiles after injury.
Methods
Materials
Powdered GTE was provided by Unilever BestFoods (Englewood, NJ). Its composition was verified by ultraviolet high performance liquid chromatography (HPLC-UV) to contain 30% (w/w) total catechins (48% EGCG, 31% EGC, 13% ECG, 8% EC) and 4.6% caffeine.30
Diet treatment
All procedures were approved by the Institutional Animal Care and Use Committee at The Ohio State University. Female Sprague Dawley rats (250 g; Harlan Laboratory) were randomized to the following dietary treatments (n = 18/group): purified control diet devoid of GTE (D11112201) or containing GTE at 2% (w/w) (D15022601). The pelleted diet contained 65% energy from carbohydrate, 20% energy from protein, and 15% energy from fat. GTE was provided at 2% (w/w) based on our prior studies demonstrating that this dietary level protects against liver injury associated with NAFLD in rodents.30,32–34 GTE at 2% also closely approximates intake from epidemiological studies suggesting that green tea (≥ 10 servings/day) lowers the risk of liver injury and inflammation.44 Rodents were provided ad libitum access to food and water for the duration of this investigation. Although time-dependent studies have yet to assess green tea catechin accumulation in target tissues, long-term feeding of tea catechins for >9 weeks suppresses high-fat diet–induced obesity by modulating lipid metabolism.47 Therefore, to avoid profound effects on weight loss between diet groups that may confound the interpretation of SCI-induced changes in metabolic endpoints, rats were fed 2% GTE diet for just 3 weeks before injury. Experimental diets were weighed and replenished, and rat body weights were recorded every 2–3 days.
SCI
Three weeks after beginning the control or GTE diets, rats were randomized to the following groups: Uninjured +0% GTE (n = 6), Uninjured +2% GTE (n = 6), SCI +0% GTE (n = 12) and SCI +2% GTE (n = 12). Animals in the SCI groups were anesthetized with an intraperitoneal cocktail of ketamine (80 mg/kg) and xylazine (10 mg/kg) after which a dorsal laminectomy was performed at vertebral level T8. The Infinite Horizons device (Precision Systems and Instrumentation) was used to deliver a moderate (200 kD) spinal contusion, after which back muscles were sutured, and the skin was closed with wound clips. Rats were injected with 5 mL of saline subcutaneously, and then were placed in warm cages overnight. To maintain hydration and prevent infection, saline and antibiotics were administered subcutaneously for 5 days post-injury (DPI). Bladders were expressed manually twice daily until spontaneous voiding returned.
Hindlimb locomotor assessment
All animals were acclimated to handling and testing in the open field environment prior to injury. Rats were tested by two observers blinded to the treatment groups prior to injury and at days 1, 3, 7, 10, 14, 21, 28, 35 and 42 post-injury using the Basso–Beattie–Bresnahan (BBB) locomotor rating scale.48 Scores for each rat's hindlimbs were averaged and used to create group means at each day.
Tissue extraction and preparation
All rats were euthanized 9 weeks after beginning the GTE enriched diets, which was 42 DPI for the SCI groups. All rats were fasted for 8 h before euthanasia. Cardiac blood samples were collected then centrifuged for 5 min at 300g to separate the serum, which was flash frozen and stored at −80°C until analysis. Rats were exsanguinated using 0.1 M phosphate buffer solution (PBS). Once the blood was cleared from the body, small cross-sections of liver from the central lobe (∼0.5 g each) were preserved in RNAlater (ThermoFisher Scientific) for gene expression studies. The rats were then transcardially perfused with 4% paraformaldehyde (PFA) in 0.1 M PBS. Livers and spinal cords were removed, post-fixed in 4% PFA for 2 h, and then transferred to 0.2 M PB overnight. Samples were then cryoprotected for 2–3 days in 30% sucrose solution. Fixed liver and spinal cord samples were frozen on dry ice and blocked using optimal cutting temperature (OCT) solution. Frozen spinal cord and liver tissues were sectioned at 10 and 20 μm, respectively, then mounted on slides (Superfrost Plus Slides, Fisher Scientific).
Serum alanine aminotransferase (ALT), non-esterified fatty acids (NEFA), and glucose analysis
Serum ALT activity, glucose (Point Scientific), and NEFA (Wako Diagnostics) were measured using spectrophotometric clinical assays in accordance with the manufacturer's instructions as previously described.32
Liver histology
Immunohistochemistry was performed on liver tissue as previously described.10 Briefly, anti-CD11b (Ox42; Serotec, MCA275, 1:2,000) and anti-Clec4f (ThermoFisher Scientific, PA5-47396, 1:400) were used to visualize liver macrophages. Liver iron was visualized using Perls Prussian blue reagent, and liver H-ferritin (Santa Cruz, sc-376594, 1:1,000) and L-ferritin (Santa Cruz, sc-74513 1:1,000) were visualized as previously described.49,50 Oil Red O staining was used to assess the deposition and accumulation of lipid molecules in hepatic tissue as previously described.10
Spinal cord histology
Spinal cord sections spanning the rostral to caudal extent of the lesion were used for immunohistochemistry as previously described.51 Briefly, axons and white matter were visualized with anti-neurofilament (NF) antibody (DSHB, RT97, 1:2000) and eriochrome cyanine (EC), respectively. Anti-CD11b (Ox42; Serotec, MCA275, 1:2000) and anti-CD68 antibodies (Serotec, MCA341GA, 1:1000) were used to visualize macrophages/microglia. Iron was visualized using Perls Prussian blue reagent as previously described.49,50 Neurons were visualized with anti-NeuN antibody (EMD-Millipore, MAB377, 1: 25,000).
Tissue analysis and quantification
An investigator blinded to experimental treatments quantified histological outcomes with MCID imaging software (Imaging Research Inc.). Sections of liver tissue were randomly selected from each group. The proportional area of Clec4f, CD11b, L-ferritin, and H-ferritin immunoreactivity and Oil Red O stain was quantified from six random fields per section by dividing the stained “target” area by the total hepatocyte area measured in each field. Staining levels are expressed as % of total hepatocyte area. For spinal cords, the injury epicenter was identified as the section with the least white matter (EC/NF labeling); analyses of spared white matter and volume at the lesion epicenter and in 10 sections spaced 200 μm apart for a total of 2 mm analyzed rostral and caudal to the epicenter were completed using MCID software and the Cavalieri estimation.52 The total immunoreactivities for CD11b+ and CD68+ macrophages/microglia and Perls Prussian blue+ iron label were quantified at the lesion epicenter and in 10 sections spaced 200 μm apart for a total of 2 mm analyzed rostral and caudal to the epicenter.
Analysis of gene expression
Gene expression was examined in fresh liver and spinal samples as previously described.10 Briefly, total RNA was extracted using Trizol Reagent (15596018, Life Technologies), and purity was assessed using microdrop spectrophotometry. cDNA was synthesized using a Superscript III reverse transcriptase kit (Invitrogen) according to the manufacturer's instructions. Reverse transcription polymerase chain reaction (RT-PCR) was performed using Sybr green on an Applied Biosystems quantitative PCR (qPCR) instrument. All primers for inflammatory genes (tumor necrosis factor-α [TNFα], CD68, CD11b, interleukin-1α [IL-1α], and interleukin-1β [IL-1]) and iron storage genes (L-ferritin, H-ferritin) were purchased from Quantitect (Table 1). mRNA expression was normalized relative to 18S, and quantification was performed using the ΔΔ cycle threshold (CT) method.53
Table 1.
Primers for Real-Time Polymerase Chain Reaction-Based Quantification of Hepatic Gene Expression in Rats
| Name | Forward sequence | Reverse sequence |
|---|---|---|
| TNF-α | TCGTAGCAAACCACCAAGCG | ATGGCAGAGAGGAGGCTGACTTTC |
| IL-1α | TCACTCGCATGGCATGTGCTGA | TCGGGCTGGTTCCACTAGGCT |
| IL-1β | GAAGATGGAAAAGCGGTTTG | AACTATGTCCCGACCATTGC |
| CXCL1 | CAGACAGTGGCAGGGATTCA | TGACTTCGGTTTGGGTGCAG |
| CCL2 | AGCCAACTCTCACTGAAGCC | AACTGTGAACAACAGGCCCA |
| CD68 | GGATTCAAACAGGACCGACATCAG | CTACAGAGTGGACTGGAGCAAATGC |
| CD11b | GAGAACTGGTTCTGGCTTGC | TCAGTTCGAGCCTTCTT |
| L-ferritin | CGCCAGGGTTTTCCCAGTCACGAC | GGACACACTTTAACAATAGGCGAG |
| H-ferritin | GCCACTGACAAAAATGACCCC | TGGCGGCGACTAAGGAGAGGGCG |
| 18S | TTCGGAACTGAGGCCATGAT | TTTCGCTCTGGTCCGTCTTG |
TNF, tumor necrosis factor; IL, interleukin; CXCL1, chemokines C-X-C motif chemokine ligand 1.
Statistical analysis
Data (means ± SEM) were analyzed using Graph Pad Prism 7.0c software. Two way ANOVA with or without repeated measures, as appropriate, was used to examine main and GTE × SCI interactive effects for all study end-points. Tukey's post-test was used to examine group differences following statistically significant main or interactive effects. Outliers that were more than twice the standard deviation were identified for each data set and excluded from analysis. All analyses were considered statistically significant at p < 0.05.
Results
Dietary GTE did not alter serum NEFA levels but lowered elevated glucose after SCI
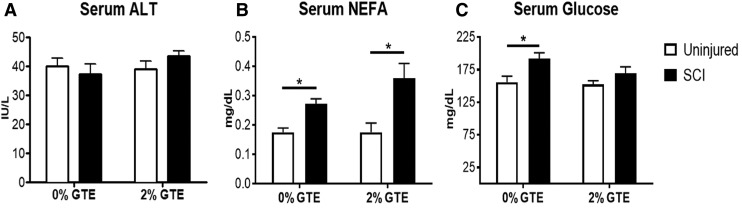
Rat body weights and food intake recorded every 2–3 days were not different (p > 0.05) between diet groups in uninjured and SCI cohorts (data not shown). Liver/body weight ratios also did not differ between injury and diet groups. Serum ALT activity, a clinical biomarker of liver injury, was unchanged by SCI and GTE (Fig. 1A). Circulating NEFAs were significantly elevated in SCI rats compared with uninjured controls (Fig. 1B), suggesting that SCI potentiates chronic adipose hydrolysis and release of triglycerides. GTE did not reduce circulating NEFAs after SCI (Fig. 1B). Because hyperglycemia is a common comorbidity in NAFLD, we measured serum glucose at the terminal time point, which revealed a significant effect of SCI on inducing a chronic rise in serum glucose. A significant rise in glucose was not observed in rats fed the GTE diet (Fig. 1C).
FIG. 1.
Serum alanine aminotransferase (ALT), non-esterified fatty acids (NEFA), and glucose after chronic spinal cord injury (SCI) in control and green tea extract (GTE) fed rats. (A) Serum ALT was not significantly different between any groups. (B) Serum NEFA and (C) glucose were significantly increased at 42 days post-injury (DPI) compared with uninjured controls (NEFA main injury effect: p = 0.0015, *p < 0.05 Tukey's post-hoc test; glucose main injury effect: p = 0.0135, *p < 0.05 Tukey's post-hoc test). GTE prevented SCI-induced increases in glucose. Bars represent + SEM.
SCI-induced chronic liver inflammation and lipid accumulation was not affected by dietary GTE
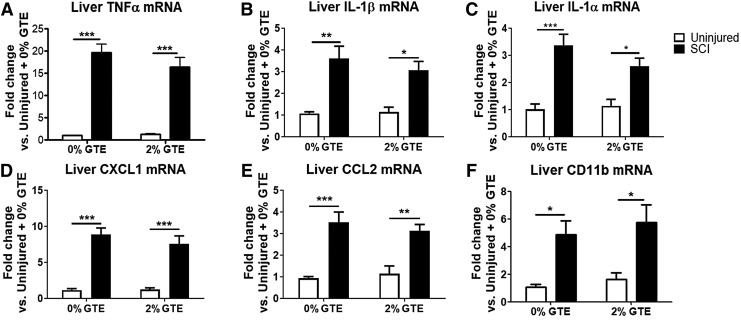
Cytokines in the liver increase by 2 h post-SCI and persist for at least 21 DPI.5,10,54,55 Here, we examined if hepatic cytokines remain elevated through 42 DPI, and if dietary GTE limited their expression. Surprisingly, hepatic inflammatory markers were still significantly increased at 42 DPI, including an ∼20-fold increase in mRNA for TNFα and a 3.5-fold increase in both IL-1β and IL-1α (Fig. 2A–C). Also elevated were mRNA for chemokines C-X-C motif chemokine ligand 1 (CXCL1) and CCL2 (Fig. 2D, E), and markers of inflammatory cell activation, including CD11b (Fig. 2F) and CD68 (data not shown). Contrary to our hypothesis, consuming dietary GTE prior to SCI and throughout the post-SCI period did not protect against elevated inflammatory gene expression in the liver (Fig. 2A–F).
FIG. 2.
Hepatic inflammatory genes are elevated at 6 weeks post-injury, but are unaffected by pretreatment with dietary green tea extract (GTE). Real-time quantitative polymerase chain reaction (qPCR) data from naïve or 42 days post-injury (DPI) livers were analyzed by two way ANOVA. A significant main effect was observed for spinal cord injury (SCI) (p < 0.05) but not GTE (p > 0.05) in all genes. (A) Tumor necrosis factor (TNF)α. (B) Interleukin (IL)-1β. (C) IL-1α. (D) Chemokines C-X-C motif chemokine ligand 1 (CXCL1). (E) CCL2. (F) CD11b, and CD68 (not shown). *p < 0.05; **p < 0.01; and ***p < 0.001 by Tukey's post-hoc test. Bars represent + SEM.
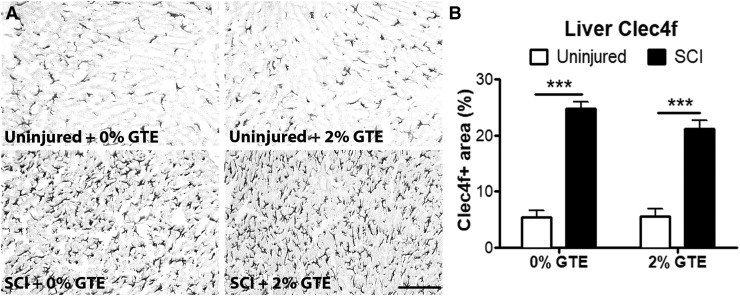
Next, Kupffer cell morphology and distribution were examined to determine if chronic SCI and/or GTE diet altered hepatocyte macrophage activation. Hepatic Clec4f, a C-lectin binding receptor expressed exclusively by differentiated Kupffer cells,56,57 was significantly increased at 42 DPI and was unaffected by prophylactic GTE (Fig 3A, B). Hepatic CD11b immunoreactivity was also significantly increased at 42 DPI (data not shown), confirming the CD11b mRNA increase seen in Figure 2. However, again there was no significant main effect of diet on CD11b immunoreactivity (data not shown).
FIG. 3.
Hepatic Kupffer cell activation was significantly increased at 42 days post-injury (DPI) and was not altered by a green tea extract (GTE)-enriched diet begun prior to spinal cord injury (SCI). (A) Representative coronal sections showing hepatic Clec4f immunoreactivity in control uninjured, GTE uninjured, control SCI, and GTE SCI livers. Scale bar = 100 μm. (B) Quantification of Clec4f immunolabeling revealed a significant main effect of injury-increased Clec4f expression at 42 DPI compared with uninjured livers (p < 0.0001, Two-way ANOVA, and ***p < 0.001 by Tukey's post-hoc test) but no significant difference between diet groups.
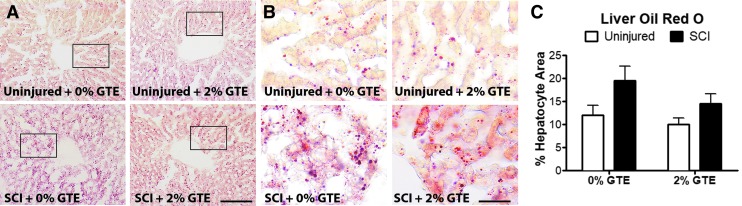
Consistent with hepatic inflammation contributing to liver steatosis,11,58,59 SCI-induced hepatic inflammation was accompanied by excess lipid accumulation, which occurred regardless of GTE treatment (Fig. 4A–C). Collectively, these data show that SCI induces NASH, and dietary GTE at the dose administered here affords no hepatoprotective benefit in mitigating these pathological responses.
FIG. 4.
Hepatic lipid accumulation increased after chronic spinal cord injury (SCI) and was not reduced by a prophylactic green tea extract (GTE)-enriched diet. (A) Representative coronal sections showing Oil Red O staining in control uninjured, GTE uninjured, control SCI, and GTE SCI livers. Scale bar = 100 μm. (B) High magnification images of boxed regions in (A). Scale bar = 25 μm. (C) Quantification of Oil Red O droplet area showed significantly increased liver fat accumulation at 42 days post-injury (DPI) compared with uninjured controls (main injury effect: p = 0.0433 by two way ANOVA). There was no significant difference between diet groups. Bars represent + SEM. Color image is available online at www.liebertpub.com/neu
GTE diet decreased hepatic iron accumulation after SCI
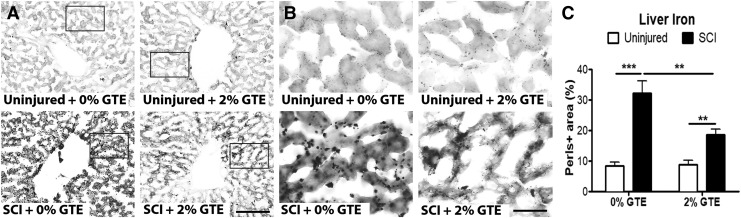
Because systemic anemia has been detected in SCI individuals and the liver is a major iron storage and iron regulatory organ, iron levels in post-SCI livers were examined histologically using the standard Perls Prussian blue stain, which labels non-heme ferric iron. Hepatic iron was significantly increased 42 DPI (Fig. 5A–C), thus providing novel evidence that SCI induces chronic iron accumulation in the liver. The GTE diet attenuated post-SCI hepatic iron increase, although iron levels were still significantly elevated compared with naive controls (Fig. 5A–C).
FIG. 5.
Spinal cord injury (SCI) induced hepatic iron accumulation was reduced by prophylactic green tea extract (GTE)-enriched diet. (A) Representative coronal sections showing Perls Prussian blue stain in control uninjured, GTE uninjured, control SCI, and GTE SCI livers. Scale bar = 100 μm. (B) High magnification images of boxed regions in (A). Scale bar = 25 μm. (C) Quantification of iron (Perls-positive area) showed significantly increased hepatic iron deposition at 42 days post-injury (DPI) compared with uninjured controls (main injury effect: p < 0.0001, ***p < 0.001, and **p < 0.01 by Tukey's post-hoc test). Liver iron deposition was significantly lower in rats fed a prophylactic GTE-enriched diet (SCI +2% GTE) compared with SCI +0% GTE (main diet effect: p = 0.0438 by two-way ANOVA; **p < 0.01 by Tukey's post- hoc test). Two way ANOVA also revealed a significant interaction between diet and injury, p = 0.0327). Bars represent +SEM.
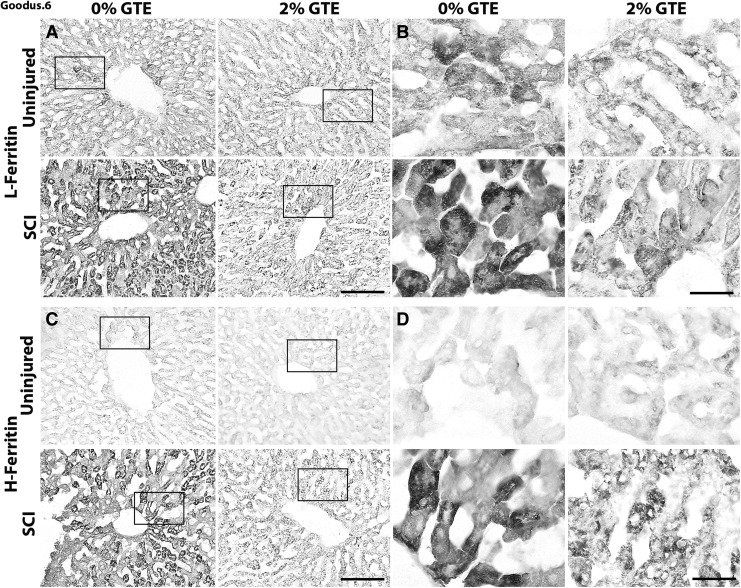
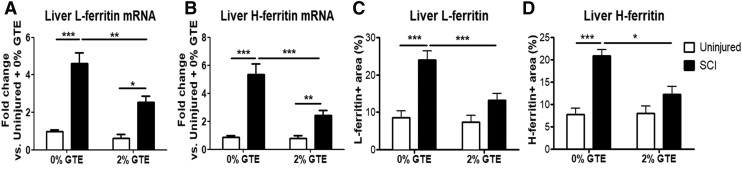
Increased intracellular iron drives expression of ferritin, the major iron storage protein. Therefore, hepatic mRNA and the expression pattern of L-ferritin and H-ferritin were examined to verify the Perls results. In accordance with elevated iron, both hepatic H- and L-ferritin mRNA and immunolabeling were significantly increased 42 days after SCI (Figs. 6 and 7). A significant SCI × GTE interactive effect indicated that, similar to the liver iron, dietary GTE significantly reduced post-SCI hepatic H- and L-ferritin mRNA and immunoreactivity (Figs. 6 and 7). In fact, L- and H-ferritin immunolabeling was not significantly increased after SCI in rats fed GTE, compared with naive rats on the GTE diet (Fig 7).
FIG. 6.
Prophylactic green tea extract (GTE) diet reduced L- and H-ferritin accumulation in livers after spinal cord injury (SCI). Representative coronal sections showing (A, B) hepatic L-ferritin and (C, D) hepatic H-ferritin in control uninjured, control GTE, control SCI, and GTE SCI livers. Scale bar = 100 μm. (B, D) High magnification images of boxed regions in (A, C). Scale bar = 25 μm.
FIG. 7.
Quantification of L- and H-ferritin gene expression and immunoreactivity in livers 42 days after spinal cord injury (SCI). Hepatic (A) L-ferritin and (B) H-ferritin mRNA levels were significantly increased at 42 days post-injury (DPI) compared with uninjured controls (L- ferritin main injury effect: p < 0.0001; H-ferritin main injury effect: p < 0.0001). L-ferritin and H-ferritin hepatic mRNA levels were significantly reduced in SCI +2% green tea extract (GTE) livers compared with SCI +0% GTE livers (L-ferritin main diet effect: p = 0.0139; H-ferritin main diet effect: p = 0.0194). Two-way ANOVA also revealed a significant interaction between diet and injury (p = 0.0139). *p < 0.05, **p < 0.01, and ***p < 0.001 by Tukey's post-hoc test. (C) Hepatic L-ferritin and (D) H-ferritin immunoreactivity increased significantly at 42 DPI compared with uninjured controls (L-ferritin main injury effect: p = 0.0102; H-ferritin main injury effect: p = 0.0211). Prophylactic GTE-enriched diet significantly reduced the SCI-induced increase in L- and H-ferritin expression (L-ferritin main diet effect: p < 0.0001; H-ferritin main diet effect: p < 0.0001). Two-way ANOVA also revealed a significant interaction between diet and injury (p = 0.0327). ***p < 0.001 by Tukey's post-hoc test. Bars represent + SEM.
GTE diet did not alter intraspinal histopathology or locomotor recovery after SCI
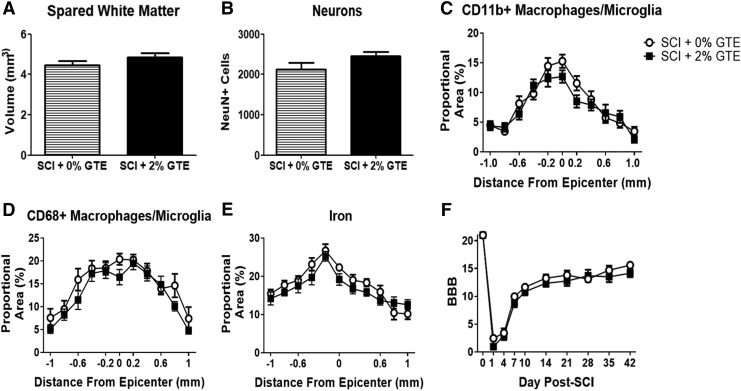
To determine if having GTE in the diet prior to and during the recovery after injury affected intraspinal tissue sparing or reduced histopathology, spinal cord cross-sections spanning the rostrocaudal extent of the injury were evaluated. GTE diet did not alter spared white matter volume (Fig. 8A), neuron sparing (Fig. 8B), spinal cord macrophage/microglia distribution and accumulation (Fig. 8C, D), or intraspinal iron accumulation (Fig. 8E). Hindlimb locomotor recovery was also not different between SCI rats receiving control or those receiving GTE diet (Fig. 8F).
FIG. 8.
Green tea extract (GTE) diet did not change spinal cord pathology or locomotor recovery after spinal cord injury (SCI). Quantification of spared white matter volume (A), NeuN+ neurons (B), Cd11b immunoreactivity (C), CD68 immunoreactivity (D), and Perls stain for iron (E) showed no significant difference between control and prophylactic GTE diet animals 42 days post-injury (DPI) throughout 2 mm of spinal cord tissue surrounding the lesion epicenter. (F) Locomotor recovery assessed with the Basso–Beattie–Bresnahan (BBB) locomotor rating scale was not different between control and GTE fed rats. Bars represent mean ± SEM.
Discussion
The metabolic state present at the time of injury likely influences metabolic changes after SCI, in either a beneficial or a detrimental fashion. Here, we tested if consuming GTE prior to SCI reduced hepatic or metabolic indices of pathology after SCI. Epidemiological studies suggest that green tea (≥10 servings/day) lowers the risk of liver injury and inflammation.44 Therefore, rats received the equivalent dose (2% GTE) for 3 weeks prior to SCI to determine if GTE's hepatoprotective effects extended to after SCI. Unfortunately, the GTE diet did not reduce hepatic pro-inflammatory gene expression or macrophage activation after SCI, although GTE did reduce hepatic iron accumulation and associated ferritin mRNA and protein expression, which, as we show here for the first time, is elevated after rodent SCI. SCI rats of both groups also exhibited increased circulating NEFAs and glucose at 42 DPI, providing further evidence that chronic SCI induces hepatic metabolic dysregulation. Notably, the rise in glucose was not significant in rats fed the GTE diet. Finally, the GTE-rich diet did not affect spinal cord pathology or locomotor recovery compared with controls. Taken together, these results suggest that whereas dietary supplementation with GTE is hepatoprotective in obesity models,31,34,47,60–62 it does not reduce SCI-induced liver and spinal cord inflammation or dyslipidemia.
The elevated hepatic lipids, inflammation, circulating glucose, and NEFAs at 42 DPI noted here are consistent with our prior work showing that SCI induces the development of NASH, an advanced stage of NAFLD.10,11 If left untreated, NASH can lead to irreversible fibrosis, cirrhosis, and hepatic carcinoma.11,59,63 Although it is unknown if NASH occurs at a higher frequency after SCI, hepatitis, cirrhosis, and hepatic carcinoma do have a significantly higher frequency in SCI individuals than in the general population.64,65 Green tea and its catechins protect against inducible NAFLD by decreasing oxidative stress, inflammatory responses, plasma aminotransferases, triglycerides, and lipoproteins.30,31,43,44 The close relationship of these factors to the development of NAFLD supported the hypothesis that dietary green tea would also protect against NAFLD-like liver pathology in chronic SCI; however, this was not observed. The mechanisms inducing NASH after SCI are unknown but likely differ from those in diet-evoked NASH that can be successfully ameliorated by GTE, such as hepatic NFκB activation and inflammation caused by metabolic endotoxemia and toll-like receptor 4 (TLR4)/ myeloid differentiation primary response 88 (MYD88) signaling.32
Novel data here show that SCI causes long-term aberrant accumulation of iron and ferritin in hepatocytes. The liver is the major regulator of systemic iron levels and iron storage;66 therefore, these data demonstrate that hepatic iron regulation is significantly disrupted for weeks after SCI in rodents. Hepatic iron overload can lead to oxidative damage and is associated with more advanced liver injury in NAFLD; further, excess hepatic iron predisposes livers to cirrhosis and hepatocellular carcinoma.67–70 Given that these are more prevalent in the SCI population, hepatic iron overload may be an important clinical indicator of pathology. Hepatic iron sequestration may also be associated with anemia, and one study of SCI individuals noted that chronic anemia was associated with increased formation of pressure sores.71,72 Therefore, better understanding of systemic iron regulation and hepatocyte dysfunction after SCI may provide important clinical targets for improving the health and longevity of SCI individuals.
Notably, hepatic iron overload in our study was significantly reduced by the GTE-rich diet. Indeed, the cytoprotective effects of tea polyphenols include iron-chelation and reducing iron absorption.73,74 Studies suggest that lowering iron absorption, especially in patients with low iron requirements, may protect against reactive oxygen species (ROS) and lipid peroxidation.73–75 Thus, dietary GTE may be an effective mechanism for reducing hepatic iron overload after SCI, although future studies are needed to determine if GTE treatment started after SCI functions similarly. Despite the reduced hepatic iron levels, other outcome measures of liver inflammation and steatosis were not reduced by 2% GTE, showing the limited effectiveness of this dose and approach in the SCI model. Two percent GTE represents consuming 10 cups of tea per day. Because this approaches typical maximum consumption, our results suggest that to test higher doses will require that different mechanisms of GTE or catechin delivery to the liver be developed. In our prior studies, the 2% dietary GTE used here protected against liver injury associated with NAFLD and NASH in rodents.30,32–34 Because SCI causes similar NASH-like chronic pathology in the liver, we predicted that this dose would produce the best chance for reducing liver pathology. However, it should be recognized that GTE may be processed differently in humans than in rodents, and conclusions are, therefore, subject to specific experimental conditions.29,36
Excess iron also accumulates in the spinal cord after injury and is thought to contribute to tissue pathology through catalytic production of hydroxyl radicals, which can kill neurons and damage membranes by lipid peroxidation.61,76–81 Iron chelators reduce spinal cord damage and promote functional improvement, although the effect has been modest in most studies.51,76–80,82 Although GTE lowered hepatic iron after SCI, it did not reduce intraspinal iron overload, which likely reflects different mechanisms of iron accumulation in the two regions. For example, intraspinal iron is thought to be largely derived from hemorrhage-induced red blood cells (RBC). Intraspinal RBCs are engulfed and destroyed by macrophages within the first 3–4 DPI, after which iron- and ferritin-positive macrophages remain long term in the lesion sites.51,76 In contrast, RBCs do not accumulate in livers after SCI, and hepatocytes, rather than macrophages, sequester iron. The signals promoting hepatic iron sequestration that were modified by the GTE diet are currently unknown. An obvious mechanism would be inflammatory signaling, but the inflammatory markers examined here were unaffected by GTE.
Our results are not in line with previous studies showing that GTE improves outcome from rodent SCI, including reduced tissue pathology and improved motor recovery.83–86 However, in those studies, GTE was given directly into the injured spinal cord, bloodstream or peritoneal cavity after SCI rather than through the diet as in this study. Other dietary supplements showing similar neuroprotection in rodents after direct injection into the injured spinal cord or bloodstream also have shown mixed results when administered through diet alone.87,88 Evidence suggests that compounds such as catechins in GTE do cross the blood–brain barrier; however, the amount of active dietary chemicals that successfully reach the central nervous system (CNS) is significantly reduced after digestion and metabolism.89–96 Therefore, the ability of diet-derived mediators to reduce CNS trauma directly may be limited compared with direct effects on more accessible organs such as the liver.
We acknowledge that although prophylactic administration of GTE is useful for examining the effect of pre-injury lifestyle on post-SCI metabolic outcome, this approach would likely have limited clinical value if begun after unanticipated incidents such as SCI. However, the simple act of adopting a healthier lifestyle by ingesting green tea, which is an effective method for improving basal liver metabolism and overall health, could have broad applications. For example, pre-treatment with GTE or perhaps other simple yet more effective methods of enhancing basal liver metabolism and decreasing liver inflammation may be beneficial for elective surgeries around the spinal cord such as tumor removal, or repair interventions, such as cell transplantations into the injured spinal cord.
Collectively, the data presented here show that SCI causes protracted liver inflammation and fat deposition, and that these are associated with chronic hepatic iron dysregulation and metabolic dysfunction, which are major health issues for the SCI community. Dietary modification is a highly translatable, cost-efficient, and generally safe approach that may provide feasible therapeutic options for combating the multiple systemic problems induced by SCI. However, as a prophylactic diet containing high amounts of GTE did not improve spinal cord tissue sparing or motor recovery, this dietary regimen alone is unlikely to have potent effects in the human SCI population. Future studies examining additional lifestyle choices, before and after SCI, are needed to further understanding of mechanisms causing systemic organ dysfunction and pathology after SCI and for designing treatments that effectively manage metabolic dysfunction in the SCI population.
Acknowledgments
The authors thank Feng Qin Yin, Rochelle Deibert, and Chureeporn Chitchumroonchokchai for excellent technical assistance. This work was funded by the National Institute of Neurological Disorders and Stroke (NINDS) P30-NS045758 (D.M.M.), R01-NS082095 (D.M.M.), Craig H. Neilsen Foundation (M.G.), United States Department of Agriculture–National Institute of Food and Agriculture (USDA-NIFA) (2014-67017-21761) (R.S.B.), and the Ohio Agricultural Research and Development Center (R.S.B.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Fleming J.C., Norenberg M.D., Ramsay D.A., Dekaban G.A., Marcillo A.E., Saenz A.D., Pasquale-Styles M., Dietrich W.D., Weaver L.C., Agerberth B., Charo J., Werr J., Olsson B., Idali F., Lindbom L., Bao F., Chen Y., Dekaban G., Weaver L., Bao F., Dekaban G., Weaver L., Barnathan E., Raghunath P., Tomaszewski J., Ganz T., Cines D., Higazi A. al-R., Bethea J., Blight A., Brandes R., Kreuzer J., Bunge R., Puckett W., Becerra J., Marcillo A., Quencer R., Carlson S., Parrish M., Springer J., Doty K., Dossett L., Chang H., Chatzipanteli K., Yanagawa Y., Marcillo A., Kraydieh S., Yezierski R., Dietrich W., Crutcher K., Gendelman H., Kipnis J., Perez-Polo J.R., Perry V., Popovich P., Weaver L., Dougherty K., Dreyfus C., Black I., Duchossoy Y., Horvat J., Stettler O., Farooque M., Isaksson J., Olsson Y., Friese M., Fugger L., Gonzalez R., Glaser J., Liu M., Lane T., Keirstead H., Greaves D., Quinn C., Seldin M., Gordon S., Gris D., Marsh D., Oatway M., Chen Y., Hamilton E., Dekaban G., Hall E., Hauben E., Schwartz M., Hauben E., Butovsky O., Nevo U., Yoles E., Moalem G., Agranov E., Hausmann O., Ito T., Oyanagi K., Wakabayashi K., Ikuta F., Jones T., Ankeny D., et al. (2006). The cellular inflammatory response in human spinal cords after injury. Brain 129, 3249–3269 [DOI] [PubMed] [Google Scholar]
- 2. Bao F., Brown A., Dekaban G.A., Omana V., and Weaver L.C. (2011). CD11d integrin blockade reduces the systemic inflammatory response syndrome after spinal cord injury. Exp. Neurol. 231, 272–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bao F., Shultz S.R., Hepburn J.D., Omana V., Weaver L.C., Cain D.P., and Brown A. (2012). A CD11d monoclonal antibody treatment reduces tissue injury and improves neurological outcome after fluid percussion brain injury in rats. J. Neurotrauma 29, 2375–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campbell S.J., Perry V.H., Pitossi F.J., Butchart A.G., Chertoff M., Waters S., Dempster R., and Anthony D.C. (2005). Central nervous system injury triggers hepatic CC and CXC chemokine expression that is associated with leukocyte mobilization and recruitment to both the central nervous system and the liver. Am. J. Pathol. 166, 1487–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell S.J., Anthony D.C., Oakley F., Carlsen H., Elsharkawy A.M., Blomhoff R., and Mann D.A. (2008). Hepatic nuclear factor kappa B regulates neutrophil recruitment to the injured brain. J. Neuropathol. Exp. Neurol. 67, 223–230 [DOI] [PubMed] [Google Scholar]
- 6. Cragg J.J., Stone J.A., and Krassioukov A.V. (2012). Management of cardiovascular disease risk factors in individuals with chronic spinal cord injury: An evidence-based review. J. Neurotrauma 29, 1999–2012 [DOI] [PubMed] [Google Scholar]
- 7. Furlan J.C., and Fehlings M.G. (2008). Cardiovascular complications after acute spinal cord injury: pathophysiology, diagnosis, and management. Neurosurg. Focus 25, E13. [DOI] [PubMed] [Google Scholar]
- 8. Anthony D.C., and Couch Y. (2014). The systemic response to CNS injury. Exp. Neurol. 258, 105–111 [DOI] [PubMed] [Google Scholar]
- 9. Gabay C., and Kushner I. (1999). Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340, 448–454 [DOI] [PubMed] [Google Scholar]
- 10. Sauerbeck A.D., Laws J.L., Bandaru V.V.R., Popovich P.G., Haughey N.J., and McTigue D.M. (2015). Spinal cord injury causes chronic liver pathology in rats. J. Neurotrauma 32, 159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farrell G.C., and Larter C.Z. (2006). Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology 43. [DOI] [PubMed] [Google Scholar]
- 12. Manns P.J., McCubbin J.A., and Williams D.P. (2005). Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Arch. Phys. Med. Rehabil. 86, 1176–1181 [DOI] [PubMed] [Google Scholar]
- 13. Nelson M.D., Widman L.M., Abresch R.T., Stanhope K., Havel P.J., Styne D.M., and McDonald C.M. (2007). Metabolic syndrome in adolescents with spinal cord dysfunction. J Spinal Cord Med. 30, Suppl. 1, S127–S139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bauman W.A., Spungen A.M., Raza M., Rothstein J., Zhang R.L., Zhong Y.G., Tsuruta M., Shahidi R., Pierson R.N., Jr., Wang J., and Gordon S.K. (1992). Coronary artery disease: metabolic risk factors and latent disease in individuals with paraplegia. Mt Sinai J Med 59, 163–168 [PubMed] [Google Scholar]
- 15. Brenes G., Dearwater S., Shapera R., LaPorte R.E., and Collins E. (1986). High density lipoprotein cholesterol concentrations in physically active and sedentary spinal cord injured patients. Arch. Phys. Med. Rehabil. 67, 445–450 [PubMed] [Google Scholar]
- 16. Kocina P. (1997). Body composition of spinal cord injured adults. Sports Med. 23, 48–60 [DOI] [PubMed] [Google Scholar]
- 17. Szlachcic Y., Adkins R.H., Adal T., Yee F., Bauman W., and Waters R.L. (2001). The effect of dietary intervention on lipid profiles in individuals with spinal cord injury. J. Spinal Cord Med. 24, 26–29 [DOI] [PubMed] [Google Scholar]
- 18. Javierre C., Vidal J., Segura R., Medina J., and Garrido E. (2005). Continual supplementation with n-3 fatty acids does not modify plasma lipid profile in spinal cord injury patients. Spinal Cord 43, 527–530 [DOI] [PubMed] [Google Scholar]
- 19. Yekutiel M., Brooks M.E., Ohry A., Yarom J., and Carel R. (1989). The prevalence of hypertension, ischaemic heart disease and diabetes in traumatic spinal cord injured patients and amputees. Paraplegia 27, 58–62 [DOI] [PubMed] [Google Scholar]
- 20. Myers J., Lee M., and Kiratli J. (2007). Cardiovascular disease in spinal cord injury. Am. J. Phys. Med. Rehabil. 86, 142–152 [DOI] [PubMed] [Google Scholar]
- 21. DeFronzo R.A. (1997). Insulin resistance: A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia and atherosclerosis. Neth. J. Med. 50, 191–197 [DOI] [PubMed] [Google Scholar]
- 22. Duckworth W.C., Jallepalli P., and Solomon S.S. (1983). Glucose intolerance in spinal cord injury. Arch. Phys. Med. Rehabil. 64, 107–110 [PubMed] [Google Scholar]
- 23. Mohr T., Dela F., Handberg A., Biering-Sørensen F., Galbo H., and Kjaer M. (2001). Insulin action and long-term electrically induced training in individuals with spinal cord injuries. Med. Sci. Sports Exerc. 33, 1247–1252 [DOI] [PubMed] [Google Scholar]
- 24. Waterland R.A., and Garza C. (1999). Potential mechanisms of metabolic imprinting that lead to chronic disease. Am. J. Clin. Nutr. 62, 179–197 [DOI] [PubMed] [Google Scholar]
- 25. Galgani J., and Ravussin E. (2008). Energy metabolism, fuel selection and body weight regulation. Int. J. Obes. (Lond). 32, Suppl. 7, S109–S119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parry S.A., and Hodson L. (2017). Influence of dietary macronutrients on liver fat accumulation and metabolism. J. Investig. Med. 65, 1102–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. LeMieux M., Al-Jawadi A., Wang S., and Moustaid-Moussa N. (2013). Metabolic Profiling in Nutrition and Metabolic Disorders. Adv. Nutr. An Int. Rev. J. 4, 548–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kanwar J., Taskeen M., Mohammad I., Huo C., Chan T.H., and Dou Q.P. (2012). Recent advances on tea polyphenols. Front. Biosci. (Elite Ed). 4, 111–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Masterjohn C., and Bruno R.S. (2012). Therapeutic potential of green tea in nonalcoholic fatty liver disease. Nutr. Rev. 70, 41–56 [DOI] [PubMed] [Google Scholar]
- 30. Bruno R.S., Dugan C.E., Smyth J.A., DiNatale D.A., and Koo S.I. (2008). Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury. J. Nutr. 138, 323–331 [DOI] [PubMed] [Google Scholar]
- 31. Park H.J., DiNatale D.A., Chung M.Y., Park Y.K., Lee J.Y., Koo S.I., O'Connor M., Manautou J.E., and Bruno R.S. (2011). Green tea extract attenuates hepatic steatosis by decreasing adipose lipogenesis and enhancing hepatic antioxidant defenses in ob/ob mice. J. Nutr. Biochem. 22, 393–400 [DOI] [PubMed] [Google Scholar]
- 32. Li J., Sapper T.N., Mah E., Moller M.V., Kim J.B., Chitchumroonchokchai C., McDonald J.D., and Bruno R.S. (2017). Green tea extract treatment reduces NFκB activation in mice with diet-induced nonalcoholic steatohepatitis by lowering TNFR1 and TLR4 expression and ligand availability. J. Nutr. Biochem. 41, 34–41 [DOI] [PubMed] [Google Scholar]
- 33. Li J., Sapper T.N., Mah E., Rudraiah S., Schill K.E., Chitchumroonchokchai C., Moller M.V., Mcdonald J.D., Rohrer P.R., Manautou J.E., and Bruno R.S. (2016). Green tea extract provides extensive Nrf2-independent protection against lipid accumulation and NFκB pro-inflammatory responses during nonalcoholic steatohepatitis in mice fed a high-fat diet. Mol. Nutr. Food Res. 60, 858–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park H.J., Lee J., Chung M., Park Y., Bower A.M., Koo S.I., Giardina C., and Bruno R.S. (2012). Green tea extract suppresses NFκB activation and inflammatory responses in diet-induced obese rats with nonalcoholic steatohepatitis 1 – 3. J Nutr. 142, 57–63 [DOI] [PubMed] [Google Scholar]
- 35. Bose M., Lambert J.D., Ju J., Reuhl K.R., Shapses S.A., and Yang C.S. (2008). The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J. Nutr. 138, 1677–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klatsky A.L., Armstrong M.A., and Friedman G.D. (1993). Coffee, tea, and mortality. Ann. Epidemiol. 3, 375–381 [DOI] [PubMed] [Google Scholar]
- 37. Crespy V., and Williamson G. (2004). h effects of green tea catechins in in vivo animal models. J. Nutr. 134, 3431–3440 [DOI] [PubMed] [Google Scholar]
- 38. Kakuda T. (2002). Neuroprotective effects of the green tea components theanine and catechins. Biol. Pharm. Bull. 25, 1513–1518 [DOI] [PubMed] [Google Scholar]
- 39. Sung H., Nah J., Chun S., Park H., Yang S.E., and Min W.K. (2000). In vivo antioxidant effect of green tea. Eur. J. Clin. Nutr. 54, 527–529 [DOI] [PubMed] [Google Scholar]
- 40. Hakim I.A., Harris R.B., Brown S., Chow H.-H.S., Wiseman S., Agarwal S., and Talbot W. (2003). Effect of increased tea consumption on oxidative DNA damage among smokers: a randomized controlled study. J. Nutr. 133, 3303S–3309S [DOI] [PubMed] [Google Scholar]
- 41. Kim J.A., Formoso G., Li Y., Potenza M.A., Marasciulo F.L., Montagnani M., and Quon M.J. (2007). Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and fyn. J. Biol. Chem. 282, 13736–13745 [DOI] [PubMed] [Google Scholar]
- 42. Friedman S.L. (2008). Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 88, 125–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kuriyama S., Shimazu T., Ohmori K., Kikuchi N., Nakaya N., Nishino Y., Tsubono Y., and Tsuji I. (2006). Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA 296, 1255–1265 [DOI] [PubMed] [Google Scholar]
- 44. Imai K., and Nakachi K. (1995). Cross sectional study of effects of drinking green tea on cardiovascular and liver diseases. BMJ 310, 693–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pezeshki A., Safi S., Feizi A., Askari G., and Karami F. (2016). The effect of green tea extract supplementation on liver enzymes in patients with nonalcoholic fatty liver disease. Int. J. Prev. Med. 7, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sakata R., Nakamura T., Torimura T., Ueno T., and Sata M. (2013). Green tea with high-density catechins improves liver function and fat infiltration in non-alcoholic fatty liver disease (NAFLD) patients: A double-blind placebo-controlled study. Int. J. Mol. Med. 32, 989–994 [DOI] [PubMed] [Google Scholar]
- 47. Bruno R.S., Dugan C.E., Smyth J.A., DiNatale D.A., and Koo S.I. (2008). Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury. J. Nutr. 138, 323–331 [DOI] [PubMed] [Google Scholar]
- 48. Basso D.M., Beattie M.S., and Bresnahan J.C. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 [DOI] [PubMed] [Google Scholar]
- 49. Schonberg D.L., and McTigue D.M. (2009). Iron is essential for oligodendrocyte genesis following intraspinal macrophage activation. Exp. Neurol. 218, 64–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schonberg D.L., Goldstein E.Z., Sahinkaya F.R., Wei P., Popovich P.G., and McTigue D.M. (2012). Ferritin stimulates oligodendrocyte genesis in the adult spinal cord and can be transferred from macrophages to NG2 cells in vivo. J. Neurosci. 32, 5374–5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sauerbeck A., Schonberg D.L., Laws J.L., and McTigue D.M. (2013). Systemic iron chelation results in limited functional and histological recovery after traumatic spinal cord injury in rats. Exp. Neurol. 248, 53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McTigue D.M., Tripathi R., Wei P., and Lash A.T. (2007). The PPAR gamma agonist Pioglitazone improves anatomical and locomotor recovery after rodent spinal cord injury. Exp. Neurol. 205, 396–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schmittgen T.D., and Livak K.J. (2008). Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108 [DOI] [PubMed] [Google Scholar]
- 54. Campbell S.J., Perry V.H., Pitossi F.J., Butchart A.G., Chertoff M., Waters S., Dempster R., and Anthony D.C. (2005). Central nervous system injury triggers hepatic CC and CXC chemokine expression that is associated with leukocyte mobilization and recruitment to both the central nervous system and the liver. Am. J. Pathol. 166, 1487–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Campbell S.J., Zahid I., Losey P., Law S., Jiang Y., Bilgen M., van Rooijen N., Morsali D., Davis A.E.M., and Anthony D.C. (2008). Liver Kupffer cells control the magnitude of the inflammatory response in the injured brain and spinal cord. Neuropharmacology 55, 780–787 [DOI] [PubMed] [Google Scholar]
- 56. Yang C.Y., Chen J.B., Tsai T.F., Tsai Y.C., Tsai C.Y., Liang P.H., Hsu T.L., Wu C.Y., Netea M.G., Wong C.H., and Hsieh S.L. (2013). CLEC4F is an inducible C-type lectin in F4/80-positive cells and is involved in alpha-galactosylceramide presentation in liver. PLoS One 8, e65070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Scott C.L., Zheng F., De Baetselier P., Martens L., Saeys Y., De Prijck S., Lippens S., Abels C., Schoonooghe S., Raes G., Devoogdt N., Lambrecht B.N., Beschin A., and Guilliams M. (2016). Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat. Commun. 7, 10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tosello-Trampont A.C., Landes S.G., Nguyen V., Novobrantseva T.I., and Hahn Y.S. (2012). Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production. J. Biol. Chem. 287, 40161–40172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Farrell G.C., Van Rooyen D., Gan L., and Chitturi S. (2012). NASH is an inflammatory disorder: Pathogenic, prognostic and therapeutic implications. Gut Liver 6, 149–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xu Y., Zhang M., Wu T., Dai S., Xu J., and Zhou Z. (2015). The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 6, 297–304 [DOI] [PubMed] [Google Scholar]
- 61. Lu C., Zhu W., Shen C.L., and Gao W. (2012). Green tea polyphenols reduce body weight in rats by modulating obesity-related genes. PLoS One E 7, e38332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yan J., Zhao Y., and Zhao B. (2013). Green tea catechins prevent obesity through modulation of peroxisome proliferator-activated receptors. Sci. China Life Sci. 56, 804–810 [DOI] [PubMed] [Google Scholar]
- 63. Sanyal A.J., Yoon S.K., and Lencioni R. (2010). The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist 15 Suppl. 4, 14–22 [DOI] [PubMed] [Google Scholar]
- 64. Imai K., Kadowaki T., Aizawa Y., and Fukutomi K. (1996). Problems in the health management of persons with spinal cord injury. J. Clin. Epidemiol. 49, 505–510 [DOI] [PubMed] [Google Scholar]
- 65. Kao C.-H., Sun L.-M., Chen Y.-S., Lin C.-L., Liang J.-A., Kao C.-H., and Weng M.-W. (2016). Risk of nongenitourinary cancers in patients with spinal cord injury: a population-based cohort study. Medicine (Baltimore). 95, e2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Anderson E.R., and Shah Y.M. (2013). Iron homeostasis in the liver. Compr. Physiol. 3, 315–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Britton L.J., Subramaniam V.N., and Crawford D.H. (2016). Iron and non-alcoholic fatty liver disease. World J. Gastroenterol. 22, 8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dongiovanni P., Fracanzani A.L., Fargion S., and Valenti L. (2011). Iron in fatty liver and in the metabolic syndrome: A promising therapeutic target. J. Hepatol. 55, 920–932 [DOI] [PubMed] [Google Scholar]
- 69. Nelson J.E., Klintworth H., and Kowdley K.V. (2012). Iron metabolism in nonalcoholic fatty liver disease. Curr. Gastroenterol. Rep. 14, 8–16 [DOI] [PubMed] [Google Scholar]
- 70. Simcox J.A., and McClain D.A. (2013). Iron and diabetes risk. Cell Metab. 17, 329–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Frisbie J.H. (2010). Anemia and hypoalbuminemia of chronic spinal cord injury: prevalence and prognostic significance. Spinal Cord 48, 566–569 [DOI] [PubMed] [Google Scholar]
- 72. Salzberg C.A., Byrne D.W., Cayten C.G., van Niewerburgh P., Murphy J.G., and Viehbeck M. (1996). A new pressure ulcer risk assessment scale for individuals with spinal cord injury. Am. J. Phys. Med. Rehabil. 75, 96–104 [DOI] [PubMed] [Google Scholar]
- 73. Hamdaoui M.H., Chahed A., Ellouze-Chabchoub S., Marouani N., Abid Z. Ben, and Hédhili A. (2005). Effect of green tea decoction on long-term iron, zinc and selenium status of rats. Ann. Nutr. Metab. 49, 118–124 [DOI] [PubMed] [Google Scholar]
- 74. Marouani N., Chahed A., Hédhili A., and Hamdaoui M.H. (2007). Both aluminum and polyphenols in green tea decoction (Camellia sinensis) affect iron status and hematological parameters in rats. Eur. J. Nutr. 46, 453–459 [DOI] [PubMed] [Google Scholar]
- 75. Ma Q., Kim E.-Y., and Han O. (2010). Bioactive dietary polyphenols decrease heme iron absorption by decreasing basolateral iron release in human intestinal Caco-2 cells. J. Nutr. 140, 1117–1121 [DOI] [PubMed] [Google Scholar]
- 76. Rathore K.I., Kerr B.J., Redensek A., Lopez-Vales R., Jeong S.Y., Ponka P., and David S. (2008). Ceruloplasmin protects injured spinal cord from iron-mediated oxidative damage. J. Neurosci. 28, 12736–12747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schultke E., Kendall E., Kamencic H., Ghong Z., Griebel R.W., and Juurlink B.H.J. (2003). Quercetin promotes functional recovery following acute spinal cord injury. J. Neurotrauma 20, 583–591 [DOI] [PubMed] [Google Scholar]
- 78. Klapka N., Hermanns S., Straten G., Masanneck C., Duis S., Hamers F.P.T., Müller D., Zuschratter W., and Müller H.W. (2005). Suppression of fibrous scarring in spinal cord injury of rat promotes long-distance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. Eur. J. Neurosci. 22, 3047–3058 [DOI] [PubMed] [Google Scholar]
- 79. Paterniti I., Mazzon E., Emanuela E., Paola R.D., Galuppo M., Bramanti P., and Cuzzocrea S. (2010). Modulation of inflammatory response after spinal cord trauma with deferoxamine, an iron chelator. Free Radic. Res. 44, 694–709 [DOI] [PubMed] [Google Scholar]
- 80. Schultke E., Kamencic H., Skihar V.M., Griebel R., and Juurlink B. (2010). Quercetin in an animal model of spinal cord compression injury: correlation of treatment duration with recovery of motor function. Spinal Cord 48, 112–117 [DOI] [PubMed] [Google Scholar]
- 81. Jeong M., Plunet W., Streijger F., Lee J.H.T., Plemel J.R., Park S., Lam C.K., Liu J., and Tetzlaff W. (2011). Intermittent fasting improves functional recovery after rat thoracic contusion spinal cord injury. J. Neurotrauma 28, 479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Schültke E., Griebel R.W., and Juurlink B.H.J. (2010). Quercetin attenuates inflammatory processes after spinal cord injury in an animal model. Spinal Cord 48, 857–861 [DOI] [PubMed] [Google Scholar]
- 83. Khalatbary A.R. (2014). Natural polyphenols and spinal cord injury. Iran. Biomed. J. 18, 120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Khalatbary A.R., Tiraihi T., Boroujeni M.B., Ahmadvand H., Tavafi M., and Tamjidipoor A. (2010). Effects of epigallocatechin gallate on tissue protection and functional recovery after contusive spinal cord injury in rats. Brain Res. 1306, 168–175 [DOI] [PubMed] [Google Scholar]
- 85. Khalatbary A.R., and Ahmadvand H. (2011). Anti-inflammatory effect of the epigallocatechin gallate following spinal cord trauma in rat. Iran. Biomed. J. 15, 31–37 [PMC free article] [PubMed] [Google Scholar]
- 86. Paterniti I., Genovese T., Crisafulli C., Mazzon E., Di Paola R., Galuppo M., Bramanti P., and Cuzzocrea S. (2009). Treatment with green tea extract attenuates secondary inflammatory response in an experimental model of spinal cord trauma. Naunyn. Schmiedebergs. Arch. Pharmacol. 380, 179–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Huang W.L., King V.R., Curran O.E., Dyall S.C., Ward R.E., Lal N., Priestley J. V., and Michael-Titus A.T. (2007). A combination of intravenous and dietary docosahexaenoic acid significantly improves outcome after spinal cord injury. Brain 130, 3004–3019 [DOI] [PubMed] [Google Scholar]
- 88. Lim S.-N., Huang W., Hall J.C.E., Michael-Titus A.T., and Priestley J.V. (2013). Improved outcome after spinal cord compression injury in mice treated with docosahexaenoic acid. Exp. Neurol. 239, 13–27 [DOI] [PubMed] [Google Scholar]
- 89. D'Archivio M., Filesi C., Di Benedetto R., Gargiulo R., Giovannini C., and Masella R. (2007). Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 43, 348–361 [PubMed] [Google Scholar]
- 90. Zhu M., Chen Y., and Li R.C. (2000). Oral absorption and bioavailability of tea catechins. Planta Med. 66, 444–447 [DOI] [PubMed] [Google Scholar]
- 91. Del Rio D., Calani L., Scazzina F., Jechiu L., Cordero C., and Brighenti F. (2010). Bioavailability of catechins from ready-to-drink tea. Nutrition 26, 528–533 [DOI] [PubMed] [Google Scholar]
- 92. Lambert J.D., Lee M.-J., Lu H., Meng X., Hong J.J.J., Seril D.N., Sturgill M.G., and Yang C.S. (2003). Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J. Nutr. 133, 4172–4177 [DOI] [PubMed] [Google Scholar]
- 93. Lee M.J., Maliakal P., Chen L., Meng X., Bondoc F.Y., Prabhu S., Lambert G., Mohr S., and Yang C.S. (2002). Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomarkers Prev. 11, 1025–1032 [PubMed] [Google Scholar]
- 94. Nakagawa K., Okuda S., and Miyazawa T. (1997). Dose-dependent incorporation of tea catechins, (−)-epigallocatechin-3-gallate and (−)-epigallocatechin, into human plasma. Biosci. Biotechnol. Biochem. 61, 1981–1985 [DOI] [PubMed] [Google Scholar]
- 95. Freund Levi Y., Vedin I., Cederholm T., Basun H., Faxén Irving G., Eriksdotter M., Hjorth E., Schultzberg M., Vessby B., Wahlund L.O., Salem N., and Palmblad J. (2014). Transfer of omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich omega-3 fatty acid preparation in patients with Alzheimer's disease: The OmegAD Study. J. Intern. Med. 275, 428–436 [DOI] [PubMed] [Google Scholar]
- 96. Mills J.D., Hadley K., and Bailes J.E. (2011). Dietary supplementation with the Omega-3 fatty acid docosahexaenoic acid in traumatic brain injury. Neurosurgery 68, 474–481 [DOI] [PubMed] [Google Scholar]