Abstract
This study assessed the effect of 12-week aerobic exercise on arterial stiffness (primary outcome), inflammation, oxidative stress, and cardiorespiratory fitness (secondary outcomes) in women with systemic lupus erythematosus (SLE). In a non-randomized clinical trial, 58 women with SLE were assigned to either aerobic exercise (n = 26) or usual care (n = 32). The intervention comprised 12 weeks of aerobic exercise (2 sessions × 75 min/week) between 40–75% of the individual’s heart rate reserve. At baseline and at week 12, arterial stiffness was assessed through pulse wave velocity (PWV), inflammatory (i.e., high-sensitivity C-reactive protein [hsCRP], tumor necrosis factor alpha [TFN-α], and inteleukin 6 [IL-6]) and oxidative stress (i.e., myeloperoxidase [MPO]) markers were obtained from blood samples, and cardiorespiratory fitness was assessed (Bruce test). There were no between-group differences in the changes in arterial stiffness (median PWV difference −0.034, 95% CI −0.42 to 0.36 m/s; p = 0.860) or hsCRP, TNF-α, IL-6, and MPO (all p > 0.05) at week 12. In comparison to the control group, the exercise group significantly increased cardiorespiratory fitness (median difference 2.26 minutes, 95% CI 0.98 to 3.55; p = 0.001). These results suggest that 12 weeks of progressive treadmill aerobic exercise increases cardiorespiratory fitness without exacerbating arterial stiffness, inflammation, or oxidative stress in women with SLE.
Keywords: exercise, physical fitness, aerobic fitness, physical function, pulse wave velocity, autoimmune diseases, vascular health, atherosclerosis, lupus, inflammatory disease
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease of an unknown etiology that predominantly affects young adult women [1], with a prevalence of up to 1 case in 1000 inhabitants in Europe [2,3]. Its prognosis has significantly improved in recent decades leading to new comorbidities, such as atherosclerotic cardiovascular diseases (CVD) [4], which have become a major cause of mortality in this population [5]. It has been shown that classical CVD risk factors (e.g., hypertension, dyslipidemia, diabetes, smoking etc.) fail to fully describe the excess of CV morbi-mortality observed in SLE [4,6], and novel CV risk factors, such as arterial stiffness, systemic inflammation, or oxidative stress, are involved [4,7,8].
Arterial stiffness is a marker of subclinical atherosclerosis that increases with age and is significantly elevated in patients with SLE compared to the general population [8,9]. Arterial stiffness reveals structural changes in the elasticity of the arteries prior to the development of clinical atherosclerosis, and is a powerful predictor of CVD independently of other CV risk factors, both in the general population [10] and in patients with SLE [11]. Therefore, attenuating the increase of arterial stiffness in this population is of clinical relevance for the early prevention of CVD.
In SLE, inflammation and tissue damage are mediated by pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6), released by recruited inflammatory cells (macrophages, myeloid dendritic cells, pathogenetic T and B cells) and immune complexes-induced complement activation [12]. These markers of systemic inflammation, as well as C-reactive protein (CRP), are known to independently predict CV events [13,14], and represent potential mechanisms explaining the excess CV morbi-mortality in SLE [15]. Oxidative stress (i.e., an imbalance in the cells due to an increase in free radicals or a decrease in antioxidants) has also shown to be involved in the development of CVD in patients with SLE [7,16]. Although behavioral interventions, such as regular exercise, might elicit significant CV benefits without many of the side effects of pharmacological treatments, they are understudied and hardly appear in the British Society for Rheumatology guidelines for the management of SLE [17].
Exercise has been shown to increase physical fitness and reduce CVD risk in many populations across the whole lifespan. The American College of Sports Medicine (ACSM) highlights the need to undertake a minimum of 150 min/week (i.e., accumulated in bouts of ≥10 min) of aerobic exercise of moderate to vigorous intensity in adults [18]. In a sample of women with SLE with mild/inactive disease, we cross-sectionally observed no association between accelerometer-assessed physical activity and arterial stiffness [19], although a higher level of cardiorespiratory fitness was related to lower age-related arterial stiffness in this population [20]. Although aerobic exercise has a promising role attenuating arterial stiffness in the general population [21], its effects in women with SLE have not been previously investigated.
Perandini et al. observed that a single bout (i.e., 30 min) of aerobic exercise (i.e., either at 50% or 70% of VO2peak) did not increase inflammation in women with either active or inactive SLE [22], and that 12 weeks of aerobic training (n = 8) tended to reduce inflammation in comparison to a control group (n = 10) [23]. Timoteo et al. [24], however, did not observe changes in IL-6 or TNF-α in the combined exercise (i.e., flexibility, resistance and aerobic training) group (n = 5) compared to the control group (n = 9). Overall, these studies had a small sample size and the exercise programs were not comprehensively described to allow replication or application in clinical practice.
The primary aim of this study was to assess the effect of a 12-week aerobic exercise intervention following the ACSM guidelines on arterial stiffness in women with SLE in comparison with usual care. Secondary aims were to assess the effects of the exercise intervention on inflammation, oxidative stress, and cardiorespiratory fitness. We hypothesized that the intervention would have a positive impact on CV risk factors and cardiorespiratory fitness [25] in women with SLE.
2. Methods
2.1. Design and Protocol Registration
This non-randomized controlled trial was registered at clinicaltrials.gov [NCT03107442] on 11 April 2017, before the enrolment of participants started (i.e., on 12 April), and no deviations occurred regarding the primary outcome and the secondary outcomes analyzed here.
2.2. Setting and Eligibility Criteria
Participants were recruited from the Systemic Autoimmune Diseases Unit of the “Virgen de las Nieves” and the “San Cecilio” University Hospitals. Women with a diagnosis of SLE according to the ACR criteria [26], a follow-up of ≥12 months, clinical and treatment stability during the previous six months, and not performing regular exercise (defined as ≥60 min/week of structured exercise) were included. Exclusion criteria were to have been under biological treatment in the previous six months or to need a prednisone dose of >10 mg/day; a background of CVD in the previous year; to present contraindications to perform exercise; other associated rheumatic conditions; pregnancy; active acute or chronic infection; neoplasms; acute renal failure; cardiac or pulmonary involvement; body mass index (BMI) >35; or not being able to read, understand, and sign written informed consent. All participants received detailed information about the study procedures, and signed written informed consent. The Research Ethics Committee of Granada approved the protocol on 11 November 2016 (reference No.: 10/2016).
2.3. Procedures
A telephone screening was conducted. Potentially eligible participants were invited to a personal screening and, if included, day 1 of the baseline examination was performed. The baseline examination comprised two assessment days. On day 1, pulse wave velocity (PWV) was assessed. Thereafter, cardiorespiratory fitness testing was performed, and socio-demographic and clinical information was collected. On day 2 (i.e., between two and four days after day 1), 8-h fasting blood samples were collected between 8:00 a.m. and 10:00 a.m. This article follows the TREND statement for improving the reporting of non-randomized experiments of behavioral and public health interventions (downloadable at EQUATOR Network: https://goo.gl/ZSyLrj; Supplementary Table S1) [27]. The funding source had no role in the study.
2.4. Interventions
2.4.1. Exercise Group
To maximize transparency and replicability, the exercise program described in this manuscript follows the Consensus on Exercise Reporting Template (CERT; Supplementary Table S2) [28]. The patients assigned to exercise performed two 75-min sessions per week during a total of 12 weeks (i.e., 24 sessions) of moderate to vigorous intensity aerobic exercise on a treadmill (BH, Serie i.RC12 Dual, Vitoria-Gasteiz, Spain) from 24 April to 14 July 2017. A scheme of the prescribed exercise intervention is presented in Table 1.
Table 1.
Summary of the prescribed training program.
| Month | Week | Weekly MVPA (min) | Session (No.) | Training Type | Total Session Time (min) | Estimated Session Time at Target Intensity (min) | Intensity (% HRR) | Series/Workout | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 90 | 1 | Continuous | 55 | ≈40 | 35–45 | 7,5’ Warm-up + 15’ 35–45% + 3’ rec + 10’ 35–45% + 3’ rec + 10’ 35–45% + 7,5’ Cool down | |
| 2 | Continuous | 65 | ≈50 | 35–45 | 7,5’ Warm-up + 2 × (15’ 35–45%/3’ rec) + 15’ 35–45% + 7,5’ Cool down | ||||
| 2 | 105 | 3 | Continuous | 65 | ≈50 | 40–50 | 7,5’ Warm-up + 20’ 40–50% + 3’ rec + 15’ 40–50% + 3’ rec + 10’ 40–50% + 7,5’ Cool down | ||
| 4 | Continuous | 75 | ≈60 | 40–50 | 7,5’ Warm-up + 2 × (20’ 40–50%/3’ rec) + 15’ 40–50% + 7,5’ Cool down | ||||
| 3 | 130 | 5 | Continuous | 75 | ≈60 | 45–55 | 7,5’ Warm-up + 25’ 45–55% + 3’ rec + 20’ 45–55% + 3’ rec + 10’ 45–55% + 7,5’ Cool down | ||
| 6 | Continuous | 85 | ≈70 | 45–55 | 7,5’ Warm-up + 2 × (25’ 45–55%/3’ rec) + 15’ 45–55% + 7,5’ Cool down | ||||
| 4 | 145 | 7 | Continuous | 85 | ≈70 | 45–55 | 7,5’ Warm-up + 30’ 45–55% + 3’ rec + 25’ 45–55% + 3’ rec + 10’ 45–55% + 7,5’ Cool down | ||
| 8 | Continuous | 90 | ≈75 | 45–55 | 7,5’ Warm-up + 1 × (40’ 45–55%/3’ rec) + 30’ 45–55% + 7,5’ Cool down | ||||
| Interval lower bound | Interval higher bound | ||||||||
| 2 | 5 | 150 | 9 | Continuous | 90 | ≈75 | 50–60 | 7,5’ Warm-up + 30’ 50–60% + 2.5’ rec + 25’ 50–60% + 2.5’ rec + 15’ 50–60% + 7,5’ Cool down | |
| 10 | Continuous | 90 | ≈75 | 50–60 | 7,5’ Warm-up + 1 × (40’ 50–60%/5’ rec) + 30’ 50–60% + 7,5’ Cool down | ||||
| 6 | 150 | 11 | Continuous | 90 | ≈75 | 55–60 | 7,5’ Warm-up + 1 × (40’ 55–60%/5’ rec) + 30’ 55–60% + 7,5’ Cool down | ||
| 12 | Interval | 90 | ≈75 | 50–55 | 60–65 | 7,5’ Warm-up + 1 × (25’ 50–55%+10’ 60–65%+5’ rec) + 1 × (25’ 50–55%+10’ 60–65%) + 7,5’ Cool down | |||
| 7 | 150 | 13 | Continuous | 90 | ≈75 | 57.5–62.5 | 7,5’ Warm-up + 1 × (45’ 57.7–62.5%/5’ rec) + 25’ 57.5–62.5% + 7,5’ Cool down | ||
| 14 | Interval | 90 | ≈75 | 52.5–57.5 | 60–65 | 7,5’ Warm-up + 1 × (20’ 52.5–57.5%+15’ 60–65%+5’ rec) + 1 × (20’ 52.5–57.5%+15’ 60–65%) + 7,5’ Cool down | |||
| 8 | 150 | 15 | Continuous | 90 | ≈75 | 55–60 | 7,5’ Warm-up + 1 × (40’ 55–60%/5’ rec) + 30’ 55–60% + 7,5’ Cool down | ||
| 16 | Interval | 90 | ≈75 | 50–55 | 60–65 | 7,5’ Warm-up + 1 × (30’ 55–60%+5’ 60–65%+5’ rec) + 1 × (30’ 55–60%+5’ 60–65%) + 7,5’ Cool down | |||
| Interval lower bound | Interval higher bound | ||||||||
| 3 | 9 | 150 | 17 | Interval | 90 | ≈75 | 55–60 | 65–70 | 7,5’ Warm-up + 2 × (15’ 55–60%+3’ 65–70%) + 5’ rec + 15’ 55–60% + 3’ 65–70% + 12’ 55–60% + 3’ 65–70% + 7,5’ Cool down |
| 18 | Interval | 90 | ≈75 | 55–60 | 65–70 | 7,5’ Warm-up + 2 × (15’ 55–60%+3’ 65–70%) + 5’ rec + 15’ 55–60% + 3’ 65–70% + 12’ 55–60% + 3’ 65–70% + 7,5’ Cool down | |||
| 10 | 150 | 19 | Interval | 90 | ≈75 | 57.5–62.5 | 65–70 | 7,5’ Warm-up + 3 × (11’ 57.5–62.5% + 3’ 65–70%) + 5’ rec + 2 × (11’ 57.5–62.5% + 3’ 65–70%) + 7,5’ Cool down | |
| 20 | Interval | 90 | ≈75 | 57.5–62.5 | 70–75 | 7,5’ Warm-up + 3 × (11’ 57.5–62.5% + 3’ 70–75%) + 5’ rec + 2 × (11’ 57.5–62.5% + 3’ 70–75%) + 7,5’ Cool down | |||
| 11 | 150 | 21 | Interval | 90 | ≈75 | 60–65 | 70–75 | 7,5’ Warm-up + 2 × (15’ 60–65%+3’ 70–75%) + 5’ rec + 15’ 60–65% + 3’ 70–75% + 12’ 60–65% + 3’ 70–75% + 7,5’ Cool down | |
| 22 | Interval | 90 | ≈75 | 60–65 | 70–75 | 7,5’ Warm-up + 2 × (15’ 60–65%+3’ 70–75%) + 5’ rec + 15’ 60–65% + 3’ 70–75% + 12’ 60–65% + 3’ 70–75% + 7,5’ Cool down | |||
| 12 | 150 | 23 | Interval | 90 | ≈75 | 60–65 | 70–75 | 7,5’ Warm-up + 5 × (5’ 60–65%+3’ 70–75%) + 5’ rec + 2 × (11’ 60–65%+3’ 70–75%) + 7,5’ Cool down | |
| 24 | Interval | 90 | ≈75 | 60–65 | 70–75 | 7,5’ Warm-up + 5 × (5’ 60–65%+3’ 70–75%) + 5’ rec + 3 × (6’ 60–65%+4’ 70–75%) + 7,5’ Cool down | |||
MVPA, moderate-to-vigorous physical activity; HRR, heart rate reserve; Rec, recovery.
The sessions took place in a quiet room of the “Virgen de las Nieves” Hospital, Granada (Spain). All sessions were performed in groups of a maximum of five persons (depending on the patients’ schedule preferences) and were supervised by both exercise professionals with a degree in Sports Sciences and residents from the Internal Medicine Department. Attendance at the sessions was registered daily and patients were contacted upon any missing session to ask for the reason and motivate them to replace it on an alternative day of the same week. Adherence to exercise is reported as the median attendance frequency and the proportion of patients attending ≥75% (i.e., 18 sessions; the minimum pre-defined attendance to assess efficacy) and ≥90% of the sessions. All the sessions began with a warm-up comprising 3–4 min of activation on the treadmill at about 35–40% of the heart rate reserve (HRR) and 3–4 min of active stretching of major muscle groups, and ended with a cool down phase of static stretching of major muscle groups and relaxation. Exercise was individually prescribed to represent moderate-to-vigorous intensity, with training intensity ranging from 40% to 75% of each patient’s HRR. The maximum heart rate (HRmax) was estimated with the formula by Tanaka et al. (HRmax = 208 − (0.7 × age)) [29]. The training (or target) heart rate (tHR) was calculated with the formula tHR = HRrest + (%HRR). Heart rate was continuously monitored during all sessions (Polar V800, Kempele, Finland). We used the session rating of perceived exertion (RPE) as a measure of subjective training load [30], and the feeling scale to assess positive affective responses experienced before and after each session [31].
The starting level was specific for each individual according to her previous exercise experience and physical fitness. During the first half of the program, only continuous exercise was performed so that the patients got used to the treadmill and felt confident at increasing intensities. Continuous sessions comprised several bouts of exertion at constant intensity, followed by a couple of minutes of recovery (i.e., rest) to drink water. During month 2, there were alternated continuous and interval sessions, and at month 3, the patients undertook interval training sessions, where there were periods of lower and periods of higher intensity efforts followed by some minutes of rest for hydration (Table 1). The progression in volume and/or intensity was patient-limited and was undertaken by increasing the treadmill speed (first) or inclination according to the symptoms and perceived exertion. There were no home-based or non-exercise components within this intervention. However, if a participant was eventually not able to attend a particular session, we provided her with a heart rate monitor and allowed recovery of that session out of the Hospital (a total of seven sessions were recovered in this fashion). Finally, the exercise intensity progressions had to be slightly modified from the initial plan. For instance, several patients perceived a 5% HRR intensity increase (i.e., from one week to another) as very heavy and difficult-to-follow. Consequently, there were weeks in which exercise intensity increased by 2.5% instead of 5% (Table 1).
2.4.2. Control Group
After the baseline evaluation, the SLE patients assigned to the (usual care) control group received verbal information about a healthy lifestyle, including physical activity guidelines and basic nutritional information.
2.5. Outcome Measures
2.5.1. Primary Outcome Measure: Arterial Stiffness
Arterial stiffness was assessed in a sitting position by PWV [9], using the Mobil-O-Graph® 24 h pulse wave analysis monitor (IEM GmbH, Stolberg, Germany), whose operation is based on oscillometry recorded by a blood pressure cuff placed on the brachial artery. The coefficient of variation (CV) of Mobil-O-Graph for consecutive PWV analyses is 3.4% and its intraclass correlation coefficient is 0.98 [0.96–0.99] [32]. This device has been largely shown to be valid and reliable for measuring PWV and central blood pressure in different populations [33,34], meets the accuracy requirements of the British Hypertension Society (BHS) standard [35], and can be recommended for clinical use [36].
2.5.2. Secondary Outcome Measures
Blood Samples and Biochemical Analyses
Fasting blood specimens for biochemical and immunological tests were collected and routinely processed by the central laboratory of our hospital. Among other measurements, they included lipids, insulin (BioRad, Marne-la-Coquette, France), and a routine biochemical profile. The homeostatic model assessment for insulin resistance (HOMA-IR) was calculated (HOMA-IR = glucose (mmol/L) × insulin (µU/L)/22.5).
Inflammatory Markers
Serum high-sensitivity CRP was assessed by an immunoturbidimetric method using the ARCHITECT cSystems (MULTIGENT CRP Vario assay); the limit of quantitation was 0.2 mg/L and the upper limit for normal serum was 5 mg/L (coefficient of variation <6%). Interleukin 6 and TNF-α, as well as myeloperoxidase (MPO; as marker of oxidative stress), were measured in plasma. Serum was initially separated by centrifugation and stored at −70 °C. Bioserum concentrations of IL-6/TNF-α (pg/mL) and MPO (ng/mL) were measured by an immunoradiometric assay using commercial kits (MILLIPLEX MAP Kit Human High Sensitivity T Cell Magnetic Bead Panel (HSTMAG-28SK) and Human Cardiovascular Disease Magnetic Bead Panel 2 (HCVD2MAG-67K)), Millipore) following the manufacturer’s instructions. Quantitative data were obtained by using the Luminex-200 system (Luminex Corporation, Austin, TX, USA), and data analysis was performed on XPonent 3.1 software (Austin, TX, USA). The detections limits were 0.73 pg/mL for IL-6, 0.43 pg/mL for TNF-a, and 0.024 ng/mL for MPO.
Cardiorespiratory Fitness
Cardiorespiratory fitness was assessed with the Bruce submaximal treadmill protocol [37]. The test comprised five increasing workload stages of 3 min each (stage 1: 2.7 km/h and 10% inclination; stage 2: 4 km/h and 12% inclination; stage 3: 5.5 km/h and 14% inclination; stage 4: 6.8 km/h and 16% inclination; stage 5: 8 km/h and 18% inclination). The test concluded when the participant achieved 85% of the individual’s HRmax, as estimated with the formula by Tanaka et al. [29]. As validated SLE-specific formulas to estimate VO2max are not available, we used the total time to reach 85% HRmax as the outcome of interest.
2.5.3. Other Measurements
All participants filled out a socio-demographic and clinical data questionnaire. Height (cm) was measured using a height gauge, weight (kg) with a bioimpedance device (InBody R20, Biospace, Seoul, Korea), and body mass index (BMI) was calculated (kg/m2). Blood pressure was measured with Mobil-O-Graph® (IEM GmbH, Stolberg, Germany) [36]. Disease activity was assessed through the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI, range 0–105 where a higher score indicates higher degree of disease activity). Physical activity was self-reported at baseline and at week 12 with the International Physical Activity Questionnaire [38].
2.6. Sample Size
The sample size was calculated for the primary outcome (i.e., PWV). Ashor et al. found an average effect of aerobic exercise on PWV of −0.63 m/s in adults aged ≥18 years [21]. A total of 52 patients (26 per group) were needed to detect an effect of −0.63 (SD 0.75) m/s, with a power of 85% and an α error of 0.05. Anticipating a maximum loss to follow-up of 15%, we aimed at recruiting a total of 60 patients.
2.7. Treatment Allocation and Blinding
Randomization was not feasible because more than half of the patients who regularly attend the Autoimmune Disease Units lived far from the Hospital and were not able to attend twice per week in case of being randomized to exercise. Therefore, participants from the city of Granada were included in the exercise group and participants living outside Granada were included in the control group. To minimize potential selection bias, we aimed to match the groups by age (±2 years), BMI (±1 kg/m2), and SLEDAI (±1 unit). The data analyzer was blinded to the patient allocation.
2.8. Statistical Analysis
The distribution of the main study variables was assessed through histogram and Q-Q plots. As the main outcomes were non-normally distributed, their descriptive characteristics were presented using the median and interquartile range instead of the mean and standard deviation and we used non-parametric tests for the main analyses. Between-group baseline characteristics were compared with the Student t-test (when normally distributed) or Kruskal-Wallis test (when non-normally distributed) for continuous variables and the Chi-square test for categorical variables. The between-group differences in the studied outcomes were assessed through quantile regression with baseline values, resting heart rate (bpm) [39], and changes in physical activity (min/week; since the change in self-reported physical activity at week 12 was >60 min higher in the control compared to the exercise group and this change was associated with changes in PWV; rPearson = −0.27, p = 0.048) as potential confounders, after checking baseline group comparisons. As we aimed at assessing efficacy, the primary analyses were defined as per-protocol, where patients from the exercise group were included if attendance at the exercise sessions was ≥75%. To assess the robustness of the results, subsequent sensitivity analyses (i.e., baseline observation carried forward (BOCF) imputation; per-protocol with minimum attendance of ≥90%, and complete-case analyses) were conducted. A blinded investigator (AS-M) undertook the data handling and all hypothesis testing. All the analyses were conducted with Stata v.13.1 (StataCorp LP., College Station, TX, USA). Statistical significance was set at p < 0.05.
3. Results
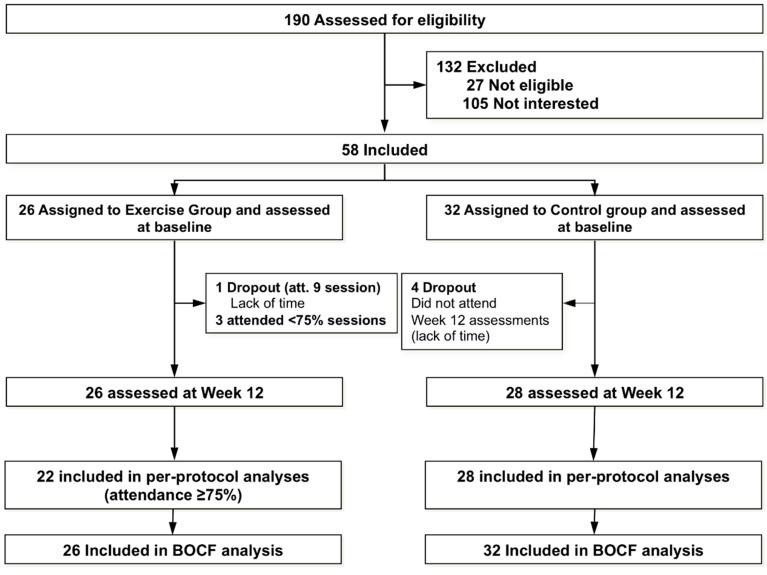
A total of 190 women with SLE were invited to participate. The flowchart of the study participants throughout the trial is presented in Figure 1. A total of 58 patients volunteered to participate, met the inclusion criteria, signed informed consent, and were finally included and assigned to either the exercise group (n = 26) or the control group (n = 32). The median attendance at the exercise intervention was 22.5 (out of 24; i.e., ~94%) sessions. A total of 22 participants (~85%) attended ≥75% of the sessions (i.e., and were included in primary analyses) and 18 (~69%) attended ≥90% of the sessions. One participant withdrew at week 5 due to severe sciatica (not associated with the exercise program). A total of four participants (i.e., 6.8% of the total sample) were lost to follow-up in the control group at week 12 and none in the exercise group. A summary of the average exercise intensity (i.e., HR) achieved at each session, RPE, and pre- and post-session affective responses are presented in Figure S1. There were no adverse events occurring during the exercise sessions.
Figure 1.
Flow chart of the study participants throughout the study.
At baseline (Table 2), the control group showed a lower resting heart rate (mean difference −9.6 bpm; p = 0.002), and higher IL-6 levels (median difference 3.09 pg/mL; p = 0.026) than the exercise group. There were no other significant between-group differences at baseline (all p > 0.05).
Table 2.
Baseline descriptive characteristics of the study participants.
| All (n = 58) | Exercise (n = 26) | Control (n = 32) | p | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age, years | 44.0 (13.9) | 43.0 (15.1) | 44.8 (13.1) | 0.618 |
| Marital status (Single/Married/Divorced; %) | 44.8/50.0/5.2 | 53.8/42.3/3.9 | 37.5/56.3/6.2 | 0.455 |
| Educational level (No studies/Primary/Secondary/University; %) | 3.4/36.2/22.4/37.9 | 0/38.5/26.9/34.6 | 6.3/34.4/18.7/40.6 | 0.521 |
| Occupational status (working/housewife/Not working; %) | 41.4/24.1/34.5 | 42.3/19.2/38.5 | 40.6/28.1/31.3 | 0.706 |
| BMI, kg/m2 | 25.2 (4.7) | 25.9 (3.4) | 24.7 (5.6) | 0.336 |
| SBP, mm/Hg | 117.7 (10.3) | 116.8 (10.0) | 118.4 (10.6) | 0.567 |
| DBP, mm/Hg | 75.5 (9.5) | 75.6 (8.8) | 75.4 (10.1) | 0.937 |
| MBP, mm/Hg | 94.8 (8.8) | 94.5 (8.3) | 95.0 (9.3) | 0.821 |
| RHR, bpm | 81.6 (11.8) | 86.9 (11.0) | 77.3 (10.7) | 0.002 |
| Insulin, mg/dL | 7.6 (3.5) | 7.7 (4.2) | 7.5 (2.8) | 0.809 |
| BP lowering drugs (%) | 15.5 | 7.7 | 21.9 | 0.138 |
| Smoke (%) | 25.9 | 15.4 | 34.4 | 0.166 |
| Alcohol (yes/no; %) | 5.2 | 7.7 | 3.2 | 0.435 |
| Menopause (%) | 39.7 | 38.5 | 40.6 | 0.867 |
| History of CVD (%) | 12.1 | 15.4 | 9.4 | 0.485 |
| Dislipemia | 17.2 | 19.2 | 15.6 | 0.718 |
| Statins | 17.2 | 23.1 | 12.5 | 0.289 |
| Hydroxicloroquine (%) | 89.7 | 96.1 | 84.4 | 0.143 |
| Dosis of Hydroxicloroquine, mg/day | 189.4 (115.8) | 187.4 (94.1) | 191.1 (132.3) | 0.905 |
| Immunosupressants (%) | 44.8 | 46.1 | 43.7 | 0.874 |
| Current corticosteroid intake (mg/day) | 3.9 (5.1) | 4.1 (6.1) | 3.7 (4.1) | 0.760 |
| Cumulative corticosteroid intake (mg) | 2947 | 2696 | 3164 | 0.511 |
| Disease duration, years | 15.4 (10.5) | 14.5 (10.4) | 16.1 (10.6) | 0.570 |
| Total PA, min/week | 90.9 (92.2) | 96.8 (97.9) | 86.3 (88.8) | 0.646 |
| SLEDAI | 0.22 (0.90) | 0.04 (0.20) | 0.38 (1.18) | 0.158 |
| SDI | 0.47 (1.11) | 0.19 (0.63) | 0.69 (1.35) | 0.092 |
| Pulse wave velocity, m/s (median, IQR) | 6.3 (5.3–7.4) | 6.3 (5.1–7.8) | 6.4 (5.5–7.3) | 0.696 |
| hsCRP, mg/L (median, IQR) | 1.46 (0.84–4.37) | 2.18 (1.2–4.37) | 1.21 (0.73–4.35) | 0.161 |
| TNF-alpha, pg/mL (median, IQR) | 15.95 (12.02–21.9) | 16.48 (12.48–21.46) | 15.18 (11.4–21.93) | 0.487 |
| IL-6, pg/mL (median, IQR) | 10.33 (7.07–13.67) | 8.18 (5.82–11.89) | 11.27 (9.21–14.14) | 0.026 |
| MPO, ng/mL (median, IQR) | 71.8 (42.8–127.1) | 60.15 (40.08–113.54) | 79.00 (56.65–161.1) | 0.241 |
| Cardiorespiratory fitness (Bruce test, min) | 8.2 (2.8) | 8.1 (2.2) | 8.3 (3.2) | 0.763 |
Values are the mean (standard deviation; SD), unless otherwise indicated. BMI, body mass index; SBP, systolic blood pressure; DBP, distolic blood pressure; MBP, mean blood pressure; CVD, cardiovascular disease; RHR, resting heart rate; PA, physical activity; SLEDAI, systemic lupus erythematosus disease activity index; SDI, systemic damage index; PWV, pulse wave velocity; hsCRP, high sensitivity C-reactive protein; TNF-α, tumor necrosis factor alpha; IL-6, interleukin-6; MPO, myeloperoxidase.
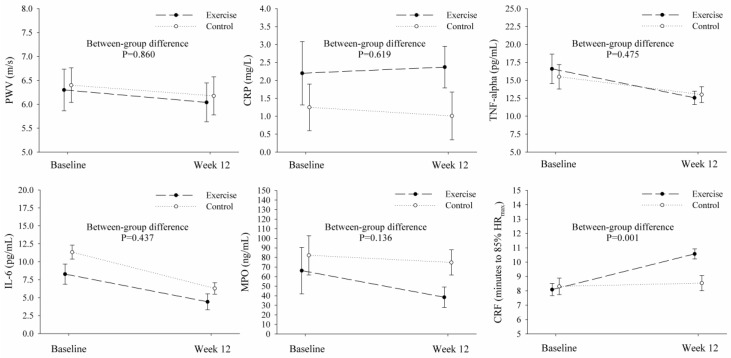
The baseline and (adjusted) follow-up values for the main study outcomes, and between-group comparisons, are presented in Figure 2. The primary analyses revealed no significant between-group differences between changes in PWV (primary aim) at week 12 (median difference −0.034, 95% CI −0.42 to 0.36 m/s; p = 0.860; Table 3), and these results were consistent across sensitivity analyses (Table 4 and Supplementary Tables S3 and S4). Regarding the secondary study aim, there were no between-group differences in the changes in hsCRP, TNF-α, IL-6, and MPO at week 12 (all p > 0.05; Table 3). Supplementary Table S5 shows a lack of between-group differences in the change from baseline to week 12 for traditional CVD risk factors such as blood pressure, insulin resistance, or BMI. In comparison to the control group, the exercise group experienced a significant increase in cardiorespiratory fitness (median difference 2.26 min, 95% CI 0.98 to 3.55; p = 0.001; Table 3). Overall, sensitivity analyses corroborated these results (Table 4 and Supplementary Tables S3 and S4).
Figure 2.
Graphical representation of the effects of the 12-week progressive aerobic exercise intervention. Results derived from the primary analyses (i.e., per-protocol with a minimum attendance of 75% for participants in the exercise group to be included). PWV, pulse wave velocity; hsCRP, high sensitivity C-reactive protein; TNF-α, tumor necrosis factor alpha; IL-6, interleukin-6; MPO, myeloperoxidase; CRF, cardiorespiratory fitness; HRmax, maximal heart rate.
Table 3.
Per-protocol (primary) analyses assessing the effects of 12-week progressive aerobic exercise on arterial stiffness, inflammation, oxidative stress, and cardiorespiratory fitness in women with systemic lupus erythematosus (participants in the exercise group were included if attendance was ≥75%).
| Change from Baseline at Week 12 | Intervention | Mean Difference (95%CI) | p | |
|---|---|---|---|---|
| Exercise (n = 22) | Control (n = 28) | |||
| Median (SE) | Median (SE) | |||
| PWV, m/s | −0.26 (0.14) | −0.22 (0.13) | −0.034 (−0.42 to 0.36) | 0.860 |
| hsCRP, mg/L | 0.17 (0.59) | −0.24 (0.55) | 0.411 (−1.25 to 2.07) | 0.619 |
| TNF-α, pg/mL | −4.04 (1.53) | −2.49 (1.41) | −1.55 (−5.87 to 2.78) | 0.475 |
| IL-6, pg/mL | −3.86 (1.04) | −5.05 (0.99) | 1.19 (−1.86 to 4.25) | 0.437 |
| MPO, ng/mL | −27.84 (9.61) | −7.49 (9.02) | −20.35 (−47.38 to 6.68) | 0.136 |
| Cardiorespiratory fitness (Bruce), min | 2.49 (0.44) | 0.22 (0.41) | 2.26 (0.98 to 3.55) | 0.001 |
The analyses were adjusted for baseline values, resting heart rate, and changes in self-reported physical activity during the study period. SE, standard error; CI, confidence interval; PWV, pulse wave velocity; hsCRP, high sensitivity C-reactive protein; TNF-α, tumor necrosis factor alpha; IL-6, interleukin-6; MPO, myeloperoxidase.
Table 4.
Sensitivity analyses: Baseline-observation carried forward imputation assessing the effects of 12-week progressive aerobic exercise on arterial stiffness, inflammation, oxidative stress, and cardiorespiratory fitness in women with systemic lupus erythematosus.
| Change from Baseline at Week 12 | Intervention | Mean Difference (95%CI) | p | |
|---|---|---|---|---|
| Exercise (n = 26) | Control (n = 32) | |||
| Median (SE) | Median (SE) | |||
| PWV, m/s | −0.25 (0.12) | −0.09 (0.12) | −0.16 (−0.51 to 0.19) | 0.365 |
| hsCRP, mg/L | −0.04 (0.36) | −0.09 (0.34) | 0.06 (−0.95 to 1.06) | 0.913 |
| TNF-α, pg/mL | −4.14 (1.85) | −3.88 (1.76) | −0.26 (−5.51 to 5.00) | 0.923 |
| IL-6, pg/mL | −3.81 (1.34) | −5.02 (1.31) | 1.22 (−2.79 to 5.22) | 0.545 |
| MPO, ng/mL | −17.11 (11.01) | −10.52 (10.84) | −6.58 (−37.97 to 24.80) | 0.676 |
| Cardiorespiratory fitness (Bruce), min | 2.71 (0.38) | 0.29 (0.36) | 2.42 (1.31 to 3.52) | <0.001 |
The analyses were adjusted for baseline values, resting heart rate, and changes in self-reported physical activity during the study period. SE, standard error; CI, confidence interval; PWV, pulse wave velocity; hsCRP, high sensitivity C-reactive protein; TNF-α, tumor necrosis factor alpha; IL-6, interleukin-6; MPO, myeloperoxidase.
4. Discussion
The main findings of this study suggest that 12 weeks of progressive treadmill aerobic exercise following the ACSM guidelines, performed between 40% and 75% of the HRR, increases cardiorespiratory fitness without exacerbating arterial stiffness, inflammation, or oxidative stress in women with SLE with mild/inactive disease, in comparison to a control group of patients with SLE that received recommendations for a healthy lifestyle. Future clinical trials with larger sample sizes are needed to enhance our understanding on how different durations, types, volumes, and exercise intensities might affect vascular health and inflammation in this population.
To the best of our knowledge, this is the first study assessing the extent to which a progressive aerobic exercise intervention of moderate to vigorous intensity, following the ACSM guidelines for aerobic exercise, might influence arterial stiffness in women with SLE. Barnes et al. [40] cross-sectionally observed that participants (mainly women) with SLE who reported exercising regularly had lower central arterial stiffness than sedentary SLE subjects and similar to that of healthy individuals. By contrast, Morillas-de-Laguno et al. observed a lack of association of accelerometer-based physical activity with PWV in women with SLE [19]. Our results did not evidence any significant change in arterial stiffness following 12 weeks of aerobic exercise, which might have different explanations. Firstly, it is possible that aerobic exercise does not actually influence arterial stiffness, although this seems unlikely in light of recent evidence suggesting that this type of training reduces arterial stiffness [21,41,42]. As it has been suggested that the volume, duration, and intensity of exercise might play a relevant role in its effects on arterial stiffness [43], it seems plausible that 12 weeks of intervention might not have been enough time to modify the elasticity of the arteries. Therefore, longer interventions are warranted to unravel the effects of exercise on arterial stiffness in SLE. Secondly, the median baseline PWV in the present study (i.e., 6.3 m/s) was far from the levels considered harmful (i.e., 10 m/s) [44]. According to Ashor et al., the effects of exercise might be more pronounced in women with SLE with higher baseline PWV [21] (e.g., with older age or at higher CVD risk).
Barnes et al. [40] observed higher hsCRP and TNF-α levels in sedentary versus physically active patients with SLE. Perandini et al. observed that a single bout (i.e., 30 min) of aerobic exercise (i.e., either at 50% or 70% of VO2peak) did not acutely increase inflammation in women with either active or inactive SLE [22], and that a 12-week aerobic exercise program reduced resting state inflammation. However, the sample size by Perandini et al. was rather low (n = 8 SLE patients), the intervention was not described with complete detail to allow replication, and their results were derived from intra-group (instead of between-group) comparisons, which might be misleading [45]. In line with our results, Timoteo et al. did not observe changes in IL-6 or TNF-α following combined (i.e., flexibility, resistance, and aerobic) exercise in a rather small study (n = 14). The results of the present study, in sum to prior evidence, suggest that aerobic exercise training does not exacerbate inflammatory markers in women with SLE with mild/inactive disease. As exercise has emerged in recent years as a highly beneficial intervention for the cardiovascular prevention of patients with SLE [46], future clinical trials should elucidate whether diverse exercise configurations might reduce inflammation in this population. It must be noted that, in animal models, aerobic exercise has been shown to protect against the cardiometabolic disturbances induced by the chronic use of corticosteroids, such as hyperglycemia, dyslipidemia, liver steatosis, and muscular hypotrophy [47]. In this line, a positive influence of exercise on the effect of corticosteroid use on CVD risk could similarly be expected in humans, although this far exceeds the purposes of the present study and is a matter of further investigation.
Oxidative stress has been implicated in the pathogenesis of CVD in both the general population and in patients with SLE [48]. Myeloperoxidase is a biomarker of oxidative stress that predicts CVD [49]. Although previous research has described an antioxidant effect of exercise [50], our results indicate no significant reduction of oxidative stress in the exercise group compared to the usual care group, although there seemed to be a non-significant trend towards reduction in the exercise group that might deserve further research. As the sample size was not calculated based on this outcome, increasing the number of participants and combining different intensity protocols would provide valuable information regarding the association of stress oxidative and exercise in SLE.
It must be noted that the exercise intensity gradually increased throughout the intervention period, and that participants’ affective responses after each exercise session was better than before the session (Figure S1). Importantly, the exercise program significantly increased cardiorespiratory fitness, as assessed by the time to achieve 85% of estimated HRmax in the Bruce test [37]. As there are no SLE-specific validated formulas to estimate VO2max, it is difficult to assess the effects of the program on VO2max. However, as a reference, if we used the formula for estimating VO2max in a healthy adult population described by Bruce et al. [37], the 2.26 minutes increase in the exercise group would translate into approximately 7.6 mL/kg × min-1 (95% CI 3.3 to 11.9; p = 0.001), which means >2 metabolic equivalents (METs). This increase in fitness is clinically relevant since a 1-MET increase is associated with a 13% to 15% reduction in CV and all-cause mortality [51], and with 10% to 30% lower adverse cardiovascular event rates [25]. Moreover, higher cardiorespiratory fitness has been shown to be related to a lower CV risk in patients with rheumatoid arthritis [52] and with lower age-related arterial stiffness in SLE [20], indicating that future studies should address the impact of long-term changes in cardiorespiratory fitness on CV health in SLE and other rheumatic populations.
This study has limitations. First, the sample size was relatively small and future studies with larger samples are needed. Second, we undertook a non-randomized design, which limits the confidence about baseline groups comparability and, despite statistical adjustment, residual confounding cannot be discarded. Future studies could overcome this limitation by performing a more pragmatic trial with home-based exercise and periodic visits to the clinic for outcome assessment. Third, only women with mild/inactive disease were included. Consequently, the results are not generalizable to men or even women with medium-high disease activity. Finally, despite the importance of comparative studies to better understand the early onset of atherosclerosis in rheumatic diseases [53], the present study failed to gather data from healthy individuals. Therefore, future studies should assess the comparative effectiveness of exercise in healthy people and patients with SLE. The study also has strengths that need to be highlighted. First, the adherence to the exercise intervention was very high, and the effects of the aerobic program on cardiorespiratory fitness underline that the program was effective. The intervention is described following the CERT guidelines [28], so as to enhance transparency and replicability, something that has been traditionally lacking in exercise-based clinical trials [54]. This allows any physician, physiotherapist, or exercise professional to use this exercise program in clinical practice or any other settings, thus increasing the transfer/dissemination of scientific knowledge to society.
In conclusion, the results of this study suggest that 12 weeks of progressive treadmill aerobic exercise following the ACSM guidelines increases cardiorespiratory fitness without exacerbating arterial stiffness, inflammation, or oxidative stress in women with SLE with mild/inactive disease, in comparison to a control group that received recommendations for a healthy lifestyle.
Future clinical trials with larger sample sizes are needed to enhance our understanding on how different durations, types, volumes, and intensities of exercise might affect vascular health and inflammation in this population. In particular, it seems interesting to assess the extent to which resistance training alone or in combination with aerobic training [42] might influence these outcomes in patients with SLE.
Acknowledgments
The authors would like to thank the study participants for their collaboration. We also gratefully acknowledge the members of the Internal Medicine Department (i.e., Juan Jiménez-Alonso, Juan Diego Mediavilla, Nuria Navarrete-Navarrete, Mónica Zamora-Pasadas, Carlos García de los Ríos, Lidia Marín Lara, Elvira Alarcón Manoja and Adriana Carlomagno) for their support during data collection, Carmen Navarro-Mateos for supervising the exercise intervention, Paloma Muñoz de Rueda for her assistance during the blood samples processing, subsequent analyses and results interpretation, and Manuel Delgado-Fernández for his involvement and thoughtful suggestions during the design of the exercise intervention. The results of the present study are part of a Doctoral Thesis conducted in the Biomedicine Doctoral Program of the University of Granada, Spain.
Supplementary Materials
The following are available online at http://www.mdpi.com/2077-0383/7/12/477/s1, Figure S1: Summary of objective (i.e., heart rate) exercise intensity, session rating of perceived exertion (RPE), and (pre- and post-session) positive affect (feeling scale) during each session of the exercise program; Table S1: TREND Statement for improving reporting of non-randomized experiments; Table S2: CERT checklist from the EJERCITALES Study physical exercise program; Table S3: Per-protocol analyses assessing the effects of 12-week progressive aerobic exercise on arterial stiffness, inflammation, oxidative stress, and cardiorespiratory fitness in women with systemic lupus erythematosus (participants in the exercise group were included if attendance ≥90%); Table S4: Sensitivity analyses: Complete case analyses assessing the effects of 12-week progressive aerobic exercise on arterial stiffness, inflammation, oxidative stress, and cardiorespiratory fitness in women with systemic lupus erythematosus (only participants with valid data were included); Table S5: Between-group comparison of the change from baseline at week 12 on traditional cardiovascular disease risk factors.
Author Contributions
Conceptualization, A.S.-M. and J.A.V.-H.; Data curation, A.S.-M., P.M.-d.-L., and J.A.V.-H.; Formal analysis, A.S.-M.; Funding acquisition, A.S.-M. and J.A.V.-H.; Investigation, A.S.-M., P.M.-d.-L., J.M.S.-S., B.G.-C., A.R.-C., C.M.-M., L.M.S.-U., and J.A.V.-H.; Methodology, A.S.-M. and J.A.V.-H.; Project administration, J.A.V.-H.; Resources, P.M.-d.-L., J.M.S.-S., B.G.-C., A.R.-C., C.M.-M., L.M.S.-U., J.L.C.R., and J.A.V.-H.; Supervision, A.S.-M., J.M.S.-S., and J.A.V.-H.; Visualization, P.M.-d.-L.; Writing—Original draft, A.S.-M.; Writing—Review & editing, A.S.-M., P.M.-d.-L., J.M.S.-S., B.G.-C., A.R.-C., C.M.-M., L.M.S.-U., J.L.C.R., and J.A.V.-H.
Funding
This work was supported by Fundación para la Investigación Biosanitaria de Andalucía Oriental (grant number: PI-0525-2016) and the Ilustre Colegio Oficial de Médicos de Granada (Premios de Investigación 2017). BG-C was supported by the Spanish Ministry of Education (FPU15/00002)
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Rees F., Doherty M., Grainge M., Davenport G., Lanyon P., Zhang W. The incidence and prevalence of systemic lupus erythematosus in the uk, 1999–2012. Ann. Rheum. Dis. 2016;75:136–141. doi: 10.1136/annrheumdis-2014-206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yee C.S., Su L., Toescu V., Hickman R., Situnayake D., Bowman S., Farewell V., Gordon C. Birmingham sle cohort: Outcomes of a large inception cohort followed for up to 21 years. Rheumatology. 2015;54:836–843. doi: 10.1093/rheumatology/keu412. [DOI] [PubMed] [Google Scholar]
- 3.Stojan G., Petri M. Epidemiology of systemic lupus erythematosus: An update. Curr. Opin. Rheumatol. 2018;30:144–150. doi: 10.1097/BOR.0000000000000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skaggs B.J., Hahn B.H., McMahon M. Accelerated atherosclerosis in patients with sle—Mechanisms and management. Nat. Rev. Rheumatol. 2012;8:214–223. doi: 10.1038/nrrheum.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannelou M., Mavragani C.P. Cardiovascular disease in systemic lupus erythematosus: A comprehensive update. J. Autoimmun. 2017;82:1–12. doi: 10.1016/j.jaut.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Esdaile J.M., Abrahamowicz M., Grodzicky T., Li Y., Panaritis C., du Berger R., Cote R., Grover S.A., Fortin P.R., Clarke A.E., et al. Traditional framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::AID-ART395>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.Nuttall S.L., Heaton S., Piper M.K., Martin U., Gordon C. Cardiovascular risk in systemic lupus erythematosus—Evidence of increased oxidative stress and dyslipidaemia. Rheumatology. 2003;42:758–762. doi: 10.1093/rheumatology/keg212. [DOI] [PubMed] [Google Scholar]
- 8.Ding F.M., Li M., Yang X., Ye Y., Kang L., Pang H., Wang Q., Xu D., Zeng X., Zhang S. Accelerated age-related arterial stiffness in systemic lupus erythematosus patients. J. Clin. Rheumatol. 2016;22:426–433. doi: 10.1097/RHU.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 9.Kerekes G., Soltesz P., Nurmohamed M.T., Gonzalez-Gay M.A., Turiel M., Vegh E., Shoenfeld Y., McInnes I., Szekanecz Z. Validated methods for assessment of subclinical atherosclerosis in rheumatology. Nat. Rev. Rheumatol. 2012;8:224–234. doi: 10.1038/nrrheum.2012.16. [DOI] [PubMed] [Google Scholar]
- 10.Laurent S., Boutouyrie P., Asmar R., Gautier I., Laloux B., Guize L., Ducimetiere P., Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.HYP.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 11.Bjarnegrad N., Bengtsson C., Brodszki J., Sturfelt G., Nived O., Lanne T. Increased aortic pulse wave velocity in middle aged women with systemic lupus erythematosus. Lupus. 2006;15:644–650. doi: 10.1177/0961203306071402. [DOI] [PubMed] [Google Scholar]
- 12.Zharkova O., Celhar T., Cravens P.D., Satterthwaite A.B., Fairhurst A.M., Davis L.S. Pathways leading to an immunological disease: Systemic lupus erythematosus. Rheumatology. 2017;56:i55–i66. doi: 10.1093/rheumatology/kew427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridker P.M., Hennekens C.H., Buring J.E., Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 14.Kaptoge S., Di Angelantonio E., Pennells L., Wood A.M., White I.R., Gao P., Walker M., Thompson A., Sarwar N., Caslake M., et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N. Engl. J. Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arida A., Protogerou A.D., Kitas G.D., Sfikakis P.P. Systemic inflammatory response and atherosclerosis: The paradigm of chronic inflammatory rheumatic diseases. Int. J. Mol. Sci. 2018;19:1890. doi: 10.3390/ijms19071890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perl A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2013;9:674–686. doi: 10.1038/nrrheum.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon C., Amissah-Arthur M.B., Gayed M., Brown S., Bruce I.N., D’Cruz D., Empson B., Griffiths B., Jayne D., Khamashta M., et al. The british society for rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology. 2018;57:e1–e45. doi: 10.1093/rheumatology/kex286. [DOI] [PubMed] [Google Scholar]
- 18.Garber C.E., Blissmer B., Deschenes M.R., Franklin B.A., Lamonte M.J., Lee I.-M., Nieman D.C., Swain D.P. American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exer. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 19.Morillas-de-Laguno P., Vargas-Hitos J.A., Rosales-Castillo A., Saez-Uran L.M., Montalban-Mendez C., Gavilan-Carrera B., Navarro-Mateos C., Acosta-Manzano P., Delgado-Fernandez M., Sabio J.M., et al. Association of objectively measured physical activity and sedentary time with arterial stiffness in women with systemic lupus erythematosus with mild disease activity. PLoS ONE. 2018;13:e0196111. doi: 10.1371/journal.pone.0196111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montalban-Mendez C., Soriano-Maldonado A., Vargas-Hitos J.A., Saez-Uran L.M., Rosales-Castillo A., Morillas-de-Laguno P., Gavilan-Carrera B., Jimenez-Alonso J. Cardiorespiratory fitness and age-related arterial stiffness in women with systemic lupus erythematosus. Eur. J. Clin. Investig. 2018;48:e12885. doi: 10.1111/eci.12885. [DOI] [PubMed] [Google Scholar]
- 21.Ashor A.W., Lara J., Siervo M., Celis-Morales C., Mathers J.C. Effects of exercise modalities on arterial stiffness and wave reflection: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 2014;9:e110034. doi: 10.1371/journal.pone.0110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perandini L.A., Sales-de-Oliveira D., Mello S., Camara N.O., Benatti F.B., Lima F.R., Borba E., Bonfa E., Roschel H., Sa-Pinto A.L., et al. Inflammatory cytokine kinetics to single bouts of acute moderate and intense aerobic exercise in women with active and inactive systemic lupus erythematosus. Exerc. Immunol. Rev. 2015;21:174–185. [PubMed] [Google Scholar]
- 23.Perandini L.A., Sales-de-Oliveira D., Mello S.B., Camara N.O., Benatti F.B., Lima F.R., Borba E., Bonfa E., Sa-Pinto A.L., Roschel H., et al. Exercise training can attenuate the inflammatory milieu in women with systemic lupus erythematosus. J. Appl. Physiol. 2014;117:639–647. doi: 10.1152/japplphysiol.00486.2014. [DOI] [PubMed] [Google Scholar]
- 24.Timoteo R.P., Silva A.F., Micheli D.C., Candido Murta E.F., Freire M., Teodoro R.B., Lima F.M., Martins Tavares Murta B., Bertoncello D. Increased flexibility, pain reduction and unaltered levels of il-10 and cd11b + lymphocytes in patients with systemic lupus erythematosus were associated with kinesiotherapy. Lupus. 2018;27:1159–1168. doi: 10.1177/0961203318768880. [DOI] [PubMed] [Google Scholar]
- 25.Ross R., Blair S.N., Arena R., Church T.S., Despres J.P., Franklin B.A., Haskell W.L., Kaminsky L.A., Levine B.D., Lavie C.J., et al. Importance of assessing cardiorespiratory fitness in clinical practice: A case for fitness as a clinical vital sign: A scientific statement from the american heart association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 26.Hochberg M.C. Updating the american college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 27.Des Jarlais D.C., Lyles C., Crepaz N. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The trend statement. Am. J. Public Health. 2004;94:361–366. doi: 10.2105/AJPH.94.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slade S.C., Dionne C.E., Underwood M., Buchbinder R. Consensus on exercise reporting template (cert): Explanation and elaboration statement. Br. J. Sports Med. 2016;50:1428–1437. doi: 10.1136/bjsports-2016-096651. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka H., Monahan K.D., Seals D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001;37:153–156. doi: 10.1016/S0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 30.Haddad M., Stylianides G., Djaoui L., Dellal A., Chamari K. Session-rpe method for training load monitoring: Validity, ecological usefulness, and influencing factors. Front. Neurosci. 2017;11:612. doi: 10.3389/fnins.2017.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardy C.J., Rejeski W.J. Not what, but how one feels: The measurement of affect during exercise. J. Sport Exer. Psychol. 1989;11:304–317. doi: 10.1123/jsep.11.3.304. [DOI] [Google Scholar]
- 32.Grillo A., Parati G., Rovina M., Moretti F., Salvi L., Gao L., Baldi C., Sorropago G., Faini A., Millasseau S.C., et al. Short-term repeatability of noninvasive aortic pulse wave velocity assessment: Comparison between methods and devices. Am. J. Hypertens. 2017;31:80–88. doi: 10.1093/ajh/hpx140. [DOI] [PubMed] [Google Scholar]
- 33.Weber T., Wassertheurer S., Rammer M., Maurer E., Hametner B., Mayer C.C., Kropf J., Eber B. Validation of a brachial cuff-based method for estimating central systolic blood pressure. Hypertension. 2011;58:825–832. doi: 10.1161/HYPERTENSIONAHA.111.176313. [DOI] [PubMed] [Google Scholar]
- 34.Weiss W., Gohlisch C., Harsch-Gladisch C., Tolle M., Zidek W., van der Giet M. Oscillometric estimation of central blood pressure: Validation of the mobil-o-graph in comparison with the sphygmocor device. Blood Press. Monit. 2012;17:128–131. doi: 10.1097/MBP.0b013e328353ff63. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien E., Pickering T., Asmar R., Myers M., Parati G., Staessen J., Mengden T., Imai Y., Waeber B., Palatini P., et al. Working group on blood pressure monitoring of the european society of hypertension international protocol for validation of blood pressure measuring devices in adults. Blood Press. Monit. 2002;7:3–17. doi: 10.1097/00126097-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Wei W., Tolle M., Zidek W., van der Giet M. Validation of the mobil-o-graph: 24 h-blood pressure measurement device. Blood Press. Monit. 2010;15:225–228. doi: 10.1097/MBP.0b013e328338892f. [DOI] [PubMed] [Google Scholar]
- 37.Bruce R.A., Kusumi F., Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am. Heart J. 1973;85:546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- 38.Craig C.L., Marshall A.L., Sjöström M., Bauman A.E., Booth M.L., Ainsworth B.E., Pratt M., Ekelund U., Yngve A., Sallis J.F., et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exer. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 39.Vargas-Hitos J.A., Soriano-Maldonado A., Martinez-Bordonado J., Sanchez-Berna I., Fernandez-Berges D., Sabio J.M. Association of resting heart rate with arterial stiffness and low-grade inflammation in women with systemic lupus erythematosus. Angiology. 2018;69:672–676. doi: 10.1177/0003319717746525. [DOI] [PubMed] [Google Scholar]
- 40.Barnes J.N., Nualnim N., Sugawara J., Sommerlad S.M., Renzi C.P., Tanaka H. Arterial stiffening, wave reflection, and inflammation in habitually exercising systemic lupus erythematosus patients. Am. J. Hypertens. 2011;24:1194–1200. doi: 10.1038/ajh.2011.143. [DOI] [PubMed] [Google Scholar]
- 41.Sardeli A.V., Gaspari A.F., Chacon-Mikahil M.P. Acute, short-, and long-term effects of different types of exercise in central arterial stiffness: A systematic review and meta-analysis. J. Sports Med. Phys. Fitness. 2018;58:923–932. doi: 10.23736/S0022-4707.17.07486-2. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y., Qi L., Xu L., Sun X., Liu W., Zhou S., van de Vosse F., Greenwald S.E. Effects of exercise modalities on central hemodynamics, arterial stiffness and cardiac function in cardiovascular disease: Systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 2018;13:e0200829. doi: 10.1371/journal.pone.0200829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shibata S., Fujimoto N., Hastings J.L., Carrick-Ranson G., Bhella P.S., Hearon C.M., Jr., Levine B.D. The effect of lifelong exercise frequency on arterial stiffness. J. Physiol. 2018;596:2783–2795. doi: 10.1113/JP275301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Townsend R.R., Wilkinson I.B., Schiffrin E.L., Avolio A.P., Chirinos J.A., Cockcroft J.R., Heffernan K.S., Lakatta E.G., McEniery C.M., Mitchell G.F., et al. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the american heart association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bland J.M., Altman D.G. Comparisons against baseline within randomised groups are often used and can be highly misleading. Trials. 2011;12:264. doi: 10.1186/1745-6215-12-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benatti F.B., Pedersen B.K. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat. Rev. Rheumatol. 2015;11:86–97. doi: 10.1038/nrrheum.2014.193. [DOI] [PubMed] [Google Scholar]
- 47.Pinheiro C.H., Sousa Filho W.M., Oliveira Neto J., Marinho Mde J., Motta Neto R., Smith M.M., Silva C.A. Exercise prevents cardiometabolic alterations induced by chronic use of glucocorticoids. Arq. Bras. Cardiol. 2009;93:400–408. doi: 10.1590/S0066-782X2009001000014. [DOI] [PubMed] [Google Scholar]
- 48.Lopez-Pedrera C., Barbarroja N., Jimenez-Gomez Y., Collantes-Estevez E., Aguirre M.A., Cuadrado M.J. Oxidative stress in the pathogenesis of atherothrombosis associated with anti-phospholipid syndrome and systemic lupus erythematosus: New therapeutic approaches. Rheumatology. 2016;55:2096–2108. doi: 10.1093/rheumatology/kew054. [DOI] [PubMed] [Google Scholar]
- 49.Ho E., Karimi Galougahi K., Liu C.C., Bhindi R., Figtree G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013;1:483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sallam N., Laher I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxid. Med. Cell Longev. 2016;2016:7239639. doi: 10.1155/2016/7239639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kodama S., Saito K., Tanaka S., Maki M., Yachi Y., Asumi M., Sugawara A., Totsuka K., Shimano H., Ohashi Y., et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 52.Metsios G.S., Koutedakis Y., Veldhuijzen van Zanten J.J., Stavropoulos-Kalinoglou A., Vitalis P., Duda J.L., Ntoumanis N., Rouse P.C., Kitas G.D. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology. 2015;54:2215–2220. doi: 10.1093/rheumatology/kev035. [DOI] [PubMed] [Google Scholar]
- 53.Gasparyan A.Y., Stavropoulos-Kalinoglou A., Mikhailidis D.P., Toms T.E., Douglas K.M., Kitas G.D. The rationale for comparative studies of accelerated atherosclerosis in rheumatic diseases. Curr. Vasc. Pharmacol. 2010;8:437–449. doi: 10.2174/157016110791330852. [DOI] [PubMed] [Google Scholar]
- 54.Slade S.C., Keating J.L. Exercise prescription: A case for standardised reporting. Br. J. Sports Med. 2012;46:1110–1113. doi: 10.1136/bjsports-2011-090290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.