Abstract
Introduction
Autosomal Dominant Polycystic Kidney Disease (ADPKD) is presumably rare in Africa. Knowledge about the disease in Nigeria is limited as demonstrated by scanty articles on the subject.
Objectives
To determine the pattern of clinical presentation and outcome of ADPKD among ADPKD patients.
Method
ADPKD subjects were prospectively studied between January 1996 and December 2010. Their demographics, clinical and investigation parameters were documented. Dependency on dialysis, renal transplant and death were the final outcomes.
Results
Forty one patients (M:F=1.3:1) with mean age of 48.6±4.6 years were studied. ADPKD was diagnosed at 2.73 cases per annum. Family history of ADPKD and hypertension were present in 56.1% and 82.9% respectively. Their mean systolic and diastolic blood pressures were 166.9 ±23.6 and 104 ±21.2 respectively.
Nocturia (78.0%) and loin pain (68.3%) were the most common presenting symptoms. Liver cysts (31.7%) and aortic regurgitation (22.0%) were the predominant extra-renal manifestations.
Twenty three (56.1%) received haemodialysis; no renal transplantation. Death rate was 51.2%. Presence of uraemia and intra-cerebral aneurysm contributed significantly to mortality.
Conclusion
ADPKD may not be so rare in Nigeria. Awareness campaign to change attitude of family members to screening and further studies using newer criteria for diagnosis of ADPKD should be conducted.
Keywords: Clinical presentation, autosomal dominant polycystic, kidney disease, Nigeria
Introduction
Autosomal Dominant Polycystic Kidney Disease (ADPKD) is the commonest form of genetically inherited kidney diseases with a prevalence rate of 1:400 to 1:1,000.1 It is generally assumed to be rare among Blacks in Africa.2,3 It ranks as the third leading cause of End Stage Renal Disease (ESRD) and accounts for 5–13.4% of patients undergoing haemodialysis in the United States and Europe. 4–6
In most developed economies, the diagnosis of ADPKD is now made as early as in the first decade of life due to advancement in gene technology contrary to resource-poor settings such as sub-Saharan Africa where the diagnosis of ADPKD is still restricted to clinical judgement and renal ultrasound. This has hampered knowledge about the disease in our setting as demonstrated by the few studies and case reports that have been published on ADPKD in Nigeria to date.7–9
We therefore set out to determine the pattern of clinical presentation and outcome of ADPKD among patients who presented to our renal unit.
Method
Consecutive patients that satisfied the inclusion criteria for ADPKD were prospectively followed up over a period of 15 years (January 1996 – December 2010) at the renal unit, Obafemi Awolowo University Teaching Hospital, Ile-Ife after due approval from the Research and Ethics Committee.
Clinical and laboratory evaluations (including medical history, physical examination and cardiac examination using echocardiogram (Vivid 7, General Electronics, USA) were conducted. The diagnosis of ADPKD was established using the Ravine criteria10, presence of hypertension, multiple liver cysts and family history of cystic kidney disease. Patients with renal cysts who did not fulfill the Ravine criteria, patients aged <18 years, those with renal malignancy, exophytic cysts, calcifications, tuberous sclerosis, or acquired simple cysts were excluded.
Pre-dialysis blood samples were obtained and assessed for full blood count and serum chemistry. Urinalysis and urine culture were carried out on samples collected under standard techniques. The glomerular filtration rate (eGFR) was estimated using the Cockroft and Gault formula.11
Ultrasound scan of the abdomen was conducted to search for presence of solid organ cysts. Cranial Computerised Tomography Scan (CAT scan) or Magnetic Resonance Imaging (MRI) were employed where necessary to rule out presence of intra-cranial aneurysm (ICA). Autopsy was carried out on all subjects who died during the study after due consent from family members. Dependency on dialysis, renal transplant and death were the final outcomes in this study.
Data was analysed using SPSS statistical software version 17. Continuous variables were expressed as mean±SD, median and range. Chi square testing was carried out to determine significance (p value < 0.05 at 95% Confidence Interval).
Results
A total of 41 patients fulfilled the diagnostic criteria. They included 23 (56.1%) males and 18 (43.9%) females (M:F=1.3:1). Their age range at the time of diagnosis was 18–80 years with mean age of 48.6±4.6 years. The median (range) follow up period for all patients was 24 (0.25–84) months. The peak age at presentation of ADPKD was between 31–40 years (table 1).
Table 1.
Parameters of ADPKD subjects in OAUTH Ile-Ife (1996–2010)
| PARAMETERS | MALE | FEMALE | ALL SUBJECTS |
| Baseline parameters | mean ±SD | mean ±SD | mean ±SD |
| Age at presentation (years) | 43.4 (±15.7) | 46.3 (±14.3) | 48.6±4.6years |
| SBP (mmHg) | 162.8 (±26.7) | 172.2 (±18.3) | 166.9 ±23.6 |
| DBP (mmHg) | 104.7 (±23.6) | 104.4 (±18.5) | 104 ±21.2 |
| Sodium (mmol/L) | 133.2 (±5.9) | 135.5 (±3.8) | 134.2±5.1 |
| Potassium (mmol/L) | 4.8 (±1.1) | 4.4 (±0.9) | 4.5±1.0 |
| Bicarbonate (mmol/L) | 20.5 (±2.3) | 20.9 (±2.5) | 20.6±2.3 |
| Urea (mmol/L) | 18.8 (±9.9) | 20.5 (±14.0) | 19.5±11.7 |
| Creatinine (umol/L) | 707 (118–2413) | 484 (76–3722) | 614 (76–3722) |
| eGFR (ml/min) | 33.2 (±22.2) | 35.5 (±25.8) | 34.2±23.6 |
| Haematocrit (%) | 27.0 (±4.8) | 25.9 (±6.1) | 26.5±5.4 |
| TWCC | 5,472.6 (±2344.9) | 5,789.3 (±2412.5) | 5,611.6±2,350 |
| Platelet | 180,671.7 (±30,233) | 214,777.8 (±139,605.9) | 195,645.1±95286.6 |
| No of HD sessions | 5.4 (±5.0) | 6.0 (±5.2) | 5.6±5.0 |
| Duration of survival (mo) | 24.0 (±24.0) | 23.2 (±20.9) | 23.6±22.4 |
| Age at death (years) | 46.6±15.8 | 45.9±14.1 | 46.3±14.7 |
| Age of survivors (years) | 44.4±16.7 | 50.3±14.8 | 47.1±15.8 |
| Age range, years (N=41) | N (%) | N (%) | N (%) |
| 15–20 | 3 (100%) | 0 | 3 (7.3%) |
| 21–30 | 1 (25%) | 3 (75%) | 4 (9.8%) |
| 31–40 | 7 (63.6%) | 4 (39.4%) | 11 (26.9%) |
| 41–50 | 4 (44.5%) | 5 (55.5%) | 9 (21.9%) |
| 51–60 | 6 (66.7%) | 3 (33.3%) | 9 (21.9%) |
| 61–70 | 1 (33.3%) | 2 (66.7%) | 3 (7.3%) |
| >70 | 1 (50%) | 1 (50%) | 2 (4.9%) |
|
JNC hypertension stage (N=41) |
|||
| pre-hypertension | 3 (7.3%) | 0 | 3 (7.3%) |
| stage 1 | 4 (9.8%) | 3 (7.3%) | 7 (17.1%) |
| stage 2 | 16 (39.0%) | 15 (36.6%) | 31 (75.6%) |
| Hypertension by age (N=38) | |||
| 15–20 | 3 (7.9%) | 0 | 3 (7.9%) |
| 21–30 | 1(2.6%) | 3 (7.9%) | 4 (10.5%) |
| 31–40 | 6 (14.8%) | 4 (10.5%) | 10 (26.3%) |
| 41–50 | 3 (7.9%) | 5 (13.2%) | 8 (21.1%) |
| 51–60 | 5 (13.2%) | 3 (7.9%) | 8 (21.1%) |
| 61–70 | 1 (2.6%) | 2 (5.3%) | 3 (7.9%) |
| >71 | 1 (2.6%) | 1 (2.6%) | 2 (5.3%) |
| Haematocrit % (N=41) | frequency | ||
| <20 | 2 (4.9%) | 5 (12.2%) | 7 (17.1%) |
| 20–29 | 14 (34.2%) | 9 (22.0%) | 23 (56.1%) |
| 30–39 | 7 (17.1%) | 4 (9.8%) | 11 (26.8%) |
| ≥40 | 0 | 0 | 0 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; N, frequency; PCV, packed cell volume; TWCC, total white cell count; HD, haemodialysis; #median/range
Their baseline parameters are presented in table 1. Their mean systolic and diastolic blood pressures were 166.9 ±23.6 and 104 ±21.2 respectively with a median serum creatinine of 844µmol/L (range 76–3,722 µmol/L). Their mean estimated glomerular filtration rate was 34.2±23.6ml/min/1.73m2.
Nocturia (78.0%), loin pain (68.3%) and facial swelling (61.0%) were the most common presenting symptoms (table 2). Liver cysts (31.7%), aortic regurgitation (22.0%) and cerebral aneurysms (14.6%) were the most common extra-renal manifestations (table 2).
Table 2.
Renal and extrarenal manifestations of ADPKD subjects in OAUTH Ile-Ife (1996–2010)
| Parameter | Total | Frequency by gender | |
| Complaints | Frequency (%) | Male | Female |
| Nocturia | 32 (78%) | 19 | 13 |
| Frothy urine | 2 (4.9%) | 1 | 1 |
| Facial swelling | 25 (61%) | 12 | 13 |
| Body swelling | 10 (24.4%) | 4 | 6 |
| Loin pain | 28 (68.3%) | 16 | 12 |
| haematuria | 15 (36.6%) | 7 | 8 |
| LOC | 4 (9.8%) | 1 | 3 |
| Oliguria | 24 (58.5%) | 14 | 10 |
| Stroke | 4 (9.8%) | 2 | 2 |
| Renal findings | |||
| Pallor | 35 (85.4%) | 18 | 17 |
| Hypertension | 36 (87.8%) | 19 | 17 |
| Hepatomegaly | 4 (9.8%) | 1 | 3 |
| Nephromegaly | 34 (82.9%) | 18 | 7 |
| LVH | 35 (85.4%) | 18 | 17 |
| Uraemia | 24 (58.5%) | 14 | 10 |
| Splenomegaly | 1 (2.4%) | 0 | 1 |
| UTI | 13 (31.7%) | 6 | 7 |
| Cystic haemorrhage | 14 (34.2%) | 8 | 6 |
| Extrarenal findings | |||
| Liver cysts | 13 (31.7%) | 6 | 7 |
| Splenic cyst | 1 (2.4%) | 0 | 1 |
| Pancreatic cyst | 2 (4.9%) | 1 | 1 |
| Aortic regurgitation | 9 (22%) | 4 | 5 |
| MVP | 0 | 0 | 0 |
| Cerebral | 6 (14.6%) | 3 | 3 |
| Aneurysm | |||
| Ovarian cyst | 0 | 0 | 0 |
LOC, loss of consciousness; LVH, left ventricular hypertrophy; UTI, urinary tract infection; MVP, mitral valve prolapsed
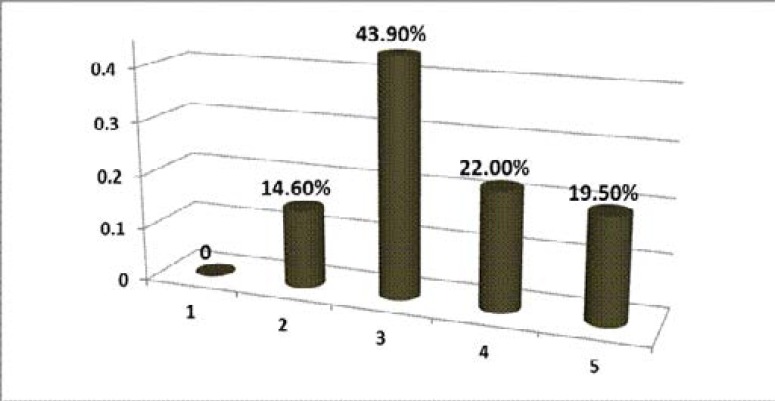
Fourteen (34.1%) experienced cystic haemorrhage. recurrent urinary tract infection was found in 13 (31.7%). End Stage Renal Disease was present in 19.5% of the subjects (figure 1).
Figure 1.
KDOQI staging of ADPKD patients in OAUTH Ile-Ife (1996–2010) HD, haemodialysis; PD, peritoneal dialysis; RRT, renal replacement therapy
A range of 1 to 7 cases were diagnosed at an average of 2.73 cases per annum (table 3). The progression of ADPKD peaked in 2006–2007 and then dropped momentarily (table 3).
Table 3.
Annual incidence of ADPKD in OAUTH Ile-Ife (1996–2010)
| Year of presentation |
Male | Female | Total |
| 1996 | 0 | 1 | 1 (2.4%) |
| 1997 | 1 | 1 | 2 (4.8%) |
| 1998 | 1 | 1 | 2 (4.8%) |
| 1999 | 0 | 2 | 2 (4.8%) |
| 2000 | 0 | 1 | 1 (2.4%) |
| 2001 | 3 | 0 | 3 (7.5%) |
| 2002 | 3 | 0 | 3 (7.5%) |
| 2003 | 1 | 0 | 1 (2.4%) |
| 2004 | 0 | 2 | 2 (4.8%) |
| 2005 | 2 | 1 | 3 (7.8%) |
| 2006 | 3 | 4 | 7 (17.1%) |
| 2007 | 4 | 3 | 7 (17.1%) |
| 2008 | 3 | 1 | 4 (9.8%) |
| 2009 | 1 | 1 | 2 (4.8%) |
| 2010 | 1 | 0 | 1 (2.4%) |
| Total | 23 | 18 | 41 (100%) |
Twenty four (58.5%) subjects presented with renal failure. Twenty three (56.1%) of them volunteered family history of chronic kidney disease. Thirty four (82.9%) had family history of hypertension. However, we could only confirm family history of kidney disease in 3 of the subjects because of reluctance of first degree relatives to participate in screening for ADPKD.
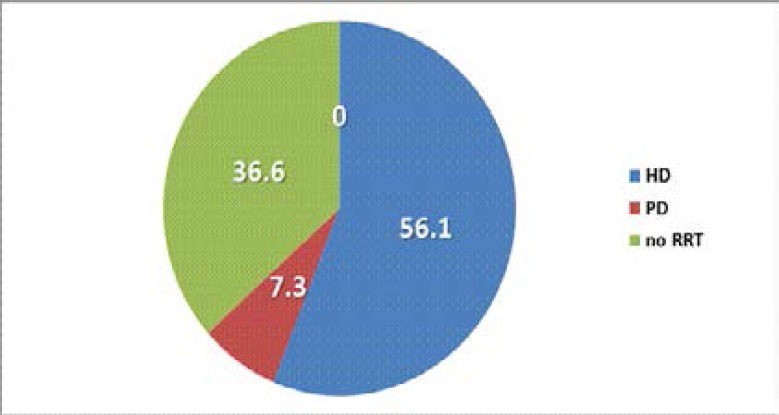
Twenty three (56.1%) received haemodialysis while none received renal transplant within the study period (figure 2). Among the 21 subjects who died during the study period, 6 (28.6%) had autopsy-confirmed complications arising from ruptured intra-cerebral aneurysm.
Figure 2.
Utilization of renal replacement therapy by ADPKD patients.
The average age at death and survival with respect to gender are as shown in table 1. The mean survival was higher among women. The presence of uraemia and intra-cerebral aneurysm contributed significantly to mortality (table 4).
Table 4.
Factors contributing to mortality among ADPKD patients in OAUTH
| Mortality factors | Alive | Dead | p value (CI, 95%) |
| Uraemia | 6 | 18 | <0.01 |
| Ruptured cerebral aneurysm | 0 | 6 | 0.01 |
| Female gender | 9 | 9 | 0.89 |
| Male gender | 11 | 12 | 0.89 |
| Left ventricular hypertrophy | 15 | 20 | 0.067 |
Discussion
General and renal manifestations
Demography: The male predominance and mean age of 48.6±4.6 years compares with findings by Chijioke et al and other authors.7,12 The peak age of incidence of ADPKD in our study (3rd and 4th) decade of life was a bit earlier than 4th to 5th decade found by Chijioke et al.7 However, since the advent of genetic mapping, the diagnosis of ADPKD may be made as early as infancy and in-utero.13 A family history of cystic kidney disease was found in 56.1% which is comparable to about 60% earlier reported. 14–15 However, first degree relatives of only seven of the subjects consented to screening for ADPKD. Three of such family groups were found to have members with history of cystic kidneys verified by history, physical examination and ultrasonographic criteria for diagnosis of ADPKD.
Renal symptoms: Nocturia and loin pain were the most common symptoms in our study. Nocturia results from inability of renal tubular cells to re-absorb salt and water and may present with normal or increased urine volume.11 It is often obtained after a careful history taking by the physician. Loin pain, on the other hand, was the main presenting complaint at presentation (68.3%) and is slightly higher than about 60% quoted by Bajwa et al.16 Acute loin pain (uni- or bilateral) may arise from cystic haemorrhage, urolithiasis, mass pressure effect and urinary tract infections.
In our study, 31.7% had urinary tract infection which is similar to 35.8% reported by Romao et al.17 It was more prevalent among females in conformity with findings by previous authors.7,17–19
Majority of the subjects (73.2%) had haematocrit below 30%. This is lower than 88.2% reported among an elderly group with ADPKD.21 The high prevalence of anaemia in our study may be linked to late presentation to the hospital as shown by 85.4% presenting between KDOQI stages 3–5 CKD. Helal et al reported uraemia among their subjects at presentation.20 This may also account for the absence of polycythaemia (commonly associated with cystic production of erythropoietin) in our subjects.
Hypertension was highly prevalent in this study. It was higher than 73% found byAlves et al. and can reach up to nearly 100% of ADPKD patients with ESRD.18,21 Majority of our patients presented with severe hypertension. It was more common among men (56.1%) in our study. In Dakar, hypertension was found in 61.1% of their male subjects who had ADPKD even though some other studies reported higher prevalence of hypertension among women.7,17–18,22
Hypertension is known to occur at an earlier age in patients with ADPKD compared to the general population.21 The peak incidence of hypertension was 31–40 years in our study which coincides with the peak age at presentation of ADPKD. This is similar to diagnosis of hypertension among male and female ADPKD subjects aged 32 and 34 years respectively by Schrier et al.23
Left ventricular hypertrophy (LVH) was found in 85.4% of all subjects, higher than 73% reported by Chapman et al.24 The occurrence rate of LVH was higher among our normotensive (40% vs 23%) and hypertensive (91.7% vs 48%) subjects when compared to findings by Chapman et al.24
Four (9.8%) of our subjects presented with loss of consciousness with radiological imaging revealing a diagnosis of haemorrhagic stroke in three of them. Stroke was found to occur in 7.6% of subjects in Brazil17 and has been associated with severe hypertension and anuerysmal sub-arachnoid haemorrhage in ADPKD patients.
Extra-renal presentations
Solid organ cysts: Polycystic liver disease ranks as the commonest extra-renal affectation by ADPKD and can occur in more than 80% of adults with the disease.25 We found that 31.7% of our subjects had polycystic liver which is similar to 31.9% and 32.4% reported previously.17,20 A lower incidence of 10.4% was reported by Alves et al in Brazil among elderly subjects.18 The occurrence of liver cysts (attributed to oestrogen-induced cystic expansion and multiplication) was higher among females in this study as previously reported by other authors.17,20,26
Cardiac valve anomalies: In our study, aortic regurgitation (AR) was the only cardiac anomaly detected by echocardiogram. The prevalence was higher than 8% and 19% reported earlier.27–28 AR can co-exist with other cardiac-specific anomalies such as mitral valve prolapse, mitral incompetence, tricuspid incompetence and tricuspid valve prolapse.27–28
Intra-cerbral aneurysms (ICA): Dunger was the first to report the association between ADPKD and ICA in 1904.29 Intra-cerebral aneurysms generally occur in 9–12% of ADPKD patients compared to 2–3% in the general population. 30 Here, we report an occurrence rate of autopsy-confirmed ICA of 14.6%. Previous autopsy series reported prevalence ranging between 2.3% and 19.7%.31–32 Other authors who used Computer Tomographic scan and magnetic resonance imaging reported occurrence rates of between 4.5% and 10.8%.33–35
ICA ruptures: Rupture of aneurysms is the most serious complication in ADPKD and may account for 7% to 13% of deaths in ADPKD.36 The average age of ICA rupture was 49.2 years in our study, higher than 41 years reported by Rivera et al.37 Only two of the subjects with ruptured ICA were diagnosed clinically. This might have been due to the sparing use of angiographic technology in our study owing to technicalities and costs.
Other extra-renal manifestations: We were able to demonstrate splenic and pancreatic cysts in our subjects but there was no evidence of abdominal hernia or diverticular disease. One author reported cysts in seminal vesicle, pancreas and arachnoid membrane as well as spinal meningeal and connective tissue abnormalities such as abdominal hernia and diverticular disease.38
Renal replacement therapy: More than half of our subjects entered into dialysis with haemodialysis as the predominant modality. Peritoneal dialysis (PD) is considered to be more frequently used and to be associated with better survival in ADPKD patients.39 In resource-poor settings however, haemodialysis is the preferred option due to scarcity of PD fluids, and associated high costs and complications. None of the subjects received kidney transplant due to costs and non-availability of volunteer donors.
Outcome: Mortality was 51.2% in our study. This is over two times the figure reported by Chijioke et al.7 Mortality was higher among males, similar to other findings.40–41 Specific causes of death could not be determined as we did not conduct autopsy for all subjects. The presence of uraemia and intra-cerebral aneurysm was associated with mortality. Dalgaard identified uraemia and cerebral haemorrhage as the commoner causes of death in a cohort of ADPKD subjects.42 Other factors that have been associated with mortality in ADPKD patients include cardiac disease and infections.40,43–44
Conclusion
The clinical presentation and outcome of ADPKD are comparable to patients from other parts of the world. There is a need for (i) public enlightenment in order to encourage relatives of ADPKD patients to comply with screening for ADPKD and, (ii) high-powered, multi-centred studies that should include use of gene technology for pre-natal and early childhood diagnosis.
Conflict of interest
No conflict of interest.
References
- 1.Torra Balcells R, Ars Criach E. Diagnóstico molecular de la poliquistosis renal autosómica dominante. Nefrologia. 2011;31:35–43. doi: 10.3265/Nefrologia.pre2010.Nov.10727. [DOI] [PubMed] [Google Scholar]
- 2.Thompson PD. Renal problems in Black South African children. Paed Nephrol. 1997;11:508–512. doi: 10.1007/s004670050330. [DOI] [PubMed] [Google Scholar]
- 3.Akinsola W, Odesanmi WO, Ogunniyi JO, Ladipo GO. Diseases causing chronic renal failure in Nigerians - a prospective study of 100 cases. Afr J Med Med Sci. 1989;18:131–137. PubMed. [PubMed] [Google Scholar]
- 4.Nunes AC, Milani V, Porsch DB, Rossato LB, Mattos CB, Roisenberg I, et al. Frequency and clinical profile of patients with polycystic kidney disease in Southern Brazil. Ren Fail. 2008;30:169–173. doi: 10.1080/08860220701810265. PubMed. [DOI] [PubMed] [Google Scholar]
- 5.Panizo N, Goicoechea M, Garcia de Vinuesa S, Arroyo D, Yuste C, Rincón A, et al. Progresión de la enfermedad renal crónica en pacientes con enfermedadpoliquística autosómica dominante. Nefrologia. 2012;32:197–205. [Google Scholar]
- 6.Corradi V, Gastaldon F, Virzì GM, de Cal M, Soni S, Chionh C, et al. Clinical pattern of adult polycystic kidney disease in a NorthEastern region of Italy. Clin Nephrol. 2009;72:259–267. doi: 10.5414/cnp72259. [DOI] [PubMed] [Google Scholar]
- 7.Chijioke A, Aderibigbe A, Olanrewaju TO, Makusidi AM, Oguntoyinbo AE, Braimoh KT. The prevalence and clinical characteristics of adult polycystic kidney disease in Ilorin, Nigeria. Port J Nephrol Hypert. 2010;24(2):1–5. [Google Scholar]
- 8.Okeahialam BN, Pam SD, Ekedigwe JE, Ekwempu CC. Familial polycystic kidney disease in Nigeria: a report of 2 cases. WAJM. 2006;3:249–251. doi: 10.4314/wajm.v25i3.28289. PubMed. [DOI] [PubMed] [Google Scholar]
- 9.Akinbodewa AA, Adejumo AO, Ogunsemoyin AO, Osasan SA, Adefolalu OA. Co existing Autosomal Dominant Polycystic Kidney Disease and nephrotic syndrome in a Nigerian patient with lupus nephritis. Ann Afr Med. 2016;15:83–86. doi: 10.4103/1596-3519.179735. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravine D, Gibson RN, Walker RG, Sheffield L, Kincaid-Smith P, Danks D. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343:824–827. doi: 10.1016/s0140-6736(94)92026-5. [DOI] [PubMed] [Google Scholar]
- 11.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369(9569):1287–1301. doi: 10.1016/S0140-6736(07)60601-1. PubMed. [DOI] [PubMed] [Google Scholar]
- 12.Yium JJ, Gabow P, Johnson A, Kimberling M, Martinez-Maldonado M. Autosomal dominant polycystic kidney disease in blacks with ESRD. Frequency, clinical course and effect of sickle cell haemoglobin. J Am Soc Nephrol. 1992;3:305–310. doi: 10.1681/ASN.V491670. [DOI] [PubMed] [Google Scholar]
- 13.MacDermot KD, Saggar-Malik AK, Economides DL, Jeffery S. Prenatal diagnosis of autosomal dominant polycystic kidney disease (PKD1) presenting in utero and prognosis for very early onset disease. J Med Genet. 1988;35(1):13–16. doi: 10.1136/jmg.35.1.13. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalgaard OZ. Bilateral polycystic disease of the kidneys: a follow-up of two hundred and eighty four patients and their families. Acta Med Scand. 1957;158:326–329. PubMed. [PubMed] [Google Scholar]
- 15.Iglesias DG, Torres VE, Offord KP, Holley KE, Beard CM, Kurland LT. Epidemiology of adult polycystic kidney disease: Olmsted County, Minnesota: 1935–1980. Am J Kid Dis. 1983;2:630–639. doi: 10.1016/s0272-6386(83)80044-4. PubMed. [DOI] [PubMed] [Google Scholar]
- 16.Bajwa ZH, Sial KA, Malik AB, Steinman TI. Pain patterns in patients with polycystic kidney disease. Kidney Int. 2004;66:1561–1569. doi: 10.1111/j.1523-1755.2004.00921.x. [DOI] [PubMed] [Google Scholar]
- 17.Romão EA, Moysés Neto M, Teixeira SR, Muglia VF, Vieira-Neto OM, Dantas M. Renal and extrarenal manifes tations of autosomal dominant polycystic kidney disease. Braz J Med Biol Res. 2006;39:533–538. doi: 10.1590/s0100-879x2006000400014. PubMed. [DOI] [PubMed] [Google Scholar]
- 18.Alves EF, Tsuneto LT, Pelloso SM, Torres PRA, Otto GLG, Silva AA, et al. Autosomal dominant polycystic kidney disease in hemodialysis patients in Southern Brazil. J Bras Nefrol. 2014;36(1):18–25. doi: 10.5935/0101-2800.20140005. [DOI] [PubMed] [Google Scholar]
- 19.Elzinga LW, Bennett WM. Miscellaneous renal and systemic complications of autosomal dominant polycystic kidney disease including infection. In: Watson ML, Torres VE, editors. Polycystic kidney disease. Oxford: Oxford Medical Publications; 1996. pp. 483–499. [Google Scholar]
- 20.Helal I, Lassoued F, Maiz HB, Kheder A. Clinical Presentation and Outcomes of Autosomal Dominant Polycystic Kidney Disease in the Elderly. Am J Med Sci and Med. 2013;1(1):18–20. [Google Scholar]
- 21.Kelleher CL, McFann KK, Johnson AM, Schrier RW. Characteristics of hypertension in young adults with autosomal dominant polycystic kidney disease compared with the general US population. Am J Hypertens. 2004;17(11 part 1):1029–1034. doi: 10.1016/j.amjhyper.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Fary Ka E, Seck SM, Niang A, Cisse MM, Diouf B. Patterns of autosomal dominant polycystic kidney diseases in black Africans. Saudi J Kidney Dis Transpl. 2010;21:81–86. [PubMed] [Google Scholar]
- 23.Schrier RW, Johnson AM, Frank MC, Chapman AB. The role of parental hypertension in the frequency and age of diagnosis of hypertension in offspring with autosomal-dominant polycystic kidney disease. Kidney Int. 2003;64:1792–1799. doi: 10.1046/j.1523-1755.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 24.Chapman BA, Johnson AM, Rainguet S, Hossack K, Gabow P, Schrier RW. Left ventricular hypertrophy In autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1997;8:1292–1297. doi: 10.1681/ASN.V881292. [DOI] [PubMed] [Google Scholar]
- 25.Bae KT, Zhu F, Chapman AB, Torres VE, Grantham JJ, Guay-Woodford LM, et al. Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Clin J Am Soc Nephrol. 2006;1:64–69. doi: 10.2215/CJN.00080605. [DOI] [PubMed] [Google Scholar]
- 26.Chapman AB. Cystic disease in women: clinical characteristics and medical management. Adv Ren Replace Ther. 2003;10:24–30. doi: 10.1053/jarr.2003.50005. [DOI] [PubMed] [Google Scholar]
- 27.Hossack KF, Leddy CF, Johnson AM, Schrier RW, Gabow PA. Echocardiographic findings in autosomal dominant polycystic kidney disease. N Engl J Med. 1988;319:907–912. doi: 10.1056/NEJM198810063191404. PubMed. [DOI] [PubMed] [Google Scholar]
- 28.Timio M, Monarca C, Pedes S, Gentili S, Verdura C, Lolli S. The spectrum of cardiovascular abnormalities in autosomal dominant polycystic kidney disease: a 10-year follow-up in a five-generation kindred. Clin Nephrol. 1992;37:245–251. [PubMed] [Google Scholar]
- 29.Dunger R. Zur Lehre von der Cystenniere, mit besonderer Beriicksichtigung ihrer Hereditat. Beitr Pathol Anat. 1904;35:445–509. [Google Scholar]
- 30.Xu HW, Yu SQ, Mei CL, Li MH. Screening for intra-cranial aneurysm in 355 patients with autosomal-dominant polycystic kidney disease. Stroke. 2011;42:204–206. doi: 10.1161/STROKEAHA.110.578740. [DOI] [PubMed] [Google Scholar]
- 31.Zeier M, Geberth S, Ritz E, Jaeger T, Waldherr R. Adult dominant polycystic kidney disease. Clinical problems. Nephron. 1988;49:177–183. doi: 10.1159/000185052. PubMed. [DOI] [PubMed] [Google Scholar]
- 32.Bigelow NH. The association of polycystic kidney disease with intracranial aneurysms and other related disorders. Am J Med Sci. 1953;225:485–494. doi: 10.1097/00000441-195305000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Chapman AB, Rubinstein D, Hughes R, Stears JC, Earnest MP, Johnson AM, et al. Intracranial aneurysms in autosomal dominant polycystic kidney disease. N Engl J Med. 1992;327(13):916–920. doi: 10.1056/NEJM199209243271303. 24. [DOI] [PubMed] [Google Scholar]
- 34.Huston J, Torres VE, Sulivan PP, Offord KP, Wiebers DO. Value of magnetic resonance angiography for the detection of intracranial aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1993;3(12):1871–1877. doi: 10.1681/ASN.V3121871. [DOI] [PubMed] [Google Scholar]
- 35.Ruggieri PM, Poulos N, Masaryk TJ, Ross JS, Obuchowski NA, Awad IA, et al. Occult intracranial aneurysms in polycystic kidney disease: screening with MR angiography. Radiology. 1994;191(1):33–39. doi: 10.1148/radiology.191.1.8134594. [DOI] [PubMed] [Google Scholar]
- 36.Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10:626–636. doi: 10.1016/S1474-4422(11)70109-0. [DOI] [PubMed] [Google Scholar]
- 37.Rivera M, Gonzalo A, Gobernadol JM, Orte L, Quereda C, Ortuno J. Stroke in adult polycystic kidney disease. Postgrad Med J. 1992;68:735–738. doi: 10.1136/pgmj.68.803.735. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 39.Reule S, Sexton DJ, Solid CA, Chen SC, Collins AJ, Foley RN. ESRD from autosomal dominant polycystic kidney disease in the United States, 2001–2010. Am J Kid dis. 2014;64:592–599. doi: 10.1053/j.ajkd.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fick GM, Johnson AM, Hammond WS, Gabow PA. Causes of death in autosomal dominant polycystic kidney disease. JASN. 1995 Jun 1;5(12):2048–2056. doi: 10.1681/ASN.V5122048. [DOI] [PubMed] [Google Scholar]
- 41.Fraile Gómez P, García-Cosmes P, Corbacho Becerra L, Tabernero Romo JM. Análisis clínico de una población con poliquistosis renal autosómica dominante. Nefrologia. 2010;30:87–94. doi: 10.3265/Nefrologia.pre2010.Jan.10211. [DOI] [PubMed] [Google Scholar]
- 42.Dalgaard OZ. Mortality Associated with Polycystic Kidney Disease. J Ins Med. 1989;21(4) [Google Scholar]
- 43.Roscoe JM, Brissenden JE, Williams EA, Cherryl AL, Silverman M. Autosomal dominant Polycystic Kidney Disease in Toronto. Kidney Int. 1993;44:1101–1108. doi: 10.1038/ki.1993.355. [DOI] [PubMed] [Google Scholar]
- 44.Singh S, Hariharan S. Renal replacement therapy in Autosomal dominant Polycystic Kidney Disease. Nephron. 1991;57:40–44. doi: 10.1159/000186213. PubMed. [DOI] [PubMed] [Google Scholar]