Abstract
Purpose
To report clinical and genetic features of a Japanese patient with end-stage retinitis pigmentosa (RP) caused by a homozygous PDE6A variant.
Methods
We performed comprehensive ophthalmic examinations. Whole exome sequencing analysis was used to investigate the RP patient with parental consanguinity. The pedigree included 4 RP patients in the two generations, which suggests presumed pseudo-autosomal dominant inheritance.
Results
A PDE6A variant (p.R653X) was identified to be homozygous and disease-causing in the patient. Homozygosity mapping revealed the homozygous region including the variant and confirmation of autosomal recessive inheritance. The patient reported night blindness at 4 years of age and exhibited typical RP fundus appearance with macula involvement during the follow-up period from at the age of 52–69 years. At the age of 52, the patient exhibited a loss of visual acuity and had severely constricted visual fields, with a further gradual deterioration of her vision until she was 69 years old. At the age of 69, funduscopy showed severe chorioretinal degeneration in the area from the posterior pole to the peripheral retina.
Conclusions and Importance
This is the first report that the PDE6A variant (p.R653X) has been identified as one of the causes of autosomal recessive RP in the Japanese population. Longitudinal natural history/end-stage findings demonstrated early-onset and a severe RP phenotype with macula involvement when the patient was in her 50s and severe chorioretinal degenerations in her late 60s.
Keywords: Retinitis pigmentosa, PDE6A, Japanese, Next generation sequencing, Chorioretinal atrophy
1. Introduction
Retinitis pigmentosa (RP) (OMIM #268000) is a heterogeneous group of inherited retinal dystrophy (IRD) characterized by night blindness, retinal degeneration with bone spicule pigmentation, constricted visual fields, and progressive disease course. The prevalence of RP is approximately 1 per 4000 persons, with more than 1 million individuals affected worldwide.1 To date, about 70 genes have been reported as causes of RP with a variable inheritance pattern.1,2
In 1995, the phosphodiesterase 6A (PDE6A) gene (OMIM *180071) was first reported to be a cause of autosomal recessive RP (arRP).3,4 The PDE6A gene encodes the alpha subunit of the cyclic guanosine monophosphate phosphodiesterase, with dysfunction of the PDE6A protein primarily affecting the rod photoreceptor cells and secondarily affecting the cone photoreceptor cells.5, 6, 7 A previous study has reported that the prevalence of PDE6A variants was about 3–4% in arRP patients in North America.8 In contrast, a large-scale molecular genetic analysis of Japanese RP patients reports that none of the RP was caused by PDE6A variants,9, 10, 11 which suggests that PDE6A-related arRP appears to be rare in the Japanese population.
In this study, we performed whole exome sequencing (WES) for one RP patient, who had a presumed pseudo-autosomal dominant inheritance pattern, and identified a PDE6A variant that was present homozygously. The purpose of this study was to report the clinical and genetic features of end-stage RP with a homozygous PDE6A variant.
2. Materials and methods
The protocol used for this study was approved by the Institutional Review Board of the Jikei University School of Medicine and the National Hospital Organization Tokyo Medical Center (approval numbers: Jikei University 24-231 6997, National Hospital Organization Tokyo Medical Center R11–087 and R14-050). This protocol adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from the participant in this study.
2.1. Clinical studies
All medical and surgical records for the patient were reviewed. The ophthalmic examinations performed in the study patient included decimal best-corrected visual acuity (BCVA), funduscopy, fluorescein angiography, visual field testing, fundus autofluorescence imaging (Spectralis HRA; Heidelberg Engineering, Heidelberg, Germany, and Optos Panoramic 200 MA; Optos PLC, Dunfermline, UK), electroretinography (ERG), spectral-domain optical coherence tomography (OCT) (OCT3 Stratus and Cirrus HD-OCT; Carl Zeiss Meditec AG, Dublin, CA, USA), and axial length using interferometry (IOL-Master, version 3.01; Carl Zeiss Meditec). The BCVA was measured using a Landolt C chart and converted to a logarithm of minimal angle of resolution (logMAR). Hand motions were converted to logMAR visual acuity values of 2.7, as previously reported.12 Rod-plus-cone ERG response was elicited by a white single flash (10 cd s/m2) produced by the Neuropack 2 system (Nihon Kohden, Tokyo, Japan) using corneal contact lens electrodes after a dark adaptation period of 30 min, as previously described.13 The rod-plus-cone ERG measurement was according to the ISCEV standard (dark-adapted 10 ERG).14 Goldmann perimetry (GP; Haag Streit, Bern, Switzerland) was used for the visual field testing. The results of the GP examination were analyzed using FIJI/ImageJ software (available at https://fiji.sc) as follows. We scanned each GP result using the same method and resolution (879 × 640 pixels) in accordance with the software. Subsequently, after tracing the V-4e isopters using the polygon selection tool, we then calculated the area. A 10-degree circular visual field in radius was used as the baseline.
2.2. Molecular genetic analysis
The genomic DNA was extracted from the leucocytes in the venous blood samples using a Gentra Puregene Blood kit (Qiagen, Hilden, Germany). WES was performed according to a previously reported method.9,15 Briefly, after comparing the variants to those in the reference human genome (hg38), we chose from the variants that remained based on the following three criteria; 1) variants could change the initial amino acid sequence with a variant cell quality over 60, 2) variants had a frequency that was less than 1% in the 1000 Genomes database, the Exome Aggregation Consortium database, the Human Genetic Variation Database, and the Tohoku Medical Megabank Organization database, and 3) variants were included in the 280 genes that have been reported to be a cause of IRD in the RetNet database (https://sph.uth.edu/retnet/) that was accessed on January 7th, 2018. The three in silico programs used to evaluate the missense variants included the PolyPhen2 (http://genetics.bwh.harvard.edu/pph2/), SIFT (http://sift.jcvi.org/www/SIFT_seq_submit2.html), and PROVEAN (http://provean.jcvi.org/index.php) programs. Any identified PDE6A variant was confirmed by Sanger sequencing, with the variant compared with the NCBI Reference Sequence (NM_000440.2).
2.3. Homozygosity analysis in chromosome 5
Single nucleotide variants and insertion/deletion variants in chromosome 5 containing the PDE6A gene were extracted from the WES data by using the HaplotypeCaller of the Genome Analysis Toolkit version 3.3–0.16 After the extraction, all variants with a genotype accuracy of over 99.9% were used for the subsequent analyses. Homozygosity or heterozygosity was determined for each nucleotide change, with the rate of homozygosity calculated in each 1 Mb of chromosome 5.
3. Results
3.1. Clinical studies
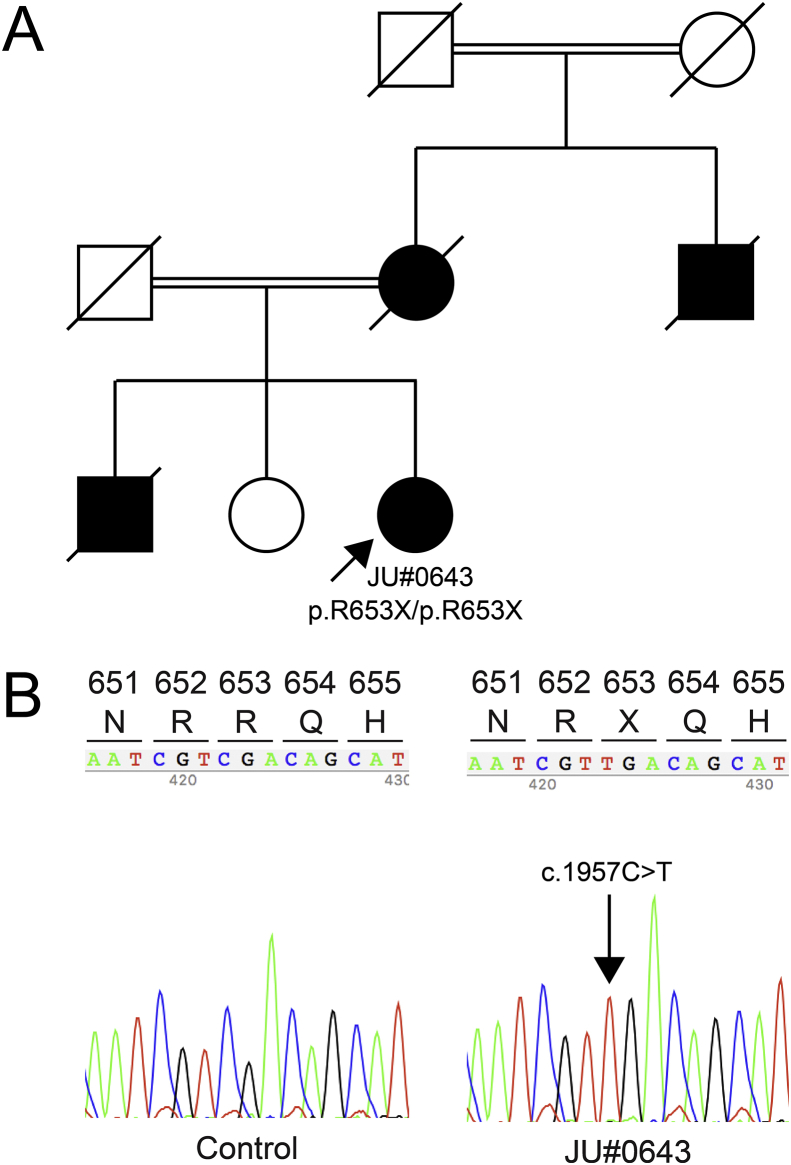
The Japanese family examined included a female proband (JU#0643) with parental consanguinity over two generations. There were four affected RP patients within the family, which was presumed to indicate a pseudo-autosomal dominant inheritance pattern (Fig. 1A). The proband was diagnosed with RP based on previous medical records, with the other family members diagnosed as affected or unaffected individuals based on the interview with the proband.
Fig. 1.
Pedigree, partial sequence data, and electroretinograms. (A) Pedigree of a Japanese family showing consanguineous marriages over two generations. (B) Partial nucleotide sequence data of a control and the proband (JU#0643). The proband carries the homozygous PDE6A variants (c.1957C > T, p.R653X, in exon 16). (C) Dark-adapted single flash electroretinography shows there was a non-recordable response in both eyes.
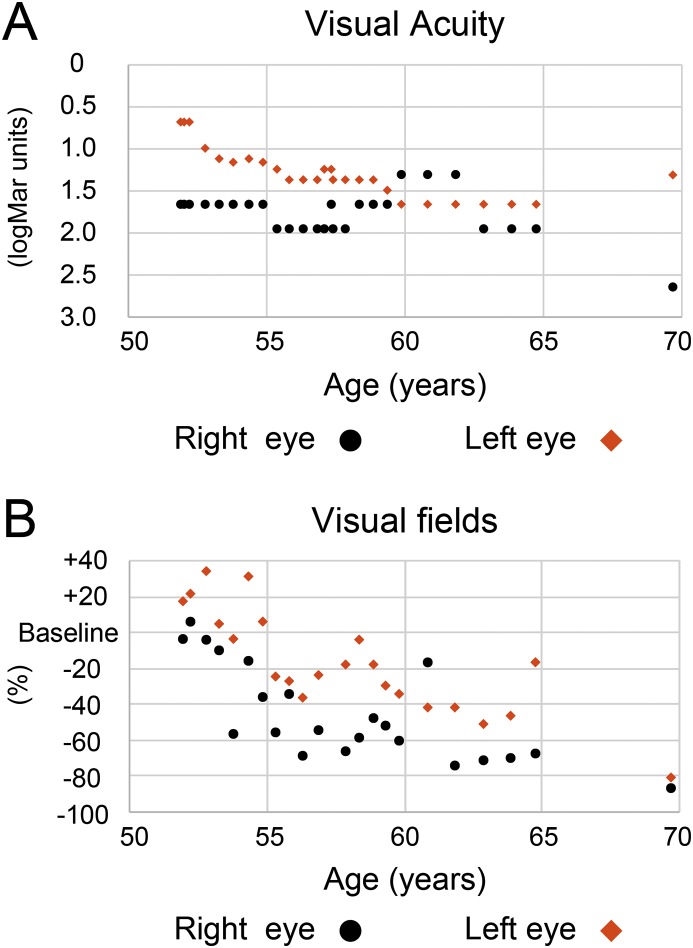
The proband was referred to the Jikei University Hospital at 52 years of age. The patient reported night blindness starting from 4 years of age. In elementary school, the BCVA of the proband was approximately 0.5 in both eyes, with her subsequent BCVA gradually decreasing. The BCVA at the first examination was 0.02 (logMAR conversion: 1.7) [-1.75 diopter (D) cylinder, +0.75 D axis 70] in the right and 0.2 (logMAR conversion: 0.8) (−3.50 D cylinder, −1.25 D axis 140) in the left eyes. Slit lamp examination indicated the presence of nuclear cataracts in both eyes. Funduscopy showed that there was retinal degeneration with bone spicule pigmentation from the mid-peripheral to peripheral retina, attenuation of the peripheral retinal vessels, and a waxy pallor of the optic disc in both eyes. Retinal appearance in the posterior pole was relatively preserved in both eyes compared with the periphery, and there was drusen-like accumulation in the right and early atrophy in the left maculae (Fig. 2A). Late-phase fluorescein angiography showed generalized hyperfluorescence due to transmission defects and chorioretinal atrophy with hypofluorescent areas by pigment clumps especially along the vascular arcades (Fig. 2B). Visual fields were severely constricted by approximately 10° by V-4e isopters in both eyes (Fig. 2C). The rod-plus-cone ERG results showed non-recordable responses in both eyes. At 57 years old, the patient underwent cataract surgery in her right eye. Axial length was 24.1 mm in each eye. Time-domain OCT images were first obtained at 58 years of age. These images showed severe retinal thinning in both eyes. At 69 years old, the patient underwent cataract surgery in her left eye. The last examination was performed at 69 years of age and showed that the BCVA was hand motion (logMAR conversion: 2.7) in the right and 0.05 (logMAR conversion: 1.3) in the left eyes. A funduscopy examination found markedly severe retinal degeneration with visible choroidal vessels in the posterior retina, waxy pallor of the optic disc, and retinal degeneration with bone spicule pigmentations from the mid-peripheral to peripheral retina (Fig. 3A and C). Fundus autofluorescence imaging showed there was a generalized loss of autofluorescence with the change in the autofluorescence of the choroidal vessels observed from the posterior pole to the peripheral retina (Fig. 3B and D). The OCT images also showed there was severe retinal thinning, which was more prominent in the outer retina. In addition, both eyes were found to have an entirely disrupted ellipsoid zone line (Fig. 3E). Furthermore, the choroidal thickness was markedly thinned in both eyes. During the 17-year follow-up period (52–69 years old), the proband exhibited progressive deterioration of her BCVA and visual fields (Fig. 4).
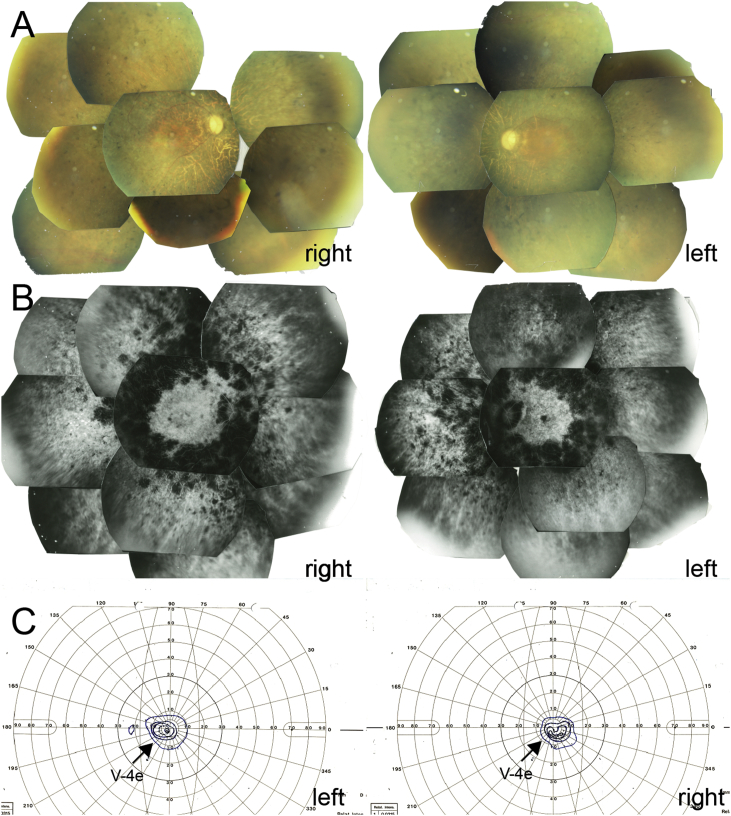
Fig. 2.
Fundus images and visual fields at 52 years old. (A) Fundus photographs show retinal degeneration with bone spicule pigmentation from the posterior pole to the peripheral retina, attenuation of the retinal vessels, and waxy pallor of the optic disc. In addition, drusen-like accumulation in the right and early atrophy in the left maculae are seen. (B) Fluorescein angiograms in the late phase show generalized hyperfluorescence due to transmission defects with hypofluorescent areas observed by the pigment clumps, especially along the vascular arcades. (C) Goldmann visual field testing shows severely constricted visual fields around 10° in the V-4e isopters.
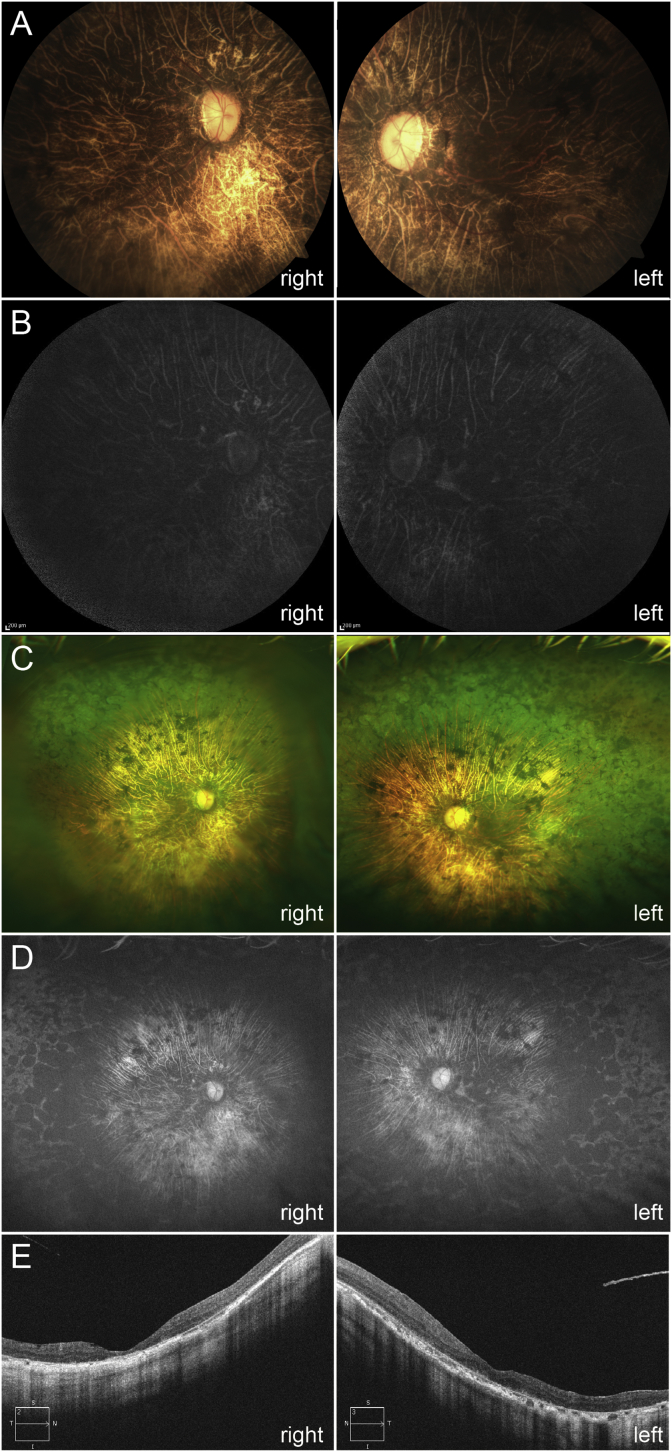
Fig. 3.
Fundus images and optical coherence tomography (OCT) at 69 years old. (A) Fundus photographs show markedly severe retinal degeneration with visible atrophic choroidal vessels in the posterior retina and waxy pallor of the optic disc. (B) Fundus autofluorescence images show a generalized loss of autofluorescence except for the choroidal vessels. (C) Wide-field fundus photographs show severe chorioretinal atrophy with bone spicule pigmentation in the area from the posterior pole to the peripheral retina. (D) Wide-field fundus autofluorescence images show there is a generalized loss of autofluorescence with the change in the autofluorescence observed in the choroidal vessels. (E) The OCT images show severe retinal thinning, which is more prominent in outer retina, along with an entirely disrupted ellipsoid zone. In addition, there is also marked thinning of the choroidal thickness.
Fig. 4.
Clinical natural history of the logMAR visual acuity and visual fields during the 17-year follow-up period. (A) Visual acuity gradually deteriorated with aging in both eyes. The cataract surgery is underwent in right eye at 57 years old and in left eye at 69 years old. (B) Visual fields with V-4e isopters also show a decreased percentage or deterioration compared with the baseline, which is defined as a 10-degree visual field in radius.
3.2. Molecular genetic analysis
Five heterozygous and two homozygous candidate variants remained after filtering the WES data of the proband (Supplementary Table S1). According to pseudo-autosomal dominant inheritance pattern, two homozygous variants [c.1957C > T (p.R653X) in the PDE6A gene and c.361G > A (p.G121S) in the TUB gene] remained as disease-causing candidates, whereas five heterozygous variants in POMGNT1, ADGRV1, CDH23, GUCY2D, and CHM genes were excluded based on genotype-phenotype correlations. The homozygous missense variant (p.G121S) in the TUB gene was also excluded as disease-causing, as only one TUB gene variant study has reported finding a homozygous loss-of-function variant, which demonstrates retinal dystrophy with obesity.17 However, our patient exhibited neither the loss-of-function variant nor obesity. In addition, the in silico programs for p.G121S predicted that the function of the TUB protein would remain. Finally the homozygous p.R653X in the PDE6A gene was determined to be disease-causing due to the consistency between the genotype and phenotype, along with the confirmation of the autosomal recessive inheritance.
3.3. Homozygosity analysis in chromosome 5
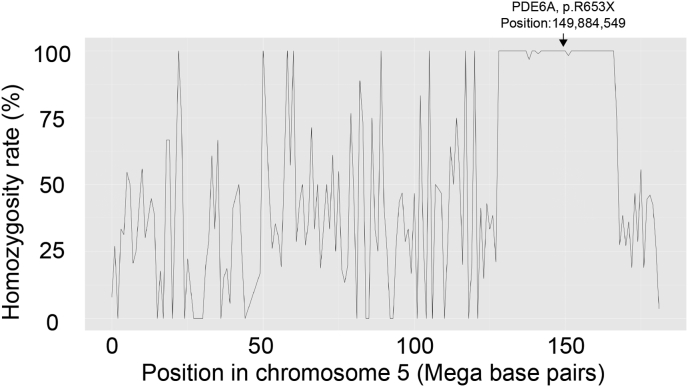
Homozygosity analysis was performed for the proband. A total of 3343 variants were extracted from chromosome 5. In the proband, 648 of 651 (99.5%) variants came from position 127,457,282 to position 167,755,050 and showed homozygosity. This region contained the area of the PDE6A gene (chromosome 5; position 149,857,956 to 149,944,793, NC_000005.10 Reference GRCh38.p7/hg38) including the identified PDE6A variant (p.R653X) (chromosome 5; position 149884549) (Fig. 5), which was supported by the consanguineous marriage.
Fig. 5.
Homozygosity mapping of chromosome 5. Single nucleotide variants [648 of 651 (99.5%)] from position 127,457,282 to position 167,755,050 show homozygosity, which includes the PDE6A variant (p.R653X) (chromosome 5; position 149884549).
4. Discussion
Our current study investigated the genetic and clinical features of a single end-stage RP patient in a consanguineous Japanese family in whom WES identified a homozygous PDE6A variant (p.R653X). There have been no previous reports of longitudinal natural history/end-stage findings of any PDE6A-associated RP patient. Here, our patient exhibited progressive deterioration of her BCVA and visual fields during a 17-year follow-up period (52–69 years old).
To date, all reported phenotypes with PDE6A variants have been classified as arRP. These patients are characterized by primary degeneration of the rod photoreceptor cells and subsequent degeneration of the cone photoreceptor cells.5, 6, 7 Two school-aged cases with PDE6A variants have been reported as early-onset RP.5,18 In one case, a 5-year-old child with the homozygous p.R653X variant (the same variant in our case) exhibited a loss of visual fields, diminished visual acuity, a typical fundus appearance of RP and reduced a-wave and b-wave amplitudes in ERG, although there were no ophthalmic images described.18 In the other case, an 8-year-old boy with a homozygous 2-bp deletion exhibited night blindness, typical RP fundus appearance with pale optic discs and markedly reduced ERG responses.5 Phenotypes in adult RP patients with the PDE6A variants exhibit similar clinical characteristics to our proband and these previously studied cases. These characteristics include onset in early childhood, retinal degeneration with bone spicule pigmentation from mid-peripheral to peripheral retina, attenuation of peripheral retinal vessels, distinguished ERG responses of both rod and cone function, and a severely constricted visual field until reaching their 20s–30s.6,7,19, 20, 21, 22 Furthermore, macula involvements, which are often accompanied by macular edema and atrophy, have been reported in most of these types of cases. These involvements are observed until subjects reach their 20s–30s, with further progression noted in some older patients.6,7,19, 20, 21, 22 There have also been a few cases of patients in their 60s–70s with the PDE6A variants that resulted in legal blindness.7 The longitudinal natural history of our patient showed that the visual function gradually deteriorated throughout a 17-year follow-up. These findings are consistent with the expected clinical course that is based on the natural history of previously reported cases from short-term clinical presentations of different PDE6A-associated RP patients.6,7,19, 20, 21, 22
The PDE6A protein consists of the PDE6 complex in rod photoreceptor cells with other beta and gamma subunits.23,24 Experimental data obtained from animal models have clarified that the lack of PDE6A function leads to the elevation of cGMP levels due to a reduction of the photo-transduction pathway. As a result, this primarily leads to the death of rod photoreceptor cells and secondarily leads to the death of cone photoreceptor cells.25 However, the marked choroidal atrophy that we observed in our patient during her late 60s (Fig. 3) has never been reported as a PDE6A-associated RP phenotype. Furthermore, the primary or secondary effect of the PDE6A dysfunction on the choroid remains unknown. It is possible that the choroidal atrophy might occur during the end stage of limited cases with PDE6A variants. Another possibility is that, although pathogenicity of the heterozygous missense variant [c.1463G > A (p.R488Q)] in the CHM gene (Supplementary Table S1), which is responsible for the X-linked choroideremia, has yet to be clarified, the variant could affect the choroidal atrophy by acting as a genetic modifier effect, as female carriers of the CHM variants are known to exhibit patchy chorioretinal degeneration.26,27
This study was a single case report, and no examinations were performed in other family members. There is a possibility of other inheritance pattern although pseudo-dominant inheritance pattern had a high probability because of parental consanguinity and the results of homozygosity mapping (Fig. 5). In addition, there was a limitation regarding unavailability of segregation of identified PDE6A and CHM variants and phenotypic evaluation of other RP family members. In order to clarify the clinical features of PDE6A-associated RP, especially the choroidal changes, additional cases will be needed.
In conclusion, this is the first report that the PDE6A variant (p.R653X) has been shown to be a cause of arRP in the Japanese population. The longitudinal natural history/end-stage findings of our proband demonstrated early-onset and a severe RP phenotype with macula involvement occurring when the patient was in her 50s and severe chorioretinal degeneration when reaching her late 60s.
Patient consent
Written informed consent was obtained from patient for publication of this case report and any accompanying images.
Acknowledgements and disclosures
Funding
This work was supported by grants from the Practical Research Project for Rare/Intractable Diseases (17ek0109282h0001) from the Japan Agency for Medical Research and Development (AMED) to TI, and the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (17K11434 and 17K11441) to TH.
Conflict of interest
The authors declare that there are no conflicts of interest regarding this paper.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
We thank the patient for participating in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2018.12.019.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hartong D.T., Berson E.L., Dryja T.P. Retinitis pigmentosa. Lancet. 2006;368(9549):1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 2.Dias M.F., Joo K., Kemp J.A. Molecular genetics and emerging therapies for retinitis pigmentosa: basic research and clinical perspectives. Prog Retin Eye Res. 2018;63:107–131. doi: 10.1016/j.preteyeres.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Dryja T.P., Finn J.T., Peng Y.W., McGee T.L., Berson E.L., Yau K.W. Mutations in the gene encoding the alpha subunit of the rod cGMP-gated channel in autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci U S A. 1995;92(22):10177–10181. doi: 10.1073/pnas.92.22.10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang S.H., Pittler S.J., Huang X., Oliveira L., Berson E.L., Dryja T.P. Autosomal recessive retinitis pigmentosa caused by mutations in the alpha subunit of rod cGMP phosphodiesterase. Nat Genet. 1995;11(4):468–471. doi: 10.1038/ng1295-468. [DOI] [PubMed] [Google Scholar]
- 5.Nair P., Hamzeh A.R., Malik E.M., Oberoi D., Al-Ali M.T., Bastaki F. Novel PDE6A mutation in an Emirati patient with retinitis pigmentosa. Oman J Ophthalmol. 2017;10(3):228–231. doi: 10.4103/ojo.OJO_213_2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bocquet B., Marzouka N.A., Hebrard M. Homozygosity mapping in autosomal recessive retinitis pigmentosa families detects novel mutations. Mol Vis. 2013;19:2487–2500. [PMC free article] [PubMed] [Google Scholar]
- 7.Kjellstrom U., Veiga-Crespo P., Andreasson S., Ekstrom P. Increased plasma cGMP in a family with autosomal recessive retinitis pigmentosa due to homozygous mutations in the PDE6A gene. Invest Ophthalmol Vis Sci. 2016;57(14):6048–6057. doi: 10.1167/iovs.16-19861. [DOI] [PubMed] [Google Scholar]
- 8.Dryja T.P., Rucinski D.E., Chen S.H., Berson E.L. Frequency of mutations in the gene encoding the alpha subunit of rod cGMP-phosphodiesterase in autosomal recessive retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1999;40(8):1859–1865. [PubMed] [Google Scholar]
- 9.Katagiri S., Akahori M., Sergeev Y. Whole exome analysis identifies frequent CNGA1 mutations in Japanese population with autosomal recessive retinitis pigmentosa. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0108721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin Z.B., Mandai M., Yokota T. Identifying pathogenic genetic background of simplex or multiplex retinitis pigmentosa patients: a large scale mutation screening study. J Med Genet. 2008;45(7):465–472. doi: 10.1136/jmg.2007.056416. [DOI] [PubMed] [Google Scholar]
- 11.Oishi M., Oishi A., Gotoh N. Comprehensive molecular diagnosis of a large cohort of Japanese retinitis pigmentosa and Usher syndrome patients by next-generation sequencing. Invest Ophthalmol Vis Sci. 2014;55(11):7369–7375. doi: 10.1167/iovs.14-15458. [DOI] [PubMed] [Google Scholar]
- 12.Grover S., Fishman G.A., Anderson R.J. Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older. Ophthalmology. 1999;106(9):1780–1785. doi: 10.1016/S0161-6420(99)90342-1. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi T., Omoto S., Takeuchi T., Kozaki K., Ueoka Y., Kitahara K. Four Japanese male patients with juvenile retinoschisis: only three have mutations in the RS1 gene. Am J Ophthalmol. 2004;138(5):788–798. doi: 10.1016/j.ajo.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 14.McCulloch D.L., Marmor M.F., Brigell M.G. ISCEV Standard for full-field clinical electroretinography (2015 update). Documenta ophthalmologica. Adv Ophthalmol. 2015;130(1):1–12. doi: 10.1007/s10633-014-9473-7. [DOI] [PubMed] [Google Scholar]
- 15.Katagiri S., Yoshitake K., Akahori M. Whole-exome sequencing identifies a novel ALMS1 mutation (p.Q2051X) in two Japanese brothers with Alstrom syndrome. Mol Vis. 2013;19:2393–2406. [PMC free article] [PubMed] [Google Scholar]
- 16.McKenna A., Hanna M., Banks E. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borman A.D., Pearce L.R., Mackay D.S. A homozygous mutation in the TUB gene associated with retinal dystrophy and obesity. Hum Mutat. 2014;35(3):289–293. doi: 10.1002/humu.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Carro R., Corton M., Sanchez-Navarro I. Panel-based NGS reveals novel pathogenic mutations in autosomal recessive retinitis pigmentosa. Sci Rep. 2016;6:19531. doi: 10.1038/srep19531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riazuddin S.A., Zulfiqar F., Zhang Q. Mutations in the gene encoding the alpha-subunit of rod phosphodiesterase in consanguineous Pakistani families. Mol Vis. 2006;12:1283–1291. [PubMed] [Google Scholar]
- 20.Tsang S.H., Tsui I., Chou C.L. A novel mutation and phenotypes in phosphodiesterase 6 deficiency. Am J Ophthalmol. 2008;146(5):780–788. doi: 10.1016/j.ajo.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan S.Y., Ali S., Naeem M.A. Splice-site mutations identified in PDE6A responsible for retinitis pigmentosa in consanguineous Pakistani families. Mol Vis. 2015;21:871–882. [PMC free article] [PubMed] [Google Scholar]
- 22.Chebil A., Falfoul Y., Habibi I., Munier F., Schorderet D., El Matri L. [Genotype-phenotype correlation in ten Tunisian families with non-syndromic retinitis pigmentosa] J Fr Ophtalmol. 2016;39(3):277–286. doi: 10.1016/j.jfo.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Kaupp U.B., Niidome T., Tanabe T. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989;342(6251):762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- 24.Chen T.Y., Peng Y.W., Dhallan R.S., Ahamed B., Reed R.R., Yau K.W. A new subunit of the cyclic nucleotide-gated cation channel in retinal rods. Nature. 1993;362(6422):764–767. doi: 10.1038/362764a0. [DOI] [PubMed] [Google Scholar]
- 25.Wensel T.G., Zhang Z., Anastassov I.A. Structural and molecular bases of rod photoreceptor morphogenesis and disease. Prog Retin Eye Res. 2016;55:32–51. doi: 10.1016/j.preteyeres.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syed N., Smith J.E., John S.K., Seabra M.C., Aguirre G.D., Milam A.H. Evaluation of retinal photoreceptors and pigment epithelium in a female Carrier of choroideremia. Ophthalmology. 2001;108(4):711–720. doi: 10.1016/s0161-6420(00)00643-6. [DOI] [PubMed] [Google Scholar]
- 27.Bonilha V.L., Trzupek K.M., Li Y. Choroideremia: analysis of the retina from a female symptomatic Carrier. Ophthalmic Genet. 2008;29(3):99–110. doi: 10.1080/13816810802206499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.