Abstract
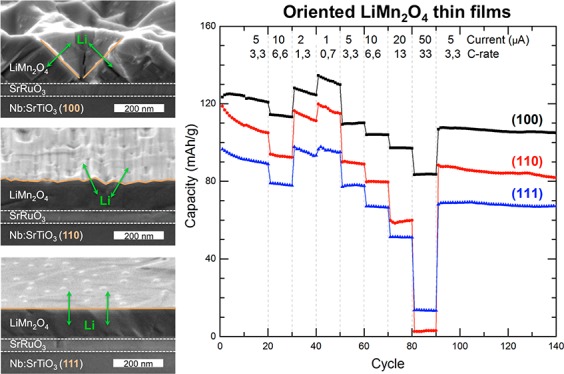
A promising cathode material for rechargeable batteries is LiMn2O4, which exhibits higher operating voltage, reduced toxicity and lower costs as compared to commonly used LiCoO2 cathodes. However, LiMn2O4 suffers from limited cycle life, as excessive capacity fading occurs during battery cycling due to dissolution of Mn into the acidic electrolyte. Here, we show that by structural engineering of stable, epitaxial LiMn2O4 thin films the electrochemical properties can be enhanced as compared to polycrystalline samples. Control of the specific crystal orientation of the LiMn2O4 thin films resulted in dramatic differences in surface morphology with pyramidal, rooftop or flat features for respectively (100), (110), and (111) orientations. All three types of LiMn2O4 films expose predominantly ⟨111⟩ crystal facets, which is the lowest energy state surface for this spinel structure. The (100)-oriented LiMn2O4 films exhibited the highest capacities and (dis)charging rates up to 33C, and good cyclability over a thousand cycles, demonstrating enhanced cycle life without excessive capacity fading as compared to previous polycrystalline studies.
Keywords: battery cathode, LiMn2O4, thin films, epitaxy, crystal orientation, spinel, pulsed laser deposition, cycle life
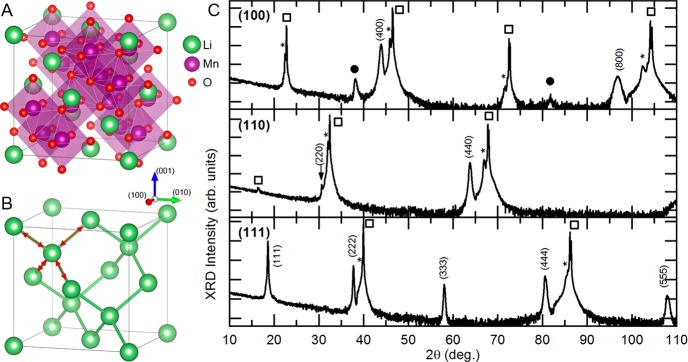
Since their introduction in the 1990s, lithium-ion batteries have become the main power source for portable electronics and power tools applications. As society transitions toward electric and zero emission mobility, next-generation electric cars require lithium batteries with superior energy and power density, without compromising safety and environmental concerns.1,2 The cycle life and lifetime are dependent on the nature of the interfaces between the electrodes and electrolyte, whereas safety is a function of the stability of the electrode materials and their interfaces with electrolyte.3−5 Existing batteries, using conventional layered oxide cathodes, are not only reaching their power and energy density limits, but their application in electric mobility and large applications is also limited by their inadequate cycle life and inherently poor safety features.6 On the other hand, spinel LiMn2O4, has emerged as a promising cathode material for next-generation lithium batteries7,8 because of its relatively high operating voltage (4.1 V vs Li) and comparable energy density (theoretically 148 mAh/g, typical 120 mAh/g) combined with low cost and absence of direct environmental or safety hazards. In spinel LiMn2O4 (space group Fd3̅ m), Li and Mn occupy tetrahedral (8a) and octahedral (16d) sites in the intervening cubic close-packed array of oxygen atoms (32e sites) (Figure 1a). The edge-shared octahedral Mn2O4 host framework provides structural stability and interconnects face-shared tetrahedral lithium (8a) sites and empty octahedral (16c) sites (Figure 1b). These interconnected pathways allow the three-dimensional diffusion of lithium ions within the Mn2O4 framework, making LiMn2O4 suitable for high power application. The lithium (de)intercalation at (8a) tetrahedral sites results into the characteristic ∼4 V voltage plateau without distorting the spinel symmetry. Interestingly, this Mn2O4 framework can further host lithium into empty octahedral (16c) sites, resulting in a 3 V voltage plateau, almost doubling its capacity (theoretical capacity of Li2Mn2O4 is 285 mAh/g) while undergoing a cubic to tetragonal phase transition. Furthermore, the operating voltage of LiMn2O4 can be increased to ∼5 V by partially substituting Mn with Ni in the Mn2O4 framework.9
Figure 1.
(a) Schematics of the spinel LiMn2O4 crystal structure, and (b) the structural network for lithium diffusion within the LiMn2O4 crystal. (c) Out-of-plane XRD measurements of 110 nm LiMn2O4 epitaxial thin films on 50 nm SrRuO3-coated Nb-SrTiO3 substrates with different crystal orientations: (100), (110), and (111). Nb-SrTiO3 substrate peaks are indicated by □, and SrRuO3 are indicated by *, whereas minor contributions of Mn2O3 phase are given by •.
Despite these advantageous properties, LiMn2O4 cathodes suffer from fading capacity and poor cycle life performance.10 The origin of this capacity loss was attributed to two factors: first, the onset of Jahn–Teller distortion in deeply discharged electrodes,1,11,12 and second, the dissolution of Mn ions from the Mn2O4 framework.13 The Jahn–Teller distortion, accompanied by the cubic to tetragonal phase transition, irreversibly damages the structural integrity of the spinel framework during deep cycling down to ∼3 V and causes permanent capacity loss. However, this Jahn–Teller distortion can be avoided by limiting the charging and discharging to the ∼4 V plateau. Whereas, Mn dissolution causing continuous loss of active material and consequently blocking of 3D lithium diffusion pathways, impedes the overall cell performance and remains the key limitation for using LiMn2O4 cathodes.2 Previous studies have suggested that acidification of electrolyte, caused by reaction of hexafluorophosphate (LiPF6) salt in electrolyte with H2O, coupled with oxygen loss at the cathode surface, to be the origin of Mn dissolution.3,4,13 The underlying mechanism can be understood via a disproportional reaction of Mn3+ generating soluble Mn2+: 4H+ + 2Li (Mn3+Mn4+)O4→ 3λ-Mn4+O2 + Mn2+ + 2Li+ + 2H2O. Various strategies have been suggested to mitigate the Mn dissolution of LiMn2O4, such as aliovalent doping, surface coating, nanostructuring, mixed phase synthesis.3,5−10,14−20 Although these strategies have indisputably shown significant enhancement in LiMn2O4 performance, it remains far from the desired level for usage in applications. Studies have shown that the specific crystal facet in contact with the electrolyte plays an important role in the electrochemical reactions occurring at the cathode surface for single crystalline nanowires,21 truncated structures22 and thin films.23 It was concluded that, as the ⟨111⟩ crystal facet possesses the lowest surface energy and the most dense Mn atom arrangement, it can form a stable solid electrolyte interphase (SEI) layer and mitigate Mn dissolution, thus improving cycling stability. However, the (100)- and (110)-oriented facets were regarded to be better aligned to the lithium diffusion channels, thus able to increase discharge capacities and to facilitate high rate capabilities.24
Therefore, perfect control on the interfacial properties between the electrodes and electrolyte is needed but remains a great challenge. Detailed understanding of the electrochemical behavior of specific crystal facets of battery materials can only be obtained when a single type of crystal orientation interfacing the electrolyte can be synthesized. This crucial requirement can be achieved by epitaxial thin film technology, in which the flat surface and restricted lattice plane of the thin film cathode simplify the reaction mechanism at such highly ordered cathode-electrolyte interface. Most studies on LiMn2O4 thin films have investigated polycrystalline samples, while only limited experimental research has been performed on single crystalline thin films.23,25−30 Characterization of such epitaxial thin films has previously been focused on the structural properties, and only few reports have shown electrochemical properties by clear redox peaks in the cyclic voltammetry, and discharge capacities of ∼125 mAh/g with clear plateau regions in the charge–discharge curves.27,29,30 Detailed insight into the relation between the specific crystal orientation toward the adjacent electrolyte and its electrochemical behavior has been lacking, which has hampered the successful development of high-quality LiMn2O4 cathode with high cyclability. A detailed study by Hirayama et al. concluded from surface X-ray diffraction measurements that a solid-electrolyte interface (SEI) was present on both (111) and (110) surfaces, although the (110) surface was less stable and indicated a higher Mn dissolution.26 So far the electrochemical performance was only reported for LiMn2O4 thin films grown on (111)-oriented SrTiO3 substrates,27,30 where an additional Li3PO4 coating was added to prevent a phase transition of the surface region and to suppress Mn dissolution and desorption of oxygen from the surface.
Here, we show that by structural engineering of stable, epitaxial LiMn2O4 thin films the electrochemical properties can be controlled and enhanced as compared to polycrystalline samples. By changing the crystal orientation of the underlying single crystalline substrate ((100), (110) and (111)) we can control the specific orientation of the LiMn2O4 thin film and, therefore, the cathode surface toward the adjacent electrolyte. All three types of LiMn2O4 films exhibit surfaces exposing predominantly ⟨111⟩ crystal facets, the lowest energy state surface for this spinel structure, which results in dramatic differences in surface morphology with pyramidal, rooftop or flat features for respectively (100), (110), and (111) LiMn2O4 films. Interestingly, the (100)-oriented films exhibited the highest capacities, (dis)charging rates up to 33C, and good cyclability over a thousand cycles, demonstrating enhanced cycle life without excessive capacity fading as compared to polycrystalline studies.20
Epitaxial engineering is used in this study to control the crystal orientation of LiMn2O4 thin films, which enables a unique insight into the relation between electrochemistry and crystal directionality, not obtainable in single crystals or polycrystalline samples. LiMn2O4 thin films were grown by pulsed laser deposition (PLD) on various single crystal Nb-doped (0.5 wt %) SrTiO3 (Nb:STO) substrates with different crystal orientations ((100), (110) and (111)). All LiMn2O4 (LMO) thin films were deposited under the same conditions and have a thickness of ∼110 nm. A 50 nm SrRuO3 (SRO) layer was deposited as an intermediate layer to enhance the electrical transport between the LMO cathode and the conducting Nb:STO substrate.27
The structural quality of the LMO films was investigated by X-ray diffraction (XRD) analysis, as shown in Figure 1c. The three types of LMO films grown on Nb:STO substrates with different orientations exhibit coherent growth in which the out-of-plane crystal orientation of the films is aligned with the orientation of the substrate. The LMO(111) and LMO(110) films show the presence of highly crystalline epitaxial layers, with a lattice parameter of ∼8.25 Å, without any impurity phase, in good agreement with previous studies of LMO growth on STO(111) and STO(110) substrates.23,27 This suggests that the PLD process parameters (e.g., temperature, pressure, laser energy density, target composition) were optimized successfully to correct for any loss of volatile lithium during ablation, nucleation or growth. Interestingly, the LMO films with (100)-orientation show minor contributions of a secondary phase, although all three LMO films were grown during the same deposition procedure. The extra peaks suggest the presence of a small amount of Mn2O3,31 which will have a negligible effect on the electrochemical performance of the (100)-oriented LiMn2O4 thin films, as the anodic reduction and cathodic oxidation reactions in Mn2O3 take place below ∼1.3 V.31,32 This is far below the potential window in our measurements of 3.6–4.5 V. In our case the coexistence of this lithium deficient phase could be due to the enhanced lithium volatility at the (100) surface of LiMn2O4.18
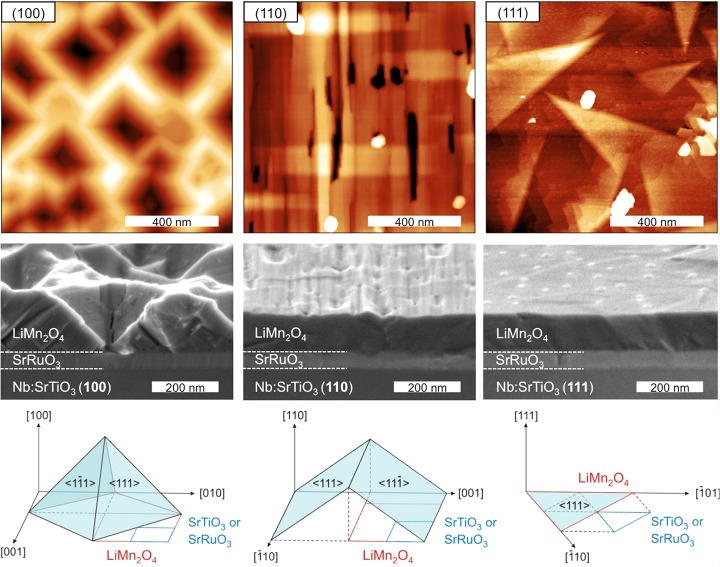
The alignment of the out-of-plane crystal orientation for all types of LMO films, suggests an epitaxial relation between the crystal structures of the deposited LMO films and the underlying Nb:STO substrates, although large differences exist between spinel LMO (a = 8.25 Å) and perovskite STO (a = 3.90 Å). The observed preferred orientation of the LMO films was confirmed by detailed analysis of the in-plane orientation by XRD (not shown) and the surface morphology through atomic force microscopy (AFM), see Figure 2. The surface of the LMO(100) film exhibits square-like structures with significant height differences (RMS ≈ 45 nm), which is in good agreement with previously observed octahedron spinel structures.22,33 Such pyramidal spinel structures consist of ⟨111⟩ crystal facets on all four sides with an occasional presence of a truncated top of the pyramid exhibiting a (100) crystal facet. The LMO(110) film forms a layer with rooftop-like structures and a lower surface roughness (RMS ≈ 5 nm), caused by the anisotropic nature of the (110)-plane which favors diffusion of atoms along the [1̅10] direction as compared to the [001] direction.34 This results in elongated ⟨111⟩ crystal facets exposed on the surface, which are all aligned in the same direction. Finally, the LMO(111) film forms a layer with triangle-like structures exhibiting a very low surface roughness (RMS ≈ 1.5 nm). The triangular shape corresponds to the (111) plane in a cubic structure, for which two different types of in-plane triangle orientations can be observed. Therefore, all three types of LMO films with different out-of-plane orientations ((100), (110) and (111)) exhibit surfaces exposing predominantly ⟨111⟩ crystal facets, which confirms that this is the lowest energy state surface of the spinel crystal structure.22
Figure 2.
AFM (top) and SEM (middle) analysis of the surface morphology of 110 nm LiMn2O4 thin films on SrRuO3-coated Nb-SrTiO3 substrates with crystal orientations (100), (110) and (111). SEM images are taken after extensive electrochemical cycling and subsequent cleaning of the surfaces. Schematics (bottom) are shown of the expected crystal facets for the different surface morphologies.
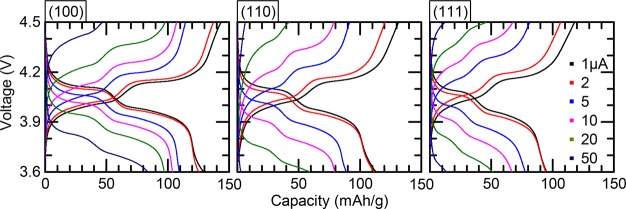
To study the dependence of the lithium transport on the specific crystal orientation of the LMO films, the lithium intercalation characteristics of the LMO thin films were measured by galvanostatic charge–discharge analysis of electrochemical cells against lithium metal with a liquid electrolyte. Figure 3 shows charge–discharge curves for the LMO films with different orientations ((100), (110), and (111)) for various currents, resulting in (dis)charge rates in the range 0.7–33C. The characteristic voltage plateaus for these epitaxial LMO thin films are in good agreement with bulk LMO charge–discharge profiles.7 The total discharge capacity for the slowest rate of 0.7C was the highest for the (100)-oriented LMO film (∼129 mAh/g), whereas the (110)- and (111)-oriented LMO films exhibit lower discharge capacities of respectively ∼113 and ∼95 mAh/g. The large surface area of the (100)-oriented LMO film, caused by pyramidal surface morphology, is considered to cause enhanced lithium kinetics as compared to the other crystal orientations. The crystal facets on all films are predominantly ⟨111⟩, which eliminates any possible effect from local variations in crystal facets. The enhanced lithium kinetics for the (100)-oriented LMO films is also demonstrated by the large capacities still achievable during (dis)charging when using higher rates. The used relatively high currents stress the material more and make the variations in lithium intercalation for the different crystal orientations more pronounced. For currents of 20 μA (∼13C), the discharge capacities for the (110)- and (111)-oriented films drop to ∼50 mAh/g, whereas the (100)-oriented film still exhibits double the capacity (∼100 mAh/g). The initial drop in discharge capacity after the first charge–discharge cycle may be attributed to anionic reaction occurring at upper voltage cutoff combined with irreversible dissolution of surface lithium and manganese.16 Furthermore, it is interesting to note that initially at low currents, all films show a slightly higher charge capacity compared to the discharge. Although the exact origin is still unclear, the difference in charge–discharge capacities are within acceptable Coulombic efficiency limits.
Figure 3.
Charge–discharge analysis of 110 nm LiMn2O4 films with different crystal orientations ((100), (110) and (111)) for various currents (1, 2, 5, 10, 20, and 50 μA). A potentiostatic period of 5 min is used to ensure complete charge or discharge before the next step.
The rate dependence of the discharge capacity is shown in more detail in Figure 4 for the LMO films with different crystal orientations. After the initial 20 charge–discharge cycles with 3.3C the films are consecutively cycled at various rates in the range 0.7–33C before finishing the sequence with the final 40 cycles with 3.3C. The results show the stability of the LMO films during substantial cycling, as well as the enhanced performance of the (100)-oriented film as compared to the other orientations. The cycle performance of the (100), (110), and (111)-oriented LMO thin films was compared after 140 cycles at various C-rates by their discharge capacities of respectively 87, 78, and 76%, as well their Coulombic efficiencies of respectively 97, 94, and 97%. Interestingly, at the highest rate of 33C, the (100)-oriented film still exhibits a capacity of about 84 mAh/g, whereas the capacities of the (110)- and (111)-oriented films have been almost reduced to zero. The observed variation in surface area between the differently oriented films (about 50% more surface area for (100)-oriented films as compared to (110)- and (111)-oriented films, see Figure 2), cannot explain this dramatic difference in lithium kinetics. Therefore, it is suggested that the actual difference in lithium intercalation along different crystal orientations within the LiMn2O4 crystal structure is more pronounced at higher rates when limitations in ionic (lithium) and electric transport become more apparent.
Figure 4.
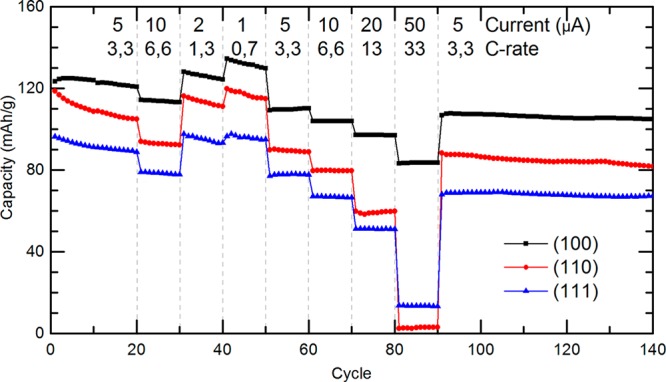
Rate performance analysis of 110 nm LiMn2O4 thin films on SrRuO3-coated Nb-SrTiO3 substrates with different crystal orientations ((100), (110), and (111)) for various currents, and corresponding C rates.
Although the conventional understanding of the Li diffusion in LiMn2O4 is three-dimensional in which the Li ions hop over the 8a and 16c sites along the interconnected pathways, these zigzagging chains form lithium diffusion channels in specific directions.35 Previous studies have suggested that although the (111)-oriented facets exhibit the lowest surface energy,18,36 the (100)- and (110)-oriented facets are better aligned to the lithium diffusion channels, thus increasing discharge capacities and facilitating high rate capabilities.24 Our results demonstrate that very stable LiMn2O4 thin films with ⟨111⟩ surface facets exhibit much higher rate capabilities for the (100)-direction as compared to the (110)-direction. Theoretical modeling would provide detailed insight into the diffusion mechanism in which the ease of lithium diffusion through the bottleneck is studied. This oxygen triangle is formed at the contact face between the tetrahedron about 8a and the octahedron about 16c and depends on the displacement of the O atoms, which can vary in differently oriented epitaxial thin films.
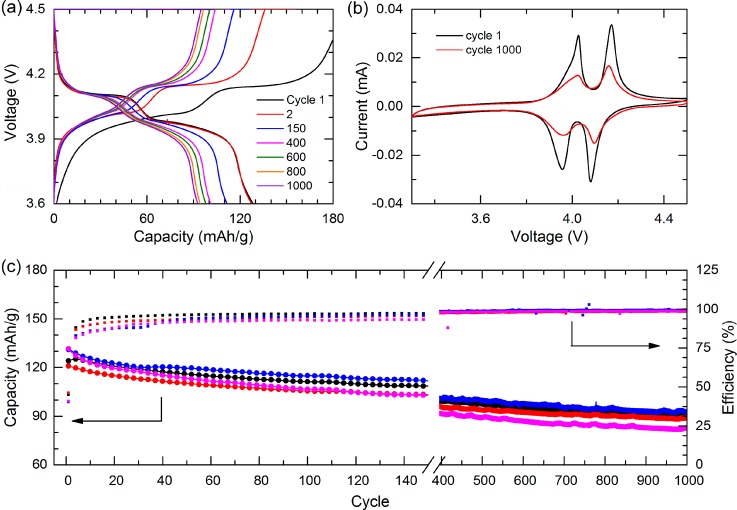
The cycle life of such high-performance (100)-oriented LMO films was investigated for several cells during prolonged battery cycling at similar conditions, see Figure 5. The electrochemical behavior of all four cells exhibited good uniformity with initial capacities of about 120–130 mAh/g, which still provided capacities of about 90 mAh/g after a thousand cycles. The stability of the voltage plateaus over the full thousand cycles is shown in Figure 5a, b, and indicates the unchanged internal resistance during the complete prolonged cycling. Figure 5c displays the enhanced cycle life performance for (100)-oriented LMO films with significantly high capacity and Coulombic efficiency over thousand cycles as compared to previous studies on bulk and polycrystalline LMO for which the capacity drops below 80% within 50 cycles.10,20 The very stable cycle performance with very minimal changes in the internal resistance, as can be seen for the (100)-oriented LiMn2O4 thin films in the constant voltage plateaus in the charge–discharge curves (Figure 5a) and constant peak positions in the cyclic voltammetry (Figure 5b), was confirmed by electrochemical impedance spectroscopy (EIS) experiments during extensive cycling (data not shown).
Figure 5.
Cycle life analysis of four 110 nm (100)-oriented LiMn2O4 thin films on SrRuO3-coated Nb-SrTiO3 substrates. The evolution of (a) charge–discharge and (b) cyclic voltammetry behaviors are shown during prolonged cycling. (c) Discharge capacity (circles) and Coulombic efficiency (squares) are given for a thousand cycles. During the measurements a current of 5 μA was used, which provided a (dis)charge rate of 3.3 C.
In conclusion, structural engineering enables improved control over the electrochemical properties of LiMn2O4 thin films, which is unique for epitaxial thin films and cannot be obtained in single-crystal or polycrystalline samples. Control of the specific crystal orientation of the LMO thin films resulted in dramatic differences in surface morphology with pyramidal, rooftop or flat features for respectively (100), (110) and (111) orientations. All three types of LMO films exhibit surfaces exposing predominantly ⟨111⟩ crystal facets, which is predicted to be the lowest energy crystallographic surface for this spinel structure. The (100)-oriented LMO films exhibited the highest capacities and (dis)charging rates up to 33C, and good cyclability over a thousand cycles, demonstrating enhanced cycle life without excessive capacity fading as compared to previous polycrystalline studies.
Experimental Methods
The LiMn2O4 and SrRuO3 layers were grown by pulsed laser deposition (PLD) at 600 °C on Nb-doped (0.5 wt %) single crystalline SrTiO3 (100, 110 or 111) substrates from sintered Li2Mn2O4 (100 wt % excess Li2O) and SrRuO3 targets, using a KrF excimer laser operating at 248 nm at a repetition rate of 2 Hz. The Nb-SrTiO3 substrates were annealed at 950 °C for 1.5 h in an oxygen flow of 150 mL/min. The oxygen pressure during growth was 0.13 mbar, while the laser energy fluence was 2.3 J cm–2 for the growth of both LiMn2O4 and SrRuO3. After deposition, the thin films were cooled to room temperature in an oxygen pressure of 0.13 mbar at a rate of 10 °C min–1.
The crystal structure, surface morphology, and thickness of the thin films were investigated by X-ray diffraction (PANalytical X’Pert PRO diffractometer), atomic force microscopy (Bruker ICON Dimension Microscope), and scanning electron microscopy (Zeiss Merlin HR-SEM), respectively.
For electrochemical characterization the films were transferred to an argon atmosphere glovebox (<0.1 ppm of H2O and O2) and placed on a hot plate for ∼10 min at 125 °C to remove any water content. Subsequently, they are positioned in an electrochemical EC-ref cell by EL-CELL and combined with a glass fiber separator of 1 mm thickness, 0.6 mL electrolyte with 1 M LiPF6 in 1:1 ethylene carbonate dimethyl carbonate (EC:DMC) and lithium metal anode. The electrochemical measurements were performed at 22 °C using a BioLogic VMP-300 system in a two-electrode setup in which the samples were cycled galvanostatically between 3.6 and 4.5 V with currents of 1, 2, 5, 10, 20, and 50 μA, corresponding to C rates of approximately 0.7, 1.3, 3.3, 6.6, 13, and 33C, respectively. A potentiostatic period of 5 min is used to ensure complete charge or discharge before the next step.
Acknowledgments
T.A.H., D.M.C., D.P.S., and M.H. acknowledge support by The Netherlands Organization for Scientific Research (NWO) under the VIDI grant nr. 13456.
The authors declare no competing financial interest.
References
- Dahn J. R., Ehrlich G. M.. Handbook of Batteries, 4 ed.; McGraw Hill: New York, 2011. [Google Scholar]
- Nitta N.; Wu F. X.; Lee J. T.; Yushin G. Li-ion battery materials: present and future. Mater. Today 2015, 18, 252–264. 10.1016/j.mattod.2014.10.040. [DOI] [Google Scholar]
- Luntz A. C.; Voss J.; Reuter K. Interfacial challenges in solid-state Li ion batteries. J. Phys. Chem. Lett. 2015, 6, 4599–604. 10.1021/acs.jpclett.5b02352. [DOI] [PubMed] [Google Scholar]
- Wang K. X.; Li X. H.; Chen J. S. Surface and interface engineering of electrode materials for lithium-ion batteries. Adv. Mater. 2015, 27, 527–45. 10.1002/adma.201402962. [DOI] [PubMed] [Google Scholar]
- Yuan Y. F.; Amine K.; Lu J.; Shahbazian-Yassar R. Understanding materials challenges for rechargeable ion batteries with in situ transmission electron microscopy. Nat. Commun. 2017, 8, 15806. 10.1038/ncomms15806. [DOI] [Google Scholar]
- Lin F.; Markus I. M.; Nordlund D.; Weng T. C.; Asta M. D.; Xin H. L.; Doeff M. M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 3529. 10.1038/ncomms4529. [DOI] [PubMed] [Google Scholar]
- Thackeray M. M. Manganese oxides for lithium batteries. Prog. Solid State Chem. 1997, 25, 1–71. 10.1016/S0079-6786(97)81003-5. [DOI] [Google Scholar]
- Lee M. J.; Lee S.; Oh P.; Kim Y.; Cho J. High performance LiMn2O4 cathode materials grown with epitaxial layered nanostructure for Li-ion batteries. Nano Lett. 2014, 14, 993–9. 10.1021/nl404430e. [DOI] [PubMed] [Google Scholar]
- Ma J.; Hu P.; Cui G. L.; Chen L. Q. Surface and Interface Issues in Spinel LiNi0.5Mn1.5O4: Insights into a Potential Cathode Material for High Energy Density Lithium Ion Batteries. Chem. Mater. 2016, 28, 3578–3606. 10.1021/acs.chemmater.6b00948. [DOI] [Google Scholar]
- Gummow R. J.; de Kock A.; Thackeray M. M. Improved capacity retention in rechargeable 4 V lithium/lithium-manganese oxide (spinel) cells. Solid State Ionics 1994, 69, 59–67. 10.1016/0167-2738(94)90450-2. [DOI] [Google Scholar]
- Van der Ven A.; Marianetti C.; Morgan D.; Ceder G. Phase transformations and volume changes in spinel LixMn2O4. Solid State Ionics 2000, 135, 21–32. 10.1016/S0167-2738(00)00326-X. [DOI] [Google Scholar]
- Thackeray M. M.; David W. I. F.; Bruce P. G.; Goodenough J. B. Lithium Insertion into Manganese Spinels. Mater. Res. Bull. 1983, 18, 461–472. 10.1016/0025-5408(83)90138-1. [DOI] [Google Scholar]
- Hunter J. C. Preparation of a new crystal form of manganese dioxide: λ-MnO2. J. Solid State Chem. 1981, 39, 142–147. 10.1016/0022-4596(81)90323-6. [DOI] [Google Scholar]
- Lee M. J.; Lho E.; Bai P.; Chae S.; Li J.; Cho J. Low-Temperature Carbon Coating of Nanosized Li1.015Al0.06Mn1.925O4 and High-Density Electrode for High-Power Li-Ion Batteries. Nano Lett. 2017, 17, 3744–3751. 10.1021/acs.nanolett.7b01076. [DOI] [PubMed] [Google Scholar]
- Kim D. K.; Muralidharan P.; Lee H. W.; Ruffo R.; Yang Y.; Chan C. K.; Peng H.; Huggins R. A.; Cui Y. Spinel LiMn2O4 nanorods as lithium ion battery cathodes. Nano Lett. 2008, 8, 3948–52. 10.1021/nl8024328. [DOI] [PubMed] [Google Scholar]
- Bhandari A.; Bhattacharya J. Review—Manganese Dissolution from Spinel Cathode: Few Unanswered Questions. J. Electrochem. Soc. 2017, 164, A106–A127. 10.1149/2.0101614jes. [DOI] [Google Scholar]
- Myung S.-T.; Lee K.-S.; Kim D.-W.; Scrosati B.; Sun Y.-K. Spherical core-shell Li[(Li0.05Mn0.95)0.8(Ni0.25Mn0.75)0.2]2O4 spinels as high performance cathodes for lithium batteries. Energy Environ. Sci. 2011, 4, 935–939. 10.1039/c0ee00298d. [DOI] [Google Scholar]
- Amos C. D.; Roldan M. A.; Varela M.; Goodenough J. B.; Ferreira P. J. Revealing the Reconstructed Surface of Li[Mn2]O4. Nano Lett. 2016, 16, 2899–906. 10.1021/acs.nanolett.5b03926. [DOI] [PubMed] [Google Scholar]
- Jiao F.; Bao J.; Hill A. H.; Bruce P. G. Synthesis of ordered mesoporous Li-Mn-O spinel as a positive electrode for rechargeable lithium batteries. Angew. Chem., Int. Ed. 2008, 47, 9711–6. 10.1002/anie.200803431. [DOI] [PubMed] [Google Scholar]
- Park S. B.; Shin H. C.; Lee W. G.; Cho W. I.; Jang H. Improvement of capacity fading resistance of LiMn2O4 by amphoteric oxides. J. Power Sources 2008, 180, 597–601. 10.1016/j.jpowsour.2008.01.051. [DOI] [Google Scholar]
- Hosono E.; Kudo T.; Honma I.; Matsuda H.; Zhou H. Synthesis of single crystalline spinel LiMn2O4 nanowires for a lithium ion battery with high power density. Nano Lett. 2009, 9, 1045–51. 10.1021/nl803394v. [DOI] [PubMed] [Google Scholar]
- Kim J. S.; Kim K.; Cho W.; Shin W. H.; Kanno R.; Choi J. W. A truncated manganese spinel cathode for excellent power and lifetime in lithium-ion batteries. Nano Lett. 2012, 12, 6358–65. 10.1021/nl303619s. [DOI] [PubMed] [Google Scholar]
- Hirayama M.; Sonoyama N.; Ito M.; Minoura M.; Mori D.; Yamada A.; Tamura K.; Mizuki J.; Kanno R. Characterization of electrode/electrolyte interface with X-ray reflectometry and epitaxial-film LiMn2O4 electrode. J. Electrochem. Soc. 2007, 154, A1065–A1072. 10.1149/1.2778853. [DOI] [Google Scholar]
- Jiang C.; Tang Z.; Wang S.; Zhang Z. A truncated octahedral spinel LiMn2O4 as high-performance cathode material for ultrafast and long-life lithium-ion batteries. J. Power Sources 2017, 357, 144–148. 10.1016/j.jpowsour.2017.04.079. [DOI] [Google Scholar]
- Sonoyama N.; Iwase K.; Takatsuka H.; Matsumura T.; Imanishi N.; Takeda Y.; Kanno R. Electrochemistry of LiMn2O4 epitaxial films deposited on various single crystal substrates. J. Power Sources 2009, 189, 561–565. 10.1016/j.jpowsour.2008.10.037. [DOI] [Google Scholar]
- Hirayama M.; Ido H.; Kim K.; Cho W.; Tamura K.; Mizuki J.; Kanno R. Dynamic structural changes at LiMn2O4/electrolyte interface during lithium battery reaction. J. Am. Chem. Soc. 2010, 132, 15268–76. 10.1021/ja105389t. [DOI] [PubMed] [Google Scholar]
- Suzuki K.; Kim K.; Taminato S.; Hirayama M.; Kanno R. Fabrication and electrochemical properties of LiMn2O4/SrRuO3 multi-layer epitaxial thin film electrodes. J. Power Sources 2013, 226, 340–345. 10.1016/j.jpowsour.2012.11.008. [DOI] [Google Scholar]
- Gao X.; Ikuhara Y. H.; Fisher C. A. J.; Moriwake H.; Kuwabara A.; Oki H.; Kohama K.; Yoshida R.; Huang R.; Ikuhara Y. Structural Distortion and Compositional Gradients Adjacent to Epitaxial LiMn2O4 Thin Film Interfaces. Adv. Mater. Interfaces 2014, 1, 1–10. 10.1002/admi.201470048. [DOI] [Google Scholar]
- Ikuhara Y. H.; Gao X.; Huang R.; Fisher C. A. J.; Kuwabara A.; Moriwake H.; Kohama K. Epitaxial Growth of LiMn2O4 Thin Films by Chemical Solution Deposition for Multilayer Lithium-Ion Batteries. J. Phys. Chem. C 2014, 118, 19540–19547. 10.1021/jp504305q. [DOI] [Google Scholar]
- Suzuki K.; Hirayama M.; Kim K.; Taminato S.; Tamura K.; Son J. Y.; Mizuki J.; Kanno R. Interfacial Analysis of Surface-Coated LiMn2O4 Epitaxial Thin Film Electrode for Lithium Batteries. J. Electrochem. Soc. 2015, 162, A7083–A7090. 10.1149/2.0111513jes. [DOI] [Google Scholar]
- Xia H.; Wan Y. H.; Yan F.; Lu L. Manganese oxide thin films prepared by pulsed laser deposition for thin film microbatteries. Mater. Chem. Phys. 2014, 143, 720–727. 10.1016/j.matchemphys.2013.10.005. [DOI] [Google Scholar]
- Qiu Y.; Xu G.-L.; Yan K.; Sun H.; Xiao J.; Yang S.; Sun S.-G.; Jin L.; Deng H. Morphology-conserved transformation: synthesis of hierarchical mesoporous nanostructures of Mn2O3 and the nanostructural effects on Li-ion insertion/deinsertion properties. J. Mater. Chem. 2011, 21, 6346–6353. 10.1039/c1jm00011j. [DOI] [Google Scholar]
- Wang F. X.; Wang X. W.; Chang Z.; Zhu Y. S.; Fu L. J.; Liu X.; Wu Y. P. Electrode materials with tailored facets for electrochemical energy storage. Nanoscale Horiz. 2016, 1, 272–289. 10.1039/C5NH00116A. [DOI] [PubMed] [Google Scholar]
- Antczak G.; Ehrlich G. Jump processes in surface diffusion. Surf. Sci. Rep. 2007, 62, 39–61. 10.1016/j.surfrep.2006.12.001. [DOI] [Google Scholar]
- Ishizawa N.; Tateishi K. Diffusion of Li atoms in LiMn2O4 – A structural point of view. J. Ceram. Soc. Jpn. 2009, 117, 6–14. 10.2109/jcersj2.117.6. [DOI] [Google Scholar]
- Kim S.; Aykol M.; Wolverton C. Surface phase diagram and stability of (001) and (111) LiMn2O4 spinel oxides. Phys. Rev. B: Condens. Matter Mater. Phys. 2015, 92, 115411. 10.1103/PhysRevB.92.115411. [DOI] [Google Scholar]