Abstract
Diabetes mellitus is one of the most common chronic diseases in the United States and peripheral neuropathy (PN) affects at least 50% of diabetic patients. Medications available for patients ameliorate symptoms (pain), but do not protect against cellular damage and come with severe side effects, leading to discontinued use. Our research group uses differentiated SH-SY5Y cells treated with advanced glycation end products (AGE) as a model to mimic diabetic conditions and to study the mechanisms of oxidative stress mediated cell damage and antioxidant protection. N-acetylcysteine (NAC), a common antioxidant supplement, was previously shown by our group to fully protect against AGE-induced damage. We have also shown that 3H-1,2-dithiole-3-thione (D3T), a cruciferous vegetable constituent and potent inducer of nuclear factor (erythroid-derived 2)- like 2 (Nrf2), can significantly increase cellular GSH concentrations and protect against oxidant species–induced cell death. Paradoxically, D3T conferred no protection against AGE-induced cell death or neurite degeneration. In the present study we establish a mechanism for this paradox by showing that D3T in combination with AGE increased oxidant species generation and depleted GSH via inhibition of glutathione reductase (GR) activity and increased expression of the NADPH generating enzyme glucose-6-phosphate dehydrogenase (G6PD). Blocking NADPH generation with the G6PD inhibitor dehydroepiandrosterone was found to protect against AGE-induced oxidant species generation, loss of viability, and neurite degeneration. It further reversed the D3T potentiation effect under AGE-treated conditions. Collectively, these results suggest that strategies aimed at combating oxidative stress that rely on upregulation of the endogenous antioxidant defense system via Nrf2 may backfire and promote further damage in diabetic PN.
Abbreviations: AGE, Advanced glycation end products; ANOVA, Analysis of variance; ARE, Antioxidant response element; BSA, Bovine serum albumin; D3T, 3H-1,2-dithiole-3-thione; DCFH-DA, 2′,7′-dichlorofluorescin diacetate; DHEA, Dehydroepiandrosterone; DMEM, Dulbecco's Modified Eagle Medium; FBS, Fetal bovine serum; G6P, Glucose-6-phosphate; G6PD, Glucose-6-phosphate dehydrogenase; GPX, Glutathione peroxidase; GR, Glutathione reductase; GSH, Reduced glutathione; GSSG, Oxidized glutathione; HRP, Horseradish peroxidase; Keap1-HKO, Keap1-hepatocyte knockout mice; MTT, 3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium bromide; NAC, N-acetylcysteine; NADPH, Nicotinamide adenine dinucleotide phosphate; NOX, NADPH oxidase; Nrf2, Nuclear factor (erythroid-derived 2) like 2; PN, Peripheral neuropathy; RA, Retinoic acid; RAGE, Receptor for AGE; ROS, Reactive oxygen species; SOD, Superoxide dismutase; 6-PGA, 6-phosphogluconate
Keywords: Peripheral neuropathy, Diabetes, D3T, NAC, Redox balance, Oxidative stress
Graphical abstract
Highlights
-
•
Glutathione(GSH) is key to protecting peripheral neurons from diabetes-induced damage.
-
•
Inducers of Nrf2 usually upregulate glutathione biosynthesis and reduction.
-
•
Paradoxically a Nrf2 inducer exacerbates diabetes-induced damage via GSH oxidation.
-
•
Diminished GSH reductase activity, despite induction, appears to account for paradox.
1. Introduction
Diabetes mellitus, a disease hallmarked by hyperglycemia, is one of the most common chronic diseases worldwide with new diagnoses on an upward trajectory. By some estimates, 30.3 million Americans live with diabetes [1]. The high incidence of diabetes poses both a public health as well as a financial concern. Direct annual medical costs for diabetes treatment in recent years has reached $176 billion [2]. Therefore, there has been growing interest in identifying new and easily accessible preventive compounds that may alleviate diabetic health complications.
Peripheral neuropathy (PN) is one of the most common health complications in type 1 and 2 diabetics, with oxidative stress implicated as a major contributor to diabetes-induced PN [3]. An underlying cause of oxidative stress-induced complications in diabetes, including PN, is the accumulation of Advanced Glycation End Products (AGEs) in circulation and tissues [4], [5], [6], [7], [8]. The products of non-enzymatic glycation of proteins, AGEs accumulate in peripheral nerves of diabetic patients [9]. Through binding the receptor for AGE (RAGE) in target tissues, AGEs stimulate oxidative stress through downstream activation of NAD(P)H oxidase in a PKCδ-dependent manner [10], [11]. The resulting increase in oxidant species facilitates damage in peripheral neurons [4]. Given the above mechanism of AGE-induced PN, dietary compounds with antioxidant properties have been proposed as potential remediating agents.
Dietary compounds with antioxidant properties may provide benefit through a variety of mechanisms [12], [13], [14]. Some compounds including ascorbate, N-acetylcysteine (NAC) or alpha-tocopherol have intrinsic antioxidant potential. These compounds can either directly neutralize reactive oxygen species (ROS), or in the case of NAC, provide the necessary substrate for synthesis of essential cellular antioxidants such as glutathione (GSH). Other compounds, including 3H-1,2-dithiole-3-thione (D3T), generate an antioxidant effect through stimulating endogenous cellular antioxidant defenses. In the case of D3T, the antioxidant response involves activation of Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) through several potential mechanisms [15], [16], [17], [18]. Nrf2, a transcription factor, controls expression of a wide array of target genes including glutathione synthesizing enzymes, quinone reductases, glutathione reductase (GR), as well as NADPH generating enzymes such as glucose-6-phosphate dehydrogenase (G6PD) [14], [19], [20], [21]. Reduced NADPH resulting from G6PD upregulation serves as a cofactor for GR mediated recycling of oxidized glutathione (GSSG) back to its reduced form (GSH). However, NAD(P)H oxidases (a major source of AGE-induced oxidative stress) [4], [9] may also potentially utilize reduced NADPH generated through Nrf2 activation to increase ROS such as superoxide, stressing the importance of mechanistic context when assessing the benefit of individual antioxidant and phytochemical compounds.
The variable mechanism of action of different antioxidants suggests some compounds may be more suitable than others in protection against diabetic PN. In particular, previous studies from our laboratory demonstrated variable effects of antioxidant compounds on AGE-induced oxidative stress and neurite morphology in the differentiated SH-SY5Y cell model. NAC offered protection from neurite loss as well as protection from DNA and protein oxidation [22]. On the other hand, D3T, a potent Nrf2 inducer, had a paradoxical impact on redox balance in SH-SY5Y cells exposed to AGE. D3T co-treatment promoted AGE-induced oxidant species formation, did not maintain GSH levels (compared to BSA control group), and did not protect from AGE-induced neurite loss [23]. In the present studies, the mechanism underlying D3T's paradoxical effect on oxidative stress in the presence of AGEs was investigated. It was hypothesized that D3T mediated induction of NADPH generating antioxidant enzyme G6PD promotes AGE-induced oxidative damage.
2. Methods
2.1. Materials
Dulbecco's Modified Medium (DMEM), Ham's F12 Medium, penicillin-streptomycin, and fetal bovine serum (FBS) were purchased from Invitrogen (Waltham, MO). All standard cell culture flasks and plates were purchased from ThermoFisher Scientific (Waltham, MO). Black 24-well culture plates for fluorescence analysis were purchased from Cellvis (Sunnyvale, CA). All reagents such as retinoic acid (RA), dehydroepiandrosterone (DHEA), bovine serum albumin (BSA), NAC, D3T, and reagents for GSH, ROS, and GR activity analysis were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated.
2.2. Cell Culture
SH-SY5Y cells were purchased from ATCC (Manassas, VA) and were cultured initially in growth media (DMEM, 10% FBS, 1% penicillin-streptomycin, 8 mM glucose) for 24 h to allow for adherence. Following adherence, growth media was replaced with differentiating media (1:1 DMEM: F12, 1% FBS, 8 mM glucose, 10 µM retinoic acid) for five days to allow for neuronal cell phenotype development. Treatments for all experiments took place following the five-day differentiation period. Cells treated with NAC or D3T had 1 mM NAC (<1% PBS) or 100 µM D3T (<1% DMSO) added to the media 24 h prior to 5 mg/mL BSA or AGE treatment. This treatment was then replenished in BSA and AGE treatment groups for 24 h. For DHEA treated cells, 100 µM DHEA (< 1% DMSO) was added to the media 24 h prior to BSA or AGE treatment and replenished during the 24-h BSA or AGE treatment.
2.3. Preparation of AGE-BSA and BSA concentration
AGE-BSA was prepared as was previously reported in our lab [23]. Briefly, 5 mg/mL BSA in PBS was incubated with 33 mM glycolaldehyde dimer for 20 h at 37 °C. Control BSA was treated in the same manner, but without the addition of glycolaldehyde. Both control and glycated BSA were then concentrated via ammonium sulfate precipitation. Ammonium sulfate was added to glycated BSA and control solutions (80% ammonium sulfate solution), and then centrifuged at 10,000 g for 30 min. Precipitated proteins were resuspended with the minimal amount of PBS required for resuspension. Solutions were then dialyzed in PBS for three days, with daily replacement of PBS. Following dialysis, solutions were sterile filtered. The final concentrations of both glycated and control BSA were approximately 15 mg/mL.
2.4. GSH assay
Reduced GSH, as well as its oxidized form GSSG, were quantified using a colorimetric based assay as described by Rahman et al. [24]. SH-SY5Y cells were plated in 6-well plates (9 cm2) at a concentration of 0.4 × 106. Each well was then pretreated with either NAC, D3T, or vehicle (<1% DMSO or PBS) for 24 h followed by co-treatment and addition of AGE-BSA or BSA-control for an additional 24 h. Absorption was measured using a Bio-Tek Powerwave X200 (Winooski, VT) spectrophotometer (abs. 412 nm). All values were normalized to protein content using a BCA assay kit (Pierce, Rockford, IL).
2.5. General Oxidative Stress Assay
General intracellular oxidative stress was measured as described previously [25]. Briefly, SH-SY5Y cells were plated on black coated 24-well plates (1.9 cm2) at a concentration of 8 × 104 cells per well. After differentiation, each well was pre-treated with indicated compounds for 24 h, followed by an additional 24-h co-treatment with either AGEs or BSA control. At the end of 48 h, media was gently removed and 200 µL of 20 µM 2′,7′-dichlorofluorescin diacetate (DCFH-DA) in PBS was added to each well. Fluorescence was measured after a 20-min incubation at 37 °C and 5% CO2 using a Synergy H1 Multi-Mode Reader (ex/em. 485/530). Fluorescence units were further normalized to viable cell number using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich, St. Louis, MO). Though this method has been used to measure general oxidative stress [26], significant limitations exist. The method does not quantify specific oxidant species. The intermediate DCF semiquinone free radical, formed via one-electron oxidation of DCFH, reacts with molecular oxygen to form superoxide. This can result in an artifactual amplification of fluorescence intensity [27], [28].
2.6. Glutathione reductase activity
GR activity was measured as described by Mannervik [29]. Briefly, SH-SY5Y cells were plated in 6-well plates (9 cm2) at a concentration of 4.0 × 105. Each well was then pretreated with either NAC, D3T, or vehicle (<1% DMSO or PBS) for 24 h followed by co-treatment with AGE-BSA or BSA-control for an additional 24 h. Absorption was measured using an Agilent Cary 60 (Santa Clara, CA) spectrophotometer (abs. 340 nm) at 30 °C over a five-minute period. Activity for each sample was normalized to total DNA. DNA content was measured with 1 µL of sample using a Thermo Scientific NanoDrop 2000 (Waltham, MA) spectrophotometer (abs. 260 & 280 nm). Activity is reported as % of BSA control.
2.7. Cell viability assay
Cell viability was assessed after indicated treatment and duration using MTT, according to the manufacturer's instructions. Briefly, SH-SY5Y cells were plated and differentiated in 24-well plates. At the end of the specified time point, cell media was removed and replaced with low glucose (1 g/L) serum free media containing 500 µg/mL MTT. Cells were incubated for 2 h. Formazan product was dissolved using DMSO and quantified using Bio-Tek Powerwave X200 spectrophotometer (abs. 540 nm).
2.8. Western blotting
All Western blots were run using a Mini-PROTEAN Tetra Cell system (Bio-rad, Hercules, CA). Both 10% and 4–20% gradient polyacrylamide gels were used. For gel electrophoresis, the running buffer was made up of 25 mM Tris, 192 mM glycine, and 0.1% (w/v) SDS at pH 8.3. Gels were run at constant voltage (200 v) for 30–50 min. For protein transfer to nitrocellulose membranes, transfer buffer was made up of 25 mM Tris, 0.2 M glycine, and 20% methanol at pH 8.5. A constant voltage (90 v) for 60 min was maintained for transfers. The transfer buffer was refrigerated prior to use, and the entire apparatus was kept cold (approx. 4 °C) during protein transfer. Following transfer, blots were incubated based on antibody manufacturer recommendations. For G6PD, GR, and β-actin quantification, blots were incubated for 12 h on a plate shaker at 4 °C with a polyclonal rabbit antibody (1:1000) (Cell Signaling, Danvers, MA) for G6PD, monoclonal mouse antibody (2 µg/mL) (Sigma-Aldrich, St. Louis, MO) for GR, and monoclonal rabbit (1:1000) (Cell Signaling, Danvers, MA) for β-actin. Both anti-rabbit and anti-mouse secondary antibodies (1:1000) were HRP-linked, and an HRP-detection kit followed by photo-development was used (Cell Signaling, Danvers, MA). All G6PD and GR bands were normalized to their corresponding β-actin bands and results are reported as % of β-actin.
2.9. Neurite Quantification
At the completion of experiments, photos were taken to document morphological changes of differentiated SH-SY5Y cells. Images were captured at 10x magnification using a Zeiss Axio Vert. A1 microscope (Oberkochen, Germany). A Zeiss AxioCam ICm1 camera was used to take photos using Zeiss Zen2 imaging software. All images were 1388 × 1038 pixels, and exposure time for all photos was 32.03 ms. Images were then analyzed using ImageJ (NIH) software with NeuronJ (ImageScience) plugin. Individual neurites were traced, and neurite length was reported as length in pixels. Neurites present in the field of view were quantified from three to four independent experiments for each treatment group. Exclusion criteria included counting only neurites with clearly defined start and end locations. Neurites that were shorter than the width of the cell body were not counted. For each treatment group, a minimum of 100 total neurites was quantified. To quantify the impact of treatments on the longest neurites, the number of neurites greater than or equal to the average length of neurites in the BSA control group (115 pixels) was calculated for each treatment group.
2.10. Statistical Methods
Values are presented as mean ±S.E.M. Comparison of means was conducted using analysis of variance (ANOVA) followed by multiple comparisons of means (Dunnett or Tukey HSD), with P < 0.05 considered statistically significant.
3. Results
3.1. Impact of D3T and NAC on GSH status
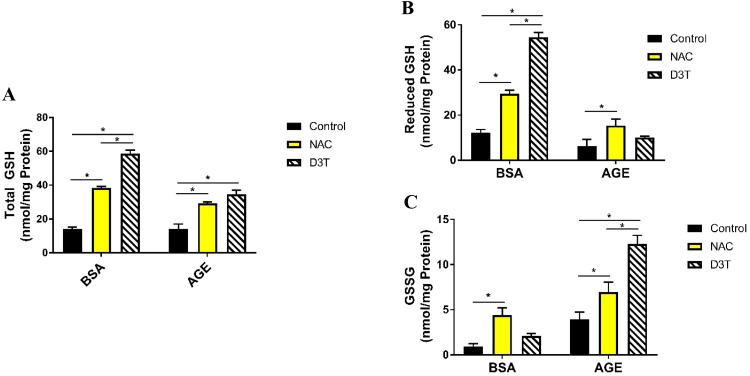
Previously, Pazdro and Burgess demonstrated that both NAC and D3T increase total GSH in the absence of AGEs, while only NAC exerted a protective effect on GSH in the presence of AGEs [22], [23]. To further evaluate this variable effect in SH-SY5Y cells, reduced GSH and its oxidized form GSSG were measured in cells treated with NAC or D3T under AGE challenge or BSA control conditions. Both NAC and D3T significantly increased total GSH in BSA and AGE groups (Fig. 1A). In addition, NAC and D3T increased reduced GSH levels under BSA-control conditions by 241% and 444%, respectively (Fig. 1B). Importantly, D3T treatment increased reduced GSH in the absence of AGEs to a greater extent than NAC. This is consistent with the mechanism of action of D3T through Nrf2 induction, where both GSH synthesis and NADPH-dependent recycling of GSSG via GR are upregulated [30]. Hence reduced GSH, in response to D3T, is increased not only through increased synthesis, but also through increased recycling of oxidized GSSG back to its reduced form. In contrast, NAC provides antioxidant protection primarily through providing cysteine as substrate for GSH synthesis [31], independent of NADPH-mediated recycling of GSSG. Consistent with this mechanism, GSSG levels were upregulated in the absence of AGE with NAC treatment while D3T treatment did not significantly increase GSSG under the same conditions (Fig. 1C). When challenged with AGE, D3T treated SH-S5Y5 cells showed no significant increase in GSH (Fig. 1B) but a drastic increase in GSSG (Fig. 1C), showing a pro-oxidative effect of D3T specific to AGE challenge conditions. NAC treatment, on the other hand demonstrated a significant increase in both reduced GSH and GSSG in the presence of AGEs, consistent with AGE-mediated pro-oxidative effect and the protective role of NAC (Fig. 1B and C).
Fig. 1.
Glutathione redox state of differentiated SH-SY5Y cells. (A) NAC and D3T treatment increase total GSH. (B) D3T does not protect reduced GSH under AGE-treated conditions. (C) D3T exacerbates AGE-induced increase in GSSG. Differentiated cells were treated with 1 mM NAC or 100 µM D3T for 24 h prior to 5 mg/mL BSA or AGE treatment. NAC and D3T were replenished during BSA and AGE treatment. Total GSH and GSSG concentrations were normalized to total protein. Reduced GSH was calculated by subtracting 2x GSSG from total GSH. Data were analyzed by two-way ANOVA with Tukey's post-hoc analysis from 3 independent experiments. Bars linked with an * are significantly different (p < 0.05).
3.2. Impact of D3T on cell viability and general oxidative stress
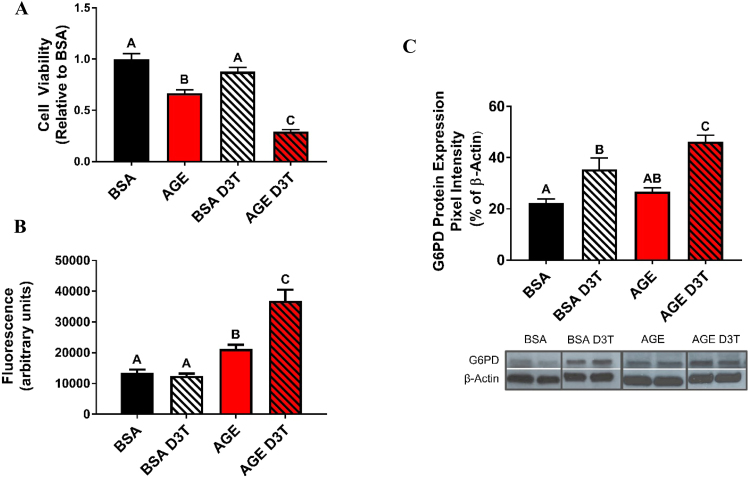
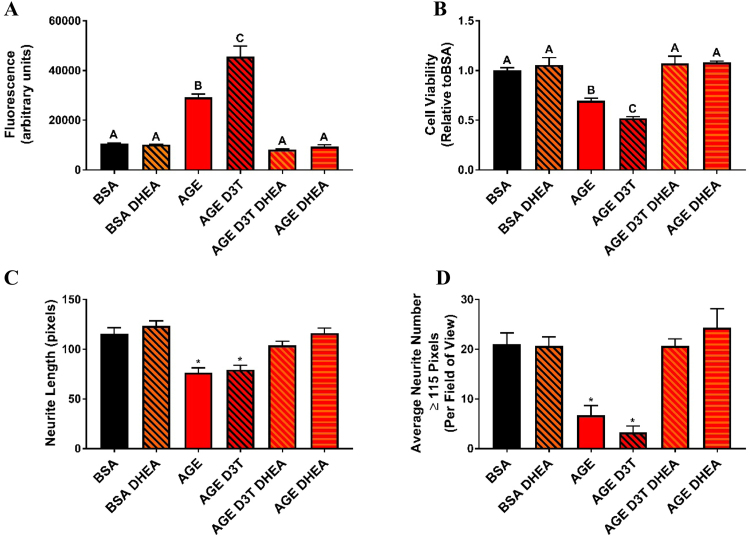
To further assess the paradoxical effect of D3T upon AGE treatment, cell viability after vehicle or D3T and AGE challenge was assessed. SH-SY5Y cells treated with AGEs alone had a significant decrease in cell viability (Fig. 2A and Sup. Fig. 1). While D3T had no effect on cell viability in the absence of AGEs, treatment of SH-SY5Y cells with AGEs and D3T resulted in a potentiated effect of AGEs on decreased cell viability (Fig. 2A and Sup. Fig. 1). Levels of oxidative stress were further analyzed to specifically investigate the potentiation effect of D3T on AGE-induced oxidative stress. Consistent with cell viability data, AGE treatment alone significantly increased general oxidative stress while D3T treatment amplified that effect (Fig. 2B).
Fig. 2.
D3T treatment exacerbates AGE-induced damage. (A) D3T exacerbates AGE-induced viability loss. Differentiated cells were treated with 100 µM D3T for 24 h prior to 5 mg/mL BSA or AGE treatment. D3T was replenished during BSA and AGE treatment. Viability was assessed using an MTT assay and results are presented as values relative to control (BSA) group. (B) D3T exacerbates AGE-induced oxidative stress. Differentiated cells were treated with 100 µM D3T for 24 h prior to 5 mg/mL BSA or AGE treatment. D3T was replenished during BSA and AGE treatment. Data were analyzed by one-way ANOVA with Tukey's post-hoc analysis from 4 to 5 independent experiments. Bars with superscript letters different from each other are significantly different (p < 0.05). (C) D3T treatment results in increased G6PD protein expression. Differentiated cells were treated with 100 µM D3T for 24 h prior to 5 mg/mL BSA or AGE treatment. D3T was replenished during BSA and AGE treatment. Blots shown are representative samples of each group. G6PD and β-actin bands appear at approximately 60 kDa and 45 kDa molecular weights, respectively. Data were analyzed by one-way ANOVA with Tukey's post-hoc analysis from 3 independent experiments. Bars not linked by a superscript letter are significantly different from each other (p < 0.05).
3.3. The effect of D3T on G6PD
Given the potentiation effect of D3T on AGE-induced oxidative stress, and the mechanism of action of AGE through activation of NAD(P)H oxidase, it was hypothesized that D3T promotes AGE-induced oxidative stress through increased generation of reduced NADPH. G6PD, the rate-limiting enzyme of the pentose phosphate pathway, is one of the major contributors to the cellular reduced NADPH pool. It is further a Nrf2 target gene, suggesting that D3T may upregulate G6PD via its Nrf2-mediated mechanism of action. To assess the effect of D3T on G6PD, G6PD protein expression was measured after a D3T treatment in AGE challenge or BSA control conditions. D3T significantly increased G6PD protein expression under BSA control conditions. Interestingly, AGE treatment alone also upregulated G6PD expression relative to BSA-control. Finally, D3T and AGE treatment demonstrated an additive effect on G6PD expression (Fig. 2C). These findings were consistent with the hypothesis that D3T potentiates AGE-induced oxidative stress via increased G6PD-mediated generation of NADPH that feeds NAD(P)H oxidase and sustains its generation of superoxide.
3.4. The effect of D3T on glutathione reductase
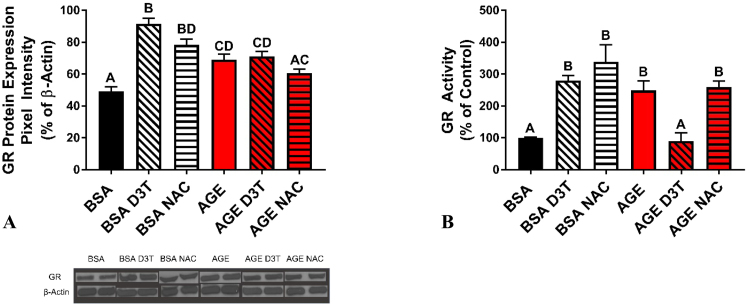
Reducing potential generated by G6PD in the form of NADPH may be utilized by NAD(P)H oxidase or GR, among other enzymes. The potentiation impact of D3T on AGE-induced oxidative stress may therefore result from both upregulating NADPH generation as well as hindering other enzymes that would normally utilize NADPH. To explore this possibility, the impact of D3T treatment on GR was investigated, since GR is a major NADPH utilizing enzyme in the context of antioxidant defense. D3T treatment under BSA control conditions increased GR protein expression (Fig. 3A), likely due to its Nrf2 inducing properties and consistent with the profound increase in reduced GSH resulting from D3T treatment in the absence of AGEs. AGE treatment alone also increased GR protein expression, and co-treatment with D3T resulted in no further change (Fig. 3A). Interestingly, GR activity did not correlate with protein expression in cells challenged with AGE and treated with D3T. In this scenario, D3T treatment unexpectedly resulted in a significant decrease in GR activity (Fig. 3B).
Fig. 3.
Glutathione reductase protein expression and activity. (A) Impact of NAC and D3T treatment on GR protein expression. Differentiated cells were treated with 1 mM NAC or 100 µM D3T for 24 h prior to 5 mg/mL BSA or AGE treatment. NAC and D3T were replenished during BSA and AGE treatment. Blots shown are representative samples of each group. GR and β-actin bands appear at approximately 52 kDa and 45 kDa molecular weights, respectively. (B) D3T treatment reduces GR activity in AGE-challenged cells. Differentiated cells were treated with 1 mM NAC or 100 µM D3T for 24 h prior to 5 mg/mL BSA or AGE treatment. NAC and D3T were replenished during BSA and AGE treatment. Results are presented as a % of the control (BSA) group. Data were analyzed by one-way ANOVA with Tukey's post-hoc analysis from 3 independent experiments. Bars with superscript letters different from each other are significantly different (p < 0.05).
3.5. The effect of DHEA on cell viability and general oxidative stress
To test the relationship between G6PD and oxidative stress under AGE treatment, an uncompetitive inhibitor of G6PD (DHEA) was utilized [32]. Treatment of SH-SY5Y cells with AGEs in the presence of DHEA reversed the effect of AGEs on increasing oxidative stress (Fig. 4A). Furthermore, DHEA treatment reversed the effect of D3T on potentiating AGE-induced oxidative stress. Cell viability was also measured in response to D3T and AGE treatment in the presence or absence of DHEA. As confirmed previously (Fig. 1C), D3T potentiated the effect of AGE on cell viability (Fig. 4B). Co-treatment with DHEA rescued SH-SY5Y cells from AGE-induced and AGE-D3T-induced decrease in cell viability (Fig. 4B). Collectively these studies confirmed the importance of G6PD in AGE-induced oxidative stress. They further implicated G6PD in the potentiation effect of D3T on the pro-oxidative outcomes of AGE treatment.
Fig. 4.
DHEA treatment protects against AGE and AGE-D3T-induced damage. (A) DHEA inhibits AGE and AGE-D3T-induced oxidative stress. (B) G6PD inhibition protects against AGE and AGE-D3T-induced viability loss. Differentiated cells were treated with 100 µM D3T and/or 100 µM DHEA for 24 h prior to 5 mg/mL BSA or AGE treatment. D3T and DHEA were replenished during BSA and AGE treatment. Data were analyzed by one-way ANOVA with Tukey's post-hoc analysis from 3 to 4 independent experiments. Bars with superscript letters different from each other are significantly different (p < 0.05). (C) DHEA treatment protects against AGE and AGE-D3T-induced neurite retraction. (D) DHEA treatment protects against AGE and AGE-D3T-induced loss of long neurites. Differentiated cells were treated with 100 µM D3T and/or 100 µM DHEA for 24 h prior to 5 mg/mL BSA or AGE treatment. D3T and DHEA were replenished during BSA and AGE treatment. A minimum of 100 neurites per group were analyzed. Neurite length and long neurite number in 10x images was quantified using Image J software with NeuronJ plugin. Data were analyzed by one-way ANOVA with Dunnett's post-hoc analysis. Bars with an * are significantly different (p < 0.05) compared to control (BSA).
3.6. The effect of DHEA on neurite morphology
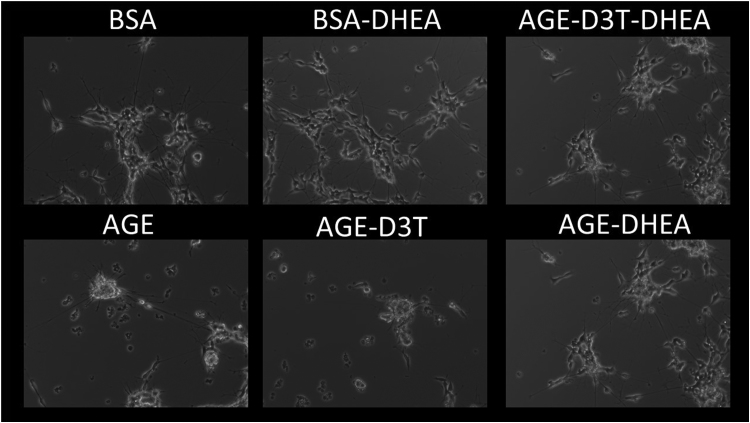
To assess the importance of G6PD in AGE-mediated neurite functional outcomes, neurite length and average number of neurites per field of view were analyzed in the presence and absence of DHEA. Consistent with previous studies, AGE treatment compromised neurite morphology as measured by average neurite length (Fig. 4C) and average neurite number per field of view (Fig. 4D). While D3T did not further potentiate the effect of AGE on neurite morphology, it is possible a ceiling effect was approached with AGE treatment alone (Fig. 4D). Interestingly, inhibition of G6PD via DHEA rescued SH-SY5Y cells from AGE-induced neurite damage (Figs. 4C-D and 5), demonstrating the importance of G6PD in the AGE-mediated mechanism of PN.
Fig. 5.
DHEA protects against AGE-induced loss of long neurites and retraction. Differentiated cells were treated with 100 µM D3T and/or 100 µM DHEA for 24 h prior to 5 mg/mL BSA or AGE treatment. D3T and DHEA were replenished during BSA and AGE treatment. Representative 10x images of those quantified using Image J software with NeuronJ plugin.
4. Discussion
The present studies evaluated the mechanisms responsible for the potentiation effect of D3T on AGE-induced oxidative stress. Pazdro and Burgess have previously shown that treatment with NAC was able to protect both viability and morphology of SH-SY5Y cells stressed with AGEs [22]. NAC appeared to confer protection by maintaining intracellular reduced GSH concentrations. The Nrf2 activator, D3T, induces GSH synthesis and was also expected to protect against AGE-induced cell damage. However, treating with D3T led to exacerbation of damage under AGE stress even though under control conditions a significant increase in reduced GSH was observed [23]. The critical observation from the Pazdro and Burgess study, that D3T potentiated oxidative stress only under AGE-challenged conditions, led us to explore this mechanism in greater detail.
D3T has been shown to be a potent Nrf2 inducer [33] and G6PD is a Nrf2 responsive gene [34]. Traditionally, Nrf2 activation is expected to protect against oxidative stress through its coordinated regulation of metabolic and antioxidant enzyme expression. In particular, the downstream upregulation of pentose phosphate pathway enzymes (including G6PD) is critical for production of NADPH to maintain endogenous antioxidants such as GSH, thioredoxin, and glutaredoxin [35]. In the liver, the major detoxification organ, Nrf2-null mice are prone to oxidative stress induced liver injury [36]. Keap1-hepatocyte knockout mice (Keap1-HKO), a model for increased liver Nrf2 activation, have been shown to be more resistant to acetaminophen hepatotoxicity [37]. Keap1-HKO mice also have significantly higher G6PD mRNA expression which corresponds to a significant increase in liver NADPH concentration [19]. These observations suggest that Nrf2-induced increase in NADPH generation enzymes can lead to protection by reduction of oxidative stress [19]. Similarly, in our studies, upregulation of antioxidant enzymes with D3T treatment in the absence of AGEs allows for increased intracellular GSH and its maintenance in its reduced form by GR.
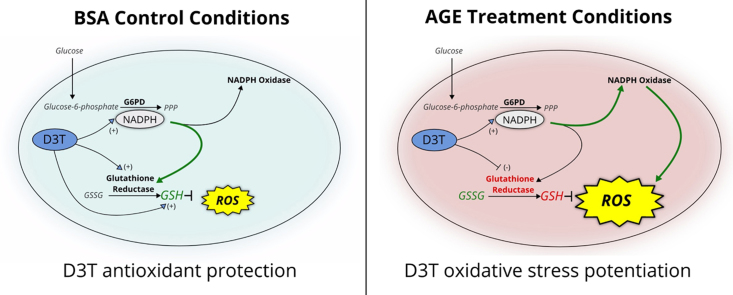
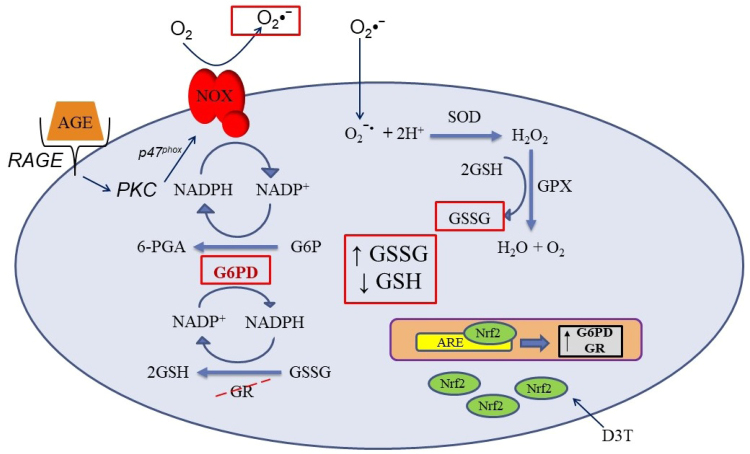
We propose that this scenario reverses in the AGE-challenge group where the same “protective” mechanism of action by D3T potentiates the pro-oxidative effects of AGE. Through increasing G6PD, D3T may increase NADPH generation that can provide reducing equivalents for AGE-activated NAD(P)H oxidase, leading to the rise in superoxide generation and oxidative stress (Fig. 2B). This is supported by results previously reported by our group which showed that inhibition of NAD(P)H oxidase with diphenyleneiodonium chloride resulted in abatement of the pro-oxidative effect of both AGE and AGE-D3T treatment [23]. G6PD-derived NADPH leading to increased oxidative stress via NAD(P)H oxidase has also been observed in the failing heart [38], [39]. The reason why increased G6PD expression led to exacerbation [38], [39] of oxidative stress rather than reduction [19] remained unanswered. For our study, we provide one potential explanation with the results from the AGE-D3T combination treatment. GR activity was unexpectedly suppressed under AGE-D3T treatment conditions, therefore redox balance was not maintained, and cell morphology and viability deteriorated (Fig. 6). This effect was reversed by inhibiting G6PD with DHEA, suggesting that the catalytic activity of G6PD was mediating the pro-oxidant effect (Fig. 4A)
Fig. 6.
Mechanism of the D3T paradox. Receptor for advanced glycation end products (RAGE); advanced glycation end product (AGE); NAD(P)H oxidase (NOX); glucose-6-phosphate dehydrogenase (G6PD); glucose-6-phosphate (G6P); 6-phosphogluconate (6-PGA); glutathione reductase (GR); glutathione peroxidase (GPX); superoxide dismutase (SOD); antioxidant response element (ARE); nuclear factor (erythroid-derived 2)-like 2 (Nrf2); 3H-1,2-dithiole-3-thione (D3T). AGE-challenge results in activation of RAGE, downstream activation of NOX, and generation of superoxide. Superoxide enters the cells and undergoes dismutation to hydrogen peroxide. Hydrogen peroxide is reduced to water and molecular oxygen at the expense of GSH by GPX. These conditions result in the observed increase in GSSG and decrease in GSH. Addition of D3T further exacerbates this condition by upregulation of G6PD, leading to increased production of reducing equivalents (NADPH) that are then used by NOX to generate more superoxide. Furthermore, D3T blunts upregulation of GR activity, which leads to depletion of GSH, promotes build-up of GSSG, and disrupts redox balance.
Determining the mechanism of inactivation of GR, and potentially other antioxidant defense enzymes not quantified in these studies, is critical to understanding the observed paradox. Peroxynitrite is an oxidant species that has been shown to inhibit GR activity [40], [41], and as nitric oxide synthase is also a NADPH dependent enzyme [42], this potential mechanism for inactivation should be explored in future studies.
The pathogenesis of PN has been strongly linked with excess production of oxidant species and the disruption of the redox state [43], leading to complications such as axonal degeneration [44]. Previously in our laboratory, Pazdro and Burgess quantified loss of neurite number in RA differentiated SH-SY5Y cells after AGE challenge as a marker of neurite deterioration [22], [23]. We expanded on this method, and quantified neurite length and long neurite number. This was done to observe the effects of antioxidants on protection against neurite retraction and protection of long neurites upon AGE-challenge. We focused on the long neurites because in PN the longest neurons are generally the first to get damaged [43], [44]. AGE-challenged cells showed significant deterioration of neurite structure, with mean neurite length dropping from 115 to 76 pixels (Fig. 4C). However, G6PD inhibition with DHEA was able to completely rescue neurite length under both AGE and AGE-D3T challenge (Fig. 4C). An even more dramatic change was observed with AGE and AGE-D3T challenge on the average number of long neurites. The BSA control group contained an average of 21 long neurites per field of view, and this number dropped significantly to 6.8 and 3.3 for AGE and AGE-D3T groups, respectively (Fig. 4D). Again, DHEA treatment completely protected neurites in both groups (Fig. 4D). These results further bring to light the critical role of G6PD and GR in maintaining GSH redox balance to prevent neurite degeneration in diabetic PN.
In conclusion, results of this study continue to support previous data from our laboratory that GSH is critical for maintaining neurites in differentiated SH-SY5Y cells. We show that under AGE-challenge conditions, D3T further disrupts redox balance and induces oxidative stress. This potentiation effect is due to upregulation of the Nrf2 responsive gene G6PD, together with a decrease in the activity of GR despite higher protein amounts. Botanical Nrf2 inducers, such as D3T, are seen as protective against diseases with an oxidative stress component [43]. However, our results suggest that Nrf2 inducing phytochemicals may exacerbate AGE-mediated damage via differential impact on antioxidant defense enzymes. Further studies in different cell lines and animal models will need to be conducted with phytochemicals such as D3T to better understand the translational nature of these results.
Acknowledgements
We are grateful to Dr. Jessica Ellis and her lab members for use of their cell culture facility and assistance with Western blot techniques and analysis. We would like to thank Janice Y. Lee for her assistance with statistical analysis and review of this manuscript, and Mark Fioretto for his assistance with imaging. Furthermore, we would like to thank Connor Koelsch for his help with sample analysis.
Acknowledgments
Funding
This work was supported by funds from Purdue University, Department of Nutrition Science, United States.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.101078.
Appendix A. Supplementary material
Supplementary material
References
- 1.Centers for Disease Control and Prevention, National Diabetes Statistics Report Centers for Disease Control and Prevention., Atlanta, GA, 2017.
- 2.Anon . Vol. 36. American Diabetes Association; 2013. Economic costs of diabetes in the U.S. in 2012; pp. 1033–1046. (1797 LP-1797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldman E.L., Nave K.-A., Jensen T.S., Bennett D.L.H. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017;93:1296–1313. doi: 10.1016/j.neuron.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent A.M., Perrone L., a Sullivan K., Backus C., Sastry A.M., Lastoskie C., Feldman E.L. , Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology. 2007;148:548–558. doi: 10.1210/en.2006-0073. [DOI] [PubMed] [Google Scholar]
- 5.Lukic I.K., Humpert P.M., Nawroth P.P., Bierhaus A. The RAGE pathway: activation and perpetuation in the pathogenesis of diabetic neuropathy. Ann. N. Y. Acad. Sci. 2008;1126:76–80. doi: 10.1196/annals.1433.059. [DOI] [PubMed] [Google Scholar]
- 6.Wada R., Yagihashi S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann. N. Y. Acad. Sci. 2005;1043:598–604. doi: 10.1196/annals.1338.067. [DOI] [PubMed] [Google Scholar]
- 7.Vlassara H. The age-receptor in the pathogenesis of diabetic complications. Diabetes Metab. Res. Rev. 2001;17:436–443. doi: 10.1002/dmrr.233. 〈http://www.ncbi.nlm.nih.gov/pubmed/11757079〉 (Accessed 23 September 2014) [DOI] [PubMed] [Google Scholar]
- 8.Sato T., Iwaki M., Shimogaito N., Wu X., Yamagishi S.-I., Takeuchi M. TAGE (toxic AGEs) theory in diabetic complications. Curr. Mol. Med. 2006;6:351–358. doi: 10.2174/156652406776894536. 〈http://www.ncbi.nlm.nih.gov/pubmed/16712480〉 (Accessed 23 September 2014) [DOI] [PubMed] [Google Scholar]
- 9.Jack M., Wright D. Role of advanced glycation endproducts and glyoxalase I in diabetic peripheral sensory neuropathy. Transl. Res. 2012;159:355–365. doi: 10.1016/j.trsl.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nitti M., Furfaro A.L., Traverso N., Odetti P., Storace D., Cottalasso D., Pronzato M.A., Marinari U.M., Domenicotti C. PKC delta and NADPH oxidase in AGE-induced neuronal death. Neurosci. Lett. 2007;416:261–265. doi: 10.1016/j.neulet.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Nitti M., d’Abramo C., Traverso N., Verzola D., Garibotto G., Poggi A., Odetti P., Cottalasso D., Marinari U.M., Pronzato M.A., Domenicotti C. Central role of PKCδ in glycoxidation-dependent apoptosis of human neurons. Free Radic. Biol. Med. 2005;38:846–856. doi: 10.1016/j.freeradbiomed.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Lee M.T., Lin W.C., Yu B., Lee T.T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals - A review. Asian-Australas. J. Anim. Sci. 2017;30:299–308. doi: 10.5713/ajas.16.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chikara S., Nagaprashantha L.D., Singhal J., Horne D., Awasthi S., Singhal S.S. Oxidative stress and dietary phytochemicals: role in cancer chemoprevention and treatment. Cancer Lett. 2018;413:122–134. doi: 10.1016/j.canlet.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Kumar H., Kim I.-S., More S.V., Kim B.-W., Choi D.-K. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat. Prod. Rep. 2014;31:109–139. doi: 10.1039/c3np70065h. [DOI] [PubMed] [Google Scholar]
- 15.Li K.-R., Yang S.-Q., Gong Y.-Q., Yang H., Li X.-M., Zhao Y.-X., Yao J., Jiang Q., Cao C. 3H-1,2-dithiole-3-thione protects retinal pigment epithelium cells against Ultra-violet radiation via activation of Akt-mTORC1-dependent Nrf2-HO-1 signaling. Sci. Rep. 2016;6:25525. doi: 10.1038/srep25525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong J., Yan D., Chen S. Stabilization of Nrf2 protein by D3T provides protection against ethanol-induced apoptosis in PC12 cells. PLoS One. 2011;6:e16845. doi: 10.1371/journal.pone.0016845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia Z., Zhu H., Trush M.A., Misra H.P., Li Y. Generation of superoxide from reaction of 3H-1,2-dithiole-3-thione with thiols: implications for dithiolethione chemoprotection. Mol. Cell. Biochem. 2008;307:185–191. doi: 10.1007/s11010-007-9598-z. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi M., Li L., Iwamoto N., Nakajima-Takagi Y., Kaneko H., Nakayama Y., Eguchi M., Wada Y., Kumagai Y., Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell. Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu K.C., Cui J.Y., Klaassen C.D. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol. Sci. 2011;123:590–600. doi: 10.1093/toxsci/kfr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baird L., Dinkova-Kostova A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Gordon G.B. A strategy for cancer prevention: stimulation of the Nrf2-ARE signaling pathway. Mol. Cancer Ther. 2004;3:885–893. 〈http://www.ncbi.nlm.nih.gov/pubmed/15252150〉 (Accessed 21 March 2018) [PubMed] [Google Scholar]
- 22.Pazdro R., Burgess J.R. Differential effects of α-tocopherol and N-acetyl-cysteine on advanced glycation end product-induced oxidative damage and neurite degeneration in SH-SY5Y cells. Biochim. Biophys. Acta. 2012;1822:550–556. doi: 10.1016/j.bbadis.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Pazdro R., Burgess J.R. The antioxidant 3H-1,2-dithiole-3-thione potentiates advanced glycation end-product-induced oxidative stress in SH-SY5Y cells. Exp. Diabetes Res. 2012;2012:137607. doi: 10.1155/2012/137607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahman I., Kode A., Biswas S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2007;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 25.Wilmanski T., Zhou X., Zheng W., Shinde A., Donkin S.S., Wendt M., Burgess J.R., Teegarden D. Inhibition of Pyruvate Carboxylase by 1α,25-dihydroxyvitamin D promotes oxidative stress in early breast cancer progression. Cancer Lett. 2017 doi: 10.1016/j.canlet.2017.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiniers M.J., van Golen R.F., Bonnet S., Broekgaarden M., van Gulik T.M., Egmond M.R., Heger M. Preparation and practical applications of 2′,7′-dichlorodihydrofluorescein in redox assays. Anal. Chem. 2017;89:3853–3857. doi: 10.1021/acs.analchem.7b00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalyanaraman B., Darley-Usmar V., Davies K.J.A., Dennery P.A., Forman H.J., Grisham M.B., Mann G.E., Moore K., Roberts L.J., Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic. Biol. Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonini M.G., Rota C., Tomasi A., Mason R.P. The oxidation of 2′,7′-dichlorofluorescin to reactive oxygen species: a self-fulfilling prophesy? Free Radic. Biol. Med. 2006;40:968–975. doi: 10.1016/j.freeradbiomed.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 29.Mannervik B. Measurement of glutathione reductase activity. Curr. Protoc. Toxicol. 1999;00:7.2.1–7.2.4. doi: 10.1002/0471140856.tx0702s00. [DOI] [PubMed] [Google Scholar]
- 30.Jia Z., Zhu H., Li Y., Misra H.P. Cruciferous nutraceutical 3H-1,2-dithiole-3-thione protects human primary astrocytes against neurocytotoxicity elicited by MPTP, MPP(+), 6-OHDA, HNE and acrolein. Neurochem. Res. 2009;34:1924–1934. doi: 10.1007/s11064-009-9978-8. [DOI] [PubMed] [Google Scholar]
- 31.Marmolino D., Manto M. Past, present and future therapeutics for cerebellar ataxias. Curr. Neuropharmacol. 2010;8:41–61. doi: 10.2174/157015910790909476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon G., Mackow M.C., Levy H.R. On the mechanism of interaction of steroids with human glucose 6-phosphate dehydrogenase. Arch. Biochem. Biophys. 1995;318:25–29. doi: 10.1006/abbi.1995.1199. [DOI] [PubMed] [Google Scholar]
- 33.Kwak M.K., Itoh K., Yamamoto M., Sutter T.R., Kensler T.W. Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3H-1, 2-dimethiole-3-thione. Mol. Med. 2001;7:135–145. 〈http://www.ncbi.nlm.nih.gov/pubmed/11471548〉 (Accessed 14 March 2018) [PMC free article] [PubMed] [Google Scholar]
- 34.Thimmulappa R.K., Mai K.H., Srisuma S., Kensler T.W., Yamamoto M., Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. 〈http://www.ncbi.nlm.nih.gov/pubmed/12234984〉 (Accessed 23 May 2014) [PubMed] [Google Scholar]
- 35.Veskoukis A.S., Margaritelis N.V., Kyparos A., Paschalis V., Nikolaidis M.G. Spectrophotometric assays for measuring redox biomarkers in blood and tissues: the NADPH network. Redox Rep. 2018;23:47–56. doi: 10.1080/13510002.2017.1392695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aleksunes L.M., Manautou J.E. Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicol. Pathol. 2007;35:459–473. doi: 10.1080/01926230701311344. [DOI] [PubMed] [Google Scholar]
- 37.Okawa H., Motohashi H., Kobayashi A., Aburatani H., Kensler T.W., Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem. Biophys. Res. Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- 38.Gupte S.A., Levine R.J., Gupte R.S., Young M.E., Lionetti V., Labinskyy V., Floyd B.C., Ojaimi C., Bellomo M., Wolin M.S., Recchia F.A. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J. Mol. Cell. Cardiol. 2006;41:340–349. doi: 10.1016/j.yjmcc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Gupte R.S., Vijay V., Marks B., Levine R.J., Sabbah H.N., Wolin M.S., Recchia F.A., Gupte S.A. Upregulation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase activity increases oxidative stress in failing human heart. J. Card. Fail. 2007;13:497–506. doi: 10.1016/j.cardfail.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Savvides S.N., Scheiwein M., Bohme C.C., Arteel G.E., Karplus P.A., Becker K., Schirmer R.H. Crystal structure of the antioxidant enzyme glutathione reductase inactivated by peroxynitrite. J. Biol. Chem. 2002;277:2779–2784. doi: 10.1074/jbc.M108190200. [DOI] [PubMed] [Google Scholar]
- 41.Barker J.E., Heales S.J.R., Cassidy A., Bolaños J.P., Land J.M., Clark J.B. Depletion of brain glutathione results in a decrease of glutathione reductase activity; an enzyme susceptible to oxidative damage. Brain Res. 1996;716:118–122. doi: 10.1016/0006-8993(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 42.Knowles R.G., Moncada S. Nitric oxide synthases in mammals. Biochem. J. 1994;298(Pt 2):249–258. doi: 10.1042/bj2980249. 〈http://www.ncbi.nlm.nih.gov/pubmed/7510950〉 (Accessed 1 November 2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edwards J.L., Vincent A.M., Cheng H.T., Feldman E.L. Diabetic neuropathy: mechanisms to management. Pharmacol. Ther. 2008;120:1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinnreich M., Taylor B.V., Dyck P.J.B. Diabetic neuropathies. Neurologist. 2005;11:63–79. doi: 10.1097/01.nrl.0000156314.24508.ed. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material