Abstract
Introduction
Limited data exists on histologically confirmed cancers and tuberculosis in rural Malawi, despite the high burden of both conditions. One of the main reasons for the limited data is the lack of access to pathology services for diagnosis. We reviewed histopathology results of patients in Neno District, one of the poorest rural districts in Malawi, from May 2011 to July 2017, with an emphasis on cancers and tuberculosis.
Methods
This is a retrospective descriptive study reviewing pathology results of samples collected at Neno health facilities and processed at Kamiza Pathology Laboratory. Data was entered into Microsoft Excel and cleaned and analysed using Stata 14.
Results
A total of 532 specimens were collected, of which 87% (465) were tissue biopsies (incision or core biopsies), and 13% (67) were cytology samples. Of all specimens, 7% (n=40) of the samples had non-diagnostic results. Among the results that were diagnostic (n=492), 37% (183) were malignancies, 33% (112) were infections and inflammatory conditions other than tuberculosis, 20% (97) were benign tumours, 7% (34) were tuberculosis, 4% (21) were pre-malignant lesions, 5% (23) were normal samples, and 4% (22) were other miscellaneous conditions. Among the malignancies (n=183), 62% (114) were from females and 38% (69) from males. Among females, almost half of the cancers were cervical (43%, n= 49), followed by Kaposi sarcoma (14%, n=16), skin cancers (9%, n=10), and breast cancer (8%, n=9). In males, Kaposi sarcoma was the most common cancer (35%, n=24), followed by skin cancers (17%, n=12). About 75% (n=137) of the cancers occurred in persons aged 15 to 60 years.
Conclusion
Histopathology services at a rural hospital in Malawi provides useful diagnostic information on malignancies, tuberculosis and other diagnoses, and can inform management at the district level.
Keywords: malignancies, Tuberculosis, Malawi, Neno, pathology
Introduction
Pathological confirmation of some of the major chronic communicable and chronic non-communicable diseases (NCDs) is essential in low- and middle-income countries to facilitate early diagnosis and treatment of these conditions1,2. The accessibility and availability of pathology in Malawi should be prioritised, particularly as Malawi begins to face a double burden of chronic communicable and chronic NCDs. HIV, which has accelerated the burden of some cancers and extra-pulmonary tuberculosis (TB), still ranks among the top ten causes of morbidity in Malawi3–5. Additionally, NCDs, especially cancers, are becoming common in Malawi and are considered the second most common cause of death, after HIV and AIDS3. The Malawi National Non Communicable Diseases and Injuries Poverty Commission, focused on Non-Communicable Diseases and Injuries (NCDIs), was launched in late 2016. The Commission found that the burden of NCDs falls disproportionately on the young, with 60% of the disability-adjusted life year (DALY) burden occurring in those under 40, compared to 18% in high-income countries6. Using global burden of disease data and expert opinion, the Commission prioritised 37 NCDIs as critical to address for the health of Malawians. Among these conditions, 7 were malignancies7.
Data on pathologically confirmed cancers and TB is limited in Malawi for several reasons. Firstly, public pathology laboratories are located in central hospitals, geographically and logistically separated from district hospitals. Presently, pathology laboratories are located in only Lilongwe and Blantyre, the two main cities of Malawi that are far from most districts8. Secondly, there are long turnaround times and missing results from some laboratories. For example, in a study looking at cervical cancer cases within a large referral hospital in Blantyre, approximately half of the cases who submitted cervical biopsies received their pathology results, with about half of those patients receiving their results two months after submitting samples9. Finally, patients using some laboratories are required to pay for the services in order to facilitate a quick turnaround time. In a context such as Malawi, where as much as half of the population is poor, many patients cannot afford to pay10,11. Due to these reasons, pathology-based diagnosis of cancers is limited in Malawi. In the last 10 years, less than 20% of cancers were confirmed histologically in palliative care and cancer clinics in Malawi12–14,17.
In Neno District, one of the most rural districts in Malawi, Partners In Health (PIH) have supported pathology services for patients attending public medical services since 2011. We hypothesise that the pathology results obtained may have a role in improving diagnosis for suspected extra-pulmonary TB and/or malignancies in this rural district. Therefore, we describe here the results obtained from pathology examination of specimens obtained in Neno District, Malawi, from May 2011 to July 2017, with an emphasis on malignancies and TB. Specifically, we aim to contribute to the knowledge gap regarding the significant burden of malignancies and extra pulmonary TB in Malawi. We present data from a rural setting, which may be one of the first districts in Malawi to have extensive pathology results. We explore the sample turnaround time, diagnostic yield, sample types, and results obtained from all the pathologies during this period.
Methods
Setting
This is a descriptive, retrospective study conducted in the rural district of Neno, in the southwest zone of Malawi. With an estimated population of 165,000 in 201715, Neno has two hospitals and 12 primary health facilities. Most of the samples presented in this study were collected at the two hospitals, given that the hospitals had qualified staff, materials, and resources to routinely collect and send samples for pathological examination. The two hospitals are public and therefore free for all at the point of care.
Sample collection
Since 2011, the district has routinely collected and sent samples to Kamiza Pathology Laboratory for pathological examination. Kamiza Pathology Laboratory is a Blantyre-based private laboratory located at least two hours—just over 100 kilometres—away from Neno District. The type and sources of samples were recorded on a standardized laboratory form at the hospital level. Upon collection of the samples, all samples were sent to Kamiza Pathology Laboratory depending on the next available transport. Currently, single specimens cost 17,820 Malawian Kwacha (MWK) (equivalent to 24 USD in October 2017) and 11,000 MWK (equivalent to 15 USD in October 2017) for histology and cytology examination, respectively.
At the laboratory, samples were accessioned with a unique histology or cytology number. The histology samples were processed, paraffin embedded, and Haematoxylin and Eosin (H&E) stained. The cytology samples were alcohol fixed or air dried and stained with Pap or Diff Quick stain, as appropriate. The slides were read, and reports generated.
After pathological processing of the samples and interpretation of the slides, all results were sent to Neno as soft copies through email to facilitate quick decision making. Hard copies were then collected by the Neno staff weekly.
Data management, outcomes, and analysis
All results from May 2011 to July 2017 were retrieved from pathology laboratory forms and entered into a Microsoft Excel database. We included basic demographic characteristics of the patients, sample turnaround time, site and specimen types, and final diagnosis of the pathology examination. As there can be variations in the definition of turnaround time, we defined turnaround time as the time the sample was collected in Neno to the time the sample results were reported on the form. This turnaround time combined times from sample collection by the health workers, temporary storage at Neno laboratory, transportation to Kamiza pathology, laboratory sample processing, and the time to results being reported by the laboratory. We categorized sample types as either histology or cytology. Histology samples included open and core needle biopsies. Cytology samples included fine needle aspiration and fluid examination of the specimens.
All results were classified as either diagnostic or non-diagnostic. Non-diagnostic results were where the samples were not representative, were inadequate, or were not useful to make the diagnosis and required repetition of the sample collection. All clinically meaningful results were defined as diagnostic results and were further sub-classified as malignant, premalignant, benign lesion, tuberculosis, infection and inflammatory, other miscellaneous conditions, and normal results. With specific emphasis on malignant and tuberculosis samples, we describe sample site and type, gender, and age category (0–14 years, 15–60 years, and over 60 years) of the cases.
All data were entered into Microsoft Excel. Data cleaning and analysis occurred in Stata 14 by StatCorp LP. We used descriptive statistics to describe our outcomes.
Ethical considerations
The study was covered by the National Health Sciences Research Committee protocol #1216 and the local Ministry of Health.
Results
Between May 2011 and July 2017, 532 specimen results were reviewed. The average turnaround time for results was 3.7 days (N= 531, range: 0–35 days). Of all specimens, 87% (n=465) were histology samples and 13% (n=67) were cytology samples.
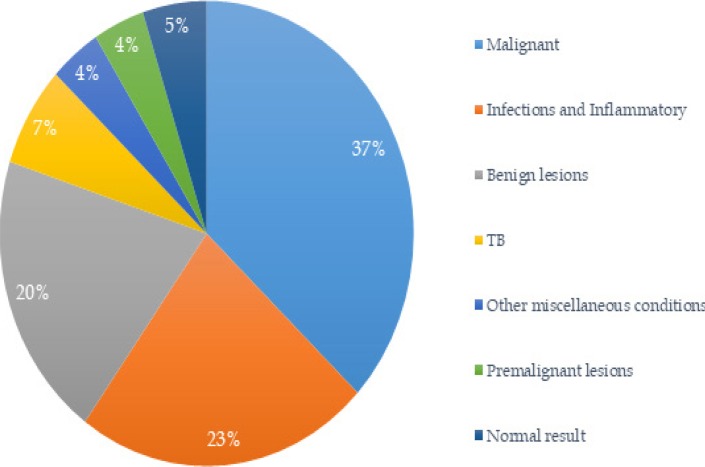
About 92% (n=492) of all samples were diagnostic. Among diagnostic results, 37% (n=183) were malignant, 23% (n=112) were infections and inflammatory conditions, 20% (n=97) were benign lesions, 7% (n=34) were tuberculosis, 4% (n=22) were due to other miscellaneous conditions, and 4% (n=21) were premalignant lesions (Figure 1). Only 5% (n=23) had normal results. Among the non-diagnostic samples (n=40), 65% (n=26) were histology and 35% (n=14) were cytology.
Figure 1.
Pathology diagnostic results in Neno
Of the diagnostic results that were identified as premalignant lesions (n=21), 76% (n=16) were lesions from the cervix, followed by atypical endometrial dysplasia (14%, n=3) and dysplasia of skin (10%, n=2).
The most common sample collection sites for malignancies were skin (30%, n=55), cervix (27%, n=50), lymph nodes (16%, n=30), breast (5%, n=9), and penis (3%, n=6), contributing to over 80% of all biopsied sites. In both sexes, cervical cancer (27%, n=49), Kaposi sarcoma (22%, n=40), cancers of the skin (12%, n=22), Hodgkin's lymphoma (5%, n=10), secondary lymph node cancers (5%, n=10), non-Hodgkin's lymphoma (5%, n=9), and breast cancer (5%, n=9) were the most common cancers (Table 1). By age category, 75% of all cancers occurred in patients between the ages of 15–60 years. Females accounted for 63% (n=114) of all cancer patients.
Table 1.
Common malignancies in Neno
| Type of cancer | Prevalence | Gender | Age | |||
| N (%) | Female (N, %) |
Male (N, %) |
Below 15 (N, %) |
15–60 (N, %) |
Over 60 (N, %) |
|
| Cervix | 49 (26.8) | 49 (43.0) | 0 (0) | 0 (0) | 35 (25.5) | 13 (40.6) |
| Kaposi sarcoma |
40 (21.8) | 16 (14.0) | 24 (34.8) | 3 (30) | 35 (25.5) | 1 (3.1) |
| Skin cancer | 22 (12.0) | 10 (8.8) | 12 (17.4) | 1 (10) | 15 (10.9) | 6 (18.8) |
| Hodgkin's lymphoma |
10(5.5) | 2(1.8) | 8(11.6) | 3(30) | 4(2.9) | 2(6.3) |
| Secondary lymph node malignancies |
10 (5.5) | 6 (5.3) | 4 (5.8) | 1 (10) | 4(2.9) | 5(15.6) |
| non-Hodgkin's lymphoma |
9 (4.9) | 3(2.6) | 6(8.7) | 0(0) | 9(6.6) | 0(0) |
| Breast | 9 (4.9) | 9 (7.9) | 0 (0) | 0 (0) | 8 (5.8) | 1 (3.1) |
| Penis | 6 (3.3) | 0 (0) | 6 (8.7) | 0 (0) | 5 (3.6) | 1 (3.1) |
| Female genital cancers* |
6(3.3) | 6(5.3) | 0(0) | 0(0) | 6(4.4) | 0(0) |
| Liver | 4(2.2) | 3(2.6) | 1(1.5) | 0(0) | 3(2.2) | 1(3.1) |
| Bone | 4 (2.2) | 3 (2.6) | 1 (1.5) | 2 (20) | 2 (1.5) | 0 (0) |
| Gastrointestinal cancers** |
4(2.2) | 4 (3.5) | 0 (0) | 0 (0) | 4 (2.9) | 0 (0) |
| Others | 10 (5.5) | 3(2.6) | 7 (10.1) | 0 (0) | 7 (5.1) | 1 (3.1) |
| Total | 183 (100) | 114(100) | 69 (100) | 10 (100) | 137 (100) | 32 (100) |
Cervical cancer was the most common cancer in females, contributing to nearly half of all cancers (43%, n=49). The next most common cancers in females were Kaposi sarcoma (14%, n=16) and skin cancer (9%, n=10). Breast cancer, lymphomas, secondary lymph node cancers, and other cancers contributed to less than 8% of all cancers in females. In contrast, Kaposi sarcoma was the most common cancer in males, contributing to over one third of all cancers (35%, n=24), followed by skin cancer (17%, n=12) and Hodgkin's lymphoma (12%, n=8). The rest of the cancers contributed to 10% or less of the total cancers males. Only two cases—both females—were found to have oesophageal cancer.
Based on morphological subtypes, there was diversity around the types of cervical, skin, and secondary lymph node cancers. For cervical cancers (n=49), 84% (n=41) were squamous cell carcinoma, 14% (n=7) were adenocarcinoma, and 2% (n=1) were not specified. For skin cancers (n=22), 64% (n=11) were malignant melanomas, followed by squamous cell carcinoma (32%, n=7) and basal cell carcinoma (9%, n=2)—the rest were other types of cancers. Squamous cell carcinoma was the most common type of secondary lymph node cancer, contributing to half of all cancers (50%, n=5), with adenocarcinoma (n=2) and other non-specified carcinomas (n=3) representing the rest of the samples.
A TB diagnosis was made in 34 samples, or 7% of the specimens (Table 2). The most common site for TB was the lymph node, contributing to 56% (n=19) of all TB cases. Most patients diagnosed with TB were between 15–60 years old.
Table 2.
Tuberculosis cases in Neno
| Type of TB | Number of Total TB cases |
Gender | Age | |||
| N, % | Female (N, %) |
Male (N, %) |
Below 15 (N, %) |
15–60 (N, %) |
Over 60 (N, %) |
|
| Lymph node | 19 (55.9) | 9 (47.4) | 10 (66.7) | 4 (44.4) | 14 (60.9) | 1 (50) |
| Skin and soft tissue |
7 (20.6) | 5 (26.3) | 2 (13.3) | 3 (33.3) | 4 (17.4) | 0 (0) |
| Abdomen | 3 (8.8) | 1 (5.3) | 2 (13.3) | 1 (11.1) | 2 (8.7) | 0 {0) |
| Chest | 3 (8.8) | 2 (10.5) | 1 (6.7) | 0 (0) | 2 (8.7) | 1 (50) |
| Breast | 2 (5.9) | 2 (10.5) | 0 (0) | 1 (11.1) | 1 (4.3) | 0 (0) |
| Total | 34 (100) | 19 (100) | 15 (100) | 9 (100) | 23 (100) | 2 (100) |
Discussion
To our knowledge, this is the first study to describe routine pathology results for the diagnosis of cancers and TB in a rural district of Malawi. This data adds to the body of literature about TB, premalignant conditions, and cancers in Malawi, particularly amongst a rural and impoverished population.
In this study, cancers alone contributed to 4 out of 10 samples that were sent for pathological analysis. By adding premalignant and TB cases, nearly half of all biopsies in Neno were diagnostic for these conditions. The high degree of these diagnoses suggests little waste in the pathology support as it is set up in Neno District.
Although it was not possible to investigate pre-biopsy diagnosis of patients in this study, we know from one study in Blantyre that cancer can be clinically misdiagnosed as TB due to inaccessibility of pathology services in pre-referral and mainly rural facilities16. In the Blantyre study, TB, which was diagnosed mainly on clinical suspicion, resulted in wrong diagnoses and delays in cancer diagnoses by up to 5 months. If pathology services were not available in Neno, it is possible that some of the cancer patients included in this study would have been similarly misdiagnosed and experienced delays. Similarly, we do not have pre-biopsy diagnoses for the patients diagnosed with TB in this study, so we cannot ascertain the likelihood of whether these patients would have been treated presumptively for TB or if they would have remained undiagnosed. However, TB case notification rates in both Neno and Malawi generally are lower than expected, and we posit that biopsy as an additional case-finding mechanism could contribute to an increase in case notification rates3.
Based on the high yield of results in this study, we recommend that clinically suspected lesions in a rural district should be biopsied and sent for pathological examination, to ensure an accurate diagnosis and hopefully impact treatment decisions. As much as possible, we would encourage abandoning clinical suspicion of cancers in favour of pathological confirmation to allow for accurate cancer and TB diagnosis. It is therefore essential that district hospitals in Malawi have access to high-quality and timely pathological services to achieve this goal.
The turnaround time in Neno is much lower than that reported in other studies in urban areas, where average times have been reported to be as high as 18 days for paid samples and 43 days for non-paid samples in one study in Blantyre10. As Neno District has no dedicated transport for pathology samples to Blantyre, the district relies on transport support from both the Ministry of Health and PIH, who routinely transport samples with any vehicle that is going to Blantyre. Additionally, the results from the laboratory are immediately emailed to the Neno laboratory and clinical teams. This allows the team to have the results as soon as they are reported.
In this review, cervical cancer and Kaposi sarcoma were the two main cancers in both sexes, with cervical cancer being most common in females and Kaposi Sarcoma being most common in males. This is similar to a population-based cancer prevalence study in Malawi, as well as other comparable studies, although our sample is likely biased toward lesions more likely to be biopsied in a district hospital setting12–14. However, in these studies, oesophageal cancer is often reported as the third most common cancer13,14. In fact, one study at a referral hospital estimated that oesophageal cancer was the most common cancer18. However, in our study, only two cases of oesophageal cancer were found within the time period studied. This discrepancy between Neno and data from across the rest of the country may be due to an inability in our facilities to obtain oesophageal samples, since endoscopy is not performed at many public district hospitals. This is therefore an important area of future study and intervention.
With the difficulties in diagnosing TB—particularly extra pulmonary TB—biopsies provide an alternative method, especially for accessible masses like lymph nodes19,20. As evidenced in this study, 7% of our cases had a diagnosis of TB. Additionally, biopsies may help to reduce the misdiagnosis of TB when patients actually have malignancies, a scenario reported in Blantyre16.
Although we explored some demographic characteristics of TB and malignancy patients in our sample, we did not ascertain the HIV status of the cases. There is evidence describing the rise of non-AIDS defining cancers (as more people are on antiretroviral drugs), although AIDS defining cancers continue to pose challenges in countries with a high HIV burden17,21,22. In Malawi, a country where 8.8% of adults (15–49 years) live with HIV23, this information linking cancers to HIV status is critical in order to inform better clinical care. We are now documenting HIV status in samples that are sent for analysis.
As a descriptive study, this review of biopsy cases did not explore if patients who received a diagnosis of cancer, pre-malignant lesions, or TB, received appropriate clinical care. However, from prior experience, patients with a diagnosis of Kaposi sarcoma receive chemotherapy in Neno District and outcomes have been shown to be excellent24,25. Additionally, patients with other cancers, apart from Kaposi sarcoma, are referred to Queen Elizabeth Central Hospital's Oncology Unit, the nearest referral facility.
Finally, this is laboratory- and facility-based data and therefore captures only patients that presented at health facilities. This has limitations in terms of generalisation to the cancer picture in the population of Neno. Additionally, the biopsies were performed based on the individual skills of health care workers, thus potentially limiting the ability to make diagnoses of other cancers such as oesophageal cancer.
Conclusions
The results presented here describe the burden and distribution of histologically confirmed cancer and tuberculosis at district level health facilities in rural Malawi. Since TB, premalignant lesions, and cancers, contribute to nearly half of all pathology samples from a rural hospital, we advocate for routine use of pathology at all districts in Malawi.
Acknowledgments
We thank all the clinical staff from Neno District health facilities who, in an effort to provide better care, collected the samples for pathological analysis. We also extend gratitude to all PIH and Ministry of Health workers and support staff for their tireless efforts in helping patients access better quality care.
Conflict of interest
Dr Steve Kamiza is the owner of Kamiza Pathology Laboratory. The pathology services in Neno was set up in 2011 as part of routine clinical services and Kamiza Pathology Laboratory was chosen based on closest available pathology laboratory during that time. The decision to inform all authors, including Dr. Steve Kamiza, of this manuscript's design and preparation was made after July 2017 and data from 2011 to July 2017 was analysed. Therefore, Dr Steve Kamiza had no influence on changing the turnaround time or any of the results of this study prior to July 2017, as he was not aware that the results from the laboratory will be analysed and submitted for publication.
All other authors have no other conflicts of interest to declare.
Author contributions
CK conceptualized the study and performed data cleaning and analysis. AP and FM collected and entered the data. CK wrote the first draft. All authors contributed to the study and approved the final version for publication.
Funding
The payment for the pathology services for Neno patients was provided by PIH as a part of strengthening public services for patient care in Neno, Malawi. The authors did not receive any funding for data collection, cleaning, manuscript writing, or submission of the article.
References
- 1.Fleming KA, Naidoo M, Wilson M, Flanigan J, Harton S, Kuti M, et al. An essential pathology package for low- and middle-income countries. Am J Clin Pathol. 2017;147(1):15–32. doi: 10.1093/AJCP/AQW143. [DOI] [PubMed] [Google Scholar]
- 2.Morhason-bello IO, Odedina F, Rebbeck TR, Harford J, Dangou J, Denny L, et al. Challenges and opportunities in cancer control in Africa : a perspective from the African Organisation for Research and Training in Cancer. Lancet Oncol. 2013;14:e141–e151. doi: 10.1016/S1470-2045(12)70482-5. [DOI] [PubMed] [Google Scholar]
- 3.Government of the Republic of Malawi, author. Health Sector Strategic Plan II 2017–2022: Towards Universal Health Coverage. Lilongwe: Government of the Republic of Malawi; 2017. [Google Scholar]
- 4.Rubinstein P, Aboulafia D, Zloza A. Malignancies in HIV/AIDS:from Epidemiology to Therapeutic Challenges. AIDS. 2014;28(4):553–465. doi: 10.1002/wrna.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6–11. doi: 10.1097/COH.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cundale K, Wroe E, Matanje-Mwagomba BL, Muula AS, Gupta N, Berman J, et al. Reframing noncommunicable diseases and injuries for the poorest Malawians: The Malawi national NCDI poverty commission. Malawi Med Journal. 2017;29(2):194–197. doi: 10.4314/mmj.v29i42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wroe EB, Cundale K, Kasomekera N, Masamba L, Gopal S, Crampin M, et al. Prioritizing Non-Communicable Diseases and Injuries Amongst the Poorest in Malawi: Where's Cancer? Second Malawi Cancer Consortium; 2017, 28–29 August. Lilongwe: Malawi cancer consortium; 2017. [Google Scholar]
- 8.Gopal S, Krysiak R, Liomba NG, Horner M, Shores CG, Alide N, et al. Early Experience after Developing a Pathology Laboratory in Malawi, with Emphasis on Cancer Diagnoses. PLoS One. 2013;8(8):6–13. doi: 10.1371/journal.pone.0070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudd P, Gorman D, Meja S, Mtonga P, Jere Y, Chidothe I, et al. Cervical cancer in southern Malawi : A prospective analysis of presentation, management, and outcomes. Malawi Med J. 2017;29(2):124–129. doi: 10.4314/mmj.v29i2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masamba L, Mtonga P, Kalilani-Phiri, Bychkovsky B. Cancer Pathology Turnaround Time at Queen Elizabeth Central Hospital, the Largest Referral Center in Malawi for Oncology Patients. J Glob Oncol. 2017:1–6. doi: 10.1200/JGO.2015.000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Statistical Office, author. Integrated Household Survey 2010–2011: Household and Social Economic Characteritics Report. Zomba: National Statistical Office; 2012. [Google Scholar]
- 12.Msyamboza K, Dzamalala C, Mdokwe C, Kamiza S, Lemerani M, Dzowela T, et al. Burden of cancer in Malawi; common types, incidence and trends: National population-based cancer registry. BMC Res Notes. 2012;5(1):149. doi: 10.1186/1756-0500-5-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukhula V, Sibale D, Tarmahomed L, Dzamalala C, Msyamboza K, Chasimpha S. Characterising cancer burden and quality of care at two palliative care clinics in Malawi. Malawi Med J. 2017;29(2):130–135. doi: 10.4314/mmj.v29i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Msyamboza KP, Manda G, Tembo B, Thambo C, Chitete C, Mindiera C, et al. Cancer survival in Malawi: A retrospective cohort study. Pan Afr Med J. 2014;19:234. doi: 10.11604/pamj.2014.19.234.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Statistical Office, author. Malawi population and housing census. Zomba: National Statistical Office; 2008. [Google Scholar]
- 16.Masamba LPL, Jere Y, Brown ERS, Gorman DR. Tuberculosis Diagnosis Delaying Treatment of Cancer: Experience From a New Oncology Unit in Blantyre, Malawi. J Glob Oncol. 2016;2(1):26–29. doi: 10.1200/JGO.2015.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moses A, Mwafongo A, Chikasema M, Kafantenganji L, Stanely C, Chimzukira E, et al. Risk factors for common cancers among patients at Kamuzu Central Hospital in Lilongwe, Malawi : A retrospective cohort study. Malawi Med J. 2017;29(2):136–141. doi: 10.4314/mmj.v29i2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mtonga P, Masamba L, Milner D, Shulman LN, Nyirenda R, Mwafulirwa K. Biopsy case mix and diagnostic yield at a Malawian central hospital. Malawi Med J. 2013;23(3):62–64. [PMC free article] [PubMed] [Google Scholar]
- 19.Mabedi C, Kendig C, Liomba G, Shores C, Chimzimu F, Kampani C, et al. Causes of cervical lymphadenopathy at Kamuzu Central Hospital. Malawi Med J. 2014;26(1):16–19. [PMC free article] [PubMed] [Google Scholar]
- 20.Purohit M, Mustafa T. Laboratory diagnosis of extra-pulmonary tuberculosis (EPTB) in resource-constrained setting: State of the art, challenges and the need. J Clin Diagnostic Res. 2015;9(4):EE01–EE06. doi: 10.7860/JCDR/2015/12422.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adebamowo CA, Casper C, Bhatia K, Mbulaiteye SM, Sasco AJ, Phipps W, et al. Challenges in the Detection, Prevention, and Treatment of HIV-Associated Malignancies in Low- and Middle-Income Countries in Africa Clement. J Acquir Immune Defic Syndr. 2015;67(1) doi: 10.1097/QAI.0000000000000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hübel K. HIV Associated Malignancies. Oncol Res Treat. 2017;40:80–81. doi: 10.1159/000456716. [DOI] [PubMed] [Google Scholar]
- 23.National Statistical Office Malawi and ICF, author. Malawi Demographic and Health survey 2015–2016. Zomba, Malawi and Rockville, Maryland, USA: National Statistical Office and ICF; 2017. [Google Scholar]
- 24.Herce ME, Kalanga N, Wroe EB, Keck JW, Chingoli F, Tengatenga L, et al. Excellent clinical outcomes and retention in care for adults with HIV-associated Kaposi sarcoma treated with systemic chemotherapy and integrated antiretroviral therapy in rural Malawi. J Int AIDS Soc. 2015:18. doi: 10.7448/IAS.18.1.19929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herce ME, Elmore SN, Kalanga N, Keck JW, Wroe EB, Phiri A, et al. Assessing and responding to palliative care needs in rural sub-Saharan Africa: Results from a model intervention and situation analysis in Malawi. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0110457. [DOI] [PMC free article] [PubMed] [Google Scholar]