Abstract
Pretilachlor is a systemic herbicide belonging to chloroacetamide group which is being used in rice fields for the control of annual weeds. The acute toxicity bioassay of pretilachlor was carried out by probit analysis method and the safe level was estimated by multiplying the 96 h LC50 with different application factors. The 12, 24, 48, 72 and 96 h LC50 values were obtained as 11.12, 9.55, 8.57, 7.11 and 5.84 mg L–1 respectively while the safe values found ranges 0.05 to 0.58 mg L–1. Buccal movements as well as the number of burst swimming movements in fishes exposed to pretilachlor were found to be increased significantly in response to all concentrations of the herbicides tested. Number of feeding attempts reduced significantly while irregular responses in fishes were observed regarding their attempt to form group after exposure to herbicide. As a whole, exposure of fishes to pretilachlor showed significant effects on all studied behaviours. So it can be concluded that the herbicide pretilachlor is toxic to fishes and its excess use should be avoided.
Keywords: Ecology, Zoology
1. Introduction
During the last decade continuous use of herbicides into the agricultural activities has resulted in frequent detection of these chemicals in both surface and ground water which can impose adverse effect on non-target organisms (Gilliom, 2007). Among these non target organisms, fishes are the most important and played significant roles in assessing potential risk associated with contamination in aquatic environment (Lakra and Nagpure, 2009). They served as bio-indicators of environmental pollution and permit early detection of aquatic environmental problems (Lopez-Barea, 1996; Van Der Oost et al., 2003).
The contamination of aquatic ecosystems by chloroacetamide herbicides like acetochlor, butachlor, metolachlor, alachlor and pretilachlor has gained increased attention in last few years after they have been identified as probable carcinogens. Acetochlor and alachlor can cause tumors in the nasal turbinates, butachlor causes stomach tumors, and metolachlor causes liver tumors (Coleman et al., 2000; Dearfield et al., 1999). Most of the studies have demonstrated the toxicity of chloroacetamide herbicides to fish and other aquatic organisms (Geng et al. 2005, 2010; Ateeq et al., 2002; Peebua et al., 2007; Chang et al., 2011). Butachlor disrupt the reproductive process and affect the thyroid and sex steroid hormones in Zebra fish (Chang et al., 2011). Studies have demonstrated the presence of chloroacetamide herbicides in surface water (Hladik et al., 2008), soil (Chao et al., 2007), and sediment (Xue et al., 2005). It has been reported that butachlor is moderately toxic and irritating for R. rutilus caspicus and S. lucioperca (Mohammad and Hedayati, 2017). Although various works have been done so far to evaluate the toxicity of butachlor, metachlor, acetochlor and alachlor, the literature regarding the toxic effects induced by pretilachlor is relatively scarce. Pretilachlor (2-chloro-2′,6′-diethyl-N-[2-propoxyethyl] acetanilide) is a systemic herbicide which is being used extensively in rice fields for control weeds. This herbicide inhibits cell division via preventing the synthesis of long chain fatty acids (Kaushik et al., 2006). Ali and Mohamad (2013) reported that the toxicity of pretilachlor on Gambusia increases with increasing concentration and exposure time. The investigations on pretilachlor have focused on removal of pretilachlor from the environment and the effect of pretilachlor on soil microorganisms (Saha et al., 2012; Toan et al., 2013).
Behavioural changes resulting from exposure of chemicals provide more sensitive early warnings than standard test methods (Smith and Logan, 1997). In fishes, behavioural responses to herbicides remain relatively scarce. Measurements on the swimming-oriented responses, opercular movement, feeding attempt, jerk swimming, and schooling are among the most common tests used in behavioural toxicology of fish.
Like other chloroacetamide herbicides pretilachlor may be toxic for fishes and various assays are required for its toxicity evaluation. For short term lethality assays many different fish species have been used based on their ease of culture, ecological relevance and economic importance. The fish species Clarias batrachus was selected for the present study because of wide distribution in the freshwater environment, non-invasive, availability throughout the season and easy acclimation to laboratory conditions. In addition to above, the fish is a bottom dweller which makes it to be in contact with xenobiotics in water as well as in sediment.
For the studies on toxicity of chemicals on animals most of the test guidelines recommend use of measured concentrations of the test chemical (OECD, 1992; USEPA, 1996; ASTM, 2007). But ecotoxicological models generally have large data requirements so must be based on use of both measured and nominal concentrations of testing chemical. As per ecotox database, 66% of such studies are based on nominal concentrations (ECOTOX; http://www.epa.gov/ecotox/). Raimondo et al. (2009) compared toxicity data on fishes reported as nominal and measured concentrations and developed an interspecies correlation estimation model for 12 pairs of fish species. They found that they were not significantly different for any species pairs and models developed from one concentration type predicted the reported toxicity of the other with high certainty. They concluded that acute toxicity test results reported as either measured or nominal concentrations may be included in ecotoxicological models. As the reports regarding the acute toxicity of pretilachlor on C. batrachus is lacking in available literature first we had chosen the nominal concentration of herbicide in the present study which will be followed by measured concentration in near future.
In view of the above the present study has been carried out to evaluate the acute toxicity of herbicide pretilachlor on fresh water teleost Clarias batrachus (Linnaeus) and to observe four behavioural activities i.e number of buccal movement per minute, number of feeding attempt per 5 minute, number of burst swimming per 5 minute and number of occurrence of grouping per 5 minute after herbicide exposure.
2. Materials and methods
2.1. Ethics statement
In the present study test fishes were obtained from local markets. Experiments were conducted according to guidelines approved by the relevant authorities in India (Indian National Science Academy and Indian Council of Medical Research) and all the experimental protocols were approved by the departmental research committee of department of Zoology, Guru Ghasidas University, Bilaspur (India).
2.2. Experimental fish and herbicide
The fishes of an average weight 10 ± 251 gm and length 8 ± 557 cm were selected to carry out acute toxicity bioassay. Selected fish specimens were bathed in 0.05% potassium permanganate (KMnO4) to avoid skin infections. Before commencement of the experiments the fishes were kept for one month in glassy aquarium (60 cm × 30 cm × 30 cm size) filled with dechlorinated tap water for their acclimatization to laboratory conditions. During this period they were fed with boiled eggs. The physico-chemical characteristics of water were determined as per APHA guidelines (2012) and was replaced every 24 h to remove waste materials. The aquarium water was maintained with temperature = 28 °C ± 1.0 °C, pH = 6.5 ± 0.2 units, total hardness = 28 ± 0.05 mg/l, dissolved oxygen = 6.6 ± 0.01 mg/l, salinity = 0.6 ± 0.02 ppt. Day and light period maintained through the entire experiment was 12:12 h. Commercial formulation of pretilachlor (CAS No. 51218-49-6) with trade name Rifit (50 % EC), manufactured by Syngnta India limited, India was used for toxicity testing.
All chemicals used in the present study were at least of analytical grade.
2.3. Acute toxicity bioassay and determination of sub lethal concentration
The LC10-90 value of pretilachlor was determined through acute toxicity bioassay using C. batrachus in a semi-static laboratory system according to the OECD guideline No 203. The solution was added to the aquarium (60 × 30 × 30 cm in size with 40 L capacity) following the method of Pluta (1989). The aquarium water containing fresh herbicide mixed was replaced after every 24 h. A group of 10 fish specimens were randomly exposed to each of the predefined pretilachlor concentrations (1.0, 3.9, 5.9, 7.9, 9.9 and 11.9 mg L−1). Another set of 10 fish in aquarium water without herbicide were used a control. The experiments were set in triplicate to obtain the various LC values. Probit analysis method was used to determine the LC10-90 values of pretilachlor for 12, 24, 48, 72 and 96 h to C. batrachus (Finney, 1971). The safe level estimates of the herbicides were drawn according to Sprague (1971), CWQC (1972), NAS/NAE (1973), IJC (1977), CCREM (1991) and Hart et al. (1948). Herbicide in water was determined by Liquid chromatography Equipment (C18 reverse phase, ultraviolet detector with 5 μm granulometry and 30 cm equivalent length, LOD: 0.05 μg L−1 with recovery % of 99%).
2.4. Behavioural responses
For study of behavioural response the 15 minute test was conducted after each exposure. The behaviour end points chosen were number of buccal movement per minute, number of feeding attempt per 5 minute, number of burst swimming per 5 minute and number of occurrence of grouping per 5 minute.
The data obtained were subjected to one way analysis of variance (ANOVA) by statistical package SPSS (Version 16).
3. Results
The LC50 values (with 95% confidence limits) of different concentrations of pretilachlor were determined and are represented in Table 1. Significant differences in (p < 0.05) in LC10–90 values were observed for different times of exposures. As the exposure time increases the median lethal concentration (LC50) decrease.
Table 1.
Lethal concentrations of pretilachlor at various exposure time for C. batrachus.
| Point | Concentrations (mg L−1) at various exposure times (95% confidence intervals) |
||||
|---|---|---|---|---|---|
| 12 h | 24 h | 48 h | 72 h | 96 h | |
| LC10 | 4.91a | 4.65a | 2.34b | 1.31c | 1.21c |
| LC20 | 5.59a | 5.46a | 3.48b | 2.24c | 1.18d |
| LC30 | 7.47a | 5.25b | 3.56c | 2.34d | 1.78e |
| LC40 | 09.48a | 07.89b | 5.88c | 3.98d | 2.45e |
| LC50 | 11.12a | 9.55b | 8.57c | 7.11d | 5.84e |
| LC60 | 11.18a | 9.25b | 7.98c | 5.58d | 4.08e |
| LC70 | 13.22a | 11.49b | 11.47b | 9.12c | 6.28d |
| LC80 | 15.56a | 13.12b | 13.21b | 11.01c | 9.45d |
| LC90 | 16.00a | 14.27b | 12.03c | 12.24c | 10.09d |
Lethal concentration values in rows with different letters significantly differ at p < 0.05.
3.1. Estimation of safe level
The safe level of herbicide pretilachlor to fish C. batrachus is presented in Table 2. The values of safe level varied from 0.05 to 0.58 mg L–1
Table 2.
Safe level estimates of pretilachlor to C. batrachus.
| 96 h LC50 (mg. L−1) | Method | Calculation/application factor | Safe level (mg L−1) |
|---|---|---|---|
| 5.84 | Hart et al. | 48 h LC50 × 0.03 × S2 where S = 24 h LC50/48 h LC50 |
0.316 |
| Sprague | 0.1 | 0.584 | |
| CWQC | 0.01 | 0.0584 | |
| NAS/NAE | 0.1–0.00001 | 0.5839 | |
| CCREM | 0.05 | 0.292 | |
| IJC | 5% of 96 h LC50 | 0.292 |
3.2. Behavioural response of fishes
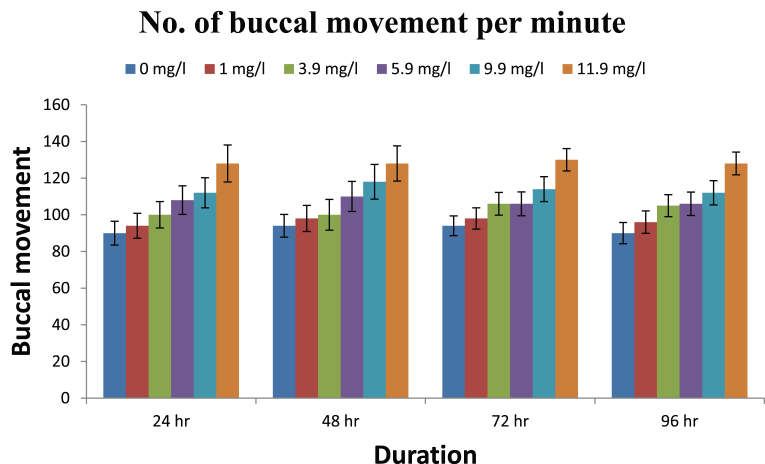
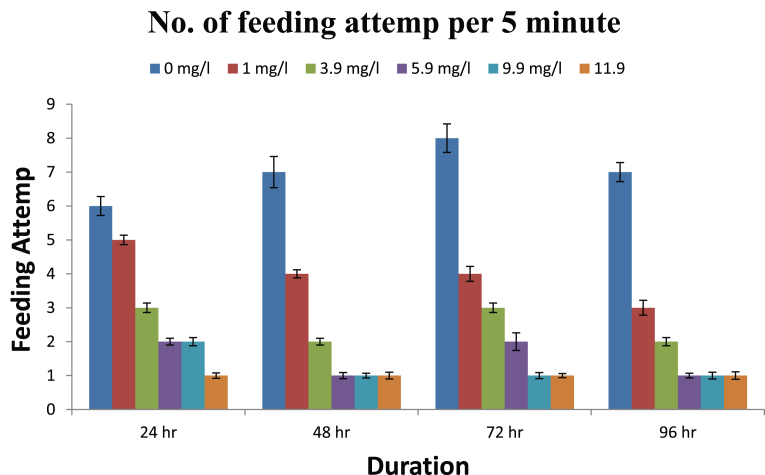
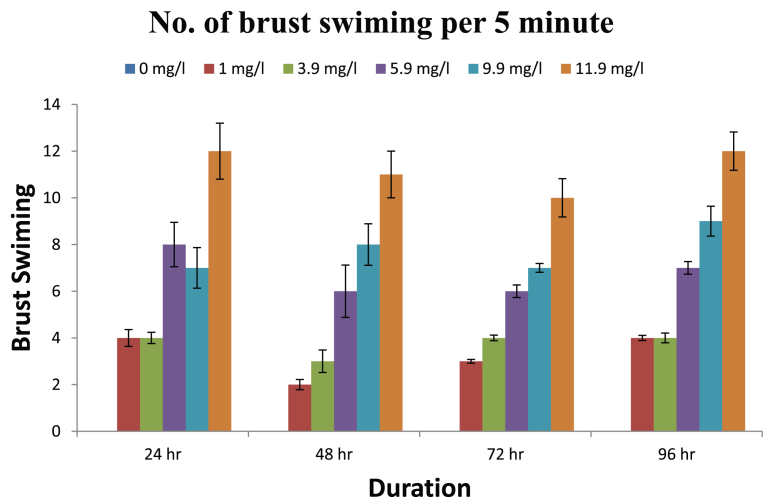
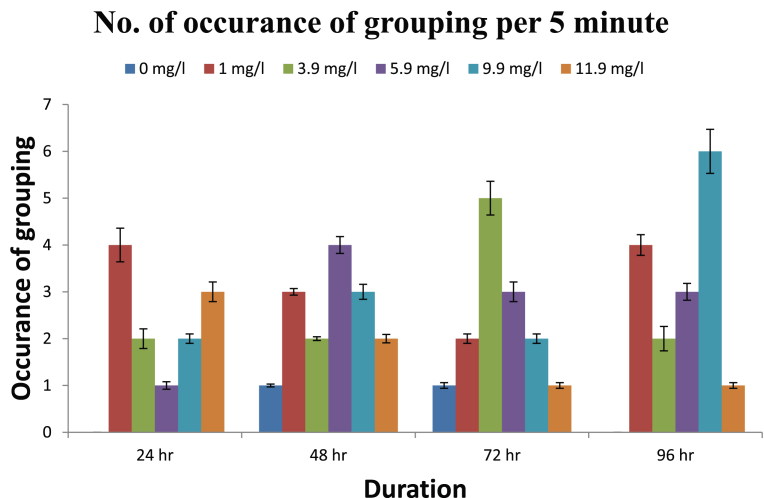
The observed behavioural responses of fishes are shown in Figs. 1, 2, 3, and 4. The buccal movements in fishes exposed to pretilachlor were found to be increased significantly at all tested concentrations (Fig. 1). Number of feeding attempts was reduced significantly in exposed fishes (Fig. 2). Similarly a significant increase in the number of burst swimming reactions was observed which increase with increasing concentration of the herbicide (Fig. 3). However no particular trend in tendency to form group was observed in exposed fishes (Fig. 4).
Fig. 1.
Buccal movements in fishes exposed to different concentration of herbicide pretilachlor.
Fig. 2.
Feeding attempt in fishes exposed to different concentration of herbicide pretilachlor.
Fig. 3.
Burst swimming in fishes exposed to different concentration of herbicide pretilachlor.
Fig. 4.
Group formation in fishes exposed to different concentration of herbicide pretilachlor.
4. Discussion
The present study was carried out to evaluate the acute toxicity and behavioural responses in Clarias batrachus (Linnaeus) exposed to herbicide pretilachlor. The results clearly indicated that exposure of fishes to pretilachlor based herbicide resulted in increased mortality with increasing concentration of the herbicide. The 96 h LC50 value reported in the present study for commercial formulation of pretilachlor is lower that the value reported by Ali and Mohammad in gambusia (2013). However it is higher than the value obtained by Maryam et al. (2013) in grass carp. These differences in obtained values of LC50 for different species were may be due to age, size and health (Abdul-Farah et al., 2004) or the existing physiological parameters of water (Eaton and Gilbert, 2008). A large difference in the values of safe level (ranged from 0.05 to 0.58 mgL–1) was obtained which indicates that it is difficult to decide the acceptable concentration of pretilachlor for C. batrachus based on the present study.
Behavioural changes are one of the most sensitive indicators of potential toxic effect in fishes (Banaee et al., 2011) but very few works has been done so far regarding the behavioural sensitivity of the fish to the pesticides used in agricultural fields. Behavioural alterations were observed in fishes exposed to glyphosate-based herbicides (Langiano and Martinez, 2008; Lushchak et al., 2009; Nwani et al., 2010; Shiogiri et al., 2012), and butachlor (Chang et al., 2011). Similar to the observations of previous workers the present study demonstrates that fish C. batrachus display immediate and various behavioural responses to herbicide pretilachlor. Four behavioural endpoints, related to burst swimming reactions, buccal movements, feeding attempts, and grouping were studied because these endpoints are easily accessible and thus helpful in assessment of sub-lethal effects of contaminants in fish and can also be used as an indicator of acute changes in the chemical environment.
The buccal movements in fishes exposed to pretilachlor were found to be increased significantly in response to all concentrations of the herbicides tested. Buccal movements are directly associated with respiratory movement so it can be concluded that sub acute exposure to herbicide adversely effects the process of respiration in fishes similar to the conclusions made by Saglio and Trijasse (1998) in case of atrazine. Burst swimming reactions in fishes are result of stressful condition (Fuiman, 1986). A significant increase in the number of burst swimming reactions was observed in fishes exposed to all concentration of herbicides. Similarly number of feeding attempts in fishes reduced significantly in exposed fishes. This decreased feeding attempt may be directly related to gustatory sensitivity of fishes to contaminants. Very few literatures is available in this regard however it has been reported in Cyprinus carpio that the nerve innervating terminal buds in the lip region was sensitive to stimulation by high concentration of benthiocarb, isoprothiolane and fenitrothion (Ishida and Kobayashi, 1996). Irregular responses in fishes were observed regarding their attempt to form group after their exposure to herbicides which need further studies to for periods longer than the present study. On the basis of results obtained it can be concluded that the herbicide pretilachlor is toxic to fishes and its use should be in controlled manner to avoid its long term harmful effects in the environment.
Declarations
Author contribution statement
Sushant Verma: Conceived and designed the experiments; Analyzed and interpreted the data.
Rakesh Soni: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
The authors received no funding from an external source.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Ali S., Mohamad R.I. Effect of pretilachlor on the mortality of fish Gambusia. World J. Zool. 2013;8(3):336–339. [Google Scholar]
- APHA . American Public Health Association; Washington: 2012. Standerd Methods for the Examination of Water and Waste Water. [Google Scholar]
- ASTM . American Society for Test and Materials; Philadelphia: 2007. Standard Guide for Conducting Acute Toxicity Tests with Fishes, Macroinvertebrates, and Amphibians. [Google Scholar]
- Ateeq B., Abul-Farah M., Ali M.N., Ahmad W. Induction of micronuclei and erythrocyte alterations in the catfish Clarias batrachus by 2,4-dichlorophenoxyacetic acid and butachlor. Mutat. Res. 2002;518:135–144. doi: 10.1016/s1383-5718(02)00075-x. [DOI] [PubMed] [Google Scholar]
- Banaee M., Sureda A., Mirvaghefi A.R., Ahmadi K. Effects of diazinon on biochemical parameters of blood in rainbow trout (Oncorhynchus mykiss) Pestic. Biochem. Physiol. 2011;99(1):1–6. [Google Scholar]
- Chang J., Liu S., Zhou S., Wang M., Zhu G. Effects of butachlor on reproduction and hormone levels in adult zebrafish (Danio rerio) Exp. Toxicol. Pathol. 2011;65(1-2):205–209. doi: 10.1016/j.etp.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Chao L., Zhou Q.X., Chen S., Cui S., Wang M.E. Single and joint stress of acetochlor and Pb on three agricultural crops in northeast China. J. Environ. Sci. China. 2007;19:719–724. doi: 10.1016/s1001-0742(07)60120-x. [DOI] [PubMed] [Google Scholar]
- Coleman S., Linderman R., Hodgson E., Rose R.L. Comparative metabolism of chloroacetamide herbicides and selected metabolites in human and rat liver microsomes. Environ. Health Perspect. 2000;108:1151–1157. doi: 10.1289/ehp.001081151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearfield K.L., McCarroll N.E., Protzel A., Stack H.F., Jackson M.A., Waters M.D. A survey of EPA/OPP and open literature on selected pesticide chemicals II. Mutagenicity and carcinogenicity of selected chloroacetanilides and related compounds. Mutat. Res. 1999;443:183–221. doi: 10.1016/s1383-5742(99)00019-8. [DOI] [PubMed] [Google Scholar]
- Eaton D.L., Gilbert S.G. Casarett & Doull's Toxicology: The Basic Science of Poisons. eighth ed. McGraw-Hill Medical; New York: 2008. Principles of toxicology; pp. 11–43. [Google Scholar]
- Farah M.A., Ateeq B., Ali M.N., Sabir R., Ahmad W. Studies on lethal concentrations and toxicity stress of some xenobiotics on aquatic organisms. Chemosphere. 2004;55(2):257–265. doi: 10.1016/j.chemosphere.2003.10.063. [DOI] [PubMed] [Google Scholar]
- Finney D.T. Cambridge University Press; Cambridge: 1971. Probit Analysis. [Google Scholar]
- Fuiman L.A. Burst-swimming performance of larval zebra danios and the effects of diel temperature fluctuations. Trans. Am. Fish. Soc. 1986;115(1):143–148. [Google Scholar]
- Geng B., Lin L., Zhang Q., Zhung B. Genotoxicity of the pesticide dichlorvos and herbicide butachlor on Rana zhenhaiensis tadpoles. Asian Hepatol. Res. 2010;1(2):118–122. [Google Scholar]
- Geng B.R., Yao D., Huang H., Xue Q., Lian Y., Zheng Z. Acute toxicities and effects of dichlorovos and butachlor to Rana japonica tadpoles and its growth. Herpetol. Sin. 2005;10:127–132. [Google Scholar]
- Gilliom R.J. Pesticides in streams and groundwater. Environ. Sci. Tech. 2007;41:3408–3484. doi: 10.1021/es072531u. [DOI] [PubMed] [Google Scholar]
- Hart W.B., Weston R.F., Dermann J.G. An apparatus for oxygenating test solutionin which fish are used as test animals for evaluating toxicity. Trans. Am. Fish Soc. 1948;75:288. [Google Scholar]
- Hladik M.L., Bouwer E.J., Roberts A.L. Neutral chloroacetamide herbicide degradates and related compounds in Midwestern United States drinking water sources. Sci. Total Environ. 2008;390:155–165. doi: 10.1016/j.scitotenv.2007.09.042. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Kobayashi H. Avoidance behavior of carp to pesticides and decrease of the avoidance threshold by addition of sodium lauryl sulfate. Fish Sci. 1995;61:441–446. https://www.jstage.jst.go.jp/article/fishsci1994/61/3/61_3_441/_pdf [Google Scholar]
- Kaushik S., Inderjit Steribig J.C., Cedergreen N. Activities of mixtures of soil-applied herbicides with different molecular targets. Pest Manage. Sci. 2006;62:1092–1097. doi: 10.1002/ps.1285. [DOI] [PubMed] [Google Scholar]
- Lakra W.S., Nagpure N.S. Genotoxicological studies in fish: a review. Indian J. Anim. Sci. 2009;79:93–98. [Google Scholar]
- Langiano V.C., Martinez C.B.R. Toxicity and effects of glyphosate-based herbicide on the Neotropical fish Prochilodus Lineatus. Comp. Biochem. Physiol. 2008;147:221–223. doi: 10.1016/j.cbpc.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Lopez-Barea J. Biomarkers to detect environmental pollution. Toxicol. Lett. 1996;88:77–79. [Google Scholar]
- Lushchak O., Kubrak O., Storey M., Storey K.B., Lushchak I. Low toxic herbicide roundup induces mild oxidative stress in goldfish tissue. Chemosphere. 2009;76:932–937. doi: 10.1016/j.chemosphere.2009.04.045. [DOI] [PubMed] [Google Scholar]
- Maryam P., Mehdi M., Morteza S., Masood F., Abbasali Z., Firouz A. Determination of the acute toxicity of pretilachlor on liver and gill issues as well as glucose and cortisol levels in fingerling grass carps (Ctenopharyngodon idella) J. Fish. Aquat. Sci. 2013;8(6):721–726. [Google Scholar]
- Mohammad F.V., Hedayati A. Acute toxicity of butachlor to Rutilus rutilus caspicus and Sander lucioperca in vivo condition. Transylv. Rev. Syst. Ecol. Res. 2017;19(3):85–92. [Google Scholar]
- Nwani C.D., Lakra W.S., Nagpur N.S., Kumar R., Kushwaha B., Srivastava S.K. Mutagenic and genotoxic effects of carbosulfan in fresh water air breathing fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Food Chem. Toxicol. 2010;48:202–208. doi: 10.1016/j.fct.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Organization for economic cooperation and development (OECD) OECD; France: 1992. Guideline for the Testing of Chemicals: Fish, Acute Toxicity Test, Document 203. [Google Scholar]
- Peebua P., Kosiyachinda P., Pokethitiyook P., Kruatrachue M. Evaluation of alachlor herbicide impacts on Nile tilapia (Oreochromis niloticus) using biochemical biomarkers. Bull. Environ. Contam. Toxicol. 2007;78(2):138–148. doi: 10.1007/s00128-007-9027-8. [DOI] [PubMed] [Google Scholar]
- Raimondo S., Vivian D.N., Barron M.G. Standardizing acute toxicity data for use in ecotoxicology models: influence of test type, life stage, and concentration reporting. Ecotoxicology. 2009;18:918–928. doi: 10.1007/s10646-009-0353-y. [DOI] [PubMed] [Google Scholar]
- Saglio P., Trijasse S. Behavioral responses to atrazine and diuron in goldfish. Arch. Environ. Contam. Toxicol. 1998;35(3):484–491. doi: 10.1007/s002449900406. https://eurekamag.com/pdf/003/003051129.pdf [DOI] [PubMed] [Google Scholar]
- Saha S., Dutta D., Karmakar R., Ray D.P. Structure-toxicity relationship of chloroacetanilide herbicides: relative impact on soil microorganisms. Environ. Toxicol. Pharmacol. 2012;34:307–314. doi: 10.1016/j.etap.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Shiogiri N.S., Paulino M.G., Carraschi S.P., BAraldi F.G., Cruz C., FErnandes M.N. Acute exposure to glyphosate-based herbicide affects the gills and liver of the Neotropical Fish Piaractus mesopotamicus. Environ. Toxicol. Pharmacol. 2012;34:388–396. doi: 10.1016/j.etap.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Smith E.H., Logan D.T. Linking environmental toxicology, ethology and conservation. In: Clemmons J.R., Buchholz R., editors. Behavioral Approaches to Conservation in the Wild. Cambridge University Press; Cambridge: 1997. pp. 277–302. [Google Scholar]
- Toan P.V., Sebesvari Z., Blasing M., Rosendahl I., Renaud F.G. Pesticide management and their residues in sediments and surface and drinking water in the Mekong Delta. Vietnam Sci. Total Environ. 2013;452–453:28–39. doi: 10.1016/j.scitotenv.2013.02.026. [DOI] [PubMed] [Google Scholar]
- USEPA . Environmental Protection Agency; Washington: 1996. Ecological Effects Test Guidelines. OPPTS 850.1075 Fish Acute Toxicity Test, Freshwater and Marine. EPA 712-C-96-118. [Google Scholar]
- Van Der Oost R., Beyer J., Vermeulen N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ. Toxicol. Pharmacol. 2003;13:57–149. doi: 10.1016/s1382-6689(02)00126-6. [DOI] [PubMed] [Google Scholar]
- Xue N., Xu X., Jin Z. Screening 31 endocrine-disrupting pesticides in water and surface sediment samples from Beijing Guanting reservoir. Chemosphere. 2005;61:1594–1606. doi: 10.1016/j.chemosphere.2005.04.091. [DOI] [PubMed] [Google Scholar]