Abstract
Introduction
Acute and chronic insomnia can exacerbate type 2 diabetes mellitus (T2DM). We investigated suvorexant (an anti-insomnia drug that targets the orexin system) effects on sleep architecture and glucose metabolism in T2DM patients with insomnia.
Materials and methods
This 7 day open-label, single-arm, intervention trial included 18 subjects with T2DM and insomnia. After 1 day acclimatization, daily glucose levels, sleep architecture, and autonomic nervous function were evaluated by continuous glucose monitoring (CGM), single-channel electroencephalography, and accelerometry, respectively.
Results
Suvorexant treatment for 3 days significantly increased total sleep time and sleep efficiency, with partial suppression of sympathetic nerve activity. CGM-measured 24 h mean glucose level decreased significantly from 157.7 ± 22.9 to 152.3 ± 17.8 mg/dL, especially in the early glucose surge after the midnight nadir (from 28.3 ± 15.0 to 18.2 ± 9.9 mg/dL), and until supper with a significant improvement in homeostasis model assessment of insulin resistance from 4.0 ± 2.8 to 2.9 ± 1.6, respectively.
Conclusions
Suvorexant treatment for insomnia of subjects with T2DM significantly improved CGM-measured daily glycemic control, which was associated with changes in sympathomimetic tone and/or improved insulin sensitivity. The amelioration of insomnia may therefore be a target for improving glycemic control in T2DM patients with insomnia.
Abbreviations: T2DM, type 2 diabetes mellitus; CGM, continuous glucose monitoring; HbA1c, glycated hemoglobin A1c; EEG, electroencephalography; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; PSQI, Pittsburgh Sleep Quality Index; REM, rapid eye movement; AHI, Apnea–Hypopnea Index; HOMA-IR, homeostasis model assessment of insulin resistance; HRV, heart rate variability; CVR-R, coefficient of variation of RR intervals; SDNN, standard deviation of the NN (i.e., R-R) intervals; HR, heart rate; bpm, beats per minute; IQR, interquartile range; SD, standard deviation; AUC, area under the curve; BMI, body mass index; CPR, C-peptide immunoreactivity; IRI, immunoreactive insulin; eGFR, estimated glomerular filtration ratio; SAS, Sleep Apnea Syndrome
Keywords: Type 2 diabetes mellitus, Therapy for insomnia, Glycemic control, Insulin resistance, Dawn phenomenon, Autonomic nervous function
Introduction
Aging is associated with higher prevalence of both type 2 diabetes mellitus (T2DM) and insomnia. Thus, the increasing number of elderly patients with T2DM is accompanied by an increase in the prevalence of insomnia in T2DM patients [1]. Insomnia and DM have been shown to exacerbate each other, and this results in higher rates of coexistence [1], [2]. This is supported by findings that poorer sleep quality is independently associated with a higher prevalence [3] and incidence [4] of DM in the general population, and with higher glycated hemoglobin A1c (HbA1c) in T2DM patients [5], [6]. The previous studies estimated sleep quality by using self-reported questionnaires [2], [3]; however, our recent study, using single-channel electroencephalography (EEG) to provide a precise estimate of sleep quality, demonstrated that poorer sleep quality was independently associated with higher HbA1c in T2DM patients [7]. These previous studies were of cross-sectional observational design and so causality could not be determined between sleep quality and glycemic control.
It has been reported that short-term sleep restriction induced changes in metabolic and endocrine function in healthy subjects, including in carbohydrate metabolism [8], [9]. An intervention to improve sleep quality might therefore improve glycemic control in T2DM patients. Thus, it is important to elucidate whether insomnia as a treatment target can improve glycemic control.
Continuous glucose monitoring (CGM) now allows a precise assessment of 24 h glycemic changes, making it possible to examine whether an intervention to treat insomnia could improve 24 h glycemic control in T2DM patients with insomnia [10]. Suvorexant, a dual orexin receptor antagonist, suppresses wakefulness and promotes sleep without affecting any neural systems other than the orexin system [11]. It therefore selectively ameliorates insomnia, unlike the commonly used benzodiazepines or non-benzodiazepine receptor agonists. This study examined whether suvorexant improved EEG-assessed sleep quality, CGM-assessed glycemic control, and sympathetic tone in T2DM patients with insomnia.
Subjects and Methods
Study design
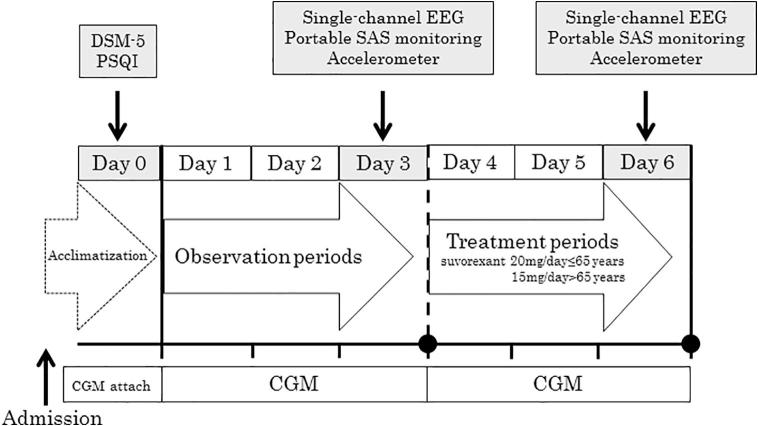
This was a 7 day, open-label, single-arm, intervention trial, performed at a single institution, the Diabetes Center of Osaka City University Hospital (Fig. 1). All eligible participants were hospitalized to minimize the impact of diet on glycemic control. Each subject's food intake was maintained at a constant level of approximately 28 kcal per ideal body weight (kg), with 60% of calories from carbohydrates. Although all the subjects suffered from insomnia, none had any history of medication for insomnia before admission. On admission (Day 0), a CGM device was attached to each subject for acclimatization [12], and the intervention trial was started on Day 1. Details have previously been reported [12], [13]. On Days 1–3, the subjects received no medication for insomnia. At 10:00 PM on Days 4–6, just before the subjects went to bed, they were given suvorexant at a daily dose of 15 mg (for those aged >65 years) or 20 mg (aged ≤65 years), according to the Japanese formulary. The type and dose of DM medications and daily caloric intake were kept unchanged throughout the 7 day study period.
Fig. 1.
Outline of the study protocol. On Day 0, the subject’s insomnia was assessed according to DSM-5 and PSQI and the subject was acclimatized to CGM. On Days 1–3, the subject took no insomnia medication. On Days 4–6, suvorexant (15 or 20 mg for subjects 65 and ≤65 years, respectively) was taken at 10:00 PM just before bedtime. On Days 3 and 6, the subject underwent measurements using single-channel EEG, portable SAS monitoring, and an accelerometer. The subject’s DM medications and daily caloric intake were kept unchanged throughout the study period. CGM, continuous glucose monitoring; DM, diabetes mellitus; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; EEG, electroencephalography; PSQI, Pittsburgh Sleep Quality Index.
Subjects
Japanese subjects with T2DM and insomnia (n = 18, 5 men and 13 women) were enrolled between March 2015 and March 2016 at the Diabetes Center of Osaka City University Hospital. The diagnosis of insomnia disorder was based on meeting the criteria of the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) (DSM-5) [14]. The diagnosis of T2DM was based on a history of DM or meeting the criteria for DM as defined in the Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus [15].
The median age and duration of DM of the subjects were 71 and 7 years, respectively. All were receiving multiple medications; these included dietetic therapy alone (n = 5) and oral hypoglycemic agents including biguanide (n = 6), sulfonylurea (n = 4), dipeptidyl peptidase-4 inhibitor (n = 7), and α-glucosidase inhibitor (n = 3). Subjects who had been taking any sleep-promote drug and insulin therapy at least three months before this study were excluded from the present study.
Blood was drawn after an overnight fast, and blood chemistry was measured by a routine standard method. HbA1c was determined using routine high-performance liquid chromatography and a latex agglutination immunoassay, and results were expressed as values equivalent to those in the National Glycohemoglobin Standardization Program [16].
All subjects provided written informed consent prior to participation. This study was approved by the Institutional Ethics Committees at Osaka City University Graduate School of Medicine (Approval No. 3063) and was conducted in accordance with the principles of the Declaration of Helsinki.
Subjective assessment of sleep architecture
Sleep architecture was assessed subjectively and objectively. The subjective assessment was by a sleep diary [7], [17] and the Pittsburgh Sleep Quality Index (PSQI). On Days 1–6, the subjects were encouraged to complete the sleep diary in the evening before going to bed and in the morning upon awakening. They recorded their total sleep time by noting the time they tried to go to sleep (bed time) and the time they finally awoke for the day (wake time), as well as the number of minutes it took them to fall asleep (sleep latency) and the total number of minutes they spent awake after they initially fell asleep (arousal time during sleep). The total sleep time for each night was then calculated as the total time between bed time and wake time minus the sleep latency and wake time after sleep onset [18]. Sleep efficiency for each night was then calculated as the total sleep time taken as a percentage of the total time spent in bed. The mean values of self-reported total sleep time, sleep latency, arousal time during sleep, and sleep efficiency were compared between Days 1–3 and Days 4–6 (after suvorexant administration).
Insomnia was also assessed on Day 0 by the PSQI, an 18-item self-report questionnaire designed to measure sleep quality and quantity over the preceding month, as we described previously [19]. A global score >5 indicates poor sleep quality [19].
Objective assessment of sleep architecture and the Apnea–Hypopnea index
On Days 3 and 6 (the third days of the observation and treatment periods), each subject underwent overnight monitoring using a single-channel EEG (SLEEPSCOPE; SleepWell Co., Osaka, Japan). Each night recording was divided into 30 s sequential periods, which were manually classified as rapid eye movement (REM) sleep and non-REM sleep and further classified as light (stage 1/2) or deep (stage 3/4) sleep, as previously described [7].
On the same nights, the Apnea–Hypopnea Index (AHI) was evaluated using a portable monitor that measured respiratory flow pressure, oxygen saturation, and heart rate (SAS2100; Nihon Kohden Corp., Tokyo, Japan), as previously reported [7], [20]. The AHI is defined as the number of apnea and hypopnea episodes per hour of sleep [21], [22].
Measurement and analysis of the dawn phenomenon, insulin resistance, and daily glucose fluctuations
To assess daily glucose level fluctuations, each subject's glucose level was monitored continuously from Day 1 to Day 6 using a CGM device (iPro2 Professional CGM; Medtronic, Northridge, PA, USA). The dawn phenomenon (Δdawn, mg/dL), stochastic spontaneous glucose fluctuations during the early morning period after an overnight fast, was quantified by subtracting the nocturnal glucose nadir from the glucose value observed just before breakfast [23]; the dawn phenomenon was considered to be present if the glucose level increased by ≥20 mg/dL, as previously reported [23], [24]. Insulin resistance was assessed using the homeostasis model assessment of insulin resistance (HOMA-IR), which has been widely used in large population studies [25], [26]. This is calculated according to the following formula [27]: HOMA-IR = fasting plasma insulin (μU/L) × fasting plasma glucose (nmol/L)/22.5.
Measurement and analysis of autonomic nervous function
Heart rate variability (HRV) was used as a noninvasive measure of autonomic nervous system modulation of cardiac activity. This was measured using an accelerometer (Activtracer AC-301 (ACT); GMS Inc., Tokyo, Japan) on Days 3 and 6, as previously described [28]. The coefficient of variation of RR intervals (CVR-R) and the standard deviation of the NN (i.e., R-R) intervals (SDNN) were calculated according to the recommendations for the clinical use of HRV [29]. SDNN is considered to reflect all the cyclic components responsible for HRV [29].
Statistical analyses
Data are expressed as number (%), median (interquartile range; IQR), or mean ± standard deviation (SD), as appropriate. Changes in values before and after the insomnia therapy were compared statistically using paired Wilcoxon tests and Fisher’s exact test. The statistical analysis was performed using the Stat View V system (Abacus Concepts, Berkeley, CA, USA) on an Apple computer. P values < 0.05 were considered statistically significant.
Results
Baseline clinical variables
The baseline clinical characteristics of the 18 subjects are shown in Table 1. The median (IQR) values for age, DM duration, HbA1c, and body mass index were 71 (46–81) years, 7 (0–34) years, 7.5% (6.1–10.0%), and 26.2 (19.7–41.0) kg/m2, respectively. The median AHI and PSQI scores were 8.0 (1.0–39.4) and 7 (2–15), respectively. The number of subjects exhibiting insomnia (PSQI >5) and obstructive sleep apnea (AHI ≥5) on admission were 12/18 (67%) and 11/18 (61%), respectively. All characteristics parameters were no significant difference between men and women.
Table 1.
Baseline characteristics of all patients at the start of the study.
| Total (n = 18) | Male (n = 5) | Female (n = 13) | p | |
|---|---|---|---|---|
| Age (years) | 71 (46–81) | 62 (59–71) | 72 (46–81) | n.s. |
| BMI (kg/m2) | 26.2 (19.7–41.0) | 26.3 (22.4–36.6) | 26.1 (19.7–41.0) | n.s. |
| Smoking status (current/past/never) | 4/2/12 | 4/1/0 | 0/1/12 | n.s. |
| Alcohol intake (yes/no) | 6/12 | 3/2 | 3/10 | n.s. |
| DM duration (years) | 7 (0–34) | 5 (0–34) | 9 (0–20) | n.s. |
| Diabetic neuropathy, n (%) | 7 (38.9) | 2 (40.0) | 5 (38.5) | n.s. |
| HbA1c (%) | 7.5 (6.1–10.0) | 7.3 (6.5–8.7) | 7.5 (6.1–10.0) | n.s. |
| Fasting CPR (ng/mL) | 2.6 (1.6–4.8) | 3.2 (1.8–3.7) | 2.3 (1.6–4.8) | n.s. |
| Fasting IRI (μU/mL) | 8.6 (3.8–34.0) | 7.9 (5.8–34.0) | 8.6 (3.8–26.2) | n.s. |
| HOMA-IR | 4.0 ± 2.8 | 4.2 ± 3.4 | 4.0 ± 2.7 | n.s. |
| eGFR (mL/min/1.73 m2) | 70.1 (49.9–118.1) | 84.1 (49.9–118.1) | 69.7 (50.8–96.3) | n.s. |
| Urinary protein (−/±/+/2+/3+) | 12/5/0/1/0 | 2/3/0/0/0 | 10/2/0/1/0 | n.s. |
| AHI (/hour) | 8.0 (1.0–39.4) | 3.3 (1.0–39.4) | 8.6 (1.8–39.0) | n.s. |
| PSQI | 7 (2–15) | 8 (5–12) | 7 (2–15) | n.s. |
Data are expressed as the mean ± SD or n (%) and median values (range) are shown for variables with skewed distributions.
And data are analyzed by the Mann-Whitney U test or Fisher’s exact test.
BMI, body mass index = weight (kilograms)/[height (meters)]2; DM, diabetes mellitus; HbA1c, glycated hemoglobin A1c; CPR, C-peptide immunoreactivity; IRI, immunoreactive insulin; HOMA-IR, homeostasis model assessment of insulin resistance; eGFR, estimated glomerular filtration ratio; AHI, apnea hypopnea index; PSQI, Pittsburgh sleep quality index; n,s, not significant; SD, standard deviation.
Changes in sleep architecture and autonomic nervous system activity
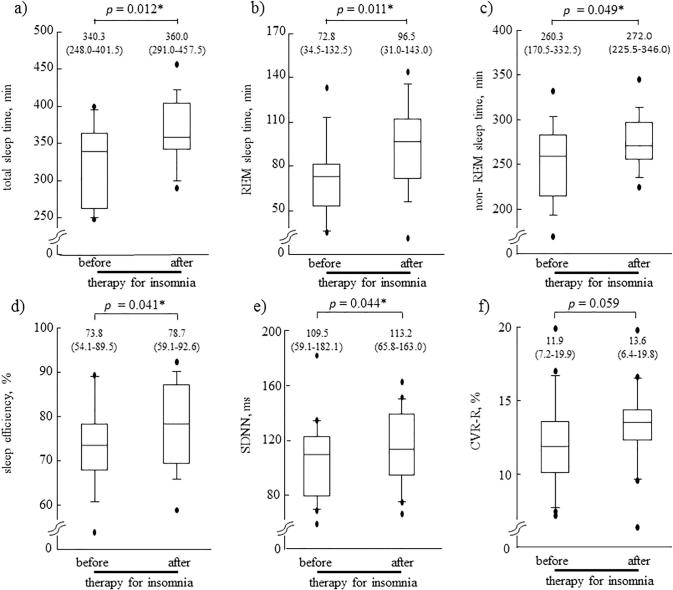
Table 2 and Fig. 2 show the changes in sleep architecture and autonomic nerve function measured on Days 3 and 6, before and after treatment with suvorexant for insomnia. The sleep diary data revealed significant increases in median total sleep time, from 360 to 410 min (p = 0.028), and median sleep efficiency, from 77.6% to 88.0% (p = 0.044), after treatment with suvorexant; however, sleep latency and arousal time during sleep did not change significantly. The EEG data revealed that treatment with suvorexant significantly increased the median total sleep time from 340.3 to 360.0 min (p = 0.012), with increases in both REM sleep time, from 72.8 to 96.5 min (p = 0.011), and non-REM sleep time, from 260.3 to 272.0 min (p = 0.049). As a result, median sleep efficiency increased significantly from 73.8% to 78.7% (p = 0.041). However, median sleep latency and arousal during sleep remained unchanged after suvorexant treatment.
Table 2.
The change of Sleep diary, Single-channel EEG, Portable monitor and Accelerometer parameters before and after therapy for insomnia.
| Before therapy | After therapy | p | |
|---|---|---|---|
| Sleep diary | |||
| Total sleep time (min) | 360 (260–540) | 410 (320–480) | 0.028* |
| Sleep latency (min) | 50 (0–180) | 30 (0–160) | 0.244 |
| Arousal time during sleep (min) | 30 (0–150) | 20 (0–80) | 0.254 |
| Sleep efficiency (%) | 77.6 (52.2–93.3) | 88.0 (59.1–100.0) | 0.044* |
| Single-channel EEG | |||
| Total sleep time (min) | 340.3 (248.0–401.5) | 360.0 (291.0–457.5) | 0.012* |
| Sleep latency (min) | 27.5 (11.0–151.5) | 43.5 (6.0–133.5) | 0.814 |
| Arousal time during sleep (min) | 58.0 (2.0–121.0) | 41.5 (9.0–154.5) | 0.388 |
| REM sleep time (min) | 72.8 (34.5–132.5) | 96.5 (31.0–143.0) | 0.011* |
| Non-REM sleep time (min) | 260.3 (170.5–332.5) | 272.0 (225.5–346.0) | 0.049* |
| Sleep efficiency (%) | 73.8 (54.1–89.5) | 78.7 (59.1–92.6) | 0.041* |
| Portable monitor | |||
| AHI (/hour) | 8.0 (1.0–39.4) | 10.6 (1.2–38.6) | 0.981 |
| Accelerometer | |||
| SDNN (ms) | 109.5 (59.1–182.1) | 113.2 (65.8–163.0) | 0.044* |
| CVR-R (%) | 11.9 (7.2–19.9) | 13.6 (6.4–19.8) | 0.059 |
| HR (bpm) | 71.0 (55.4–85.0) | 70.8 (55.0–81.7) | 0.164 |
Data are expressed as the median (range) of 18 subjects with T2DM for each 3 days of observation periods and of treatment periods. And data are analyzed by the paired Wilcoxon-tests. *p < 0.05.
EEG, electroencephalogram; REM, rapid eye movement; AHI, apnea hypopnea index; SDNN, the standard deviation of the NN (i.e., R-R) interval; CVR-R, the coefficient of variation of RR intervals; ms, millisecond; HR, heart rate; bpm, beats per minute; T2DM, type 2 diabetes mellitus.
Fig. 2.
Comparison of EEG-assessed sleep quality and autonomic nervous system function before and after insomnia therapy in 18 subjects with T2DM and insomnia. Treatment with suvorexant significantly increased the median total sleep time (p = 0.012) with increases in both REM sleep time (p = 0.011) and non-REM sleep time (p = 0.049). As a result, median sleep efficiency increased significantly (p = 0.041). As well as, median SDNN increased significantly (p = 0.044), and median CVR-R showed a tendency to increase (p = 0.059). *p < 0.05 before versus after therapy (Wilcoxon signed-rank test). EEG, electroencephalography; T2DM, type 2 diabetes mellitus; REM, rapid eye movement; SDNN, standard deviation of the NN (R-R) interval; CVR-R, coefficient of variation of R-R intervals.
After the treatment with suvorexant, median SDNN increased significantly from 109.5 to 113.2 ms (p = 0.044), and median CVR-R showed a tendency to increase, from 11.9% to 13.6%, although this did not reach statistical significance (p = 0.059). The median heart rate did not change appreciably, from 71.0 to 70.8 bpm (p = 0.164). There was no significant change in AHI.
Changes in daily glucose fluctuations and glycemic parameters
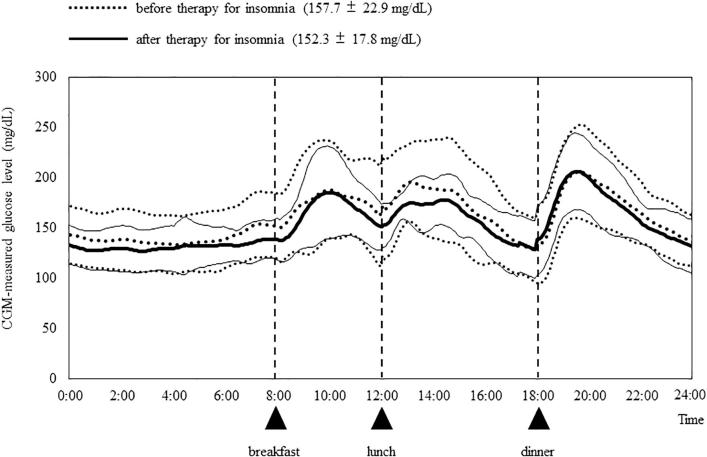
Fig. 3 shows the mean 24 h glucose levels across all the subjects for the 3 days before (Days 1–3) and 3 days after (Days 4–6) treatment with suvorexant, and the analyzed CGM data are shown in Table 3. There were no significant differences in average glucose levels between Days 1, 2, and 3 or between Days 4, 5, and 6 (data not shown).
Fig. 3.
CGM-measured glucose levels before and after therapy for insomnia. Following treatment with suvorexant, the mean glucose levels of the 18 subjects decreased pre-breakfast, with this decrease maintained until just before dinner. CGM, continuous glucose monitoring.
Table 3.
The change of parameters estimated by CGM and HOMA-IR before and after therapy for insomnia.
| CGM measured glucose level | Before therapy | After therapy | p |
|---|---|---|---|
| 24-h mean glucose level (mg/dL) | 157.7 ± 22.9 | 152.3 ± 17.8 | 0.010* |
| SD over 24-h (mg/dL) | 32.8 ± 9.9 | 28.9 ± 9.5 | 0.015* |
| Nocturnal nadir time (mg/dL) | 118.1 ± 22.4 | 122.1 ± 19.8 | 0.306 |
| Pre-breakfast time (mg/dL) | 146.9 ± 28.6 | 139.9 ± 19.4 | 0.028* |
| Magnitude of dawn phenomenon (mg/dL) | 28.3 ± 15.0 | 18.2 ± 9.9 | 0.002* |
| Dawn phenomenon (≧20 mg/dL), n (%) | 14(78) | 8(44) | 0.086 |
| AUC for glycemic variability | |||
| AUC (0000–2400) (mg/dL·min) | 227001 ± 32883 | 219554 ± 25674 | 0.047* |
| AUC (0000–0800) (mg/dL·min) | 64212 ± 11223 | 63477 ± 8191 | 0.353 |
| AUC (0800–1200) (mg/dL·min) | 42961 ± 7241 | 40306 ± 5750 | 0.025* |
| AUC (1200–1800) (mg/dL·min) | 60048 ± 10733 | 55451 ± 6594 | 0.018* |
| AUC (1800–2400) (mg/dL·min) | 61621 ± 9127 | 59472 ± 8221 | 0.184 |
| HOMA-IR | 4.0 ± 2.8 | 2.9 ± 1.6 | 0.044* |
Data are expressed as the mean ± SD of 18 subjects with T2DM for each 3 days of observation periods and of treatment periods. And data are analyzed by the paired Wilcoxon-tests except for presence of dawn phenomenon, which was analyzed by Fisher’s exact test. *p < 0.05.
CGM, continuous glucose monitoring; HOMA-IR, homeostasis model assessment of insulin resistance; AUC, area under the curve; SD, standard deviation; T2DM, type 2 diabetes mellitus.
A visual comparison of the 24 h glucose curves in Fig. 3 suggests that treatment with suvorexant resulted in a reduction in mean glucose levels over the period from midnight until dinner in the evening, with a narrower range in glucose fluctuations during this period. After treatment, the 24 h mean glucose level and glucose level at pre-breakfast decreased significantly from 157.7 to 152.3 mg/dL (p = 0.010) and from 146.9 to 139.9 mg/dL (p = 0.028), respectively. The SD for the 24 h glucose level, which reflects the amount of fluctuation, also decreased significantly, from 32.8 to 28.9 mg/dL (p = 0.015). The improvement of glycemic control by treatment with suvorexant was confirmed by a significant decrease in the area under the 24 h glucose level curve (AUC) from 227001 to 219554 mg/dL·min (p = 0.047). The AUCs for the periods 08:00–12:00 and 12:00–18:00 also decreased significantly, but those for 18:00–24:00 and 00:00–08:00 did not.
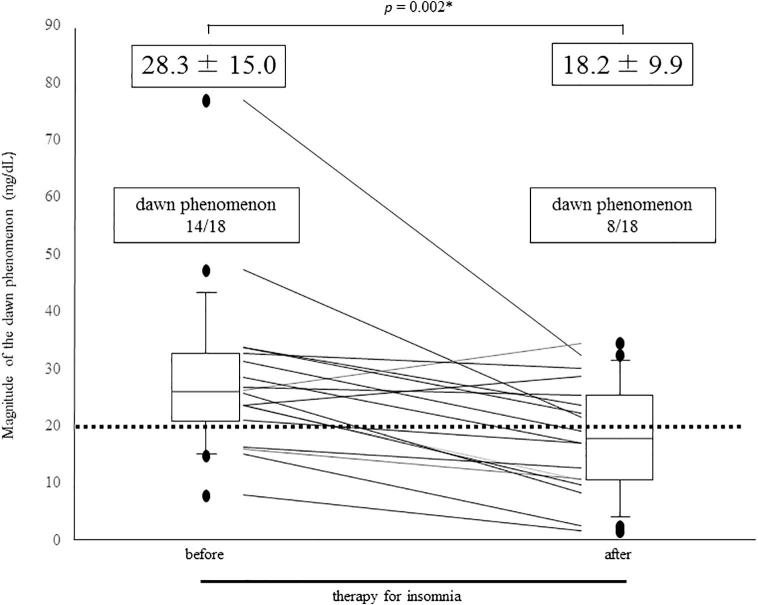
The mean of increase in glucose levels from the night nadir until just before breakfast (the dawn phenomenon) decreased significantly after treatment with suvorexant from 28.3 to 18.2 mg/dL (p = 0.002) (Fig. 4). Before treatment, 14 subjects (78%) were positive for the dawn phenomenon (with an elevation >20 mg/dL); this decreased to 8 subjects (44%) after treatment (Fig. 4). HOMA-IR values significantly decreased after treatment from 4.0 to 2.9 (p = 0.044). (Table 3)
Fig. 4.
Comparison of the dawn phenomenon before and after insomnia therapy in the 18 subjects. Mean values of the dawn phenomenon significantly decreased after insomnia therapy (*p = 0.002; Wilcoxon signed-rank test). Of the 14 subjects who initially exhibited a positive dawn phenomenon (a morning glucose elevation >20 mg/dL), only 8 remained positive after the insomnia treatment.
Discussion
This study demonstrated that treatment for insomnia with suvorexant improved both sleep quality and glycemic control in the subjects with T2DM and insomnia. The HRV assessments of the subjects' autonomic nervous function suggested that the improvement in glycemic control may be influenced by the reduction of sympathomimetic activity with suvorexant. It is known that suvorexant, a dual orexin receptor antagonist, suppresses wakefulness and promotes sleep without affecting neural systems other than the orexin system [11], unlike benzodiazepines and non-benzodiazepine receptor agonists. This strongly suggested that improving sleep quality with suvorexant by itself could improve glycemic control in subjects with T2DM suffering from insomnia, possibly due in part to improved sympathetic nerve activity.
In this study, treatment with suvorexant resulted in significant improvement in 24 h glycemic control, with significantly reduced glucose level fluctuation. Of note, glycemic control was especially improved during the period from midnight to just before breakfast (the dawn phenomenon) and between breakfast and dinner.
The dawn phenomenon is caused by a spontaneous elevation in serum epinephrine and norepinephrine, as well as an increase in cortisol associated with the transition from sleeping to wakefulness. It has been previously reported that nocturnal increases in sympathetic nervous system activity, in addition to growth hormone, may be of importance in the development of the dawn phenomenon in patients with type 1 diabetes [30]. In this study, the increases in SDNN and CVR-R suggested that suvorexant treatment suppressed sympathetic nerve activity as well as improving sleep quality. On the basis of the sequence of events previously reported [7], it is possible that it was the improvement in sleep quality with suvorexant that suppressed sympathetic nerve activity, thereby improving the dawn phenomenon.
Increased sympathetic nerve activity is associated with increased insulin resistance in T2DM patients [31], so it is possible that the reduced insulin resistance with suvorexant treatment, as shown by the decrease in HOMA-IR, may be accounted for by the suppression of sympathetic nerve activity. We have previously reported a cross-sectional study in which poor sleep quality estimated by EEG was independently associated with poor glycemic control and increased insulin resistance in subjects with T2DM, although causality could not be determined [7]. However, taken together with the sequence of events observed in the present study, these findings suggest that insomnia in T2DM patients may impair glycemic control by increasing sympathetic activity and thereby increasing insulin resistance. Thus, tackling the insomnia could be a definite target for improving glycemic control in T2DM patients with insomnia.
The midnight surge in serum cortisol could induce the dawn phenomenon [32]. It has also been reported that daytime serum cortisol levels were inversely correlated with total sleep time in patients with chronic insomnia [33] and that T2DM patients, among whom there is a high prevalence of insomnia, showed upregulation of hypothalamic–pituitary–adrenal axis activity [34]. Therefore, although it is possible that suvorexant treatment for insomnia might improve glycemic control in subjects with T2DM, we observed that serum cortisol at 8:00 AM and overnight urinary free cortisol did not change significantly after the insomnia therapy (data not shown).
Suvorexant, an orexin receptor antagonist, was selected as the sleep-promoting drug for the present study because of its effect on sleep quality was more selective than that of benzodiazepines and non-benzodiazepine receptor agonists [35]. It has been reported that suvorexant promoted both REM and non-REM sleep in normal C57BL/6 mice [36], [37]. Each day, the orexin system is activated during the awake period and inactivated during the sleeping period [38]. Because of the stimulatory effect of orexin on food intake and sympathetic-driven hepatic glucose production [39], [40], it is possible that insomnia-induced activation of the orexin system may impair the regulation of glucose homeostasis [41]. However, the present study kept the subjects' food intake constant throughout the whole 7 day study period, so it can be discounted that the improved glycemic control was a result of reduced food intake after suvorexant administration [42]. Furthermore, we did not change drug regimens which might affect glucose metabolism. Among the 18 enrolled patients, 7, 6, 4, and 3 patients have been taking dipeptidyl peptidase-4 inhibitor, biguanide, sulfonylurea, and α-glucosidase inhibitor, respectively. There was no significant change in improved glycemic control between those with and without each anti-diabetic drug, negating the interaction of suvorexant with antidiabetic drug to augment their hypoglycemic effect.
Basal sympathetic tone is abnormally increased in T2DM patients [43]. It has been reported that disordered sleep with decreases in slow-wave sleep may elevate nocturnal catecholamine levels [44], which suggests that suvorexant might improve sympathetic tone (supported by the reductions in SDNN and CVR-R in the present study). Although it is possible that suvorexant centrally suppresses sympathetic nerve activity at the rostral ventrolateral medulla [45], it has been shown that it took a long time for suvorexant to suppress sympathetic nerve activity in diabetic db/db mice [46].
This study had some limitations. First, the sample size was small, and all the subjects were ethnically Japanese. Second, the study was performed in a hospital setting, but CGM are ideally conducted at home within a daily routine environment; however, to minimize the influence of hospitalization and the CGM measurements on sleep architecture, the subjects were acclimatized by performing CGM the 1 day before the start of the study. Furthermore, hospitalization was needed to keep caloric intake and its time constant during study period in the present study.
The strengths of this study include consistency in daily caloric intake throughout the study period and evaluation of the effect of suvorexant on glycemic control after objective confirmation of improved sleep architecture and assessment of sympathetic tone using EEG and an accelerometer, respectively.
Conclusion
In conclusion, this study demonstrated that suvorexant treatment for insomnia of subjects with T2DM may improve glycemic control, as measured by CGM, by attenuating the early morning glucose level surge and then maintaining this decreased level until supper. The findings suggested that the improved glycemic control could be accounted for by the improvement in insomnia by itself, the attenuated sympathomimetic tone, and/or improved insulin sensitivity, although a direct effect of suvorexant on glycemic control could not be totally discounted. Thus, this study raised the possibility that amelioration of insomnia may be a target for improving glycemic control in T2DM patients with insomnia.
Acknowledgments
Acknowledgement
There are no acknowledgments to this manuscript to be declared.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcte.2018.12.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Skomro R.P., Ludwig S., Salamon E., Kryger M.H. Sleep complaints and restless legs syndrome in adult type 2 diabetics. Sleep Med. 2001;2:417–422. doi: 10.1016/s1389-9457(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 2.Ayas N.T., White D.P., Al-Delaimy W.K., Manson J.E., Stampfer M.J., Speizer F.E. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson P.M., Rööst M., Engström G., Hedblad B., Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care. 2004;27:2464–2469. doi: 10.2337/diacare.27.10.2464. [DOI] [PubMed] [Google Scholar]
- 4.Yaggi H.K., Araujo A.B., McKinlay J.B. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 5.Ohkuma T., Fujii H., Iwase M., Kikuchi Y., Ogata S., Idewaki Y. Impact of sleep duration on obesity and the glycemic level in patients with type 2 diabetes: the Fukuoka Diabetes Registry. Diabetes Care. 2013;36:611–617. doi: 10.2337/dc12-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knutson K.L., Ryden A.M., Mander B.A., Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 7.Yoda K., Inaba M., Hamamoto K., Yoda M., Tsuda A., Mori K. Association between poor glycemic control, impaired sleep quality, and increased arterial thickening in type 2 diabetic. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiegel K., Leproult R., Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 9.Donga E., van Dijk M., van Dijk J.G., Biermasz N.R., Lammers G.J., van Kralingen K.W. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. Clin Endocrinol Metab. 2010;95:2963–2968. doi: 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- 10.Gómez A.M., Henao Carrillo D.C., Muñoz Velandia O.M. Devices for continuous monitoring of glucose: update in technology. Med Devices (Auckl) 2017;10:215–224. doi: 10.2147/MDER.S110121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubey A.K., Handu S.S., Mediratta P.K. Suvorexant: the first orexin receptor antagonist to treat insomnia. J Pharmacol Pharmacother. 2015;6:118–121. doi: 10.4103/0976-500X.155496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urata H., Mori K., Emoto M., Yamazaki Y., Motoyama K., Morioka T. Advantage of insulin glulisine over regular insulin in patients with type 2 diabetes and severe renal insufficiency. J Ren Nutr. 2015;25:129–134. doi: 10.1053/j.jrn.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Wada N., Mori K., Nakagawa C., Sawa J., Kumeda Y., Shoji T. Improved glycemic control with teneligliptin in patients with type 2 diabetes mellitus on hemodialysis: evaluation by continuous glucose monitoring. J Diabetes Complications. 2015;29:1310–1313. doi: 10.1016/j.jdiacomp.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Seow L.S.E., Verma S.K., Mok Y.M., Kumar S., Chang S., Satghare P. Evaluating DSM-5 insomnia disorder and the treatment of sleep problems in a psychiatric population. J Clin Sleep Med. 2018;14:237–244. doi: 10.5664/jcsm.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;26:s5–s20. [DOI] [PubMed]
- 16.Kashiwagi A., Kasuga M., Araki E., Oka Y., Hanafusa T., Ito H. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi H., Masaki C., Makino M., Yoshida M., Mukaibo T., Kondo Y. Management of sleep-time masticatory muscle activity using stabilisation splints affects psychological stress. J Oral Rehabil. 2013;40:892–899. doi: 10.1111/joor.12110. [DOI] [PubMed] [Google Scholar]
- 18.Matthews K.A., Patel S.R., Pantesco E.J., Buysse D.J., Kamarck T.W., Lee L. Similarities and differences in estimates of sleep duration by polysomnography, actigraphy, diary, and self-reported habitual sleep in a community sample. Sleep Health. 2018;4:96–103. doi: 10.1016/j.sleh.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buysse D.J., Reynolds C.F., 3rd, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 20.Hirota T., Morioka T., Yoda K., Toi N., Hayashi N., Maruo S. Positive association of plasma leptin with sleep quality in obese type 2 diabetes patients. J Diabetes Invest. 2018;9:1100–1105. doi: 10.1111/jdi.12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapur V.K., Auckley D.H., Chowdhuri S., Kuhlmann D.C., Mehra R., Ramar K. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry R.B., Budhiraja R., Gottlieb D.J., Gozal D., Iber C., Kapur V.K. Academy of sleep medicine. rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. deliberations of the sleep apnea definitions task force of the American Academy of sleep medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carroll M.F., Schade D.S. The dawn phenomenon revisited: implications for diabetes therapy. Endocr Pract. 2005;11:55–64. doi: 10.4158/EP.11.1.55. [DOI] [PubMed] [Google Scholar]
- 24.Monnier L., Colette C., Dejager S., Owens D. Magnitude of the dawn phenomenon and its impact on the overall glucose exposure in type 2 diabetes: is this of concern? Diabetes Care. 2013;36:4057–4062. doi: 10.2337/dc12-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antuna-Puente B., Disse E., Rabasa-Lhoret R., Laville M., Capeau J., Bastard J.P. How can we measure insulin sensitivity/resistance? Diabetes Metab. 2011;37:179–188. doi: 10.1016/j.diabet.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Mojiminiyi O.A., Abdella N.A. Effect of homeostasis model assessment computational method on the definition and associations of insulin resistance. Clin Chem Lab Med. 2010;48:1629–1634. doi: 10.1515/CCLM.2010.303. [DOI] [PubMed] [Google Scholar]
- 27.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Kadoya M., Koyama H., Kanzaki A., Kurajoh M., Hatayama M., Shiraishi J. Plasma brain-derived neurotrophic factor and reverse dipping pattern of nocturnal blood pressure in patients with cardiovascular risk factors. PLoS One. 2014;9 doi: 10.1371/journal.pone.0105977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.No authors listed. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996;17:354–381. [PubMed]
- 30.Campbell P.J., Bolli G.B., Cryer P.E., Gerich J.E. Sequence of events during development of the dawn phenomenon in insulin-dependent diabetes mellitus. Metabolism. 1985;34:1100–1104. doi: 10.1016/0026-0495(85)90153-2. [DOI] [PubMed] [Google Scholar]
- 31.Masuo K., Rakugi H., Ogihara T., Esler M.D., Lambert G.W. Cardiovascular and renal complications of type 2 diabetes in obesity: role of sympathetic nerve activity and insulin resistance. Curr Diabetes Rev. 2010;6:58–67. doi: 10.2174/157339910790909396. [DOI] [PubMed] [Google Scholar]
- 32.Atiea J.A., Aslan S.M., Owens D.R., Luzio S. Early morning hyperglycaemia “dawn phenomenon” in non-insulin dependent diabetes mellitus (NIDDM): effects of cortisol suppression by metyrapone. Diabetes Res. 1990;14:181–185. [PubMed] [Google Scholar]
- 33.D'Aurea C., Poyares D., Piovezan R.D., Passos G., Tufik S., Mello M.T. Objective short sleep duration is associated with the activity of the hypothalamic-pituitary-adrenal axis in insomnia. Arq Neuropsiquiatr. 2015;73:516–519. doi: 10.1590/0004-282X20150053. [DOI] [PubMed] [Google Scholar]
- 34.Chan O., Inouye K., Riddell M.C., Vranic M., Matthews S.G. Diabetes and the hypothalamo-pituitary-adrenal (HPA) axis. Minerva Endocrinol. 2003;2:87–102. [PubMed] [Google Scholar]
- 35.Uslaner J.M., Tye S.J., Eddins D.M., Wang X., Fox S.V., Savitz A.T. Orexin receptor antagonists differ from standard sleep drugs by promoting sleep at doses that do not disrupt cognition. Sci Transl Med. 2013;5:179ra44. doi: 10.1126/scitranslmed.3005213. [DOI] [PubMed] [Google Scholar]
- 36.Betschart C., Hintermann S., Behnke D., Cotesta S., Fendt M., Gee C.E. Identification of a novel series of orexin receptor antagonists with a distinct effect on sleep architecture for the treatment of insomnia. J Med Chem. 2013;56:7590–7607. doi: 10.1021/jm4007627. [DOI] [PubMed] [Google Scholar]
- 37.Etori K., Saito Y.C., Tsujino N., Sakurai T. Effects of a newly developed potent orexin-2 receptor-selective antagonist, compound 1 m, on sleep/wakefulness states in mice. Front Neurosci. 2014;8:8. doi: 10.3389/fnins.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gotter A.L., Winrow C.J., Brunner J., Garson S.L., Fox S.V., Binns J. The duration of sleep promoting efficacy by dual orexin receptor antagonists is dependent upon receptor occupancy threshold. BMC Neurosci. 2013;14:90. doi: 10.1186/1471-2202-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakurai T. The role of orexin in motivated behaviours. Nat Rev Neurosci. 2014;15:719–731. doi: 10.1038/nrn3837. [DOI] [PubMed] [Google Scholar]
- 40.Tsuneki H., Wada T., Sasaoka T. Role of orexin in the central regulation of glucose and energy homeostasis. Endocr J. 2012;59:365–374. doi: 10.1507/endocrj.ej12-0030. [DOI] [PubMed] [Google Scholar]
- 41.Spiegel K., Tasali E., Leproult R., Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutton E.L. Profile of suvorexant in the management of insomnia. Drug Des Devel Ther. 2015;9:6035–6042. doi: 10.2147/DDDT.S73224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huggett R.J., Scott E.M., Gilbey S.G., Stoker J.B., Mackintosh A.F., Mary D.A. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation. 2003;108:3097–3101. doi: 10.1161/01.CIR.0000103123.66264.FE. [DOI] [PubMed] [Google Scholar]
- 44.Irwin M., Thompson J., Miller C., Gillin J.C., Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab. 1999;84:1979–1985. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 45.Lee Y.H., Dai Y.W., Huang S.C., Li T.L., Hwang L.L. Blockade of central orexin 2 receptors reduces arterial pressure in spontaneously hypertensive rats. Exp Physiol. 2013;98:1145–1155. doi: 10.1113/expphysiol.2013.072298. [DOI] [PubMed] [Google Scholar]
- 46.Tsuneki H., Kon K., Ito H., Yamazaki M., Takahara S., Toyooka N. Timed inhibition of orexin system by suvorexant improved sleep and glucose metabolism in type 2 diabetic db/db mice. Endocrinology. 2016;157:4146–4157. doi: 10.1210/en.2016-1404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.