Abstract
Introduction
Severe morning stiffness with painful involvement of the girdles are often referred by patients with Interstitial Lung Disease (ILD), but the association between ILD and Polymyalgia Rheumatica (PMR) is rarely reported. The purpose of the work is to describe a series of patients classified as having PMR with ILD.
Material and methods
We retrospectively enrolled patients with a diagnosis of PMR referred to our center during the previous year for respiratory symptoms. Data concerning clinical and serological manifestations suggesting Connective Tissue Disease (CTD), High-Resolution Chest Tomography (HRCT), and Pulmonary Function Tests (PFTs) were systematically collected in order to verify the diagnosis.
Results
Fifteen out of seventeen PMR patients had ILD. Ten patients had a confirmed diagnosis of PMR, while in five patients a CTD was discovered. Seven patients showed a severe restrictive pattern at PFTs requiring oxygen supplementation (five with PMR and two with CTD). In thirteen patients pulmonary symptoms started before or together with muscular symptoms. Regarding HRCT patterns, patients showed a Nonspecific Interstitial Pneumonia in nine cases, Usual Interstitial Pneumonia (UIP) and possible UIP in two and three cases, and a single case of Organizing Pneumonia and Combined Pulmonary Fibrosis and Emphysema Syndrome.
Conclusions
Lung involvement should be evaluated in PMR patients, especially if asthenia is poorly responsive to low doses of steroids. In these cases, the diagnosis should be re-evaluated in depth, looking for a seronegative Rheumatoid Arthritis, a clinically amyopathic myositis or Interstitial Pneumonia with Autoimmune features.
Keywords: Interstitial lung disease, Polymyalgia rheumatica, Connective tissue disease, Antisynthetase syndrome, Myositis, Interstitial pneumonia with autoimmune features
1. Introduction
Polymyalgia Rheumatica (PMR) is a common disease characterized by an old age onset, severe morning stiffness and inflammatory involvement of pelvic and scapular girdles with pain. It is associated with elevation of Erythrocyte Sedimentation Rate (ESR) and C Reactive Protein (CRP) but not of Creatine Phosphokinase (CPK) or Lactic Dehydrogenase (LDH) [1]. Differential diagnosis may be challenging, and it should be distinguished from other inflammatory conditions, mainly late onset of Rheumatoid Arthritis (RA). Moreover, a possible association of PMR with fever, malaise, peripheral arthritis, Remitting Symmetric Seronegative Synovitis with Pitting Edema (RS3PE) and Giant Cell Arteritis (GCA) should be mentioned [1].
Currently, lung function in PMR has not been adequately studied: in a first study, five out of 26 patients showed PMR and GCA with restrictive disease or Interstitial Lung Disease (ILD). Considering this association, the authors supposed that GCA could be the causative agent rather than PMR [2]. Besides, a recent retrospective study demonstrated high prevalence of ILD, especially with ground glass opacities (GGOs), in PMR patients despite the association with RA [3]. Unfortunately, in this work, the exclusion criteria for other concomitant inflammatory diseases were not clearly reported.
The aim of this work is to describe our experience with a group of patients with a diagnosis of PMR who were referred to our center due to respiratory symptoms, revealing an associated ILD, in order to highlight a possible association and discuss a potential explanation.
2. Material and methods
We considered retrospectively all patients with a diagnosis of PMR referred to our center between January 2017 and May 2018.
From the first visit, patients' data were systematically collected in our database.
The initial diagnosis of PMR was controlled by rheumatologists (GS, DS, FP, NDP) and discussed by our Multidisciplinary Team (MDT), composed also by pulmonologists (SET, AV, MP, CV) and a radiologist (SP). During MDT for each patient was evaluated the possibility of an alternative diagnosis able to explain ILD, the assessment of its severity as well as its management.
The new proposed criteria of Dasgupta B. et al. demonstrated better performance in discriminating PMR from other inflammatory diseases; for this reason they were used instead to the previous one to verify the diagnosis of PMR [1,4]. The diagnosis of CTD was made according to specific classification criteria. However, currently Antisynthetase Syndrome (AS) does not have validated criteria. Therefore, the classification is made according to Connors proposed criteria [5].
General data contemplated smoking habit, occupation, dyspnea, cough, signs and symptoms of Connective Tissue Disease (CTD).
All patients performed general blood tests, including complete blood count, creatinine, CPK, LDH, ESR, CRP, transaminases, Antinuclear Antibodies, Extractable Nuclear Antigens (ENA) antibodies, anti-Double Stranded DNA antibody, Lupus Anticoagulant, Anti Neutrophil Cytoplasmic Antibodies. The ENA antibodies panel included: SSA/Ro 60kd, SSA/Ro 52kd, SSB/La, anti Topoisomerase I, Anti Centromere, Anti Jo1, Anti Smith, anti Ribonucleoprotein, anti Pm/Scl. The SSA/Ro are frequently combined with anti t-RNA synthetase antibodies (ATSA), that are specific for inflammatory myositis. In case of SSA/Ro positivity, elevation of CPK and/or LDH, ANA positivity with cytoplasmic or nucleolar pattern, a research for ATSA and myositis associated antibodies (different by anti Jo1) was performed [6]. These antibodies were anti Mi2, anti Ku, anti SRP, anti EJ, anti OJ, anti PL7, anti PL12.
Pulmonary Function Tests (PFTs) (SentrySuite 2.15.147 CareFusion Germany 234 GmbH Leibnizstrasse 7 D-97204 Hoechberg) were performed according to their specific guidelines [[7], [8], [9]] by pulmonologists who, if clinically indicated, suggested performing a High-Resolution Chest Tomography (HRCT) for diagnosis of ILD.
The ILD classification was made by radiologists expert in ILD, evaluating the prevalent pattern through the presence of GGOs, reticulations, bronchiectasis and honeycombing [10].
Nailfold Videocapillaroscopy (NVC) (VideoCap 3.0 Reuma, DS Medica Viale Monza 133 20125 Milan, Italy) was performed and interpreted semiquantitatively by rheumatologists [11]. NVC was performed in all patients despite a referred Raynaud Phenomenon (RP).
Pulmonology and rheumatologic visits, blood analysis as reported above, PFTs, HRCT and NVC are exams performed routinely by our team in all ILD patients. In case of specific alterations, a second line of specific exams will be performed. I.e. four patients that referred sicca syndrome performed a Schirmer Test for the diagnosis of Sjögren Syndrome (SjS).
Missing data were less than 1%. The study was conducted according to the declaration of Helsinki and written informed consent from patients was obtained.
3. Results
In the last 16 months, fifteen out of seventeen Caucasian patients referred to our unit with an initial diagnosis of PMR showed an ILD. None of these patients had an associated GCA or RS3PE.
All patients referred to our center complaining of asthenia, shortness of breath and dry cough unresponsive to low doses of corticosteroids (prednisone 5–10mg). Three patients with a dosage of 10mg of prednisone referred a good control of the PMR symptoms, while the other patients with lower dosage of steroids showed already significant morning stiffness, aching of the scapular and limb girdles as well as a significant elevation of both ESR and CRP.
In the re-evaluation of the diagnosis, a specific autoimmune disease was discovered in five cases: three patients with Rheumatoid Arthritis (RA), one patient with SjS classified according to specific criteria [12,13] and one patient with high titre positivity for PL7 and Mi2 classified as an AS. In other patients, the diagnosis of PMR was confirmed.
Patients had a median (I-III quartiles) age of 70 (66.5–76) years, with a slight prevalence of female gender (9 women, 6 men, ratio 1.5). Four of them were heavy smokers. Median time of the respiratory symptoms onset before the diagnosis of ILD was 24 (7.5–42) months, while the morning stiffness onset before the diagnosis of PMR had a median of 9 (6–24) months.
The majority of patients referred the onset of respiratory symptoms before or at the same time (range of three months) as the polymyalgia ones. Only 2 patients, in which morning stiffness preceded the clinical onset of ILD, were affected by RA and SjS.
Impaired PFTs were seen in ten patients, of which seven (four with PMR, three with CTD) needed oxygen support.
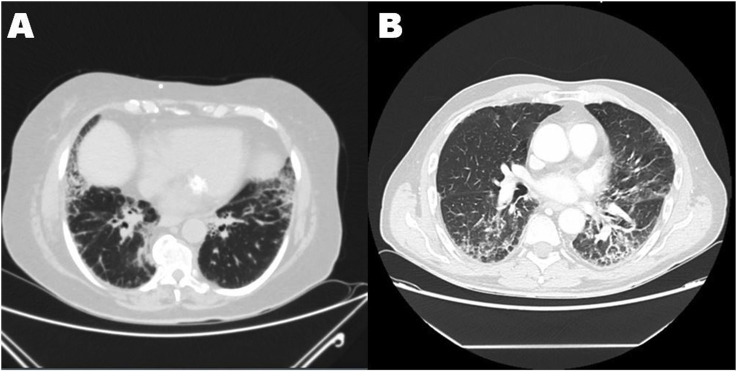
Regarding HRCT pattern, patients with ASS and SjS showed Nonspecific Interstitial Pneumonia (NSIP), subjects with RA presented one case of NSIP and two cases of Usual Interstitial Pneumonia (UIP) pattern. In patients with confirmed diagnosis of PMR we found 5 cases of NSIP, 3 cases of possible UIP and a single case of Organizing Pneumonia (OP) and a Combined Pulmonary Fibrosis and Emphysema syndrome (CPFE). In Fig. 1 an example of patients' HRCT is showed.
Fig. 1.
Interstitial Lung Disease with a Nonspecific Interstitial Pneumonia pattern in patients initially diagnosed as Polymyalgia Rheumatica. The diagnosis was corrected in patient A in Antisynthetase Syndrome, while in patient B was confirmed.
NVC resulted positive in patients with SjS (active pattern) and ASS (late pattern). However, neither of the patients referred RP. Complete patients' characteristics are reported in Table 1.
Table 1.
Clinical and serological features of patients with initial diagnosis of PMR.
| S | Sex | Age | Smoke (p/y) | Lung symptoms (m) | Muscle symptoms (m) | Clinical sign | PMR score | FVC | FEV1 | TI | DLCO | 6MWT | O2Tp | AAb | NVC | HRCT pattern | Final Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 60 | 0 | 84 | 60 | RP, SS | 6 | 129 | 121 | 83 | 90 | 420 | No | None | N | OP | PMR |
| 2 | F | 62 | 0 | 1 | 2 | none | 6 | 83 | 91 | 90 | 34 | 100D | Yes | None | N | NSIP | PMR |
| 3 | F | 66 | 0 | 10 | 24 | PF, SS | 5§ | 91 | 101 | 92 | 52 | 400 D | Yes | ANA 1:320; RF↑; SSA; SSB | A | NSIP | SjS |
| 4 | M | 66 | 70 | 6 | 5 | Fe | 5 | 114 | 109 | 78 | 80 | 425 D | No | ANA 1:160 | N | NSIP | PMR |
| 5 | M | 67 | 0 | 60 | 6 | Fe | 4§ | 61 | 60 | 77 | 58 | 450 D | Yes | ANA 1:80, ACPA↑ | N | UIP | RA |
| 6 | F | 67 | 0 | 24 | 6 | none | 5 | 129 | 121 | 83 | 90 | 375 | No | ANA 1:640 | N | NSIP | PMR |
| 7 | M | 68 | 100 | 6 | 24 | PIA | 3§ | 114 | 99 | 89 | 46 | 475 D | Yes | RF↓; ACPA↑ | N | UIP | RA |
| 8 | M | 70 | 25 | 60 | 12 | none | 6 | 120 | 122 | 79 | 76 | 375 | No | none | N | UIP-P | PMR |
| 9 | F | 71 | 4 | 12 | 12 | SS | 8§ | 94 | 101 | 88 | 47 | 400 D | Yes | none | N | UIP-P | PMR |
| 10 | F | 71 | 15 | 24 | 24 | PIA | 3§ | 79 | 66 | 81 | 98 | NA | No | none | N | NSIP | RA |
| 11 | M | 76 | 45 | 36 | 6 | none | 6 | 63 | 66 | 79 | 47 | NA | Yes | none | N | CPFE | PMR |
| 12 | F | 77 | 0 | 9 | 6 | SS, Fe | 5§ | 81 | 90 | 86 | 67 | NA | No | ANA 1:160 SSA, Mi2, PL7/PL12 | L | NSIP | ASS |
| 13 | F | 79 | 0 | 6 | 9 | none | 6 | 86 | 103 | 94 | 81 | 300 | No | none | N | NSIP | PMR |
| 14 | F | 82 | 0 | 36 | 6 | RP | 5 | 111 | 91 | 85 | 49 | 200 | Yes | none | N | NSIP | PMR |
| 15 | M | 76 | 1 | 48 | 48 | none | 6 | 106 | 119 | 87 | 49 | 450 | No | none | N | UIP-P | PMR |
Legend: 6MWT: 6 minutes walking test; A: Active pattern; AAb: auto-antibodies ACPA: Anti Citrullinated Protein Antibodies; ANA: Antinuclear Antibodies; ASS: Anti Synthetase Syndrome; CPFE: Combined Pulmonary Fibrosis and Emphysema; D: desaturation; DLCO: Diffusion Lung For Carbon Monoxyde; F: Female; Fe: Fever; FVC: Forced Vital Capacity; HRCT: High-Resolution Chest Tomography; L: late pattern; M: Male; M: Months; N: normal; NSIP: Non Specific Interstitial Pneumonia; NVC: Nailfold Videocapillaroscopy; OP: Organizing Pneumonia; O2Tp: Oxygen supplement therapy; P/y: pack/years; p-ANCA: peripheral- Anti Neutrophil Cytoplasmic Antibodies; PF: Puffy Fingers; PIA: Peripheral Inflammatory Arthritis; PMR: Polymyalgia Rheumatica; PMR score: points reached according to the Dasgupta Classification Criteria for PMR; RA: Rheumatoid Arthritis; RF: Rheumatoid Factor; RP: Raynaud Phenomenon; S: subject; SS: Sicca Syndrome; SjS: Sjögren Syndrome; TI: Tiffenau Index UIP: Usual Interstitial Pneumonia; UIP-P: possible UIP.
↑ = High Titre, ≥ at least two times above the upper limit.
↓ = Low Titre,> to the upper limit but < two times of increase.
§ = with ultrasound of shoulders and hips.
Regarding the disease management, considering the lack of literature regarding the association of PMR and ILD, it was decided in MDT to treat PMR patients with a UIP-like pattern with anti fibrotic therapy for ILD and a prednisone 10mg/daily (slowly tapered to 2.5–5mg/daily) for the management of PMR. In fact, a low dose of steroids is generally dramatically able to control PMR [1]. The other patients with a UIP inconsistent HRCT pattern were treated with high dosage of prednisone (37.5 mg slowly tapered to a minimum of 12.5mg) and Azathioprine 150mg/daily.
Finally, a one-year follow up is available for 5 patients with a final diagnosis of ILD-PMR (from the remaining subjects, 2 were lost in follow up while for 3 patients this data is currently not available). No new clinical, NVC or serological features of autoimmunity were found. In all patients PMR symptoms appeared solved, with a stable normalization of both ESR and CRP. From the respiratory point of view, PFTs resulted stable in 2 patients (P4 and P8). Patient P1, P13 and P14 showed an impairment of PFTs (P1 reduction of 10% of both FVC and DLCO; P13 reduction of 20% of both FVC and DLCO; P14 reduction of 20% of DLCO). Anyway, the oxygen support result unnecessary for P1 and P13, while P14 needed an increase of the litres per minute of Oxygen (data not shown in the table).
4. Discussion
The majority of patients with an initial diagnosis of PMR referred to our center for respiratory symptoms revealed an associated ILD. Generally, clinical symptoms of ILD preceded or occurred together with PMR, while in two subjects PMR preceded lung symptoms: in these patients the final diagnosis was RA in one case and SjS in another. In general, ILD severity appeared considerable, given that seven patients needed oxygen therapy.
This data could explain the lack of evidence in literature of ILD associated to PMR. The treatment of the ILD, specially without a UIP definite pattern in HRCT, is generally based on high dosage of steroids for a long time. This therapy is usually successful for the treatment of PMR, hiding its manifestation. However, from these preliminary data, it could be reckless to suppose a direct etiologic role of PMR in causing ILD. PMR criteria present high sensitivity but low specificity, with a low capacity for distinguishing PMR from other inflammatory conditions, mainly autoimmune, infective or neoplastic diseases [14]. It is particularly difficult to distinguish a PMR from a late onset of Seronegative RA. The association of ILD and PMR symptoms should lead to the reconsideration of the diagnosis, looking for an unrecognized CTD. In our cohort, all patients systematically underwent diagnostic re-assessment and a definite rheumatic diagnosis able to explain ILD was made in five cases. In three cases, as described in literature, a diagnosis of RA was made. In other two cases, the diagnosis was guided by positivity for anti-Ro/SSA 52k, a myositis-associated antibody [14]. In the first case, the final diagnosis was SjS, while in the second case, the patient was diagnosed as having ASS, after the finding of Mi2, PL7 and PL12 positivity. PL7 and PL12 are antibodies directed against tRNA synthetase and are associated with progressive ILD and amyopathic or subclinical myositis [15]. ASS is a syndrome characterized by the classic triad myositis, inflammatory arthritis and ILD, in which RP, fever, calcinosis, cutaneous sclerosis and mechanic's hands may be associated [16]. Considering these data, the observation of positive NVC only in patients with anti Ro/SSA 52k positivity without associated RP could not be random. NVC was reported positive only in about 10% of patients with SjS [17], but increasingly data suggest NVC as a useful diagnostic tool for myositis, even in patients with SSA and without RP [18]. Therefore, a silent myositis also in patient with SjS could be hypothesized.
On the other hand, ten patients had confirmation of diagnosis of PMR, and none of these showed a definite UIP pattern in HRCT. Although it is impossible to exclude definitively a specific diagnosis, it should be noted that UIP is the predominant ILD pattern in RA [19]. Indeed, a fascinating speculation may be that a subset of PMR patients may have also a myositis without serological or electromyographic signs of muscular involvement.
The possibility of a “Silent myositis” is not remote: in fact, a definition of Clinical Amyopathic Dermatomyositis (CADM) was needed for the definition of a subset of about 20% of patients with typical dermatologic involvement, similar risk of ILD and malignancy reported for classic DM but with an absent or subclinical myositis [20]. Obviously, if for CADM cutaneous manifestations can lead to a diagnosis, a similar subtype of other forms of autoimmune myositis is actually hard to classify. Hopkinson et al. described some cases of PM manifesting with normal levels of CPK and LDH, initially confused with PMR [21]. However, the above-mentioned data about CADM suggest that subsequent increasing of CPK and LDH are not necessary to suppose an inflammatory autoimmune myositis.
Finally, all patients with confirmed diagnosis of PMR satisfied the “Interstitial Pneumonia with Autoimmune Features” (IPAF) classification criteria: despite the absence of serological findings, they had a compatible HRCT pattern and PMR symptoms are able to satisfy the clinical domain [22]. In our opinion, these patients should be classified as IPAF, treated according to their disease severity and HRCT pattern and followed prospectively in order to evaluate new clinical or serologic signs able to reach a classification for specific CTD. The increased attention to the possible association of PMR symptoms and ILD could clarify if it is actually sustained by a common pathogenic pathway.
Limitations of this study are the small number of patients and a possible selection bias, considering that our unit is the regional referral center for rare lung diseases. It seems reasonable that patients refer to a specific clinical unit based on the problem perceived as predominant.
5. Conclusions
In conclusion, both ILD and PMR symptoms can be associated with late onset of CTD and they can occur together. Therefore, an associated ILD should be suspected in PMR patients, particularly if asthenia is not resolved by low doses of corticosteroids. In these cases respiratory symptoms should be investigated and studied in depth, looking for an underlying CTD.
Compliance with ethical standards
The work was conducted according to the declaration of Helsinki. Written informed consent was obtained from all individual participants included in the study.
Disclosures
This work did benefit of approval and funding by the Departmental Project #A, “molecular, clinical and instrumental early markers in metabolic and chronic-degenerative disease” of the Clinical and Experimental Medicine Dpt, University of Catania (Italy).
Conflict of interest
CV is part of the F. Hoffmann-La Roche Ltd. Scientific board. He has received consulting fees and/or speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, F. Hoffmann-La Roche Ltd and Menarini. The authors GS, DS, SET, AV, MP, FP, NDP, SP declare that they have no conflict of interest.
Acknowledgments
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2018.12.014.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dasgupta B., Cimmino M.A., Kremers H.M., Schmidt W.A., Schirmer M., Salvarani C., Bachta A., Dejaco C., Duftner C., Jensen H.S., Duhaut P., Poór G., Kaposi N.P., Mandl P., Balint P.V., Schmidt Z., Iagnocco A., Nannini C., Cantini F., Macchioni P., Pipitone N., Del Amo M., Espigol-Frigolé G., Cid M.C., Martinez-Taboada V.M., Nordborg E., Direskeneli H., Aydin S.Z., Ahmed K., Hazleman B., Silverman B., Pease C., Wkefield R.J., Lugmani R., Abril A., Michet C.J., Marcus R., Gonter N.J., Maz M., Carter R.E., Crowson C.S., Matteson E.L. Provisional classification criteria for polymyalgia rheumatica: a European League against Rheumatism/American College of Rheumatology collaborative initiative. Arthritis Rheum. 2012;64(2012):943–954. doi: 10.1002/art.34356. [DOI] [PubMed] [Google Scholar]
- 2.Acritidis N.C., Andonopoulos A.P., Galanopoulou V., Drosos A.A., Constantopoulos S.H. Pulmonary function of non-smoking patients with giant cell Arteritis and/or polymyalgia rheumatica; a controlled study. Clin. Rheumatol. 1988;7:231–236. doi: 10.1007/BF02204460. PMID: 3416567. [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita S., Aoki T., Takahashi H., Oki H., Hayashida Y., Saito K., Tanaka Y., Korogi Y. Thin-section chest CT findings in polymyalgia rheumatica: a comparison between with and without rheumatoid arthritis. Clin. Imag. 2016;40:382–385. doi: 10.1016/j.clinimag.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Macchioni P., Boiardi L., Catanoso M., Pazzola G., Salvarani C. Performance of the new 2012 EULAR/ACR classification criteria for polymyalgia rheumatica: comparison with the previous criteria in a single-centre study. Ann. Rheum. Dis. 2014;73:1190–1193. doi: 10.1136/annrheumdis-2013-204167. [DOI] [PubMed] [Google Scholar]
- 5.Connors G.R., Christopher-Stine L., Oddis C.V., Danoff S.K. Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest. 2010;138:1464–1474. doi: 10.1378/chest.10-0180. [DOI] [PubMed] [Google Scholar]
- 6.Scirè C.A., Gonzalez-Gay M.A., Selva-O’Callaghan A., Cavagna L. Clinical Spectrum time course of interstitial pneumonia with autoimmune features in patients positive for antisynthetase antibodies. Respir. Med. 2017;132:265–266. doi: 10.1016/j.rmed.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Miller M.R., Hankinson J., Brusasco V., Burgos F., Casaburi R., Coates A., Crapo R., Enright P., Van Der Grinten C.P., Gustafsson P., Jensen R., Johnson D.C., MacIntyre N., McKay R., Navajas D., Pedersen O.F., Pellegrino R., Viegi G., Wanger J. ATS/ERS task force, standardisation of spirometry. Eur. Respir. J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 8.Macintyre N., Crapo R.O., Viegi G., Johnson D.C., van der Grinten C.P., Brusasco V., Burgos F., Casaburi R., Coaters A., Enright P., Gustafsson P., Hankinson J., Jensen R., McKay R., Miller M.R., Navajas D., Pedersen O.F., Pellegrino R., Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 9.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walking test. Am. J. Respir. Crit. Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 10.Raghu G., Collard H.R., Egan J.J., Martinez F.J., Behr J., Brown K.K., Colby T.V., Cordier J.F., Flaherty K.R., Lasky J.A., Lynch D.A., Ryu J.H., Swigris J.J., Wells A.U., Ancochea J., Bouros D., Carvalho C., Costabel U., Ebina M., Hansell D.M., Johkoh T., Kim D.S., King T.E., Jr., Kondoh Y., Myers J., Muller N.L., Nicholson A.G., Richeldi L., Selman M., Dudden R.F., Griss B.S., Protzko S.L., Schunemann H.J. ATS/ERS/JRS/ALAT committee on idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutolo M., Sulli A., Smith V. How to perform and interpret capillaroscopy. Best Pract. Res. Clin. Rheumatol. 2013;27:237–248. doi: 10.1016/j.berh.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Aletaha D., Neogi T., Silman A.J., Funovits J., Felson D.T., Bingham C.O., 3rd, Bimbaum N.S., Burmester G.R., Bykerk V.P., Cohen M.D., Combe B., Costenbaker K.H., Dougados M., Emery P., Ferraccioli G., Hazes J.M., Hobbs K., Huizinga T.W., Kavanaugh A., Kay J., Kvien T.K., Laing T., Mease P., Menard H.A., Moreland L.W., Naden R.L., Pincus T., Smolen J.S., Stanislawska-Niernat E., Symmons D., Tak P.P., Upchurch K.S., Vencovsky J., Wolfe F., Hawker G. Rheumatoid arthritis classification criteria: an American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2010;62(2010):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 13.Shiboski C.H., Shiboski S.C., Seror R., Criswell L.A., Labetoulle M., Lietman T.M., Rasmussen A., Scofield H., Vitali C., Bowman S.J., Mariette X., International Sjogren’s Syndrome Criteria Working Group American College of Rheumatology/European League against rheumatism classification criteria for Primary Sjögren’s Syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2016;76(2017):9–16. doi: 10.1002/art.39859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Gay M.A., Garcia-Porrùa C., Salvarani C., Oliveri I., Hunder G.G. Polymyalgia manifestations in different conditions mimicking polymyalgia rheumatica. Clin. Exp. Rheumatol. 2000;18:755–759. PMID 11138344. [PubMed] [Google Scholar]
- 15.Ghirardello A., Borella E., Beggio M., Franceschini F., Fredi M., Doria A. Myositis autoantibodies and clinical phenotypes. Autoimmun. Highlights. 2014;5:69–75. doi: 10.1007/s13317-014-0060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katzap E., Barilla-LaBarca M.L., Marder G. Antisynthetase syndrome. Curr. Rheumatol. Rep. 2011;13:175–181. doi: 10.1007/s11926-011-0176-8. [DOI] [PubMed] [Google Scholar]
- 17.Corominas H., Ortiz-Santamaria V., Castellvi I., Moreno M., Morlà E., Clavaquera T., Erra A., Martinez-Pardo S., Ordoñez S., Santo P., Reyner P., Gonzalez M.J., Codina O., Gelman M.S., Juanola-Roura X., Olivé A., Torrente-Segarra V., CapiCATgroup Nailfold capillaroscopic findings in primary Sjögren syndrome with and without Raynaud's phenomenon and/or positive anti-SSA/RO and anti-SSB/La antibodies. Rheumatol. Int. 2016;36:365–369. doi: 10.1007/s00296-015-3396-9. [DOI] [PubMed] [Google Scholar]
- 18.Sebastiani M., Triantafyllias K., Manfredi A., Gonzalez-Gay M.A., Palmou-Fontana N., Cassone G., Drott U., Delbrück C., Rojas-Serrano J., Bertolazzi C., Nuño L., Giannini M., Iannone F., Vicente E.F., Castañeda S., Selva-O'Callaghan A., Araguas E.T., Emmi G., Iuliano A., Bauhammer J., Miehle N., Parisi S., Cavagna L., Codullo V., Montecucco C., Lopez-Longo F.J., Martínez-Barrio J., Nieto-González J.C., Vichi S., Confalonieri M., Tomietto P., Bergner R., Sulli A., Bonella F., Furini F., Scirè C.A., Bortoluzzi A., Specker C., Barsotti S., Neri R., Mosca M., Caproni M., Weinmann-Menke J., Schwarting A., Smith V., Cutolo M. American and European network of antisynthetase syndrome collaborative group, Nailfold capillaroscopy characteristics of antisynthetase syndrome and possible clinical associations: results of a multi center international study. J. Rheumatol. 2018 doi: 10.3899/jrheum.180355. pii: jrheum.180355 in press. [DOI] [PubMed] [Google Scholar]
- 19.Kim E.J., Collard H.R., King T.E., Jr. Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest. 2009;136:1397–1405. doi: 10.1378/chest.09-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendewald M.J., Wetter D.A., Li X., Davis M.D. Incidence of dermato-myositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch. Dermatol. 2010;146:26–30. doi: 10.1001/archdermatol.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkinson N.D., Shawe D.J., Gumpel J.M. Polymyositis, not polymyalgia rheumatica. Ann. Rheum. Dis. 1991;50:321–322. doi: 10.1136/ard.50.5.321. PMID 2042988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambataro G., Sambataro D., Torrisi S.E., Vancheri A., Pavone M., Rosso R., Schisano M., Crimi C., Pignataro F., Fischer A., Del Papa N., Vancheri C. State of the art in Interstitial Pneumonia with Autoimmune Features (IPAF): a systematic review on retrospective studies and suggestion for further advances. Eur. Respir. Rev. 2018;27 doi: 10.1183/16000617.0139-2017. pii 170139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.