Abstract
Background
Hemophilia is an inherited genetic disease characterized by the inability to coagulate blood after injury. The rationale of the current study was to evaluate serum proteins S and C and correlate to kidney function test in hemophilic patients for early diagnosis of abnormality in renal function.
Subjects and Methods
This study was conducted on 80 males subjects divided into four groups. Group I: Control: Healthy subjects. Group II: Renal dysfunction (serum Creatinine >2mg/dl): Group III: Hemophilic patients. Group IV: Hemophilic patients with renal disorder. Serum urea, creatinine, sodium, potassium, protein C and protein S level were determined.
Resuts
Protein C and S levels showed a significant decrease in hemophilic/and with renal dysfunction (P < 0.001, p<0.001). The level of plasma protein C and S levels were positively correlated with increased urinary albumin (P < 0.01). Urinary albumin was increased about 15 folds in hemophilic patients with renal dysfunction and nephrotic patients as compared with the control group. The cut-off value in 90% patients at the hemophilic patients with renal dysfunction 70%. Positive correlations were observed between urinary albumin (r=0.66), and creatinine (r=0.73).
Conclusion
These biomarkers showed good predictive values with regard to ROC-AUC (0.41 and 0.75 for Proteins C and S, respectively).
Keywords: Hemophilia, renal dysfunction, protein C, protein S
Background
Hemophilia, is an inherited genetic disease. In this condition, the ability of one's body to make blood clots is impaired. Clotting of blood is needed to stop bleeding from injuries. As a consequence, the bleeding lasts long after an injury and even the risk of bleeding inside joints or the brain increases. People who are born with hemophilia either have little or no clotting factors. Hemophilia A and hemophilia B are the two main types. Type A occurs due to the defect in the synthesis of factor VIII, while type B is due to defect in factor IX synthesis. Both types are chromosome X linked and affect male live births. The alterations in the intrinsic pathways of blood coagulation are determined1–6.
Protein C, is blood coagulation factor IV, synthesized as inactive zymogen and activated by partial proteolysis. It plays a vital role in regulation of anticoagulation7. It also plays a role in cell death, maintaining the permeability of blood vessel walls and inflammation. This zymogenic form is a vitamin K dependent glycoprotein. The activated protein C (APC) performs these functions mainly via proteolytic inactivation of the proteins, like Factor Va and Factor VIIIa8. Protein is structurally similar to other vitamin K dependent glycoproteins that affect blood clotting, like, prothrombin, Factor VII, Factor IX and Factor X9. This protein C zymogen is activated in coagulation-thrombin, and is greatly enhanced by the presence endothelial protein C receptors. It is encoded by PROC gene10, if protein C is deficient or there is any resistance to APC, it may result in an increased risk of forming dangerous blood clots, a condition called arterial embolism. Protein S is glycoprotein, synthesized in the endothelium. This protein is vitamin K dependent. Protein S plays a role crucial in the anti-coagulation pathway as a co-factor to protein C, in rendering Factors Va and VIIIa inactive5. In humans, protein S is encoded by the PROS1 gene6, 7. The mutations in the gene PROS1 lead to deficiency of protein S level, which increases the risk of arterial embolism8,9. As mentioned earlier, among the anti-coagulation components of the human body, Protein C plays a major role. The proteins that are made inactive by APC, Factor Va and Factor VIIIa, (via proteolysis of peptide bonds in proteins Factors V and VIII respectively), act as cofactors for formation of thrombin which is required in the process of blood clotting and they form the prothrombinase complex together10. Protein S acts a co-factor in the inactivation of protein factors Va and VIIIa. Prothrombin and Factor Xa bind to protein Factor Va, and enhances the rate of thrombin production. Thus, by inactivating the protein factor Va, thrombin production is halted. Moreover, protein Factor VIII, acts as a cofactor for producing activated Factor X, which activates prothrombin into thrombin11. Protein Factor VIIIa further greatly enhances the activation of Factor X. Factor VIII is known as anti-hemophilic factor, because of the fact that it plays a major role in blood clotting. Hence, the deficiency of this Factor VIII causes hemophilia A12.
The current study aimed to evaluate serum proteins S and C and correlated them to kidney function tests in hemophilic patients for early diagnosis of abnormality in renal function to avoid its complications.
Subjects and methods
Informed consent was taken from all individuals included in this study. The study was done according to the ethical committee of King Abdulaziz University. This study was conducted on 80 male subjects admitted at the Hematology Department, King Abdulaziz University hospital. All individuals were subjected to full clinical examination (diabetes, hypertension, cardiovascular disorder. They were classified according to clinical examination to the following groups:
Group I: Control: Included 20 healthy subjects, no evidence for any symptoms of clinical disorder.
Group II: Renal diseases: Included 20 patients confirmed to have renal disorder by clinical examination and elevated serum creatinine and micro-albuminuria.
Group III: Hemophilic patients: Included 20 hemophilic patients : This group were confirmed to have hemophilic by clinical examination and hematological parameters.
Group IV: Hemophilic patients with renal disorder: Included 20 hemophilic patients with renal function disorder. A five ml blood sample was collected from each subjects. Serum was obtained by centrifugation at 4000 r.p.m for 10 minutes. Serum was stored at −80 °C until used.
The exclusion criteria include patients with diabetes, renal disease, cardiovascular disease, neoplastic, or hepatic damage and were matched for age and body mass index with no history of liver disease.
Serum samples were subjected to the following examination. Serum urea, creatinine, sodium, potassium, protein C and protein S levels, urinary albumin were determined by available kits obtained from Bio diagnostic company.
Statistical analysis
Results were expressed as mean ± SD. Sensitivity was calculated as: (patients with positive markers)/ (all patients); specificity was calculated as: (patients with negative markers)/(all patients). Results were compared using Student's t test for continuous variables and chi-square analysis. A p value ≤0.05 was considered significant. One-way ANOVA was additionally used as a confirmatory test.
Results
Table (1) showed that serum urea and creatinine levels were significantly elevated in microalbuminuria with nephrotic syndrome compared with control groups (p <0.001) for both. Serum urea and creatinine were statistically significantly elevated in hemophilic patients with renal dysfunction compared with hemophilic and renal dysfunction groups (p <0.001) for both. ANOVA analysis showed non-significant changes which were observed between nephrotic syndrome and nephrotic with hemophilic. Non-significant changes in the levels of sodium and potassium in all groups compared with the controls. Plasma protein C and S levels showed a significant decrease in hemophilic/and with renal dysfunction (P < 0.001, p <0.001). The level of plasma protein C and S levels were positively correlated with increase in urinary albumin (P < 0.01). Urinary albumin was increased about 15 folds in hemophilic patients with renal dysfunction and nephrotic and compared with control group.
Table (1).
The levels of serum urea, creatinine sodium, potassium, in all studied groups (mean ±SD)
| Patients Groups | Healthy Control n=20 |
Hemophilic n=20 |
Renal dysfunction (microalbuminuria) n=20 |
Hemophilic +Renal dysfunction n=20 |
| Parameters | ||||
| Urea (mg/dl) Mean+SD P1 value P2 value |
28.3± 2 --- --- |
31±3.4 <0.001 --- |
82+ 6 <0.001 <0.001 |
95+ 7.2 N.S <0.05 |
| Creatinine (mg/dl) Mean+SD P1 value P2 value |
0.9 ±0.02 --- --- |
1.0 +0.06 N.S --- |
2.1+ 0.08 <0.001 <0.001 |
1.9+ 0.08 <0.001 N.S |
| Sodium (meq/l) Mean+SD P1 value P2 value |
133 ±6.5 --- --- |
134+6.7 N.S --- |
136+ 8.8 N.S --- |
135+ 9.9 < N.S --- |
| Potassium (meq/l) Mean+SD P1 value |
4.5± 0.02 --- --- |
4.3±0.12 N.S --- |
4.5+ 0.03 N.S --- |
4.6+ 0.04 < N.S --- |
n = number of cases p1 value, hemophilic, renal dysfunction and hemophilic with renal dysfunction versus control. p2 value, hemophilic with renal dysfunction versus renal dysfunction.
The cut-off value in 90% patients at the hemophilic with renal dysfunction 70%. Positive correlations were observed between urinary albumin (r=0.66), and creatinine (r=0.73) while were not correlated with other laboratory markers such as plasma urea and sodium and potassium.
Receiver operating curve (ROC) analysis was performed to define the diagnostic profile of urine level markers among subjects with diabetes. The serum level of protein C showed an area under curve (AUC) of 0.92 with (sensitivity 91.0 % and specificity, 88%). Also, the protein S supported the diagnostic profile, showing an AUC of 0.75 with (sensitivity, 91.0%; specificity, 90.9%).
Discussion
Protein S aids APC in the proteolysis of Factor Va, by making three cleavages (at Arg 306, Arg 506, Arg 679). The first two cleavages make Factor Va's attraction to Factor Xa to diminish and after the cleavage at the first site, functioning of Factor V is hampered. Furthermore, Protein S binds to Factor Xa, and inhibits it from diminishing the inactivation of Factor Va, that was caused by APC13,14. Protein S also assists APC to inactivate Factor VIIIa via proteolysis (at sites Arg 336 and Arg 562), thus disabling it15,16.
Chronic renal dysfunction is considered a health problem worldwide and is a risk for increased morbidity and mortality. Early sensitive assessment of renal function is serving as a screening protocol for monitoring disease development and prognosis. Identification of biomarkers for evaluating kidney function contributes to the therapeutic protocol and avoids its complications17.
Nephropathy is the most common complication of many diseases include hemophilia and can lead to renal failure. Here, we tried to find a sensitive biomarker for diagnosis of early phase of nephropathy in hemophilic patients. Micro-albumunuria as a result of impairment of the filtration of the glomerular basement membrane is used as predictive of renal impairment. Patients were categorized into 4 groups (normal, hemophilic, nephropathy and hemophilic with nephritic syndrome). Early diagnosis of nephropathy in hemophilic patients is necessary to initiate appropriate treatment. Elevation of serum urea, creatinine and urinary albumin are the most markers that physicians depend on in diagnosis. In the present study, serum creatinine and urea were positively correlated and good index for renal impairment.
Data obtained showed that, Protein C and S levels in plasma were significantly increased in patients with nephropathy and hemophilic with nephritic syndrome compared with control or hemophilic. It was expected that this increment was probably due to the tubular phase before glomerular manifestation. This results in accordance of previous study, which stated that, there is a significant elevation in protein C and S in subjects with hemophilic and hemophilic with renal disorder compared with control subjects17–21. This suggests that the proteins C and S levels of plasma were related to tubular impairment and can be an earlier measurable marker of renal involvement before onset of albuminuria. This finding indicated that the proteins C and S levels could be an index reflecting renal tubular epithelial cells. However, plasma endogenous creatinine depends on creatinine synthesis, metabolism to creatinine and tubular clearnce. Moreover several tubular proteins are excreted even before the detection of micro-albuminuria and elevation of plasma creatinine22,23. Therefore, other biomarkers for evaluation of renal function have been found and one of them was protein C. It was suggested that proteins S and C may be one of the additional tubular markers which represent the kidney state of hemophilic patients.
It was found that, renal function was inversely associated with urine albumin excretion, which is a good predictor of decline in kidney function than albuminuria24,25. This is in agreement with the present study that showed a positive correlation between nephrotic syndrome with levels of proteins C and S. Since its excretion in urine is associated with nephritic syndrome while it is a good predictor which may detect the eventual need for renal function monitoring.
Proteins C and S were decreased in 95% at the diabetic microalbuminuric stage and 87% at the nephrotic syndrome, which indicated that it might be a useful biomarker for detecting early injury in hemophilic patients.
Conclusion
These biomarkers showed good predictive values with regard to ROC-AUC (0.41 and 0.75 for Proteins C and S respectively).
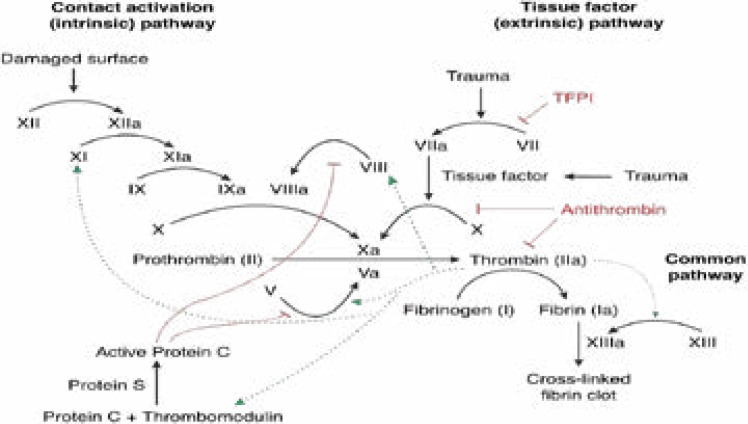
Fig.
Blood coagulation and Protein C anticoagulation pathway.
Table (2).
The levels of serum protein C and S and urinary albumin in all studied groups (mean ±SD)
| Patients Groups | Healthy control n=20 |
Hemophilic n=20 |
Renal dysfunction (microalbuminuria) n=20 |
Hemophilic +Renal dysfunction n=20 |
| Parameters | ||||
| Protein C (ug/l) Mean+SD P1 value P2 value |
42± 3.2 --- --- |
28 ±5.3 N.S --- |
33.8+ 3.8 <0.001 <0.001 |
20.4+ 3.2 <0.001 N.S |
| Protein S (U/l) Mean+SD P1 value P2 value |
56.6± 8 --- --- |
33.2±6 N.S --- |
29.7+ 5.8 <0.001 <0.001 |
19.3+ 2.2 <0.01 <0.001 |
| Urinary albumin (ug/l) Mean+SD P1 value P2 value |
Not detected --- --- |
3.9+ 0.07 --- --- |
29+ 0.07 ---- ------ |
43+ 5.2 <0.001 <0.01 |
n = number of cases p1 value, hemophilic, renal dysfunction and hemophilic with renal dysfunction versus control. p2 value, hemophilic with renal dysfunction versus renal dysfunction.
Table 3.
Receiver operating curve (ROC) analysis of investigated urine parameters as a test for diagnosis of diabetic nephropathy
| Variable | protein C | protein S | Creatinine |
| AUC | 0.9 | 0.73 | 0.92 |
| Sensitivity (%) | 89 % | 92.3% | 95.5% |
| Specificity (%) | 90.4% | 89.9% | 97% |
| AUC; area under curve |
Acknowledgment
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant No. (G-432-247-38). The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Competing interest
The authors certify that there is no actual or potential conflict of interest in relation to this article.
References
- 1.Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ. CKD: common, harmful, and treatable — World Kidney Day 2007. Am J Kidney Dis. 2007;49:175–179. doi: 10.1053/j.ajkd.2006.12.013. PubMed. [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. JASN. 2005;16:180–188. doi: 10.1681/ASN.2004070539. PubMed. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. PMC free article. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Zeeuw D, Hillege HL, de Jong PE. The kidney, a cardiovascular risk marker, and a new target for therapy. Kidney Int Suppl. 2005;98:S25–S29. doi: 10.1111/j.1523-1755.2005.09805.x. PubMed. [DOI] [PubMed] [Google Scholar]
- 5.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. PubMed. [PubMed] [Google Scholar]
- 6.Hemophilia. The Merck Manuals: The Merck Manual for Healthcare Professionals. http://www.merckmanuals.com/professional/sec11/ch136/ch136c.html#sec11-ch136-ch136d-524.
- 7.Mammen EF, Thomas WR, Seegers WH. “Activation of purified prothrombin to autoprothrombin I or autoprothrombin II (platelet cofactor II or autoprothrombin II-A)”. Thrombosis Et Diathesis Haemorrhagica. 1960 Dec;5:218–249. [PubMed] [Google Scholar]
- 8.Nicolaes GA, Dahlback B. “Congenital and acquired activated protein C resistance”. Seminars in Vascular Medicine. 2003 Feb;3(1):33–46. doi: 10.1055/s-2003-38331. [DOI] [PubMed] [Google Scholar]
- 9.Hall JA, Morton I. Concise dictionary of pharmacological agents: properties and synonyms. Kluwer Academic; [Google Scholar]
- 10.Castoldi E, Hackeng TM. “Regulation of coagulation by protein S”. Curr. Opin. Hematol. 1999;15(5):529–536. doi: 10.1097/MOH.0b013e328309ec97. (September 2008) [DOI] [PubMed] [Google Scholar]
- 11.Lundwall A, Dackowski W, Cohen E, Shaffer M, Mahr A, Dahlbäck B, Stenflo J, Wydro R. “Isolation and sequence of the cDNA for human protein S, a regulator of blood coagulation”. Proc Natl Acad Sci USA. 1986 Sep;83(18):6716–6720. doi: 10.1073/pnas.83.18.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long GL, Marshall A, Gardner JC, Naylor SL. “Genes for human vitamin K-dependent plasma proteins C and S are located on chromosomes 2 and 3, respectively”. Somat Cell Mol Genet. 1988 Jan;14(1):93–98. doi: 10.1007/BF01535052. [DOI] [PubMed] [Google Scholar]
- 13.Beauchamp NJ, Dykes AC, Parikh N, Campbell Tait R, Daly ME. “The prevalence of, and molecular defects underlying, inherited protein S deficiency in the general population”. Br J Haematol. 2004 Jun;125(5):647–654. doi: 10.1111/j.1365-2141.2004.04961.x. [DOI] [PubMed] [Google Scholar]
- 14.García de Frutos P, Fuentes-Prior P, Hurtado B, Sala N. “Molecular basis of protein S deficiency”. Thromb Haemost. 2007 Sep;98(3):543–556. [PubMed] [Google Scholar]
- 15.Mosnier LO, Griffin JH. “Protein C anticoagulant activity in relation to anti-inflammatory and anti-apoptotic activities”. Frontiers in Bioscience. 2006;11:2381–2399. doi: 10.2741/1977. [DOI] [PubMed] [Google Scholar]
- 16.Goldenberg NA, Manco-Johnson MJ. “Protein C deficiency”. Haemophilia. 2008 Nov;14(6):1214–1221. doi: 10.1111/j.1365-2516.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- 17.Mosnier LO, Griffin JH. “Protein C anticoagulant activity in relation to anti-inflammatory and anti-apoptotic activities”. Frontiers in Bioscience. 2006;11:2381–2399. doi: 10.2741/1977. [DOI] [PubMed] [Google Scholar]
- 18.Rouse RL, Zhang J, Stewart SR, Rosenzweig BA, Espandiari P, Sadrieh NK. Comparative profile of commercially available urinary biomarkers in preclinical drug-induced kidney injury and recovery in rats. Kidney Int. 2011;79:1186–1197. doi: 10.1038/ki.2010.463. PubMed. [DOI] [PubMed] [Google Scholar]
- 19.Aaltonen P, Holthöfer H. The nephrin-based slit diaphragm: new insight into the signaling platform identifies targets for therapy. Nephrol Dial Transplant. 2007;22:3408–3410. doi: 10.1093/ndt/gfm403. [DOI] [PubMed] [Google Scholar]
- 20.Kestilä M, Lenkkeri U, Männikkö M, et al. Positionally cloned gene for a novel glomerular protein-nephrin-is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. PubMed. [DOI] [PubMed] [Google Scholar]
- 21.Ruotsalainen V, Ljungberg P, Wartiovaara J, et al. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci U.S.A. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beltcheva O, Martin P, Lenkkeri U, Tryggvason K. Mutation spectrum in the nephrin gene (NPHS1) in congenital nephritic syndrome. Hum Mutat. 2001;17:368–373. doi: 10.1002/humu.1111. PubMed. [DOI] [PubMed] [Google Scholar]
- 22.Kotajima N, Kimura T, Kanda T, et al. Type IV collagen as an early marker for diabetic nephropathy in non-insulindependent diabetes mellitus. J Diabetes Complications. 2000;14:13–17. doi: 10.1016/s1056-8727(00)00064-7. [DOI] [PubMed] [Google Scholar]
- 23.Iijima T, Suzuki S, Sekizuka K, et al. Follow-up study on urinary type IV collagen in patients with early stage diabetic nephropathy. J Clin Lab Anal. 1998;12:378–382. doi: 10.1002/(SICI)1098-2825(1998)12:6<378::AID-JCLA8>3.0.CO;2-J. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinokio Y, Suzuki S, Hirai M, et al. Urinary excretion of 8-oxo-7, 8-dihydro-2 deoxyguanosine as a predictor of the development of diabetic nephropathy. Diabetologia. 2002;45:877–882. doi: 10.1007/s00125-002-0831-8. PubMed. [DOI] [PubMed] [Google Scholar]
- 25.Broedbaek K, Weimann A, Stovgaard ES, et al. Urinary 8-oxo-7,8-dihydro-2 deoxyguanosine as a biomarker in type 2 diabetes. Free Radic Biol Med. 2011;51:1473–1479. doi: 10.1016/j.freeradbiomed.2011.07.007. [DOI] [PubMed] [Google Scholar]