Abstract
There has been a steady increase in the interest towards employing nanoliposomes as colloidal drug delivery systems, particularly in the last few years. Their biocompatibility nature along with the possibility of encapsulation of lipid-soluble, water-soluble and amphipathic molecules and compounds are among the advantages of employing these lipidic nanocarriers. A challenge in the successful formulation of nanoliposomal systems is to control the critical physicochemical properties, which impact their in vivo performance, and validating analytical techniques that can adequately characterize these nanostructures. Of particular interest are the chemical composition of nanoliposomes, their phase transition temperature, state of the encapsulated material, encapsulation efficiency, particle size distribution, morphology, internal structure, lamellarity, surface charge, and drug release pattern. These attributes are highly important in revealing the supramolecular arrangement of nanoliposomes and incorporated drugs and ensuring the stability of the formulation as well as consistent drug delivery to target tissues. In this article, we present characterization of nanoliposomal formulations as an example to illustrate identification of key in vitro characteristics of a typical nanotherapeutic agent. Corresponding analytical techniques are discussed within the context of nanoliposome assessment, single particle analysis and ensuring uniform manufacture of therapeutic formulations with batch-to-batch consistency.
Keywords: Materials chemistry, Analytical chemistry, Biotechnology, Bioengineering, Biochemistry
1. Introduction
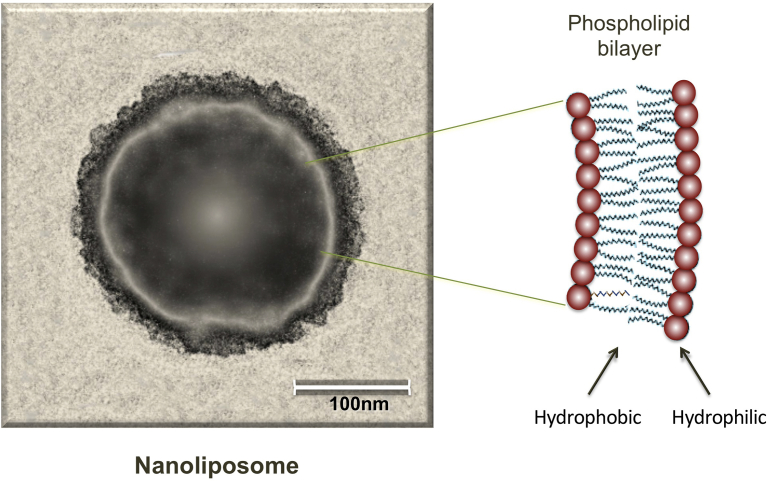
Nanoliposomes can be defined as submicrometric bilayer vesicles mainly composed of phospholipid molecules. The internal compartment of nanoliposomes is filled with an aqueous media – such as de-ionized water, a buffer or an isotonic saline solution – in which one or more hydrophilic compound(s) can be dissolved. On the other hand, lipophilic molecules can be accommodated in the lipid-phase of the bilayer vesicle [1, 2]. The word nanoliposome is derived from an older terminology known as “liposome”, which means “lipid structure” (i.e. lipos: fat, and soma: body). With the advancement in the scientific field of nanotechnology, the word nanoliposome has been introduced to exclusively refer to nanoscale lipid vesicles, since liposome is a general word covering many classes of phospholipid vesicles with diameters in the size range of tens of nanometers to several micrometers [3]. Fig. 1 depicts a single nanoliposome visualized by energy filtered transmission electron microscopy (EFTEM). Schematic enlargement of a section of the phospholipid bilayer of the nanoliposome shows positions of the hydrophilic and hydrophobic regions of the bilayer. Due to their unique structures and superior biocompatibility, nanoliposomes are being used extensively as nanocarriers for drug delivery, protein and peptide delivery, food fortification, cosmetics and gene therapy applications [4, 5, 6]. They improve the efficiency of a vast variety of bioactive agents, including pharmaceutical, nutraceutical and cosmeceutical compounds, by preserving the functionality of the encapsulated materials as well as targeting them to particular cells or tissues [4]. In addition to phospholipids, nanoliposomes can incorporate other molecules such as cholesterol, antigens, polymers and antioxidants in their structure. These excipients assist in improving the stability and shelf life of the formulation or targeting the nanoliposomes where their effect is needed in vitro or in vivo [6, 7]. An example of polymer grafted vesicles is the FDA approved PEGylated nanoliposomal doxorubicin (Doxil), which is being clinically employed for cancer treatment since 1995 [8]. The polyethylene glycol (PEG) grafting is designed to evade uptake of the nanoliposomes by the reticuloendothelial system and prolong its blood circulation time. As a consequence of this strategy, better biological compatibility, reduced drug toxicity, and higher drug efficacy are obtained with Doxil compared with the conventional doxorubicin drug with no nanoliposomes [8].
Fig. 1.
A single nanoliposome visualized by energy filtered transmission electron microscope (EFTEM). A section of the surface of the nanoliposome is schematically enlarged to show the arrangement of the phospholipid molecules in the form of a bilayer structure. Positions of the hydrophilic and hydrophobic regions of the bilayer are also indicated by arrows. Nanoliposome is composed of soybean lecithin and polyunsaturated fatty acids (fish derived DHA and EPA; 2:3 w/w) (lecithin: PUFAs 2:0.4, mass ratio), manufactured by Mozafari method [10, 11].
Medical and pharmaceutical applications of nanoliposomes can be mainly classified into diagnostic and therapeutic applications. These could be achieved by the employment of nanoliposomes containing various markers or incorporating different drugs or vaccines. Nanoliposomes can also be used as a tool, a model for cell membranes, or a reagent in the basic studies of cellular interactions, recognition processes, and mode of action of certain therapeutic agents [1, 5, 9]. In addition to the medical and pharmaceutical applications, nanoliposomes are being used for the encapsulation, delivery and controlled release of food material and nutraceuticals. These include omega fatty acids [10, 11], various dairy products [12] as well as vitamins and other health benefit compounds as recently reviewed in details by Khorasani et al. [3].
The physicochemical properties of nanoliposomes, particularly chemical composition, phase transition, morphology, size, polydispersity index, number of lamellae, surface charge, density of the ligands immobilized on their surface, and drug encapsulation efficiency, are determinative factors for their successful clinical application [9]. Here we present the advantages, disadvantages, and recent applications of a number of single particle analyzing techniques for nanoliposome characterization. An important parameter in the design and manufacture of nanoliposomal formulations is phase transition temperature (Tc) that is an indication of the thermal behavior, dynamic properties and stability of the lipid nanovesicles. Considerations pertaining to the Tc, surface charge and selection of right ingredients and solvents for the manufacture of nanoliposomes are also explained in the following sections.
2. Main text
2.1. Materials, ingredients and solvents
The main characteristics of nanoliposomes strongly depend on the selection of ingredients, solvents and co-solvents for their formulation. These properties include permeability, surface activity, electrical charge, stability, and safety of the nanovesicles. The bilayer of nanoliposomes is predominantly made up of phospholipid molecules (Fig. 1). The hydrophilic section of the phospholipids is oriented towards the internal and external aqueous phases and the hydrophobic groups associate with their counterparts on the other phospholipid molecules. Curving of the bilayer sheet into a spherical structure forms a very stable construction due to the lack of chemical interaction of the phospholipids with the aqueous medium. Therefore, it can be postulated that the mechanism of the formation of the lipidic nanovesicles is the hydrophilic–hydrophobic interactions and van der Waals forces between phospholipids and water molecules [1, 2, 9].
2.1.1. Phospholipid ingredients
The most commonly employed phospholipid for the manufacture of nanoliposomes is lecithin (phosphatidylcholine), which is immiscible with water and is inexpensively isolated from egg yolk or soy. The composition of the phospholipid ingredients and the preparation method of nanoliposomes determine if a single or multiple bilayers are formed. Fatty acids also make up nanoliposomes and their degree of saturation depends on the source. Animal sources provide more saturated fatty acids. These ingredients influence the phase transition temperature, which is the conversion from a gel to the more leaky liquid form, as explained in the next section. Sugars and large polar molecules cannot permeate through a nanoliposome bilayer. On the other hand, small lipophilic molecules can permeate through the phospholipid membrane if they are soluble in the suspension medium. Hydroxyl ions, potassium ions, protein and peptide molecules permeate very slowly [10, 11, 12, 13, 14].
The effect of phospholipid type and concentration on the size and polydispersity index of nanoliposomes was studied by different research groups in the presence and absence of cholesterol [15, 16, 17]. In an attempt to optimize the ethanol injection method for liposome preparation, Jaafar-Maalej et al. [15] reported that 50 mg/ml of Phospholipon 80H (a product of Phospholipid GmbH containing 80% hydrogenated soy phosphatidylcholine) was an optimum concentration for the preparation of egg yolk lecithin vesicles. They also reported formation of large egg-yolk lecithin vesicles at concentrations above 60 mg/ml Phospholipon 80H with a size increase from 80 to 170 nm [15]. Using a mixture of unsaturated phospholipids EPC (egg phosphatidylcholine) and EPG (egg phosphatidylglycerol) for liposome preparation, Gentine and co-workers [16] concluded that phospholipid concentrations should not exceed 20–25 mM due to their limited solubility in the solvents employed (ethanol/isopropanol) resulting in poor formulations. In another study, Sebaaly and colleagues [17] detected that at certain concentrations (ca. above 50 mg/ml) Phospholipon 80H was not soluble in ethanol and phospholipid aggregation occurred. However, decreasing phospholipid concentrations from 50 to 10 mg/ml led to an obvious decrease in vesicles size (from ca. 356 nm to ca. 210 nm). They also reported adding cholesterol to the liposomal formulations in order to improve vesicle size homogeneity [17] as explained in more details in the next section.
2.1.2. Other excipients
In addition to phospholipid molecules, nanoliposomes can incorporate other ingredients such as sterols in their structure. Sterols are important constituent of most natural membranes and their incorporation into nanoliposome bilayer can bring about major changes in the characteristics of the formulation. The most commonly employed sterol in the structure of the lipid vesicles is cholesterol. Cholesterol does not by itself form bilayer structures, however, it can be incorporated into phospholipid membranes in very high concentrations, e.g. up to 1:1 molar ratios of cholesterol to a phospholipid molecule (such as phosphatidylcholine) [18, 19]. Cholesterol is used in nanoliposome formulations in order to increase their stability by modulating the fluidity of the phospholipid bilayer. It modifies membrane fluidity by preventing crystallization of the acyl chains of the phospholipid molecules and providing steric hindrance to their movement. This phenomenon contributes to the stability of nanoliposome formulation and reduces the permeability of their bilayer membrane to solutes [20, 21]. There are also scientific reports on the effect of cholesterol in increasing the size homogeneity and improving the polydispersity index of phospholipid vesicles [17]. Studies have revealed that phospholipid composition and cholesterol content are among the major parameters to be considered in the formulation of nanoliposomal products [19, 20, 21]. Nanoliposomes made of natural and herbal ingredients are particularly receiving increasing attention in the manufacture of medicinal and nutritional products. Phospholipids and sphingolipids, along with sterols (mainly cholesterol), are the choice of ingredients that are commonly used in the preparation of nanoliposomes. These ingredients are biocompatible, biodegradable and non-toxic [20, 21, 22]. In order to prevent or at least minimize oxidation of the phospholipid ingredients, antioxidant compounds can be incorporated into the structure of the nanovesicles. A commonly used antioxidant in the formulation of liposome and nanoliposome products is alpha-tocopherol, which is a lipophilic molecule and as a result will be located in the lipidic phase of the vesicles. This antioxidant acts as a scavenger of free radicals and thereby protects the susceptible ingredients and extends the stability and shelf life of the lipid vesicles [9, 12, 19].
2.1.3. Solvents
Following rational selection of the nanoliposomal ingredients, appropriate solvents must be chosen based on the intended application, dosage form, route of administration, and method of preparation of the nanovesicles. The organic solvents generally employed in the classical methods of liposome and nanoliposome preparation (e.g. chlorinated solvents, diethyl ether, methanol or acetone) represent potential hazard to consumer's health due to their toxicity [3, 4, 7, 23]. Level of the residual organic solvents, which is acceptable in the finished product, depends on different factors such as the type of solvent and the route of administration of the therapeutic formulation. Although the organic solvents are usually removed from the product by vacuum or evaporation, trace amounts may be present in the final formulation, potentially causing toxicity and influencing the stability of the nanovesicles. In order to solve these drawbacks, employment of alternative organic solvents is being considered by scientists and researchers in the field [10, 11, 23, 24]. It has been postulated that organic solvents can cause cytotoxicity by two different mechanisms:
Molecular level cytotoxicity comprises the effects caused by organic solvents dissolved within the aqueous phase of the nanoliposomes, which may cause protein denaturation, enzyme inhibition and membrane modifications (such as membrane expansion, structure disorders and permeability alterations). Phase toxicity effects, on the other hand, include the extraction of nutrients, disruption or extraction of outer cellular components, and the limited access to nutrients caused by cell attraction to interfaces, formation of emulsions and the coating of cells [7, 23, 24]. In addition to the above-mentioned problems and drawbacks, utilization of volatile organic solvents or detergents in nanoliposome manufacture necessitates performance of two additional steps during the manufacture process:
-
1.
Removal of these solvents and detergents, and:
-
2.
Assessment of the level of residual solvents, including the potentially toxic polar and non-polar protic and aprotic solvents, co-solvents or detergents remained in the final products [7].
Results of cytotoxicity studies performed by our group, using two different toxicity evaluation protocols (i.e. MTT and NRU), have shown that even after 24 hour vacuum, nanoliposomes prepared using chloroform and methanol were significantly toxic while nanoliposomes prepared in the absence of volatile organic solvents were completely non-toxic towards Human bronchoepithelial cell line [23]. The presence of unwanted chemicals and solvents, even in small amounts, may influence the efficacy, safety, and stability of the nanoliposomal products [24]. The ‘International Conference on Harmonization’ (ICH) guideline, specific for residual solvents in pharmaceutical products (ICH 1997 [25] and ICH 2016 [26]), can be used to determine acceptable levels of the mentioned chemicals remained in the finished product. This guideline groups residual solvents into the following three classes:
class 1 includes solvents that should be avoided due to their high level of toxicity;
class 2 includes solvents the application of which should be limited; and
class 3 includes solvents with potentially low toxicity (Table 1).
Table 1.
Different classes of solvents in pharmaceutical products and their suggested concentration limits [25, 26, 27].
| Class 1 Solvents to be avoided |
Class 2 Solvents to be limited |
Class 3 Solvents with low risk |
|||
|---|---|---|---|---|---|
| Solvent | Limit (ppm) | Solvent | Limit (ppm) | Solvent | Limit (%w/w) |
| Benzene | 2 | Acetonitrile | 410 | Acetic acid | 0.5 |
| Carbon tetrachloride | 4 | Chlorobenzene | 360 | Anisole | 0.5 |
| 1,1-Dichloroethene | 8 | Chloroform | 60 | Butyl acetate | 0.5 |
| 1,2-Dichloroethane | 5 | Cyclohexane | 3880 | Formic acid | 0.5 |
| 1,1,1-Trichloroethane | 1500 | Dichloromethane | 600 | Heptane | 0.5 |
| Hexane | 290 | Isobutyl acetate | 0.5 | ||
| Methanol | 3000 | Methyl acetate | 0.5 | ||
| Sulfolane | 160 | Pentane | 0.5 | ||
| Tetralin | 100 | Propyl acetate | 0.5 | ||
| Xylene | 2170 | Tetrahydrofuran | 0.5 | ||
A commonly employed method for the quantitation of organic volatile impurities (OVIs) in pharmaceutical products is gas chromatography with flame ionization detection. Various sample preparation and injection approaches are used for the analysis of OVIs in pharmaceuticals as reviewed recently by Heydari [27]. Therefore, avoiding the use of potentially toxic solvents and detergents, e.g. by employing safe and robust procedures such as Mozafari Method, will potentially bring down the time and cost of manufacture of nanoliposomes [10, 11, 19]. This method has been successfully employed for the encapsulation of a number of different cosmeceutical, nutraceutical and pharmaceutical compounds, without employing toxic solvents or detergents [10, 11, 19, 23, 28]. Furthermore, a physiologic, safe and nontoxic polyol, such as glycerol or sorbitol, which are commonly used in pharmaceutical and food grade products, can be employed as a dispersant or co-solvent in the preparation of nanoliposomes as explained below.
2.2. Phase transition temperature
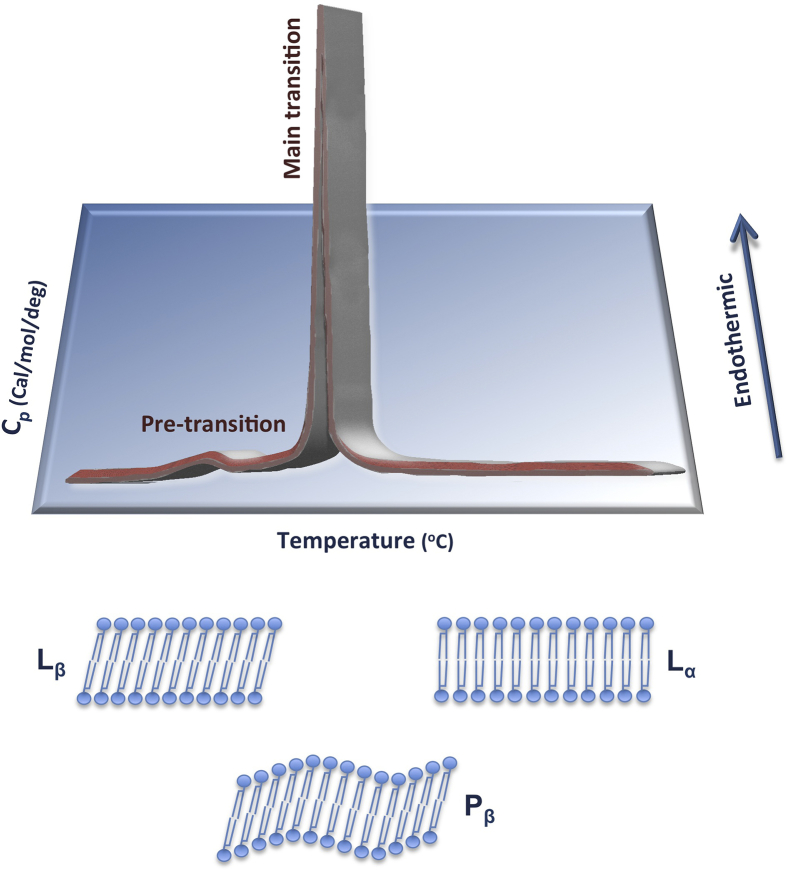
Amphipathic molecules such as detergents and phospholipids can undergo a thermotropic phase transition at temperatures much lower than their melting point. The detailed molecular nature of the major thermotropic phase transition of these long-chain amphiphilic molecules was defined first by infrared spectroscopic studies [13]. When water comes in contact with the phospholipid bilayer of nanoliposomes it diffuses into the polar (ionic) region of the bilayer only when the temperature is reached at which the hydrocarbon chains of the phospholipid molecules “melt” (the transition temperature). If the temperature becomes higher than this, there will be a simultaneous dissociation of the ionic structure by the penetration of water molecules and melting of the hydrocarbon chain region of the phospholipid molecules. The temperature of transition (Tc) depends upon the nature of the hydrocarbon chains, the polar region of the molecule, the amount of water molecules present and on any solutes dissolved in the suspension medium of the vesicles. Once the water has penetrated into the vesicle bilayer and the sample is then cooled to a temperature below the Tc, the hydrocarbon chains rearrange themselves into an orderly crystalline lattice. However, the water molecules will not necessarily be expelled from the system [13, 14]. Also known as “gel to liquid crystalline transition temperature”, Tc is a temperature at which the phospholipid bilayers of nanoliposomes lose much of their ordered packing while their fluidity increases [22] (Fig. 2).
Fig. 2.
A typical Differential Scanning Calorimetry (DSC) curve of phospholipid bilayers undergoing gel-to-liquid crystal (Lβ – Pβ – Lα) phase transition under controlled heating showing pre-transition and the main phase transition (Tc) temperatures.
In general, Tc is lowered by decreased length of the fatty acyls of phospholipid molecules, by unsaturation of the acyl chains, as well as presence of branched chains and bulky head groups (e.g. cyclopropane rings) [1, 19, 20]. An understanding of phase transitions and fluidity of phospholipid membranes is essential both in the manufacture and application of nanoliposomes. This is due to the fact that phase behavior of nanoliposomes determines important properties such as permeability, aggregation, fusion, deformability and protein binding, all of which can significantly affect the stability of the vesicles and their behavior in vitro and in vivo [20, 21]. It has been reported that phase transition temperature of the phospholipid vesicles affects the pharmacokinetics of the encapsulated drugs such as doxorubicin [29, 30]. Tc can be measured by a number of techniques including, electron spin resonance, fluorescence probe polarization and differential scanning calorimeter (DSC). Fig. 2 shows a representative graph obtained by DSC technique in which thermal behavior of a phospholipid is assessed and the pre-transition and the main transition (Tc) peaks are visible. Molecular arrangement of the phospholipid ingredients before and after Tc, and transition from the gel to liquid crystalline phase, are also schematically presented.
Nanoliposomes made of a pure phospholipid ingredient will not form at temperatures below Tc of the phospholipid molecule. This temperature requirement is reduced to some extent, but not eliminated, by the inclusion of cholesterol [19, 20, 21]. In some cases, it is recommended that nanoliposome preparation be carried out at temperatures much higher than the Tc. In the case of vesicles containing dipalmitoyl phosphatidylcholine (DPPC, Tc = 41 °C), for instance, it has been suggested that preparation procedure be carried out at 51 °C (i.e. 10 °C higher than the Tc) [21, 28]. This is in order to make sure that all the phospholipids are homogenously dispersed in the suspension medium and have sufficient flexibility to align themselves in the bilayer structure of the lipid vesicles. Following termination of the preparation process, nanoliposomes are usually allowed to anneal and stabilize for certain periods of time (e.g. 30–60 min), at a temperature above Tc, before storage [19].
There are adequate number of available phospholipids with different Tc values, which can be used in the manufacture of nanoliposomes. Table 2 lists some of the phospholipids commonly used as nanoliposome ingredients. Depending on the sensitivity of the drug or other bioactive molecules to be encapsulated, phospholipids with low Tc values can be selected to avoid the need to employ high temperatures during nanoliposome manufacturing process.
Table 2.
Most commonly used phospholipids in liposome and nanoliposome preparations and their gel to liquid crystalline transition temperatures (Tc) (from reference [1] with permission).
| Full name | Abbreviation | Tc (°C) |
|---|---|---|
| Diarachidoyl phosphatidylcholine | DAPC | 64 |
| Dilauryloyl phosphatidylcholine | DLPC | −1.5 |
| Dilauryloyl phosphatidylglycerol | DLPG | 4 |
| Dimyristoyl phosphatidic acid | DMPA | 51 (pH6.0) |
| Dimyristoyl phosphatidylcholine | DMPC | 23 |
| Dimyristoyl phosphatidylethanolamine | DMPE | 50 |
| Dimyristoyl phosphatidylglycerol | DMPG | 23 |
| Dimyristoyl phosphatidylserine | DMPS | 36 |
| Dioleoyl phosphatidylcholine | DOPC | −21 |
| Dioleoyl phosphatidylethanolamine | DOPE | −16 |
| Dioleoyl phosphatidylglycerol | DOPG | −18 |
| Dioleoyl phosphatidylserine | DOPS | −11 |
| Dioleoyltrimethyl ammonium-propane | DOTAP | 1 |
| Dipalmitoyl phosphatidic acid | DPPA | 67 (pH6.5) |
| Dipalmitoyl phosphatidylcholine | DPPC | 41 |
| Dipalmitoyl phosphatidylethanolamine | DPPE | 64 |
| Dipalmitoyl phosphatidylglycerol | DPPG | 41 |
| Dipalmitoyl phosphatidylserine | DPPS | 52 |
| Dipalmitoyl sphingomyelin | DPSPH | 41 |
| Distearoyl phosphatidylcholine | DSPC | 55 |
| Distearoyl phosphatidylglycerol | DSPG | 55 |
| Distearoyl sphingomyelin | DSSPH | 57 |
| Phosphatidylcholine (from egg) | PC | −15 to −7 |
| Phosphatidylserine | PS | 7 |
| Sphingomyelin | SPH | 32 |
2.3. Zeta potential
Besides the phase transition property of their phospholipid ingredients, the surface charge of nanoliposomes could also be varied. They can be neutral or zwitterionic (by employing phospholipids such as phosphatidylcholine, or phosphatidylethanolamine), negatively charged or anionic (when using acidic phospholipids such as phosphatidylserine, phosphatidylglycerol, phosphatidic acid, or dicetylphosphate) or they can be positively charged (by employing cationic lipids such as DOTAP, DOTMA, or stearylamine) in physiological pH ranges [1, 21]. The net charge of the nanoliposomal formulation is an important parameter in terms of vesicle interaction with bioactive molecules. Utilizing the electrostatic attraction between oppositely charged bioactive compounds and lipid vesicles is a mean to increase encapsulation or entrapment efficiency. Therefore, for efficient entrapment of a positively charged molecule or compound an anionic nanoliposome could be employed and vice versa [1, 2, 29]. From the cytotoxicity point of view, nanoliposome charge has been shown to have a very crucial role. There are many reports on the toxicity of positively-charged phospholipid vesicles [31, 32, 33, 34, 35, 36, 37]. One reason for the toxicity of cationic vesicles is believed to be the interaction of the cationic lipids with cell organelle membranes, specifically the anionic lipids making up these biomembranes. For example, in mitochondrial membranes, cardiolipin is the major anionic lipid, and interaction of this molecule with cationic species would be detrimental to the basic energy reactions of the cell [32, 33]. Another postulated mechanism, for cationic lipid-mediated toxicity in the lung, is the involvement of reactive oxygen intermediates [34]. Negatively charged vesicles, however, are reported to be less cytotoxic or completely safe when compared to their cationic counterparts [23, 37, 38]. Furthermore, it has been postulated that anionic vesicles, in general, associate more efficiently and are taken up more readily by the cells compared with neutral or zwitterionic vesicles [39, 40], although, no clear mechanism has been proposed for this observation. Consequently, most FDA-approved therapeutic lipid–drug formulations are negatively charged [30].
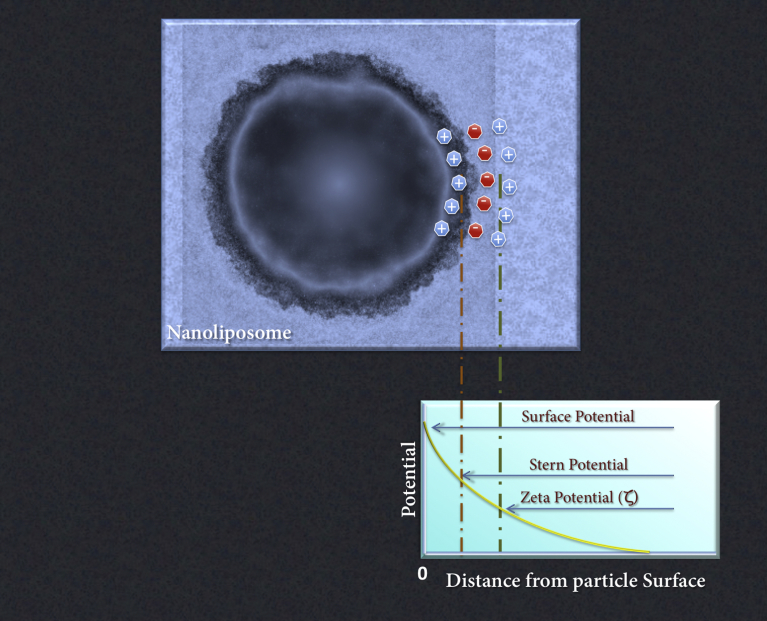
The charge density of nanoliposomal surface and the binding affinity of various ions to the lipid vesicles can be determined by measuring a parameter called “zeta potential” (ZP). The ZP of a nanoliposome is the overall charge that the nanovesicle acquires in a particular environment or suspension medium [1, 19]. In another words, ZP is the charge that develops at the interface between a particle's surface (e.g. nanoliposome surface) and its liquid medium. This parameter, which is measured in MilliVolts (mV), may arise by any of several mechanisms. Among these are the dissociation of ionogenic groups on the vesicle surface and the differential adsorption of solution ions into the surface region. The net charge at the surface of the nanoliposome affects the ion distribution in the surrounding region, increasing the concentration of counter-ions near the surface. Consequently, an electrical double layer is formed in the region of the nanovesicle-liquid interface (Fig. 3).
Fig. 3.
Schematic representation of the charge distribution around the surface of a nanoliposome and illustration of the concept of zeta potential. The electrical double layer is composed of a layer of ions strongly bound to the charged surface (i.e. Stern layer) and an adjacent region of loosely associated mobile ions and counter-ions.
2.3.1. Zeta potential and nanoliposome stability
Understanding the distinct colloidal and interfacial phenomena associated with the applications of nanovesicles requires knowledge of their ZP. Furthermore, knowledge of the ZP of a nanoliposome formulation can help to predict the fate of the formulation in vitro and in vivo. The magnitude of the measured ZP can be used to predict the stability and shelf-life of the nanoliposomal sample. If the nanoliposomes in a suspension possess a large negative or positive ZP then they will tend to repel each other and resist the formation of aggregates. However, if the phospholipid vesicles have low ZP values, i.e. close to zero, then there will be nothing to prevent the particles approaching each other and aggregate or fuse [41]. ZP is a function of the surface charge of the phospholipid vesicle, any adsorbed layer at the interface and the nature and composition of the medium in which the nanoliposome is suspended. It is usually of the same sign as the potential actually at the vesicle surface and is expressed in units of millivolts (mV). Since it reflects the effective charge on the nanoliposomal vesicles and is therefore related to the electrostatic repulsion between them, ZP has proven to be extremely relevant to the practical study and control of colloidal stability and flocculation processes. The greater the ZP the more likely the formulation is going to be stable because the charged vesicles repel each other and thus overcome the natural tendency of particles to adhere with each other, aggregate/agglomerate and/or fuse. Generally a zeta potential of above +30 mV and below –30 mV is considered a suitable threshold value for the colloidal stability of the phospholipid vesicles [16, 42, 43].
2.4. Preparation techniques
For the selection of an appropriate method for nanoliposome preparation it is essential that novel therapeutic formulations, which are initially tested in the laboratory on a small scale (e.g. microliters), are adaptable and can maintain the same characteristics when prepared in large volumes (e.g. liters) for preclinical and clinical testing. Large volumes are necessary to evaluate the nanoliposomes in an appropriate in vivo model, in order to meet the guidelines set by regulatory authorities for product licensing. Industrial-scale production of nanoliposomes for clinical applications requires not only the ability to produce sufficient quantities, but also necessitates reproducibility and rigorous adherence to quality standards as described in the Good Manufacture Practice (GMP) guidelines [30].
2.4.1. Conventional methods
A number of classical procedures have been reported in the literature for nanoliposome preparation. These include, but not limited to, the following techniques and procedures:
-
•
Thin-film hydration method (also known as Bangham method, which is the first method employed for liposome preparation synthetically) [44];
-
•
Solvent-injection technique [45];
-
•
Detergent dialysis [46];
-
•
Reversed phase evaporation [47];
-
•
Homogenization [48]; and
-
•
French pressure cell method [49].
Downsizing procedures are performed to obtain monomodal nano-sized vesicles with narrow size distribution from a heterogeneous mixture of lipid vesicles. These methods include extrusion through filters of defined pore sizes, high-pressure homogenization, freeze-thawing and sonication [4, 19, 50].
2.4.2. Effect of phospholipids and Tc temperature on nanoliposome preparation
In order to produce stable vesicles in a reproducible manner, majority of nanoliposome preparation procedures depend on the selection of right combination of phospholipid ingredients and their phase transition temperature (Tc). Generally, a pure phospholipid ingredient will not form vesicles at temperatures below the phase transition temperature of the phospholipid molecule. However, this temperature requirement is partially altered by the inclusion of cholesterol and other excipients as explained above [19, 20, 21]. With some preparation techniques, such as extrusion, microfluidization or homogenization, it is recommended that nanoliposome preparation be carried out at temperatures above the Tc. This is in order to make sure that all the phospholipid ingredients are at the “gel state” and as a result have sufficient flexibility to align themselves in the bilayer structure of the nanoliposomes. At the end of the preparation procedure, nanoliposomes should be allowed to anneal and stabilize for certain periods of time (e.g. 30–60 min), at a temperature above their Tc, before storage [2, 4, 19].
2.4.3. Adverse aspects of classical techniques
There are a number of problems associated with the above-mentioned classical preparation methods, which can be classified into the following categories:
-
•
The particle size of the vesicles is too large or has a broad size distribution (polymodal) so there is a need for post-processing procedures.
-
•
Trace amounts of the detergents or the organic solvent residues remaining in the final product is a serious issue since it not only affects the stability of some drugs (e.g. protein or polypeptide agents), but also adversely affects clinical applications.
-
•
An additional step is required to evaporate the organic solvents used during the preparation procedure to bring down the concentration of these toxic solvents.
-
•
High shear force treatments such as homogenization or sonication may have deleterious effects on the ingredients and drug molecules.
-
•
Considering that many of the phospholipid ingredients of the vesicles are sensitive to temperature, sterilization of nanoliposomal formulations can cause a problem. Therefore, there is a need for preparation methods that can be carried out in an ultraclean/sterilized environment. However, classical nanoliposome preparation techniques do not always meet this requirement.
-
•
In some procedures careful monitoring is needed to ensure batch-to-batch consistency.
-
•
Classical methods are multistep and hence they are lengthy procedures increasing the cost of manufacture.
To solve these problems, many novel preparation technologies have been devised for the preparation of nanoliposomes considering that the final product must be:
-
•
Within the uniformity requirement;
-
•
Reproducible within a defined size and charge range;
-
•
Sterile and pyrogen free;
-
•
Devoid of any potentially toxic solvent and harmful additives;
-
•
Adequately stable in storage with acceptable shelf-life; and also:
-
•
The preparation method must be time-efficient, cost-effective and scalable [2, 4, 51, 52].
In an attempt to manufacture vesicles meeting the above-mentioned criteria, Wagner et al. [53, 54] developed a cross-flow injection module in which the aqueous phase is pumped from its starting container to a collecting vessel, and the ethanolic phase is injected half-way at an injection module. This procedure could be run in a continuous manner and the manufacture scale-up merely depends on the size of the attached vessels. There are few other techniques that are based on employing ethanol as a substitute to toxic solvents e.g. methanol and chloroform [30]. Although some of these methods are readily scalable, they suffer from the fact that some lipids, phospholipids and drugs are not soluble in ethanol. Nevertheless, inadequate dissolution or mixing of the ingredients could result in heterogeneous composition, charge and size of the nanoliposomal drug formulations.
2.4.4. Scalable methods of nanoliposome preparation
There are certain therapeutic agents, including some proteins and oligonucleotides, which are sensitive to denaturation in organic solvents. Detergent dialysis method is a potentially scalable technique that could be more suitable for these agents [46]. In this method, phospholipids are mixed with a surfactant or detergent in an aqueous solution to produce micelles followed by dilution or removal of the detergent to produce lipid vesicles with the ability to incorporate proteins and oligonucleotides in their native form. Detergent dialysis method is flexible, and potentially scalable, however, it has some serious disadvantages. Encapsulation efficiency of hydrophobic compounds is low and methods employed to remove the detergent may also remove hydrophilic molecules. Another drawback is that the multistep process is time-consuming. These limitations, especially the challenge of removing residual concentrations of detergents and organic solvents, make detergent dialysis and ethanol injection methods more costly for industrial-scale preparation of nanoliposomes [30].
2.4.5. Heating method
Another technique that was devised to address scale-up, cost and toxicity problems is known as Heating method, which was developed by Mozafari et al. and first published in 2002 [55]. This method makes it possible to manufacture liposomes and nanoliposomes (in addition to some other colloidal drug delivery systems) using a single vessel in the absence of potentially toxic solvents and detergents. In brief, phospholipids and other excipients are hydrated under an inert atmosphere such as argon or nitrogen for 1–2 hours in an aqueous medium. In the next step the ingredients are mechanically stirred after adding a polyol (e.g. glycerol) as a co-solvent or dispersant at a temperature of ca. 120 °C for 30 min. This temperature makes it sure that all ingredients (especially cholesterol) are properly dissolved in the aqueous medium without the need to use organic solvents and the product will be sterile and pyrogen free. Depending on their heat sensitivity, drug compounds can be added at the high temperature or at a lower temperature after the other ingredients are uniformly dispersed [55, 56].
2.4.6. Mozafari method
Recently, Mozafari and colleagues [11, 28] developed a more robust and faster procedure (compared to the Heating method) known as “Mozafari method”. It is one of the simplest techniques for the preparation of liposomes and nanoliposomes (in addition to some other encapsulation systems). This method has been employed successfully for the encapsulation of the food-grade antimicrobial nisin [28] as well as omega fatty acids [10, 11]. Mozafari method allows manufacture of carrier systems in one-step, without the need for the pre-hydration of ingredient material, and without employing toxic solvents or detergents and harsh procedures such as homogenization or microfluidization, from small scales to the industrial scales. This method is economical and capable of manufacturing nanoliposomes, with a superior monodispersity and storage stability using a simple protocol and one, single vessel. A novel drug delivery system, known as Tocosome, composed of tocopheryl phosphates, was recently manufactured in our laboratory using Mozafari method [57]. Tocosome was employed for the encapsulation of 5-fluorouracil to improve solubility, preserve the function and decrease side-effects of the anticancer molecule [57]. A step-by-step preparation of nanoliposomes using Mozafari method and some other techniques is described in details in reference [19].
2.5. Methods of single particle probing
Before a nanoliposomal formulation could be approved for clinical utilization, it must be adequately characterized with respect of certain in vitro and in vivo attributes. The in vitro evaluations include determination of particle size, polydispersity index (PDI), charge and morphology of the formulations. There are several methods for the assessment of the size of nanoliposomes. These include light scattering, diffraction, hydrodynamic and microscopic techniques. Examples of the analytical methods used in nanoliposome research are atomic force microscopy (AFM), transmission electron microscopy (TEM), flow cytometry, coulter counter and optical density method [58, 59, 60, 61, 62]. Preferably, characterization methods have to be meaningful, clear, reproducible and fast. Microscopic techniques are widely employed to study the shape, lamellarity, surface characteristics, size and stability of nanoliposomes. Considering a statistically meaningful analysis of size distribution of a sample of nanoliposomes, methods such as light scattering, which measure the size of large number of vesicles in an aqueous medium instantly, are more useful than microscopic techniques [1, 61]. Dynamic light scattering (DLS) is a robust method, which offers adequate statistics with respect to the in situ measurements of size, PDI and ZP of nanoliposomal formulations [18, 61]. However, light scattering does not provide information about the morphology and shape of the nanoliposomes and it assumes any cluster of particles as one single object. This is while microscopic techniques provide more detailed information about the morphology of nanoliposomes.
2.5.1. Microscopic methods of analysis
They make direct observation possible; henceforth they provide information on the shape of the nanovesicles as well as presence/absence of any aggregation and/or fusion and their internal architecture. For example, freeze fracture electron microscopy can make it possible to visualize and quantify the number of bilayers (lamellarity) and internal compartments of nanoliposomes. The drawback of the microscopic techniques, however, is that the number of particles that can be studied at any certain time is limited and sample preparation can be complicated and time consuming. Consequently, the ideal approach for the characterization of nanoliposomes should be to employ more than one of the analytical techniques mentioned above.
Representative image of a nanoliposome composed of soy lecithin and containing omega 3 fish oil is depicted in Fig. 1. Nanoliposomes were prepared by Mozafari method and examined by energy filtered transmission electron microscope (EFTEM). For the microscopic analysis, nanoliposome samples were placed on a copper grid and dried under nitrogen. They were then negatively stained with 2% phosphotungstic acid (PTA) solution and dried again before being observed under EFTEM [63]. It should be noted that the contrast agents used to be able to visualize the samples are potentially toxic and hazardous chemicals. This is a drawback that some of the other microscopic techniques (e.g. TEM, SEM, etc.) also suffer from. Unfortunately, the majority of microscopic techniques involve sample manipulation procedures such as staining, labeling, fixation or vacuum, which are time demanding and may cause some alterations in the structure and/or size of the samples [58].
2.5.2. Scanning probe microscopy
There are certain imaging techniques such as scanning tunneling microscopy and atomic force microscopy, which unlike other microscopic instruments do not require extensive sample preparation procedures [60, 64]. These techniques can be used in ambient conditions with a very simple sample preparation process. These high-resolution microscopes belong to a group of imaging instruments known as scanning probe microscopes (SPM). SPM is a technique for imaging surfaces at the nanometer scale by rastering a fine probe (also known as a tip) across the surface and measuring the repulsive/attractive interactions between the tip and the surface. SPM is a general term comprising a wide variety of techniques based on different interactions between the tip and the surface. These techniques, defined by the type of interaction being measured, include atomic force microscopy (AFM), scanning tunneling microscopy (STM), Kelvin probe force microscopy (KPFM), magnetic force microscopy (MFM), electrostatic force microscopy (EFM), and Ballistic electron emission microscopy or BEEM. Among these instruments STM and AFM are the most commonly employed techniques for measuring surface roughness of micro and nanoparticles [64, 65, 66, 67]. SPM techniques can be used to study nanostructures in air or solution at ambient conditions with very high resolutions in two and three dimensions. When operated in the tapping and non-contact mode, AFM allows the observation of the morphology of nanoliposomes in their native form. Especially the intermittent contact motion of the tip (tapping) eliminates lateral or shear forces which would otherwise scrape or deform the vesicles [68, 69]. It should be noted that, liposomes and nanoliposomes might undergo changes in their shape once deposited on the substrate of an AFM. As a result of the interaction between the sample and the substrate, nanoliposome may flatten and spread out depending on its chemical composition, membrane fluidity and elasticity. This will cause errors in nanoliposome size measurements using SPM techniques [60, 61].
2.5.3. Evaluation of surface charge
In addition to morphology and size, the other important parameter to be considered in the characterization of nanoliposomal formulations is their overall electrostatic behavior as indicated by the zeta potential (ZP). Currently, there are a number of techniques available for measuring the quantity of ZP. These methods are generally based on one of the three electrokinetic effects: (i) electrophoresis, (ii) electroosmosis, and (iii) the streaming potential [70, 71]. In the electrophoresis method, ZP is determined by placing the particles in an electric field and measuring their mobility, using an appropriate microscopic technique. The electrophoretic mobility (υE) is then related to the ζ-potential at the interface of the nanovesicles employing the “Smoluchowski equation” [72, 73] as described below:
| υE = 4 π ε0εr·ζ/6 πμ·(1+κr) | (1) |
In Eq. (1), ε0 is the relative dielectric constant and εr is the electrical permittivity of a vacuum, μ is the solution viscosity, r is the particle radius and K= (2n0z2e2/εrε0kBT)1/2 is the Debye–Hückel parameter, n0 is the bulk ionic concentration, z is the valence of the ion, e is the charge of an electron, kB is the Boltzmann constant, and T is the absolute temperature (°K) [74].
By directly analyzing the electrophoretic mobility of nanoliposomes, their ZP value can also be determined using the “Henry equation” [75, 76]:
| υE = 2 εz f(Ka)/3η | (2) |
where υE is the electrophoretic mobility, ε is the dielectric constant, z is the zeta potential, η is the viscosity and f(Ka) is Henry's function. For measuring ZP in aqueous solutions of moderate electrolyte concentration, a Henry's function value of 1.5 could be employed (Smoluchowski approximation). However, if ZP is measured in a non-polar solvent, f(Ka) should be set to 1.0 (Huckel's approximation) [77]. Measurement of the ZP of nanoliposomal formulations can also be carried out using the laser Doppler velocimetry technique (LDV), named after the Austrian physicist Christian Doppler [78, 79]. LDV is a non-intrusive method in which the Doppler shift in a laser beam is employed to measure the velocity in a transparent or semi-transparent fluid. This technique is based on the Doppler effect, and the analysis of the intensity autocorrelation function of the scattered light, where the frequency of the light is altered when the source of the light is moving. The frequency of the light can be changed by diffraction of the light from a moving particle [80, 81].
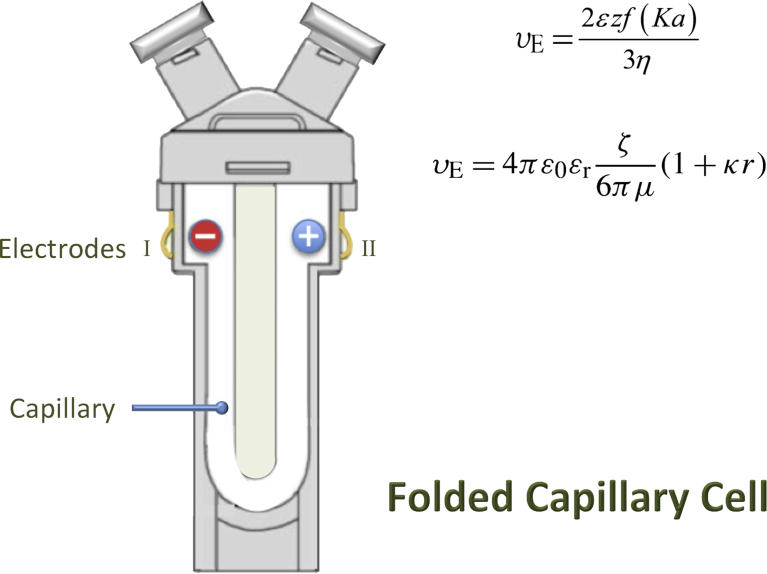
A recently developed method for the evaluation of ZP of nanoliposomes is referred to as the “diffusion barrier technique”, using laser Doppler electrophoresis [82, 83, 84, 85]. This method is particularly suitable for low sample volumes and high ionic strength buffers. A very small volume of the sample (∼20 μl) is carefully injected into the tube of the folded capillary cell fitted with the electrodes, which is prefilled with the buffer only. The nanoliposome sample will therefore be located between the first and second electrodes inside the special cell (also called a cuvette, Fig. 4). While the nanovesicles are kept separated from the electrodes, an alternating electric field is applied across the electrodes, and the sample is illuminated with temporally coherent light [85]. For an electrophoretic mobility measurement the diffusion barrier is employed to isolate the nanovesicles from the electrode surface spatially whilst maintaining electrical contact with the surface, via the buffer within which they are dispersed [83]. The combination of hindered diffusion coupled with the length of the “U” tube inside the cuvette, confine the nanoliposomes to a region where they cannot contact the electrodes during the analysis (Fig. 4). Therefore, the structure and electrophoretic mobility of the nanoliposomes will not be altered (by preventing their direct contact with the electrodes), and as the electrolyte intermediates between the electrodes and the nanovesicles in solution, electrical contact is maintained without the anodic reaction [82]. This means that the voltage can be applied for a longer period of time in order to generate more reliable data from the measurement. The buffer used in the capillary cells has to be the same buffer in which the nanoliposomes are prepared, with the same conductivity, same pH, and same additives, in order to match the original sample and the diffusion barrier as closely as possible.
Fig. 4.
The special capillary cuvette (cell) used for the measurement of zeta potential of nano structures such as nanoliposomes based on the Henry equation and Smoluchowski equation (see text for details).
There are some non-invasive laboratory instruments available, which make it possible to perform particle size, PDI and ZP analysis on the same sample. Generally called size and zeta potential analyzer instrument, they include NanoPlus (Micromeritics), Zetasizer Nano (Malvern Panalytical), SZ-100 Nanopartica (Horiba), and NanoBrook 90Plus (Brookhaven) to name a few. These advanced analytical instruments are equipped with softwares utilizing “electrophoretic mobility”, “Smoluchowski equation”, “brownian motion”, “laser diffraction” and “diffusion barrier” principles to measure size, PDI and ZP with high precision.
2.5.4. Scanning ion occlusion sensing
There are some other analytical techniques, which make assessment of size and ZP of an individual phospholipid vesicle, possible. One of these methods is referred to as the “scanning ion occlusion sensing” (SIOS). It is a nanopore-based technology that can be used for single-nanoliposome probing [86, 87]. SIOS analyzes phospholipid vesicles (e.g. liposomes and nanoliposomes) in the size range of 60 nm to few micrometers [88]. Operation mechanism of SIOS is based on the conventional Coulter counter, where individual nanoliposomes are measured as they traverse a nanopore. When a single nanoliposome passes through the tunable nanopore, a current reduction takes place as a result of an increase in electrical resistance. The magnitude of reduction of current and the frequency of the pulses are related to the particle size and concentration of the nanovesicle, respectively. Nanoliposomes are driven either by electrophoresis and electro-osmosis or by pressure generated from a pressure module [89, 90]. SIOS is a useful method to analyze multiple parameters of nanoliposomes on a particle-by-particle basis. This technique has proven to possess higher resolution in comparison with techniques such as dynamic light scattering. Furthermore, SIOS was successfully used to measure changes in the size and surface charge of phospholipid vesicles as a result of incubation in plasma [87, 89]. There are still unresolved problems, which need to be addressed with the SIOS technique. For instance, it is difficult to choose a suitable elastic pore for a polydisperse nanoliposome sample to avoid detecting several vesicles at the same time. Also, it is still a problem to detect only one single vesicle at the time and the data obtained with different nanopore sizes can hardly be compared in parallel [90].
2.5.5. Flow cytometry
An established method for the assessment of single nanoliposome diameter and size distribution is flow cytometry (FCM). This technology is widely employed in analyzing and sorting cells, bacteria, and other cell-sized particles. FCM has been applied in the analysis of multilamellar and large unilamellar vesicles (MLV and LUV). It employs light scattering to measure particles and vesicles in a continuous flow system. Nanoliposomes have to be fluorescently labeled in order to be distinguished from the impurities and noise signal. Subsequently, the scattered light at 10° angle, side scattered light at 90° angle, or fluorescence of the sample is measured. FCM is a very fast, reliable, and reproducible analytical technique. Nevertheless, when using light scattering detection, the operation of FCM can be affected by noisy signal from buffers, electronics, or optics [89, 90].
2.5.6. Nanoparticle tracking analysis
Another probing method, which is able to track and measure a single nanoliposome moving under Brownian motion, is nanoparticle tracking analysis (NTA) [91]. This high-resolution technique is effective in determining the size, size distribution, and concentration of liposomes and nanoliposomes. It could be used to measure size of the particles and vesicles within 30–1000 nm size range [92]. Samples are injected into the special cell of the instrument and then illuminated by laser light (635 nm) that passes through a liquid layer on the optical surface [92, 93]. Refraction occurs and the region in which the phospholipid vesicles are present is illuminated and visualized under microscopy. Charge-coupled device camera records a video (30 frames per second) in which the movement of vesicles under Brownian motion could be observed. Instrument's software identifies and tracks the center of each vesicle throughout the length of the video and relates it to the particle size. The hydrodynamic size and size distribution of the lipid vesicles can be calculated by Stokes–Einstein equation using particle diffusion coefficient. This method enables measurement of size of both monodisperse and polydisperse samples. Moreover, it is able to measure zeta potential of the phospholipid vesicles and detect their fluorescence signals. Disadvantages of the NTA method include its requirement for complex optimization by a skilled operator and the difficulty to identify an appropriate concentration for probing the sample [9, 94, 95]. In addition, nanoliposome characterization by the NTA method could be impeded by the refractive index of the sample [96, 97, 98, 99].
2.5.7. Total particle analysis
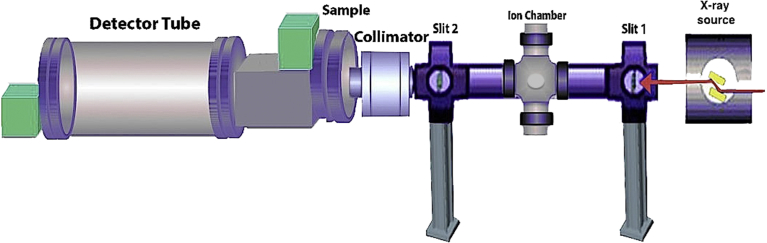
The aforementioned techniques are specialized apparatus or instrumentations currently available for single-particle analysis. This is while there are a number of analytical methodologies, which can be useful for the evaluation of total particles present in the sample under investigation. The sub-nanometer scale analysis opportunity provided by techniques such as small angle neutron and X-ray scattering (Fig. 5) is of special importance to pharmaceutical and biomedical industries [99]. As drug carrier systems become more functionalized and continue decreasing in size, the ability to elucidate details on size scales smaller than those available from optical techniques becomes extremely pertinent. Therefore, information gathered from small angle scattering aids optimizing pharmaceutical efficacy of a therapeutic formulation at its most fundamental level. Traditionally, size-exclusion chromatography (SEC), small-angle X-ray scattering (SAXS), first generations of electron microscopes, and similar techniques, have been used to characterize particle size. However, all of these methods provide information derived from averages of ensembles of particles, and none can provide biophysical data capturing precise differences in particle size from multiple heterogeneous populations [92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103]. Single-particle sizing techniques, presented and explained in this manuscript, enable analysis of individual nanoliposomes, allowing the population size distribution to be examined more accurately. Such information has the potential to provide insights into the relationship between particle size and structural composition and can prove valuable for pharmaceutical applications of nanocarrier systems.
Fig. 5.
Schematic representation of the main components of small-angle X-ray scattering (SAXS) setup.
3. Conclusions
For a successful formulation of nanoliposomes, their physical attributes including morphology, size, PDI and zeta potential need to be assessed as accurately as possible to ascertain their suitability for Human use. Characterization of nanoliposomes is of great importance in order to be able to predict and control their in vitro and in vivo behavior and to design safe and efficient products for diagnostic, therapeutic, nutritional and cosmetic use. Variations in the shape, size, PDI and ZP of the phospholipid nanovesicles in time, are monitored to assess stability and shelf life of nanoliposomal products. Several techniques are available for the characterization of nanoliposomes, and progress in the development and improvement of these methods is continuously ongoing. At present, there is hardly any analytical technique without limitations and shortcomings. Overall, the choice of technique depends on which aspect of nanoliposome characteristic need to be evaluated and what level of detail is required. Therefore, there is still requirement for the development of selective, specific and reproducible analytical techniques with high resolutions for nanoliposome analysis. Regulations and guidelines need to be set, by regulatory agencies, as for the acceptable and safe range of size, PDI and ZP for different applications and routes of administration of nanoliposomal products. Future perspective of nanoliposomal products holds great promise with respect of their unique role in improving Human life quality owing to the progress in the accuracy and sensitivity of single particle analytical techniques.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Mozafari M.R., Mortazavi S.M. Trafford Pub. Ltd; Oxford (UK): 2005. Nanoliposomes: From Fundamentals to Recent Developments. [Google Scholar]
- 2.Mozafari M.R. Liposomes: an overview of manufacturing techniques. Cell. Mol. Biol. Lett. 2005;10(4):711–719. [PubMed] [Google Scholar]
- 3.Khorasani S., Danaei M., Mozafari M.R. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. Technol. 2018;79:106–115. [Google Scholar]
- 4.Maherani B., Arab-Tehrany E., Mozafari M.R., Gaiani C., Linder M. Liposomes: a review of manufacturing techniques and targeting strategies. Curr. Nanosci. 2011;7(3):436–452. [Google Scholar]
- 5.Sharma D., Ali A.A., Trivedi L.R. An updated review on: liposomes as drug delivery system. Pharma Tutor. 2018;6(2):50–62. [Google Scholar]
- 6.Amoabediny G., Haghiralsadat F., Naderinezhad S., Helder M.N., Akhoundi Kharanaghi E., Mohammadnejad Arough J., Zandieh-Doulabi B. Overview of preparation methods of polymeric and lipid-based (niosome, solid lipid, liposome) nanoparticles: a comprehensive review. Int. J. Poly. Mat. Poly. Biomat. 2018;67(6):383–400. [Google Scholar]
- 7.Mozafari M.R., Danaei M., Javanmard R., Raji M., Maherani B. Nanoscale lipidic carrier systems: importance of preparation method and solvents. Glob. J. Nanomed. 2017;2(4):555593. [Google Scholar]
- 8.Chen C., Zhu S., Huang T., Wang S., Yan X. Analytical techniques for single-liposome characterization. Anal. Meth. 2013;5(9):2150–2157. [Google Scholar]
- 9.Danaei M., Dehghankhold M., Ataei S., Hasanzadeh Davarani F., Javanmard R., Dokhani A., Khorasani S., Mozafari M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10:57. doi: 10.3390/pharmaceutics10020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasti B., Erfanian A., Selamat J. Novel nanoliposomal encapsulated omega-3 fatty acids and their applications in food. Food Chem. 2017;230:690–696. doi: 10.1016/j.foodchem.2017.03.089. [DOI] [PubMed] [Google Scholar]
- 11.Rasti B., Jinap S., Mozafari M.R., Abd-Manap M.Y. Optimization on preparation condition of polyunsaturated fatty acids nanoliposome prepared by Mozafari method. J. Liposome Res. 2014;24:99–105. doi: 10.3109/08982104.2013.839702. [DOI] [PubMed] [Google Scholar]
- 12.Gibbs B.F., Kermasha S., Alli I., Mulligan C.N. Encapsulation in the food industry: a review. Int. J. Food Sci. Nutr. 1999;50(3):213–224. doi: 10.1080/096374899101256. [DOI] [PubMed] [Google Scholar]
- 13.Chapman D., Williams R.M., Ladbrooke B. Physical studies of phospholipids. VI. Thermotropic and lyotropic mesomorphism of some 1, 2- diacyl-phosphatidylcholines (lecithins) Chem. Phys. Lip. 1967;1(5):445–475. [Google Scholar]
- 14.Veksli Z., Salsbury N.J., Chapman D. Physical studies of phospholipids: XII. Nuclear magnetic resonance studies of molecular motion in some pure lecithin-water systems. Biochim. Biophys. Acta Biomembr. 1969;183(3):434–446. doi: 10.1016/0005-2736(69)90158-8. [DOI] [PubMed] [Google Scholar]
- 15.Jaafar-Maalej C., Diab R., Andrieu V., Elaissari A., Fessi H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010;20:228–243. doi: 10.3109/08982100903347923. [DOI] [PubMed] [Google Scholar]
- 16.Gentine P., Bubel A., Crucifix C., Bourel-Bonnet L., Frisch B. Manufacture of liposomes by isopropanol injection: characterization of the method. J. Liposome Res. 2012;22(1):18–30. doi: 10.3109/08982104.2011.584318. [DOI] [PubMed] [Google Scholar]
- 17.Sebaaly C., Greige-Gerges H., Stainmesse S., Fessi H., Charcosset C. Effect of composition, hydrogenation of phospholipids and lyophilization on the characteristics of eugenol-loaded liposomes prepared by ethanol injection method. Food Biosci. 2016;15:1–10. [Google Scholar]
- 18.Maherani B., Wattraint O. Liposomal structure: a comparative study on light scattering and chromatography techniques. J. Disp. Sci. Tech. 2017;38(11):1633–1639. [Google Scholar]
- 19.Mozafari M.R. Liposomes. Humana Press; 2010. Nanoliposomes: preparation and analysis; pp. 29–50. [DOI] [PubMed] [Google Scholar]
- 20.Szoka F., Jr., Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes) Ann. Rev. Biophys. Bioeng. 1980;9(1):467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- 21.New RRC . 1990. Liposomes: a Practical Approach. IRL Oxford, UK. [Google Scholar]
- 22.Mozafari M.R., Hasirci V. Mechanism of calcium ion induced multilamellar vesicle-DNA interaction. J. Microencapsul. 1998;15(1):55–65. doi: 10.3109/02652049809006835. [DOI] [PubMed] [Google Scholar]
- 23.Mozafari M.R., Reed C.J., Rostron C. Cytotoxicity evaluation of anionic nanoliposomes and nanolipoplexes prepared by the heating method without employing volatile solvents and detergents. Pharmazie. 2007;62(3):205–209. [PubMed] [Google Scholar]
- 24.Cortesi R., Esposito E., Gambarin S., Telloli P., Menegatti E. Preparation of liposomes by reverse-phase evaporation using alternative organic solvents. J. Microencapsul. 1999;16(2):251–256. doi: 10.1080/026520499289220. [DOI] [PubMed] [Google Scholar]
- 25.ICH . ICH; Geneva, Switzerland: 17 July 1997. Harmonized Tripartite Guideline for Residual Solvents, Step 4. [Google Scholar]
- 26.ICH . 2016. Harmonized Guideline. Impurities: Guideline for Residual Solvents. Q3C (R6) [Google Scholar]
- 27.Heydari R. Residual solvents determination in pharmaceuticals by static headspace-gas chromatography and headspace liquid-phase microextraction gas chromatography. Anal. Lett. 2012;45(13):1875–1884. [Google Scholar]
- 28.Colas J.C., Shi W., Rao V.M., Omri A., Mozafari M.R., Singh H. Microscopical investigations of nisin-loaded nanoliposomes prepared by Mozafari method and their bacterial targeting. Micron. 2007;38(8):841–847. doi: 10.1016/j.micron.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Gabizon A.A., Barenholz Y., Bialer M. Prolongation of the circulation time of doxorubicin encapsulated in liposomes containing a polyethylene glycol-derivatized phospholipid: pharmacokinetic studies in rodents and dogs. Pharm. Res. 1993;10(5):703–708. doi: 10.1023/a:1018907715905. [DOI] [PubMed] [Google Scholar]
- 30.Kraft J.C., Freeling J.P., Wang Z., Ho R.J. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014;103(1):29–52. doi: 10.1002/jps.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell P.I. Toxicity of some charged lipids used in liposome preparations. Cytobios. 1983;37(145):21–26. [PubMed] [Google Scholar]
- 32.Filion M.C., Phillips N.C. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. Biochim. Biophys. Acta Biomembr. 1997;1329(2):345–356. doi: 10.1016/s0005-2736(97)00126-0. [DOI] [PubMed] [Google Scholar]
- 33.Filion M.C., Phillips N.C. Major limitations in the use of cationic liposomes for DNA delivery. Int. J. Pharm. 1998;162(1-2):159–170. [Google Scholar]
- 34.Dokka S., Toledo D., Shi X., Castranova V., Rojanasakul Y. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharmaceut. Res. 2000;17(5):521–525. doi: 10.1023/a:1007504613351. [DOI] [PubMed] [Google Scholar]
- 35.Nagahiro I., Mora B.N., Boasquevisque C.H., Scheule R.K., Patterson G.A. Toxicity of cationic liposome-DNA complex in lung isografts. Transplant. 2000;69(9):1802–1805. doi: 10.1097/00007890-200005150-00012. [DOI] [PubMed] [Google Scholar]
- 36.Omidi Y., Barar J., Akhtar S. Toxicogenomics of cationic lipid-based vectors for gene therapy: impact of microarray technology. Curr. Drug Deliv. 2005;2(4):429–441. doi: 10.2174/156720105774370249. [DOI] [PubMed] [Google Scholar]
- 37.Chawla R.S., Kellaway I.W., Hunneyball I.M., Stevens J. The effect of liposomal charge on drug toxicity and efflux. J. Pharm. Pharmacol. 1979;31(S1):86P. doi: 10.1111/j.2042-7158.1979.tb11634.x. [DOI] [PubMed] [Google Scholar]
- 38.Welz C., Neuhuber W., Schreier H., Metzler M., Repp R., Rascher W., Fahr A. Nuclear transport of oligonucleotides in HepG2-cells mediated by protamine sulfate and negatively charged liposomes. Pharm. Res. 2000;17(10):1206–1211. doi: 10.1023/a:1026410612600. [DOI] [PubMed] [Google Scholar]
- 39.Heath T.D., Lopez N.G., Papahadjopoulos D. The effects of liposome size and surface charge on liposome-mediated delivery of methotrexate-γ-aspartate to cells in vitro. Biochim. Biophys. Acta Biomembr. 1985;820(1):74–84. doi: 10.1016/0005-2736(85)90217-2. [DOI] [PubMed] [Google Scholar]
- 40.Monkkonen J., Valjakka R., Hakasalo M., Urtti A. The effects of liposome surface charge and size on the intracellular delivery of clodronate and gallium in vitro. Int. J. Pharm. 1994;107(3):189–197. [Google Scholar]
- 41.Shi Y. University of Southern Mississippi; 2014. Self-Assembled Gold Nanoplexes for Cancer-Targeted SiRNA Delivery. MSc dissertation. [Google Scholar]
- 42.McFadyen P.E., Fairhurst D.A. British Ceramic Proceedings. Institute of Ceramics; 1993. Zeta potentials of nanoceramic materials-measurement and interpretation. 175–175. [Google Scholar]
- 43.Larsson M., Hill A., Duffy J. Suspension stability; why particle size, zeta potential and rheology are important. Ann. Trans. Nordic Rheol. Soc. 2012;20:209–214. [Google Scholar]
- 44.Chiani M., Norouzian D., Shokrgozar M.A., Azadmanesh K., Najmafshar A., Mehrabi M.R., Akbarzadeh A. Folic acid conjugated nanoliposomes as promising carriers for targeted delivery of bleomycin. Artif. Cells Nanomed. Biotech. 2018;46(4):757–763. doi: 10.1080/21691401.2017.1337029. [DOI] [PubMed] [Google Scholar]
- 45.Hashemi S.H., Montazer M., Naghdi N., Toliyat T. Formulation and characterization of alprazolam-loaded nanoliposomes: screening of process variables and optimizing characteristics using RSM. Drug Dev. Ind. Pharm. 2018;44(2):296–305. doi: 10.1080/03639045.2017.1391834. [DOI] [PubMed] [Google Scholar]
- 46.Salimi A. Liposomes as a novel drug delivery system: fundamental and pharmaceutical application. Asian J. Pharm. 2018;12(01) [Google Scholar]
- 47.Tang Y., Tang D., Zhang J., Tang D. Novel quartz crystal microbalance immunodetection of aflatoxin B1 coupling cargo-encapsulated liposome with indicator-triggered displacement assay. Anal. Chim. Acta. 2018 May 9 doi: 10.1016/j.aca.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 48.Barnadas-Rodriguez R., Sabes M. Factors involved in the production of liposomes with a high pressure homogenizer. Int. J. Pharm. 2001;213(1–2):175–186. doi: 10.1016/s0378-5173(00)00661-x. [DOI] [PubMed] [Google Scholar]
- 49.Hamilton R.L., Goerke J., Guo L.S., Williams M.C., Havel R.J. Unilamellar liposomes made with the French pressure cell: a simple preparative and semiquantitative technique. J. Lipid Res. 1980;21(8):981–992. [PubMed] [Google Scholar]
- 50.Sriwongsitanont S., Ueno M. Effect of freeze-thawing process on the size and lamellarity of PEG-lipid liposomes. Open Coll. Sci. J. 2011;4:1–6. [Google Scholar]
- 51.Huang Z., Li X., Zhang T., Song Y., She Z., Li J., Deng Y. Progress involving new techniques for liposome preparation. Asian J. Pharm. Sci. 2014;9(4):176–182. [Google Scholar]
- 52.Barenholz Y., Amselem S. Quality control assays in the development and clinical use of liposome-based formulations. In: Gregoriadis G., editor. Liposome Technology. second ed. CRC Press; Boca Raton: 1993. pp. 527–616. [Google Scholar]
- 53.Wagner A., Platzgummer M., Kreismayr G., Quendler H., Stiegler G., Ferko B., Vecera G., VorauerUhl K., Katinger H. GMP production of liposomes – a new industrial approach. J. Liposome Res. 2006;16(3):311–319. doi: 10.1080/08982100600851086. [DOI] [PubMed] [Google Scholar]
- 54.Wagner A., Vorauer-Uhl K. Liposome technology for industrial purposes. J. Drug Deliv. 2011;2011:591325. doi: 10.1155/2011/591325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mozafari M.R., Reed C.J., Rostron C., Kocum C., Piskin E. Construction of stable anionic liposome-plasmid particles using the heating method: a preliminary investigation. Cell. Mol. Biol. Lett. 2002;7(3):923–928. [PubMed] [Google Scholar]
- 56.Mortazavi S.M., Mohammadabadi M.R., Khosravi-Darani K., Mozafari M.R. Preparation of liposomal gene therapy vectors by a scalable method without using volatile solvents or detergents. J. Biotechnol. 2007;129:604–613. doi: 10.1016/j.jbiotec.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Mozafari M.R., Javanmard R., Raji M. Tocosome: novel drug delivery system containing phospholipids and tocopheryl phosphates. Int. J. Pharmaceut. 2017;528(1-2):381–382. doi: 10.1016/j.ijpharm.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 58.Ozer A.Y. Nanomaterials and Nanosystems for Biomedical Applications. Springer; Netherlands: 2007. Applications of light and electron microscopic techniques in liposome research; pp. 145–153. [Google Scholar]
- 59.Jones M.N. The surface properties of phospholipid liposome systems and their characterisation. Adv. Coll. Interface Sci. 1995;54:93–128. doi: 10.1016/0001-8686(94)00223-y. [DOI] [PubMed] [Google Scholar]
- 60.Mozafari M.R., Reed C.J., Rostron C., Hasirci V. A review of scanning probe microscopy investigations of liposome-DNA complexes. J. Liposome Res. 2005;15(1–2):93–107. doi: 10.1081/lpr-64965. [DOI] [PubMed] [Google Scholar]
- 61.Mozafari M.R., Reed C.J., Rostron C. Prospects of anionic nanolipoplexes in nanotherapy: transmission electron microscopy and light scattering studies. Micron. 2007;38(8):787–795. doi: 10.1016/j.micron.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 62.Negussie A.H., Yarmolenko P.S., Partanen A., Ranjan A., Jacobs G., Woods D., Bryant H., Thomasson D., Dewhirst M.W., Wood B.J., Dreher M.R. Formulation and characterisation of magnetic resonance imageable thermally sensitive liposomes for use with magnetic resonance-guided high intensity focused ultrasound. Int. J. Hyperther. 2011;27(2):140–155. doi: 10.3109/02656736.2010.528140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rasti B., Jinap S., Mozafari M.R., Yazid A.M. Comparative study of the oxidative and physical stability of liposomal and nanoliposomal polyunsaturated fatty acids prepared with conventional and Mozafari methods. Food Chem. 2012;135(4):2761–2770. doi: 10.1016/j.foodchem.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 64.Jiang Y., Genin G.M., Pryse K.M., Elson E.L. Atomic force microscopy of phase separation on ruptured, giant unilamellar vesicles. bioRxiv. 2018 Jan 1:250944. doi: 10.1115/1.4043871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hasegawa Y. Compendium of Surface and Interface Analysis. Springer; Singapore: 2018. Scanning tunneling microscopy; pp. 599–604. [Google Scholar]
- 66.Salapaka S.M., Salapaka M.V. Scanning probe microscopy. IEEE Contr. Syst. 2008;28(2):65–83. [Google Scholar]
- 67.Khosravi-Darani K., Pardakhty A., Honarpisheh H., Rao V.M., Mozafari M.R. The role of high-resolution imaging in the evaluation of nanosystems for bioactive encapsulation and targeted nanotherapy. Micron. 2007;38(8):804–818. doi: 10.1016/j.micron.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Albrecht T.R., Grütter P., Horne D., Rugard D. Frequency modulation detection using high-Q cantilevers for enhanced force microscope sensitivity. J. Appl. Phys. 1991;69:668–673. [Google Scholar]
- 69.Ruozi B., Belletti D., Tombesi A., Tosi G., Bondioli L., Forni F., Vandelli M.A. AFM, ESEM, TEM, and CLSM in liposomal characterization: a comparative study. Int. J. Nanomed. 2011;6:557–563. doi: 10.2147/IJN.S14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fennell Evans D., Wennerstrom H., Rajagopalan R. The colloidal domain: where physics, chemistry, biology, and technology meet. J. Colloid Interface Sci. 1995;172(2):193–197. [Google Scholar]
- 71.Sze A., Erickson D., Ren L., Li D. Zeta-potential measurement using the Smoluchowski equation and the slope of the current–time relationship in electroosmotic flow. J. Coll. Interface Sci. 2003;261(2):402–410. doi: 10.1016/S0021-9797(03)00142-5. [DOI] [PubMed] [Google Scholar]
- 72.Hong K.M., Noolandi J. Solution of the Smoluchowski equation with a Coulomb potential. I. General results. J. Chem. Phys. 1978;68(11):5163–5171. [Google Scholar]
- 73.Egorova E.M. The validity of the Smoluchowski equation in electrophoretic studies of lipid membranes. Electrophoresis. 1994;15(1):1125–1131. doi: 10.1002/elps.11501501170. [DOI] [PubMed] [Google Scholar]
- 74.Hunter R.J. vol. 2. Academic Press; 2013. (Zeta Potential in Colloid Science: Principles and Applications). [Google Scholar]
- 75.Henry D.C. The cataphoresis of suspended particles. Part I. The equation of cataphoresis. Proc. R. Soc. Lond. A. 1931;133(821):106–129. [Google Scholar]
- 76.Salgin S., Salgin U., Bahadir S. Zeta potentials and isoelectric points of biomolecules: the effects of ion types and ionic strengths. Int. J. Electrochem. Sci. 2012;7(12):12404–12414. [Google Scholar]
- 77.Sabbah M., Esposito M., Pierro P.D., Giosafatto C.V., Mariniello L. Insight into zeta potential measurements in biopolymer film preparation. J. Biotechnol. Biomat. 2016;6:e126. [Google Scholar]
- 78.Soema P.C., Willems G.J., Jiskoot W., Amorij J.P., Kersten G.F. Predicting the influence of liposomal lipid composition on liposome size, zeta potential and liposome-induced dendritic cell maturation using a design of experiments approach. Eur. J. Pharm. Biopharm. 2015;94:427–435. doi: 10.1016/j.ejpb.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 79.Kwolek U., Wojcik K., Janiczek M., Nowakowska M., Kepczynski M. Synthesis and antibacterial properties of quaternary ammonium derivative of polyethylenimine. Polimery. 2017;62:311–316. [Google Scholar]
- 80.McPhee W.C. Mc Master University; 1991. Preparation and Characterization of Temperature Sensitive Poly (N- Isopropyl Acrylamide) – Microgel Latexes. Doctoral dissertation. [Google Scholar]
- 81.Delgado A.V., Gonzalez-Caballero F., Hunter R.J., Koopal L.K., Lyklema J. Measurement and interpretation of electrokinetic phenomena. J. Coll. Interface Sci. 2007;309(2):194–224. doi: 10.1016/j.jcis.2006.12.075. [DOI] [PubMed] [Google Scholar]
- 82.Corbett J.C., Connah M.T., Mattison K. Advances in the measurement of protein mobility using laser Doppler electrophoresis – the diffusion barrier technique. Electrophoresis. 2011;32(14):1787–1794. doi: 10.1002/elps.201100108. [DOI] [PubMed] [Google Scholar]
- 83.Tucker I.M., Corbett J.C., Fatkin J., Jack R.O., Kaszuba M., MacCreath B., McNeil-Watson F. Laser Doppler electrophoresis applied to colloids and surfaces. Curr. Opin. Coll. Interface Sci. 2015;20(4):215–226. [Google Scholar]
- 84.Bhattacharjee S. DLS and zeta potential–what they are and what they are not? J. Contr. Release. 2016;235:337–351. doi: 10.1016/j.jconrel.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 85.Corbett J.C., Connah M., Mattison K. 2017 Sep 21. (Inventors). Laser Doppler Electrophoresis Using a Diffusion Barrier. United States Patent Application: US 14/258,144. [Google Scholar]
- 86.De Vrij J., Maas S.L., Van Nispen M., Sena-Esteves M., Limpens R.W., Koster A.J., Leenstra S., Lamfers M.L., Broekman M.L. Quantification of nanosized extracellular membrane vesicles with scanning ion occlusion sensing. Nanomedicine. 2013;8(9):1443–1458. doi: 10.2217/nnm.12.173. [DOI] [PubMed] [Google Scholar]
- 87.Henriquez R.R., Ito T., Sun L., Crooks R.M. The resurgence of Coulter counting for analyzing nanoscale objects. Analyst. 2004;129(6):478–482. doi: 10.1039/b404251b. [DOI] [PubMed] [Google Scholar]
- 88.Yang L., Broom M.F., Tucker I.G. Characterization of a nanoparticulate drug delivery system using scanning ion occlusion sensing. Pharm. Res. 2012;29(9):2578–2586. doi: 10.1007/s11095-012-0788-3. [DOI] [PubMed] [Google Scholar]
- 89.Kanasova M., Nesmerak K. Systematic review of liposomes' characterization methods. Monatsh. Chem. Chem. Month. 2017;148(9):1581–1593. [Google Scholar]
- 90.Chen C., Zhu S., Wang S., Zhang W., Cheng Y., Yan X. Multiparameter quantification of liposomal nanomedicines at the single-particle level by high-sensitivity flow cytometry. ACS Appl. Mater. Interfaces. 2017;9(16):13913–13919. doi: 10.1021/acsami.7b01867. [DOI] [PubMed] [Google Scholar]
- 91.Saveyn H., De Baets B., Thas O., Hole P., Smith J., Van Der Meeren P. Accurate particle size distribution determination by nanoparticle tracking analysis based on 2-D Brownian dynamics simulation. J. Coll. Interface Sci. 2010;352(2):593–600. doi: 10.1016/j.jcis.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 92.Filipe V., Hawe A., Jiskoot W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharmaceut. Res. 2010;27(5):796–810. doi: 10.1007/s11095-010-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reshetov V., Zorin V., Siupa A., D'Hallewin M.A., Guillemin F., Bezdetnaya L. Interaction of liposomal formulations of meta – tetra (hydroxyphenyl) chlorin (temoporfin) with serum proteins: protein binding and liposome destruction. Photochem. Photobiol. 2012;88(5):1256–1264. doi: 10.1111/j.1751-1097.2012.01176.x. [DOI] [PubMed] [Google Scholar]
- 94.de Morais Ribeiro L.N., Couto V.M., Fraceto L.F., de Paula E. Use of nanoparticle concentration as a tool to understand the structural properties of colloids. Sci. Rep. 2018;8(1):982. doi: 10.1038/s41598-017-18573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wright M. Drug Delivery. CRC Press; 2017 Sep 19. Application of nanoparticle tracking analysis in drug delivery; pp. 237–264. [Google Scholar]
- 96.Oeyen E., Van Mol K., Baggerman G., Willems H., Boonen K., Rolfo C., Pauwels P., Jacobs A., Schildermans K., Cho W.C., Mertens I. Ultrafiltration and size exclusion chromatography combined with asymmetrical-flow field-flow fractionation for the isolation and characterisation of extracellular vesicles from urine. J. Extracell. Vesicles. 2018;7(1):1490143. doi: 10.1080/20013078.2018.1490143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gioria S., Caputo F., Urban P., Maguire C.M., Bremer-Hoffmann S., Prina-Mello A., Calzolai L., Mehn D. Are existing standard methods suitable for the evaluation of nanomedicines: some case studies. Nanomedicine. 2018;13(5):539–554. doi: 10.2217/nnm-2017-0338. [DOI] [PubMed] [Google Scholar]
- 98.Rupert D.L., Mapar M., Shelke G.V., Norling K., Elmeskog M., Lotvall J.O., Block S., Bally M., Agnarsson B., Höök F. Effective refractive index and lipid content of extracellular vesicles revealed using optical waveguide scattering and fluorescence microscopy. Langmuir. 2018 Jun 20 doi: 10.1021/acs.langmuir.7b04214. [DOI] [PubMed] [Google Scholar]
- 99.Geertsen V., Barruet E., Gobeaux F., Lacour J.L., Tache O. Contribution to accurate spherical gold nanoparticles size determination by single particle inductively coupled mass spectrometry: a comparison with small angle X-ray scattering. Anal. Chem. 2018 Jul 16 doi: 10.1021/acs.analchem.8b01167. [DOI] [PubMed] [Google Scholar]
- 100.Blanchette C.D., Law R., Benner W.H., Pesavento J.B., Cappuccio J.A., Walsworth V., Kuhn E.A., Corzett M., Chromy B.A., Segelke B.W., Coleman M.A. Quantifying size distributions of nanolipoprotein particles with single-particle analysis and molecular dynamic simulations. J. Lipid Res. 2008;49(7):1420–1430. doi: 10.1194/jlr.M700586-JLR200. [DOI] [PubMed] [Google Scholar]
- 101.Sadat S.M., Jahan S.T., Haddadi A. Effects of size and surface charge of polymeric nanoparticles on in vitro and in vivo applications. J. Biomater. Nanobiotechnol. 2016;7(02):91–108. [Google Scholar]
- 102.Chromy B.A., Arroyo E., Blanchette C.D., Bench G., Benner H., Cappuccio J.A., Coleman M.A., Henderson P.T., Hinz A.K., Kuhn E.A., Pesavento J.B. Different apolipoproteins impact nanolipoprotein particle formation. J. Am. Chem. Soc. 2007;129:14348–14354. doi: 10.1021/ja074753y. [DOI] [PubMed] [Google Scholar]
- 103.Zollmann T., Moiset G., Tumulka F., Tampe R., Poolman B., Abele R. Single liposome analysis of peptide translocation by the ABC transporter TAPL. Proc. Natl. Acad. Sci. 2015;112(7):2046–2051. doi: 10.1073/pnas.1418100112. [DOI] [PMC free article] [PubMed] [Google Scholar]