Abstract
The increased hepatic gluconeogenesis in type 2 diabetes mellitus has often been ascribed to increased transcription of phosphoenolpyruvate carboxykinase 1, cystolic form (PEPCK1), although recent evidence has questioned this attribution. To assess the metabolic role of PEPCK1, we treated regular chow fed and high-fat fed (HFF) male Sprague-Dawley rats with a 2′-O-methoxyethyl chimeric antisense oligonucleotide (ASO) against PEPCK1 and compared them with control ASO-treated rats. PEPCK1 ASO effectively decreased PEPCK1 expression in the liver and white adipose tissue. In chow fed rats, PEPCK1 ASO did not alter adiposity, plasma glucose, or insulin. In contrast, PEPCK1 ASO decreased the white adipose tissue mass in HFF rats but without altering basal rates of lipolysis, de novo lipogenesis, or glyceroneogenesis in vivo. Despite the protection from adiposity, hepatic insulin sensitivity was impaired in HFF PEPCK1 ASO-treated rats. PEPCK1 ASO worsened hepatic steatosis, although without additional impairments in hepatic insulin signaling or activation of inflammatory signals in the liver. Instead, the development of hepatic insulin resistance and the decrease in hepatic glycogen synthesis during a hyperglycemic clamp was attributed to a decrease in hepatic glucokinase (GCK) expression and decreased synthesis of glycogen via the direct pathway. The decrease in GCK expression was associated with increased expression of activating transcription factor 3, a negative regulator of GCK transcription. These studies have demonstrated that PEPCK1 is integral to coordinating cellular metabolism in the liver and adipose tissue, although it does not directly effect hepatic glucose production or adipose glyceroneogenesis.
The regulation of hepatic glucose production is remarkably flexible, dynamically switching from disparate physiological states of feeding and fasting and maintaining normoglycemia. However, this control over hepatic glucose production fails in patients with type 2 diabetes in which increased gluconeogenesis leads to fasting hyperglycemia (1–3) and contributes to postprandial hyperglycemia (4). Much attention has focused on the transcriptional regulation of phosphoenolpyruvate carboxykinase-1 (PEPCK1; the cytosolic isoform of this enzyme). Unlike the other key gluconeogenic enzymes, PEPCK1 is primarily regulated at the transcriptional level, and numerous studies have described how islet hormones can alter the activity of transcription factors that affect PEPCK1 transcription (5–9). Also, in some experimental models (e.g., Zucker diabetic fatty rats, insulinopenic models), the development of hyperglycemia has been associated with an increase in PEPCK1. Thus, the transcriptional regulation of PEPCK1 was thought to be a key step in the pathogenesis of type 2 diabetes mellitus.
This paradigm has shortcomings. Many have reported a lack of concordance between hepatic PEPCK1 expression and glucose production. Mice lacking hepatic PEPCK1 will not develop hypoglycemia (10). Furthermore, hepatic PEPCK1 was found to only weakly regulate the rate of gluconeogenesis (11). We reported that hepatic PEPCK1 expression was not increased in rodents and humans with fasting hyperglycemia, demonstrating that increased PEPCK1 expression was not required for fasting hyperglycemia (12).
PEPCK1 is also important in white adipose tissue (WAT), where it supports glyceroneogenesis (13). Its expression and activity will be increased in fat-fed rodents compared with chow fed rodents. PEPCK1 has peroxisome proliferator-γ (PPARγ) response elements in its promoter and mutation of the PPARγ) response elements decreases adipose mass in regular chow (RC)-fed rodents (14–16). In contrast, transgenic overexpression of adipose PEPCK1 promotes obesity (17). Thus, in WAT, PEPCK1 might promote adipose lipid storage by supporting the synthesis of GA3P for fatty acid esterification into triglycerides.
The regulation of gluconeogenesis remains an active area of research. The level of gluconeogenic enzyme expression relates to the gluconeogenic capacity of the liver. In contrast, although many studies have often attributed alterations in hepatic glucose production to changes in PEPCK1 expression, as stated, the evidence has suggested that PEPCK1 is neither a strong regulator of gluconeogenesis nor increased in type 2 diabetes. To further our understanding of the role PEPCK1 has on whole body glucose and lipid metabolism, we used specific second-generation (2′-O-methoxyethyl chimeric) antisense oligonucleotides (ASOs) to decrease the expression of PEPCK1 in normal rats. Second-generation ASOs selectively silence protein expression in key metabolic tissues (e.g., liver and WAT) in normal adult animals, circumventing the developmental concerns with knockout mice. ASOs do not decrease expression in other key tissues (e.g., skeletal or cardiac muscle, brown adipose tissue, pancreas, brain) (18). We treated RC-fed and high fat-fed (HFF) adult rats with PEPCK1 ASO and performed hyperinsulinemic-euglycemic clamp studies to quantify the changes in glucose production and insulin sensitivity.
Methods and Materials
Animals
Normal male Sprague-Dawley (Envigo, Indianapolis, IN) rats weighing 300 to 325 g were housed in a 12-hour dark/light cycle, received food and water ad libitum, and acclimated to our facility for ≥3 days before use. The rats were then fed a normal rodent chow (Envigo 2018; 77% carbohydrate, 5% fat, and 18% protein) or a high-fat diet containing 27% safflower oil (Dyets 112245; 26% carbohydrate, 59% fat, 15% protein) for 4 weeks. The rats were injected twice weekly with either control ASO or PEPCK1 ASO at a dose of 75 mg/kg/week intraperitoneally for ≥4 weeks. The body weight was monitored weekly. Assessment of food intake by the rats was performed by measuring the weight of the chow before and after a 24-hour period. The rats underwent placement of jugular venous (for blood sampling) and carotid artery (for infusion) catheters ~5 to 7 days before the infusion studies and were allowed to recover to preoperative weights before the study. The rats were fasted overnight (14 to 16 hours) before the studies.
For the mouse studies, male C57BL/6 mice were given a high-fat diet and treated with ASO the same as for the rats. After the fifth ASO injection, the mouse metabolic parameters (e.g., energy expenditure, food intake) were measured using a comprehensive animal metabolic monitoring system (Columbus Instruments, Columbus, OH). The mice were allowed to acclimate for ~24 hours before data collection for the subsequent 55 hours. The institutional animal care and use committee at the Veteran’s Affairs Medical Center (West Haven, CT) and Yale University School of Medicine (New Haven, CT) approved all the studies.
Selection of ASOs
The PEPCK1 and control ASOs were designed and produced as previously described (19). The sequence [5-GCTCAAGTATGTTTTCTGTG-3 (ISIS-113309)] was selected against PEPCK1 and the sequence [5-CCTTCCCTGAAGGTTCCTCC-3 (ISIS-141923)] was selected as the control ASO.
Hyperinsulinemic-euglycemic clamp studies in Sprague-Dawley rats
After 4 weeks of ASO treatment and an overnight fast, awake, unrestrained rats underwent 4 mU/kg/min hyperinsulinemic-euglycemic clamp studies, as previously described (20). At the end of the clamp, the rats were euthanized with sodium pentobarbital injection via a jugular catheter (75 mg/kg). The tissues were harvested in situ with precooled tongs, frozen immediately in liquid N2, and stored at −80°C for subsequent analysis. Additionally, samples of liver were placed in RNAlater for RNA extraction.
Hyperglycemic clamp in Sprague-Dawley rats
To quantify the basal glycerol turnover and glycogen synthesis, we performed hyperglycemic clamps. After 4 weeks of ASO treatment and an overnight fast, basal glycerol turnover was assessed with a primed/continuous infusion (250 μL/kg/min, 50 μL/kg/min for 120 minutes) of 3 mg/mL d5-glycerol. The hyperglycemic study then began with an infusion of 20% glucose enriched to ~3% with U-13C glucose. The plasma glucose level was increased to ~200 mg/dL and maintained for 2 hours. At the end of the clamp, the rats were euthanized and tissues obtained, as described previously.
2H2O assessment of de novo lipogenesis and triglyceride-bound glycerol synthesis
The rats were labeled with deuterium oxide (Cambridge Isotope Laboratories, Inc., Andover, MA) with a 23.4 mL/kg bolus of 0.9% sodium chloride in 99% 2H2O, followed by 3 days of 5% 2H2O-enriched drinking water ad libitum. The rats were euthanized after an overnight fast.
Metabolites and hormones
Blood was collected from surgically placed catheters, as described, and immediately centrifuged in aprotinin-containing Beckman P100 tubes. Plasma supernatants were removed and stored at 4°C. Plasma glucose was measured using the glucose oxidase method on a Beckman Glucose Analyzer II. Plasma insulin and glucagon were measured using RIA (Millipore). Liver triglycerides and glycogen were measured as previously described (20).
Analysis of plasma glucose and glycerol by gas chromatography/mass spectrometry
To determine the enrichment of [6,6-2H] glucose in plasma, the samples were deproteinized with five volumes of 100% methanol, dried, and derivatized with 1:1 acetic anhydride/pyridine to produce the pentaacetate derivative of glucose. The atom percent excess (APE) of GlucoseM+2 was measured by gas chromatography/mass spectrometry analysis using a Hewlett-Packard 5890 gas chromatograph interfaced to a Hewlett-Packard 5971A mass-selective detector operating in the electron-ionization mode. GlucoseM+6 incorporation into glycogen was similarly measured from plasma and glycogen samples. For the latter analysis, glycogen was purified from liver homogenates by precipitation, dialyzed to remove free glucose, and subsequently hydrolyzed to glucose with amyloglucosidase, as previously described (21).
Adipocyte cell size analysis
Adipose tissue was fixed overnight in zinc formalin fixative before paraffin embedding and sectioning to 10-µm slices. Hematoxylin and eosin staining of sections was performed according to standard procedures (22). Microscopic images were taken, and the cell size was analyzed using CellProfiler (23) with an adipocyte membrane-specific pipeline (22).
Glycogen phosphorylase assay
The glycogen phosphorylase activity is measured by reverse direction incorporation of 14C-U glucose-1-phosphate (G1P) into glycogen. Liver tissue homogenate was mixed with assay buffer [40 mM 2-(N-Morpholino)ethanesulfonic acid (pH 6.1), 5 mM Ethylenedinitrilotetraacetic acid, 100 mM sodium fluoride, 50 mM G1P, 0.2 UCi 14C-U G1P, 0.1% BSA, 0.1% β-Mercaptoethano, 10% 1,2-dimethoxyethane (Sigma-Aldrich, St. Louis, MO), 10 mg/mL glycogen (Sigma-Aldrich)], with or without 0.5 mM caffeine and 10 mM AMP. The samples were incubated at 37°C for 15 minutes. The samples were spotted onto Whatman 3MM filter papers, which were immersed in 66% alcohol to stop the reaction. The glycogen phosphorylase activity results are shown as an activity ratio of −AMP/+AMP.
Calculations
Endogenous glucose production (EGP) was calculated using the Steele equation in steady state. Glycogen synthesized via the direct pathway was calculated as follows:
GlucoseM+6 from Glycogen is the APE of GlucoseM+6 measured from glycogen extracts (as described). Plasma GlucoseM+6 is the average APE of GlucoseM+6 over the course of the infusion study. The amount of glycogen synthesized via the indirect pathway is the difference between the glycogen content and the amount synthesized via the direct pathway.
De novo lipogenesis was assessed by quantifying the percentage of newly synthesized palmitate, as described previously (24). Glyceroneogenesis was assessed as the percentage of newly synthesized triglyceride-bound glycerol, as described previously (25).
Total RNA preparation and real-time quantitative RT-PCR analysis
Total RNA was extracted from liver samples using the RNeasy kit (Qiagen). RNA was reverse-transcribed using the Quantitect Reverse transcription kit (Qiagen). The abundance of each mRNA was assessed using real-time PCR and a SYBR Green detection system (BioRad) on an Eppendorf Realplex2 system. The list of primer pairs is provided in Table 1. The expression data for each gene of interest were normalized for the efficiency of amplification (26).
Table 1.
List of Primers Used for Quantitative PCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| PEPCK1 | ATGACACCCTCCTCCTGCAT | CAGGAAGTGAGGAAGTTTGTGG |
| PC | CGCTAGGAACTGCTGGTTGT | TCCGTGTCCGAGGTGTAAA |
| PEPCK 2 | GCACGGGTAGAAAGAAAGAC | CATGCATCCTGGGAATCTCT |
| FBP1 | ACCCAGCTGCTGAATTCGC | TGGCGCACCGCTGAC |
| G6Pc | ACTGGTTCATCTTGGTGTCCGTGA | AGGTGGAGCCAGTCTCCAATCACA |
| PGC 1α | AAAATCCAGAGAGTCATACTTGCTC | AATTTTTCAAGTCTAACTATGCAGACC |
| SREBP-1 | AACGTCACTTCCAGCTAGAC | CCACTAAGGTGCCTACAGAGC |
| PPARG | GGCCAGCATCGTGTAGATGA | GTGCCAGTTTCGATCCGTAGA |
| SCD1 | CGATCTGTGACCTGGAAAAATAAA | AATCACTGTAGATCTGATGACCTGGA |
| HPRT | TCCCAGCGTCGTGATTAGTGA | CCTTCATGACATCTCGAGCAAG |
| mGPAT | ACCCAGAGATGGGATACTGG | CGCTGAAATGGAAGGAGAG |
| Actin | CCAGATCATGTTTGAGACCTTC | CATGAGGTAGTCTGTCAGGTCC |
| GCK | GAAGACCTGAAGAAGGTGATGAGC | GTCTATGTCTTCGTGCCTTACAGG |
| SRC2 | TGATAGAGCTCTGGGGATACCA | GAACTGCTCCGGATCCAC |
| ATF3 | CCTGCAGAAGGAGTCAGAGAA | TGCAGGTTGAGCATGTAAATCA |
| TBP | GGACTCCTGTCTCCCCTACC | CTCAGTGCAGAGGAGGGAAC |
| 18s | TGGCTGAACGCCACTTGTC | TTCCGATAACGAACGAGACTCT |
Western blotting
Liver proteins were extracted in ice-cold homogenization buffer [10 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% NP 40, 0.5% sodium deoxycholate] supplemented with protease and phosphatase inhibitor cocktails (Sigma-Aldrich). Equal amounts of protein were resolved by SDS-PAGE (TGX gels; BioRad) and electroblotted onto a nitrocellulose membrane (BioRad). After blocking for 1 hour at room temperature in Tris-buffered saline with Tween 20 containing 5% (w/v) milk, the blots were incubated overnight with primary antibodies PEPCK1 (27), PEPCK2 (28), pyruvate carboxylase (29), G6PC (30), pAkt Ser 473 (31), Akt (32), Gapdh (33), caspase1 (34), pJNK Thr 183/Y185 (35), and JNK (36) at 4°C, washed, and incubated with horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature and visualized using enhanced chemiluminescence (Thermo) and a ChemiDoc MP imaging system (BioRad). For phosphorylated protein detection, the primary antibodies were prepared in Tris-buffered saline with Tween 20 with 5% BSA.
Statistical analysis
Statistical analysis of the data was performed using GraphPad Prism, version 6.0. All data are expressed as the mean ± SEM. For comparisons of two groups with a single cohort of rats (i.e., RC-fed basal or HFF), the two-tailed, unpaired Student t test was used. When multiple comparisons were performed of the same samples (i.e., measuring expression of multiple genes using real-time PCR), the Holm-Sidak correction was applied to adjust the P values. Both unadjusted and adjusted P values are reported.
Results
PEPCK ASO decreases adipose and hepatic PEPCK1 expression
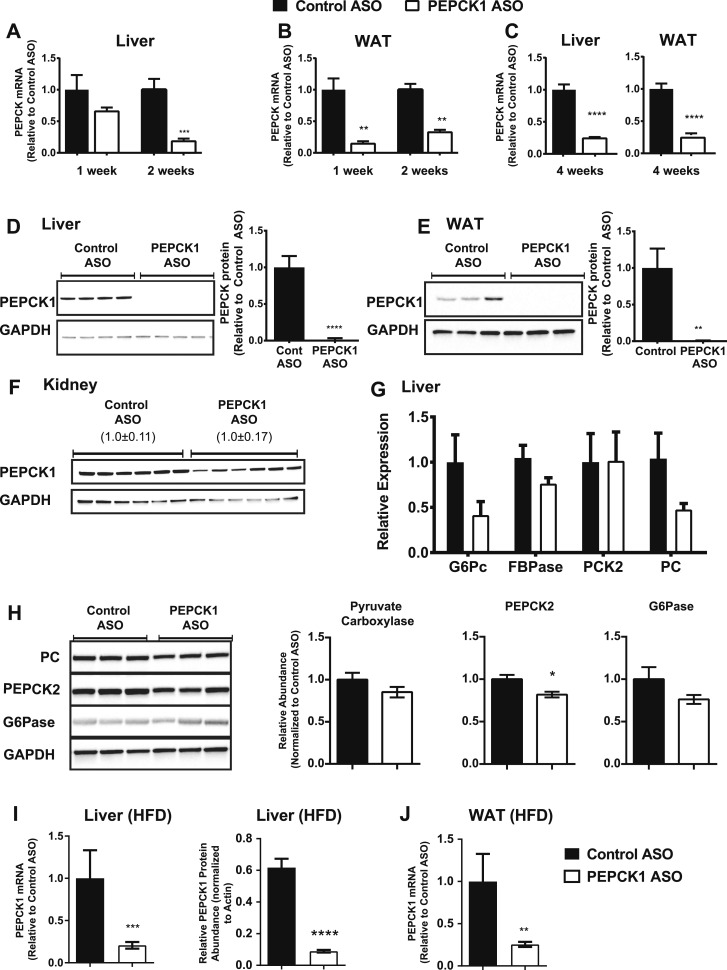
PEPCK1 ASO effectively decreased Pck1 mRNA expression in liver and WAT (Fig. 1). Hepatic Pck1 expression was reduced after 2 weeks of ASO treatment (Fig. 1A). We found a substantial reduction in Pck1 expression in WAT after even 1 week (two doses; Fig. 1B). PCK1 protein expression was decreased by ~90 and 95% in liver and WAT after 2 weeks (Fig. 1D and 1E). Pck1 mRNA expression remained decreased by ~80% in liver and ~80% in WAT after 4 weeks of treatment (Fig. 1C). PEPCK1 ASO did not affect PCK1 protein expression in the kidney (Fig. 1F). No change was found in the mRNA expression of other gluconeogenic enzymes or in PC and G6PC protein expression (Fig. 1G and 1H). A reduction was observed in PCK2 protein expression in whole cell lysates (~15% reduction; P = 0.02) that might reflect cross-reactivity of the antibody with PCK1. Finally, PEPCK1 ASO was also effective in decreasing Pck1 expression in HFF rats in both liver (∼79% decrease in mRNA and ∼88% decrease in protein) and WAT (∼75% decrease in mRNA; Fig. 1I and 1J).
Figure 1.
PEPCK1 ASO is specific and effective. (A) mRNA expression of PEPCK1 in liver and (B) WAT after 1 wk (n = 5 for control ASO and n = 6 for PEPCK1 ASO) and 2 wk (n = 6 for control ASO and n = 7 for PEPCK1 ASO) of treatment in HFF rats. (C) mRNA expression of PEPCK1 in liver and WAT after 4 wk of treatment. (D) Protein expression of PEPCK1 in liver whole cell lysates (n = 6 per group). Protein expression of PEPCK1 in (E) WAT and (F) kidney whole cell lysates. Values indicate mean ± SEM of PEPCK expression relative to GAPDH. (G) mRNA expression of other gluconeogenic enzymes (as labeled) in liver (n = 7 in each group). (H) Protein expression of other gluconeogenic enzymes in liver whole cell lysates (n = 7 for control ASO and n = 8 for PEPCK1 ASO). (I) PEPCK1 mRNA and protein expression in high-fat fed rat livers. (J) PEPCK1 mRNA expression in HFF WAT. mRNA and protein expression is normalized to a control mRNA and protein (e.g., TBP and GAPDH or actin) and expressed as fold of control ASO group. Black bars represent control ASO; white bars, PEPCK1 ASO. Data presented as mean ± SEM. *P < 0.05 vs control ASO; **P < 0.01 vs control ASO; ****P < 0.0001 vs control ASO. G6Pase, glucose 6-phosphatase; FBPase, fructose 1,6 bisphosphatase; PC, pyruvate carboxylase.
PEPCK1 ASO prevents weight gain in HFF rats
The initial and final body weights were not significantly different in the RC-fed rats treated with ASO. However, the increase in body weight was 15 g less over 4 weeks in the PEPCK1 ASO-treated rats (P < 0.05; Table 2). The epididymal WAT mass was not different between the two treatment groups in the RC-fed rats. No differences were found in plasma glucose, insulin, or lipid concentrations (Table 2) in the RC-fed rats treated with either control ASO or PEPCK1 ASO. The liver triglyceride concentration was greater in the RC rats treated with PEPCK1 ASO (2.3 ± 0.05 vs 2.8 ± 0.2 mg/g liver; n = 6 and n = 8, respectively; P = 0.04). The fasting liver glycogen concentration was not substantially different (0.40% ± 0.07% vs 0.28% ± 0.04%; n = 6 per group; P = 0.19).
Table 2.
Baseline Parameters in RC-Fed Treated With Either Control ASO or PEPCK1 ASO for 4 Weeks
| Variable | Control ASO (n = 10) | PEPCK1 ASO (n = 7) |
|---|---|---|
| Starting body weight, g | 335 ± 1.7 | 344 ± 1.8 |
| Final body weight, g | 372 ± 2.8 | 365 ± 2.9 |
| Body weight gain | 37 ± 2.0 | 22 ± 2.6a |
| Glucose, mg/dL | 105 ± 8 | 114 ± 8 |
| Insulin, µU/mL | 5.1 ± 1.8 | 5.6 ± 2.0 |
| Plasma triglycerides, mg/dL | 43 ± 6 | 49 ± 2 |
| Free fatty acids, mM | 0.52 ± 0.06 | 0.38 ± 0.01a |
P < 0.05 vs control ASO for each diet.
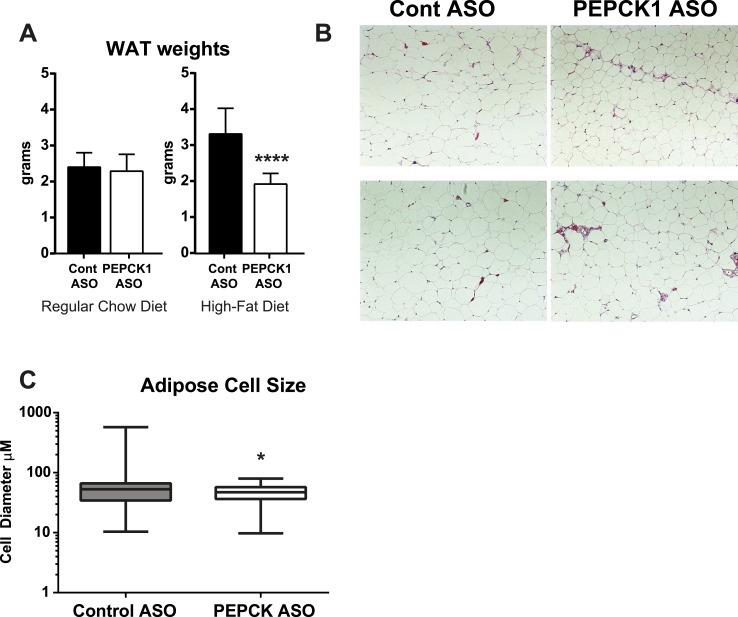
PEPCK1 ASO had a marked effect on weight gain and adiposity in the HFF rats. The weight gain was decreased by 80% (P < 0.001; Table 3) and the WAT mass by ∼40% (Fig. 2A). We found a slight reduction in the fasting plasma triglyceride concentration. No change was found in the fasting plasma fatty acid concentrations in the HFF rats treated with PEPCK1 ASO (Table 3). The plasma leptin concentration was unchanged (2.9 ± 0.14 ng/mL vs 2.8 ± 0.16 ng/mL; n = 8 per group), and the plasma adiponectin concentration was decreased by ∼25% (12.63 ± 0.82 ng/mL vs 9.1 ± 0.58 ng/mL; n = 8 per group; P = 0.003).
Table 3.
Baseline Parameters in HFF Treated With Either Control ASO or PEPCK1 ASO for 4 Weeks
| Variable | Control ASO (n = 8) | PEPCK1 ASO (n = 7) |
|---|---|---|
| Starting body weight, g | 323 ± 1.5 | 325 ± 1.6 |
| Final body weight, g | 385 ± 5.0 | 337 ± 2.7a |
| Body weight gain | 62 ± 4.1 | 12 ± 3.5a |
| Glucose, mg/dL | 114 ± 1 | 112 ± 4 |
| Insulin, µU/mL | 8.0 ± 1 | 6.1 ± 0.4 |
| Plasma triglycerides, mg/dL | 33.8 ± 2.0 | 25.9 ± 2.5b |
| Free fatty acids, mM | 0.45 ± 0.03 | 0.45 ± 0.05 |
P < 0.0001 vs control ASO for that diet.
P < 0.05 vs control ASO for that diet.
Figure 2.
PEPCK1 ASO decreases adipose tissue mass in HFF rats. (A) Epididymal WAT weights in regular chow fed (n = 10 and n = 7 for control ASO and PEPCK1 ASO) and HFF rats after 4 wk of ASO treatment (n = 8 per group). (B) Hematoxylin and eosin-stained sections of WAT after 4 wk of treatment. (C) Mean adipose cell diameter shown as box and whiskers plot, indicating the median (n = 6 per group). Data expressed as mean ± SEM. *P < 0.05 vs control ASO.
Food consumption was not different between the HFF control ASO and PEPCK1 ASO rats (230 ± 62 kcal/kg/d vs 289 ± 77 kcal/kg/d; n = 7 and n = 8, respectively; P = 0.57). Adipose histologic examination revealed a qualitative reduction in the adipocyte cell size (Fig. 2B), which was further quantified using a computer-assisted algorithm [using CellProfiler (20)]. The mean diameter was decreased by ∼25%, from 60 µm in the control ASO-treated rats to 45 µm in the PEPCK ASO-treated rats (Fig. 2C). The mRNA expression of several key genes that regulate adipose lipid metabolism was assessed. Although we found a tendency toward a reduction in SREBP1c, AGPAT, and ATGL, after adjusting for multiple comparisons, these differences were not statistically significant (Table 4). PEPCK1 is thought to regulate adipose glyceroneogenesis, which can affect the ability of adipose tissue to esterify and retain fatty acids. HFF rats treated with either control ASO or PEPCK1 ASO were given 2H2O for 3 days to quantify 2H incorporation into triglyceride-bound glycerol. 2H2O labeling in plasma water was equivalent between groups (control ASO, 4.1% ± 0.15%; vs PEPCK1 ASO, 4.3% ± 0.20%; n = 7 per group). The percentage of newly synthesized triglyceride-bound glycerol was not different between the control ASO or PEPCK1 ASO-treated rats in WAT (control ASO, 3.7% ± 0.4%; vs PEPCK1 ASO, 2.6% ± 0.4%; P = 0.07; n = 7 per group). Glycerol can also be synthesized via glycerol kinase; however, we found no difference in the expression of adipose glycerol kinase (Table 4).
Table 4.
Expression of Select Genes in HFF Rats Treated With Either Control ASO or PCK1 ASO After an Overnight Fast
| Gene | Control ASO | PEPCK1 ASO | Unadjusted P Value | Adjusted P Value |
|---|---|---|---|---|
| WAT | ||||
| SREBP1 | 1.00 ± 0.14 (8) | 0.60 ± 0.08 (8) | 0.0262 | 0.2128 |
| PPARγ | 1.00 ± 0.07 (8) | 1.41 ± 0.22 (8) | 0.0992 | 0.4070 |
| AGPAT | 1.00 ± 0.14 (8) | 0.47 ± 0.13 (8) | 0.0141 | 0.1569 |
| DGAT2 | 1.00 ± 0.19 (8) | 0.92 ± 0.34 (8) | 0.8366 | 0.8366 |
| ATGL | 1.00 ± 0.10 (8) | 0.64 ± 0.10 (8) | 0.0235 | 0.2119 |
| GK | 1.00 ± 0.17 (8) | 0.85 ± 0.14 (8) | 0.5129 | 0.8489 |
| Liver | ||||
| SREBP1 | 1.00 ± 0.18 (8) | 0.49 ± 0.10 (8) | 0.0269 | 0.2128 |
| FAS | 1.00 ± 0.17 (7) | 2.26 ± 0.12 (7) | <0.0001a | 0.0007a |
| SCD1 | 1.00 ± 0.52 (7) | 3.31 ± 0.77 (7) | 0.0284 | 0.2128 |
| mtGPAT | 1.00 ± 0.45 (8) | 1.56 ± 0.52 (7) | 0.4282 | 0.7576 |
| AGPAT | 1.00 ± 0.30 (8) | 0.13 ± 0.02 (7) | 0.0174 | 0.176 |
| DGAT2 | 1.00 ± 0.14 (8) | 0.61 ± 0.12 (8) | 0.0494 | 0.2621 |
| ATGL | 1.00 ± 0.12 (8) | 1.82 ± 0.22 (8) | 0.0058 | 0.0731 |
| GK | 1.00 ± 0.18 (8) | 1.46 ± 0.25 (8) | 0.2760 | 0.7253 |
| GCK | 1.00 ± 0.21 (8) | 0.12 ± 0.05 (8) | 0.0011a | 0.0155a |
| FOXO1 | 1.00 ± 0.16 (8) | 1.25 ± 0.22 (8) | 0.3765 | 0.7576 |
| SRC2 | 1.00 ± 0.20 (8) | 1.46 ± 0.25 (8) | 0.1746 | 0.5359 |
| ATF3 | 1.00 ± 0.21 (8) | 12.20 ± 1.79 (8) | <0.0001a | 0.0004a |
Data presented as mean ± SEM, with the number of samples in parentheses. Holm-Sidak method used to adjust for multiple comparisons. Unadjusted P values and adjusted P values are presented.
Statistically significant difference.
To better assess whether PEPCK1 ASO affected the whole body energy balance (e.g., reductions in calorie intake or increases in expenditure), we treated male C57BL/6 mice fed a high-fat diet with either control ASO or PEPCK1 ASO. The use of mice enabled a more precise assessment of the whole body energy balance using an available indirect calorimeter cage system, which does not accommodate rats. To avoid the potentially confounding influence of altered body weight, metabolic cage measurements were performed after 2 weeks of therapy, before a substantial divergence had occurred in body weight. Although the whole body fat mass was 40% lower after 2 weeks of ASO, we did not find a substantial difference in total body weight (Table 3). The reduction in adiposity could not be attributed to any differences in food consumption, energy expenditure, or respiratory quotient (Table 5). The 24-hour profiles did not show any substantial differences in the diurnal variations of these key parameters.
Table 5.
Metabolic Cage Data of Male C57BL/6 Mice After 2 Wk of HFD and Either Control ASO or PEPCK1 ASO Treatmenta
| Variable | Control ASO | PEPCK1 ASO |
|---|---|---|
| Body weight | 29.7 ± 0.97 | 28.7 ± 0.53 |
| Fat mass, % | 9.8 ± 1.35 | 6.2 ± 0.8b |
| Energy expenditure, kcal/kg/h | 16.2 ± 0.58 | 15.4 ± 0.39 |
| RQ | 0.78 ± 0.004 | 0.78 ± 0.006 |
| Feeding, g/kg/h | 1.46 ± 0.2 | 1.56 ± 0.16 |
| Activity, counts/h | 123 ± 20 | 130 ± 10 |
| Energy expenditure, kcal/kg/h | 15 ± 0.57 | 13.9 ± 0.44 |
| RQ | 0.76 ± 0.004 | 0.77 ± 0.005 |
| Feeding, g/kg/h | 0.81 ± 0.15 | 0.77 ± 0.11 |
| Activity, counts/h | 60 ± 7 | 54 ± 6 |
| Energy expenditure, kcal/kg/h | 17.7 ± 0.62 | 17.5 ± 0.35 |
| RQ | 0.8 ± 0.005 | 0.81 ± 0.007 |
| Feeding, g/kg/h | 2.32 ± 0.29 | 2.6 ± 0.24 |
| Activity, counts/h | 207 ± 38 | 231 ± 19 |
Abbreviation: RQ, respiratory quotient.
n = 6–7 per group.
P < 0.05 compared with control ASO.
PEPCK ASO results in insulin resistance
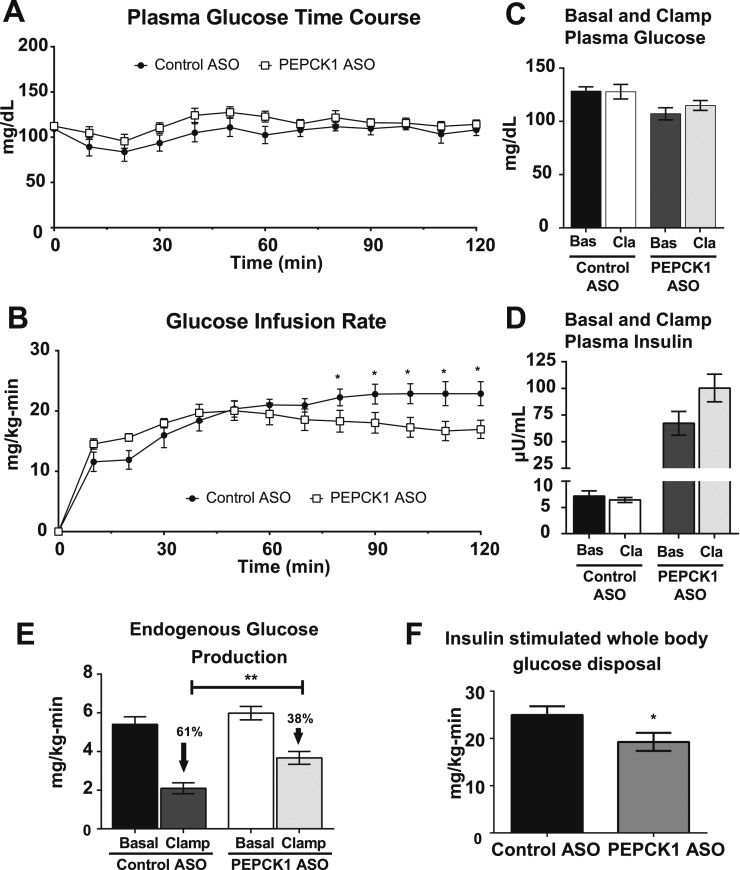
To determine whether the reduction in adiposity influenced insulin action, the HFF rats treated with control or PEPCK1 ASO underwent 4 mU/kg/min hyperinsulinemic-euglycemic clamps to quantify the changes in basal and insulin-stimulated glucose metabolism. Plasma glucose and insulin concentrations were matched during the hyperinsulinemic-euglycemic clamp (Fig. 3A and 3C). The PEPCK1 ASO-treated rats required a lower glucose infusion rate to maintain euglycemia (Fig. 3B). Basal and clamped plasma insulin concentrations were matched between groups (Fig. 3C). The basal rate of EGP (largely reflecting gluconeogenesis in rodents fasted overnight) was unchanged. However, the ability of insulin to suppress EGP was diminished, suggesting the presence of hepatic insulin resistance (Fig. 3E). We also found modest reductions in the rate of insulin-stimulated whole body glucose disposal, suggesting some degree of peripheral insulin resistance (Fig. 3F).
Figure 3.
PEPCK1 ASO treatment worsens insulin resistance in HFF rats. (A) Plasma glucose excursion during hyperinsulinemic (4 mU/kg/min)-euglycemic clamp. (B) Glucose infusion rate. (C) Basal and clamp plasma glucose taken as average of T20 to T0 min and T90 to T120 min, respectively. (D) Basal and clamp plasma insulin concentration. (E) EGP under basal and clamped conditions. (F) Insulin-stimulated whole body glucose disposal (n = 6 for control ASO and n = 7 for PEPCK1 ASO). Data expressed as mean ± SEM. *P < 0.05 vs control ASO; **P < 0.01 vs control ASO.
Fasting whole body lipolysis during the basal portion of the infusion was assessed by infusion of 2H5-glycerol. No differences were found in whole body glycerol turnover (control ASO, 3.2 ± 0.24 mg/kg/min; vs PEPCK1 ASO. 2.9 ± 0.34 mg/kg/min; n = 5 and n = 4, respectively). These data are consistent with the lack of differences between either the basal plasma fatty acid concentration (Table 3) or the plasma fatty acid concentration under hyperinsulinemic-euglycemic conditions (control ASO, 0.19 ± 0.05 mM; vs PEPCK1 ASO, 0.20 ± 0.04 mM).
PEPCK1 ASO promotes hepatic lipid accumulation in HFF rats
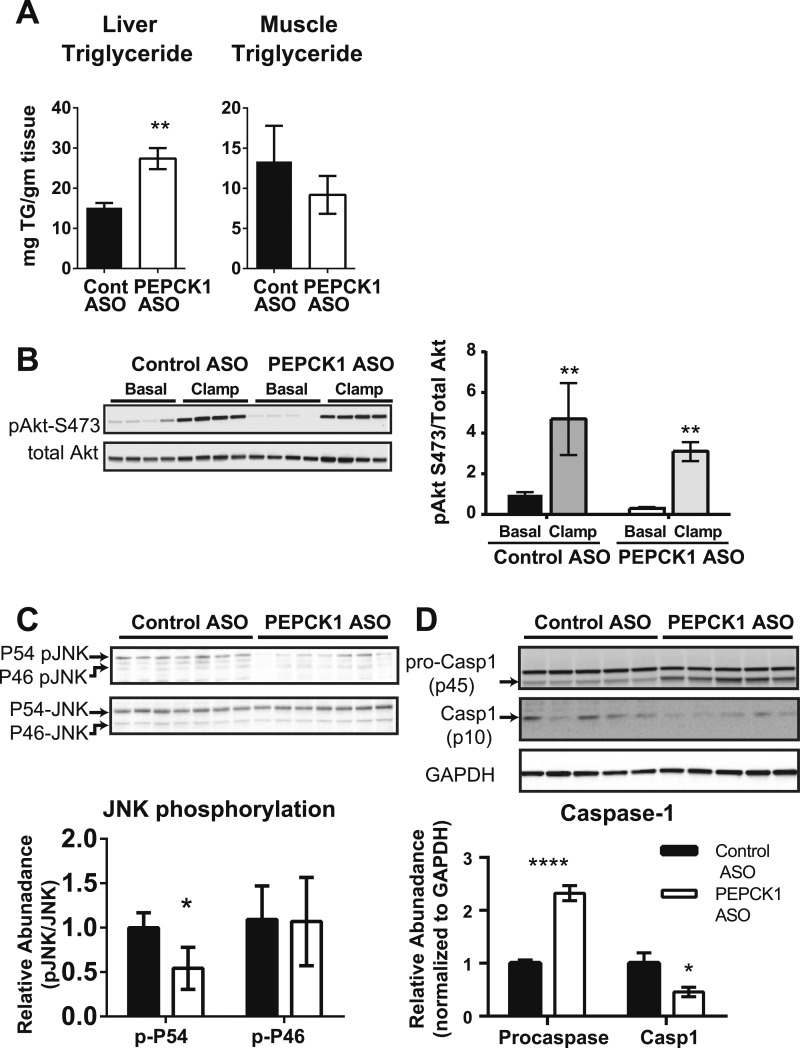
Despite the reduction in adiposity, PEPCK1 ASO increased the hepatic triglyceride content in HFF rats (Fig. 4A). The muscle triglyceride content was not changed. No differences were found in mRNA expression of SREBP1c or several key enzymes that regulate hepatic lipid content (Table 4), although the mRNA expression of fatty acid synthase was increased (Table 4). The percentage of lipids synthesized via de novo lipogenesis was not different between groups (control ASO, 2.62% ± 0.003%; vs PEPCK1 ASO, 2.63% ± 0.003%; P = 0.5; n = 7 for each group). The plasma ketone concentration was not different (0.68 ± 0.04 mM vs 0.59 ± 0.04 mM; P = 0.15). A slight reduction was found in new glycerol synthesis in the liver (control ASO, 45.3% ± 3.2%; vs PEPCK1 ASO, 35.6% ± 2.0%; P = 0.02). No difference was found in the expression of hepatic glycerol kinase (Table 4). Thus, PEPCK1 ASO treatment of HFF rats promoted hepatic steatosis without a marked impact on the hepatic lipid synthetic pathways.
Figure 4.
PEPCK1 ASO worsened hepatic steatosis but did not impair insulin signaling. (A) Liver and muscle triglyceride content from HFF rats treated with control ASO (n = 8) or PEPCK1 ASO (n = 7) after an overnight fast. (B) Akt phosphorylation at S473 under fasting (basal) and hyperinsulinemic (clamp) condition. (C) JNK phosphorylation assessed for P54 and P46 isoform. (D) Procaspase 1 and caspase 1 expression (n = 7 to 8 per group; representative blots shown for Western blots). *P < 0.05 vs control ASO; **P < 0.01 vs control ASO; ****P < 0.0001 vs control ASO.
PEPCK1 ASO does not impair hepatic insulin signaling or activate inflammatory pathways in HFF rats
The development of fatty liver can impair hepatic insulin signaling. No difference was found in insulin-stimulated hepatic Akt phosphorylation (Ser 473) between the groups (Fig. 4B). Activation of inflammatory pathways has also been associated with insulin resistance in fatty liver. Many of these inflammatory pathways converge on the activation of c-jun N terminal kinase (JNK), and JNK activation has been associated with insulin resistance (37). However, phosphorylation of the P54 isoform was decreased, and no change occurred in the phosphorylation of the P46 isoform. The significance of the preferential decrease in p54 is unclear (Fig. 4C). Inflammasome activation has been associated with nonalcoholic fatty liver disease and hepatic inflammation (38). However, we found a decrease in mature caspase 1 p10 in the PEPCK1 ASO-treated rats (Fig. 4D). Thus, the development of hepatic insulin resistance in the PEPCK1 ASO-treated rats could not be attributed to impairments in insulin signaling or activation of inflammatory pathways in the liver.
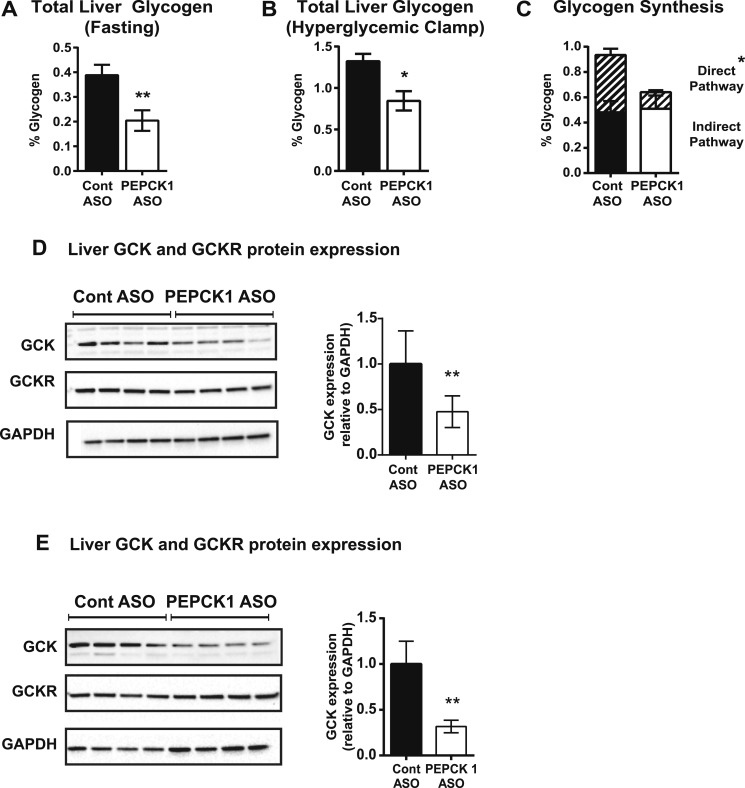
PEPCK1 ASO impairs hepatic glycogen synthesis and decreases GCK expression
We observed a ∼50% decrease in the fasting hepatic glycogen content in the HFF PEPCK1 ASO-treated rats (Fig. 5A). The PEPCK1 ASO treatment decreased the net glycogen synthesis during the hyperinsulinemic-euglycemic clamp (0.46 ± 0.13 mg/100 mg liver vs 0.02 ± 0.05 mg/100 mg liver; n = 6 and n = 7 for control ASO and PEPCK1 ASO, respectively; P < 0.01). We performed a hyperglycemic clamp in HFF rats treated with control or PEPCK1 ASO using U-13C enriched 20% dextrose to interrogate glucose incorporation into glycogen. Hyperglycemia was maintained via an infusion of 20% dextrose for 120 minutes (clamp glucose concentration: control ASO, 197 ± 5 mg/dL; vs PEPCK1 ASO, 196 ± 6 mg/dL; n = 5 and n = 4 for control ASO and PEPCK1 ASO, respectively). The plasma insulin concentrations were similar between the two groups during the hyperglycemic clamp (control ASO, 46 ± 5.1 µM/mL; vs PEPCK1 ASO, 51 ± 6.7 μU/mL; P = 0.33). The total hepatic glycogen content was decreased ∼35% in the PEPCK1 ASO-treated animals (Fig. 5B). Although plasma U-13C enrichment was similar in the control ASO and PEPCK1 ASO-treated animals throughout the hyperglycemic clamp (control ASO, 2.1% ± 0.1%; vs PEPCK1 ASO, 2.2% ± 0.1%), the enrichment in glycogen was decreased in the PEPCK1 ASO-treated animals (0.6% ± 0.1% vs 0.4% ± 0.03%; P = 0.002). A marked decrease occurred in glycogen formed via the direct pathway of glycogen synthesis without any change in glycogen synthesized via the indirect pathway (Fig. 5C). GCK regulates the incorporation of glucose into glycogen via the direct pathway. PEPCK1 ASO treatment decreased hepatic GCK mRNA expression by ∼80% (Table 4) and protein expression by ∼50% in the livers of fasted rats (Fig. 5D). At the end of the hyperglycemic clamp, GCK protein was still ∼70% lower in the PEPCK1 ASO-treated rats (Fig. 5E).
Figure 5.
PEPCK1 ASO decreased liver glycogen content. (A) Liver glycogen in HFF rats treated with control (n = 8) or PEPCK1 ASO (n = 7) after an overnight fast and (B) at the end of a hyperglycemic clamp (n = 5 and n = 4 for control ASO and PEPCK1 ASO, respectively). (C) Glycogen synthesized during the hyperglycemic clamp via the direct (hatched bars) and indirect (solid bars) pathways of glycogen synthesis. (D) Liver GCK and GCK receptor protein expression after an overnight fast. (E) Liver GCK and GCK receptor protein expression at the end of the hyperglycemic clamp. *P < 0.05 vs control ASO; **P < 0.01 vs control ASO.
The transcriptional regulation of GCK is incompletely understood. It is clearly induced by insulin and can be increased by SREBP1 (39). As fasting SREBP1 mRNA expression had a tendency to be lower, we also quantified hepatic SREBP1c mRNA expression and two key target genes (ACC and FAS) after the hyperglycemic clamp, a state that mimics the fed state. SREBP1c expression was not different (1.3 ± 0.4 vs 0.73 ± 0.1; P = 0.25; n = 5 and n = 4 for control ASO and PEPCK1 ASO, respectively). No differences were found in ACC (0.03 ± 0.002 vs 0.04 ± 0.004; P = 0.58) or FAS (0.21 ± 0.03 vs 0.24 ± 0.03; P = 0.43) expression. SRC2 (steroid receptor coactivator-2), another transcriptional activator of GCK (40), was also unchanged in fasted PEPCK1 ASO- vs control ASO-treated rats (Table 4). Several transcription factors have been shown to suppress GCK expression. FOXO1 is an insulin responsive transcription factor that can suppress GCK expression (41). However, fasting FOXO1 mRNA expression was unchanged (Table 4). Activating transcription factor 3 (ATF3) can also suppress GCK expression. Fasting hepatic ATF3 mRNA expression was increased ∼12-fold (Table 4) and might account for the suppression of hepatic GCK expression.
Discussion
The view of PEPCK1’s role as a key gluconeogenic enzyme has been largely defined by the reaction that it catalyzes and the high level of transcriptional control in response to hormonal signals. PEPCK1 mRNA expression increases with fasting and rapidly decreases in the fed state. Insulin efficiently suppresses PEPCK1 mRNA expression, although protein expression persists for a longer period (42). Adipose PEPCK1 expression is similarly regulated. Expression is increased in the fasted state and thought to support glyceroneogenesis for fatty acid re-esterification (43). Many have equated insulin-mediated suppression of PEPCK transcription to the suppression of hepatic gluconeogenesis (41, 44–48). By extension, dysregulation of hepatic gluconeogenesis in diabetic states has often been ascribed, at least in part, to the dysregulation of PEPCK.
However, several studies have demonstrated that PEPCK1 expression relates poorly to gluconeogenic or glyceroneogenic flux (11, 12, 43). Our studies quantified the metabolic effect of reducing PEPCK1 expression in key tissues of adult rats and we found several key findings. First, the HFF rodents had decreased adiposity, although adipose triglyceride-glycerol synthesis was not affected. Thus, PEPCK1 can affect adipose fat storage but does not strongly influence adipose glyceroneogenesis. Second, PEPCK1 ASO did not alter the basal glucose concentration or rates of glucose production, attesting to PEPCK1’s minimal control over hepatic glucose production. Third, although the HFF rats treated with PEPCK1 ASO were protected from obesity, they did manifest hepatic insulin resistance. The impaired ability of insulin to regulate hepatic glucose metabolism can be attributed to the decreased expression of GCK and decreased synthesis of glycogen via the direct pathway.
The protection from weight gain suggests that adipose PEPCK1 is important for adipose lipid storage. PEPCK1 is considered important for adipose glyceroneogenesis, which enables adipose lipid storage by supporting the synthesis of glycerol-3-phosphate for fatty acid esterification when dietary carbohydrate is limited (e.g., in the fasted state or with high-fat feeding) (13, 25, 49). Several genetic rodent models have supported this hypothesis. Mutation of the PPARγ response element in the PEPCK promoter (PEPCKPPARE−/−) eliminated expression specifically in WAT and resulted in a variable reduction in WAT mass (15). In contrast, transgenic overexpression of PEPCK in adipose tissue (aP2-PEPCK) promotes weight gain and adiposity in RC-fed mice (17). However, other studies have reported contrasting findings. Nye et al. (43) assessed adipose glyceroneogenesis and PEPCK1 activity in 48-hour fasted rats and “fed” rats (rats fed sucrose-supplemented water and studied during a glucose infusion). Although adipose PEPCK1 activity was markedly increased in the fasted rats, the rate of adipose glyceroneogenesis was unchanged, leading the investigators to conclude that glyceroneogenic flux might be regulated by factors other than PEPCK1 activity (e.g., substrate availability). Furthermore, studies in later generations of the PEPCKPPARE−/− mice no longer manifested the lipodystrophic phenotype and did not have any difference in triglyceride-bound glycerol synthesis (50). Although adipose PEPCK1 expression remained absent, an increase in adipose glycerol kinase activity might have provided an alternate pathway for glycerol synthesis and diminished the lipodystrophic phenotype.
The reduction in WAT mass in HFF rodents treated with PEPCK1 ASO could not be attributed to increased whole body lipolysis or lipid oxidation. Basal glycerol turnover, an index of basal lipolysis, was not different. Neither the basal plasma fatty acid concentrations nor the insulin-stimulated fatty acid concentrations were different. The fasting plasma ketone concentration, an indirect measure of lipid oxidation, was not altered. No detectable changes were found in whole body energy expenditure, food intake, or respiratory exchange ratio in the PEPCK1 ASO-treated mice, arguing against changes in energy balance or increases in lipid oxidation. We found a tendency for altered expression of genes that regulate lipid metabolism in adipose tissue. The gene expression data suggested a possible decrease in SREBP1c, AGPAT, and ATGL. Although these differences were not substantial after correcting for multiple comparisons, these data might suggest a general impairment of adipocyte function and raises the question of how this might arise after a decrease in PEPCK1 expression. We speculate that PEPCK1 might regulate the adipose tissue metabolism by regulating cataplerosis in adipocytes. It is possible that the reduction in cataplerotic flux with PEPCK1 ASO decreases mitochondrial function and impairs adipocyte function. Some precedent exists for this hypothesis. Adipose PEPCK1 expression is regulated by transcription factors that regulate adipose differentiation, such as PPARγ (51) and CCAAT/enhancer-binding protein-α (52). PEPCK1 activity is coupled to citrate synthase activity and, thus, might regulate the entry of carbons into the TCA cycle (11, 53). Also, adipose mitochondrial reactive oxygen species production and mitochondrial function have been implicated in adipose differentiation (54). Further studies are required to determine whether adipogenesis is dependent on PEPCK1 expression.
The second focus of our studies was the assessment of liver glucose metabolism in PEPCK1 ASO-treated rats. Decreasing PEPCK1 did not affect the basal rates of glucose production, consistent with previous studies (10, 11). In a more recent study, restoring hepatic PEPCK1 expression in liver-specific PEPCK1 knockout mice to only 10% of the wild-type levels restored PEPCK activity to 60% of the wild-type levels and normalized the phenotype (55). In the present study, ASO treatment decreased PEPCK1 expression in a relatively acute fashion (i.e., within 4 weeks vs a lifelong genetic deletion) but also did not affect the fasting plasma glucose concentration or rate of EGP. These data are consistent with the concept that PEPCK1 expression does not exert substantial control over hepatic glucose production.
PEPCK1 ASO did, however, affect hepatic glucose metabolism in other ways. Insulin’s ability to suppress hepatic glucose production was impaired in the PEPCK1 ASO-treated rats and was associated with increased hepatic steatosis. This recapitulates the observation of hepatic steatosis with fasting seen in PEPCK1 knockout mice. The development of hepatic steatosis has been associated with impairments of insulin signaling (56, 57). However, we did not detect any differences in insulin-stimulated Akt2 phosphorylation in the PEPCK1 ASO-treated rats. Activation of inflammatory pathways can also impair hepatic insulin action; however, neither JNK phosphorylation nor caspase 1 maturation was increased. Thus, the “usual suspects” accounting for fat-induced insulin resistance were not culpable in this model. However, both control ASO and PEPCK1 ASO-treated rats were fed a high-fat diet and both had increased liver triglycerides compared with chow-fed conditions. Thus, a limitation of the present study was that the insulin signaling in the RC-fed animals was not assessed. Therefore, the additional fat accumulation after PEPCK1 ASO treatment might not have further impaired hepatic insulin signaling. Future studies that directly compare the effect of PEPCK1 ASO in RC-fed animals to that in HFF animals could answer this question.
Hepatic insulin action directly activates hepatic glycogen synthesis. However, the HFF PEPCK1 ASO-treated rats had a decrease in hepatic glycogen content after both an overnight fast and at the end of both euglycemic and hyperglycemic clamps. This is consistent with previous reports of decreased hepatic and muscle glycogen synthesis in PEPCK1 knockout mice during a hyperglycemic clamp (58). We attributed this to a decrease in GCK expression. This decrease in GCK expression accounted for the decrease in hepatic glycogen synthesis via the direct pathway seen during the hyperglycemic clamp studies, similar to findings from humans with mutations in GCK [maturity onset diabetes of the young type 2 (59)] and mice with liver-specific GCK deletion (60). Coate et al. (61) recently reported that the development of hepatic insulin resistance in fructose-fed dogs could also be attributed to the reduction in GCK protein without detectable changes in insulin-stimulated Akt phosphorylation.
How could the decrease in GCK expression affect hepatic insulin action? The reduction in GCK decreases the formation of glucose 6-phosphate, which is both a substrate (albeit several steps removed) and an allosteric activator of glycogen synthesis (62, 63). Although GCK is important for both glycolysis and glycogen synthesis, it appears to exert a much stronger control over the latter (64), and increased expression of GCK was reported to enhance the suppression of EGP during a hyperglycemic clamp (65). The synthesis of glycogen via the indirect pathway did not appear to be affected by PEPCK1 ASO. The carbon sources for the indirect pathway are thought arise from intrahepatic glycolysis and reconstitution of three carbon precursors into glucose (66). Although the reduction in GCK should decrease intrahepatic glycolysis, it is possible that other enzymes (i.e., hexokinase 1) might still permit sufficient glycolytic flux to allow for glycogen synthesis via the indirect pathway. Alternatively, with the decrease in flux through hepatic GCK, extrahepatic glucose metabolism might play a greater role in contributing the three-carbon inputs (e.g., Cahill and Cori cycles) to support the indirect pathway of glycogen synthesis.
A direct relationship between PEPCK1 and GCK expression has been observed in other models. GCK activity and hepatic glycogen were decreased in liver-specific PEPCK1 knockout mice, although GCK expression was not measured (10). Restoration of PEPCK1 expression in liver-specific PEPCK1 knockout mice, to even a fraction of wild-type levels, was sufficient to restore GCK expression to nearly 80% of wild-type levels (55). GCK expression was increased in transgenic mice overexpressing PEPCK in the liver (67). Thus, hepatic PEPCK1 expression appears to be closely linked to GCK expression.
However, the mechanism connecting PEPCK1 and GCK is not clear. We considered some possible explanations for the reduced GCK expression. The expression of several key regulators such as SREBP1c, SRC2, and FOXO1 were not different. However, we did observe an increase in ATF3 expression. ATF3 can suppress GCK expression, possibly by antagonizing CREB-mediated transcriptional activation (68, 69). ATF3 is considered a stress responsive transcription factor that is upregulated in response to numerous stimuli. For example, CCl4, ethanol, and deprivation of amino acids have been reported to induce ATF3 (68, 70, 71). Overexpression of ATF3 in mice leads to perinatal lethality associated with suppression of gluconeogenic enzymes (72, 73). Silencing ATF3 expression has been shown to ameliorate glucose intolerance in Zucker diabetic fatty rats and improve fat oxidation (74). Thus, it is possible that decreasing PEPCK1 in HFF rats triggered the expression of ATF3 as part of a cellular stress response. The increase in ATF3 might then have suppressed GCK expression. This association merits further investigation, because it might reveal other pathways by which hepatic insulin resistance can be exacerbated.
In conclusion, the data presented have confirmed that PEPCK1 does not primarily regulate hepatic gluconeogenesis or adipose glyceroneogenesis. However, PEPCK1 does appear to play an important role in coordinating metabolism. PEPCK1 activity likely subtly regulates many metabolic processes, possibly by providing a cataplerotic egress of carbons from TCA cycle intermediates that can be coupled to the anaplerotic entry of carbons into the TCA cycle (53). The loss of PEPCK1 protects against obesity but with a substantial metabolic cost in worsening hepatic steatosis and impaired insulin-mediated hepatic glycogen synthesis via the direct pathway owing to a decrease in hepatic GCK expression. Although PEPCK has been studied for >60 years, our full understanding for the pathways controlled by this enzyme is still evolving.
Acknowledgments
We thank Yanna Kosover, Aida Groszman, and Dr. Heino Velasquez for technical assistance with these studies.
Financial Support : These studies were funded by the US Department of Veterans Affairs (Grant I01 BX000901 to V.T.S.) and the National Institute of Diabetes and Digestive and Kidney Diseases (Grants R24 DK-085638, UL1 RR-0241395, and P30 DK-079310).
Author Contributions: S.A.B., A.K.G., and V.T.S. designed the research. S.A.B., A.A.A., D.F.V., V.P., Y.R., C.C., L.P., N.T., and A.K.G. performed the experiments. S.A.B., A.K.G., and V.T.S. analyzed the data. S.A.B., A.K.G., D.F.V., and V.T.S. wrote the report.
Disclosure Summary: S.B. is an employee and shareholder of Ionis Pharmaceuticals. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- ASO
antisense oligonucleotide
- APE
atom percent excess
- ATF3
activating transcription factor 3
- EGP
endogenous glucose production
- GCK
glucokinase
- G1P
glucose-1-phosphate
- HFF
high-fat fed
- JNK
c-jun N terminal kinase
- PEPCK1
phosphoenolpyruvate carboxykinase 1 (cytosolic form)
- PPARγ
peroxisome proliferator-γ
- RC
regular chow
- WAT
white adipose tissue
References
- 1. Wajngot A, Chandramouli V, Schumann WC, Ekberg K, Jones PK, Efendic S, Landau BR. Quantitative contributions of gluconeogenesis to glucose production during fasting in type 2 diabetes mellitus. Metabolism. 2001;50(1):47–52. [DOI] [PubMed] [Google Scholar]
- 2. Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992;90(4):1323–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49(12):2063–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rizza RA. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes. 2010;59(11):2697–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413(6852):131–138. [DOI] [PubMed] [Google Scholar]
- 6. O’Brien RM, Noisin EL, Suwanichkul A, Yamasaki T, Lucas PC, Wang JC, Powell DR, Granner DK. Hepatic nuclear factor 3- and hormone-regulated expression of the phosphoenolpyruvate carboxykinase and insulin-like growth factor-binding protein 1 genes. Mol Cell Biol. 1995;15(3):1747–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakae J, Kitamura T, Silver DL, Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest. 2001;108(9):1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437(7062):1109–1111. [DOI] [PubMed] [Google Scholar]
- 9. Jurado LA, Song S, Roesler WJ, Park EA. Conserved amino acids within CCAAT enhancer-binding proteins (C/EBP(alpha) and beta) regulate phosphoenolpyruvate carboxykinase (PEPCK) gene expression. J Biol Chem. 2002;277(31):27606–27612. [DOI] [PubMed] [Google Scholar]
- 10. She P, Shiota M, Shelton KD, Chalkley R, Postic C, Magnuson MA. Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Mol Cell Biol. 2000;20(17):6508–6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, Browning JD, Magnuson MA. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 2007;5(4):313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samuel VT, Beddow SA, Iwasaki T, Zhang XM, Chu X, Still CD, Gerhard GS, Shulman GI. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with type 2 diabetes. Proc Natl Acad Sci USA. 2009;106(29):12121–12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reshef L, Hanson RW, Ballard FJ. Glyceride-glycerol synthesis from pyruvate. 1969;244:1994–2001. [PubMed] [Google Scholar]
- 14. Wan Z, Matravadia S, Holloway GP, Wright DC. FAT/CD36 regulates PEPCK expression in adipose tissue. Am J Physiol Cell Physiol. 2013;304(5):C478–C484. [DOI] [PubMed] [Google Scholar]
- 15. Olswang Y, Cohen H, Papo O, Cassuto H, Croniger CM, Hakimi P, Tilghman SM, Hanson RW, Reshef L. A mutation in the peroxisome proliferator-activated receptor gamma-binding site in the gene for the cytosolic form of phosphoenolpyruvate carboxykinase reduces adipose tissue size and fat content in mice. Proc Natl Acad Sci USA. 2002;99(2):625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olswang Y, Blum B, Cassuto H, Cohen H, Biberman Y, Hanson RW, Reshef L. Glucocorticoids repress transcription of phosphoenolpyruvate carboxykinase (GTP) gene in adipocytes by inhibiting its C/EBP-mediated activation. J Biol Chem. 2003;278(15):12929–12936. [DOI] [PubMed] [Google Scholar]
- 17. Franckhauser S, Muñoz S, Pujol A, Casellas A, Riu E, Otaegui P, Su B, Bosch F. Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes. 2002;51(3):624–630. [DOI] [PubMed] [Google Scholar]
- 18. Peng B, Andrews J, Nestorov I, Brennan B, Nicklin P, Rowland M. Tissue distribution and physiologically based pharmacokinetics of antisense phosphorothioate oligonucleotide ISIS 1082 in rat. Antisense Nucleic Acid Drug Dev. 2001;11(1):15–27. [DOI] [PubMed] [Google Scholar]
- 19. Kumashiro N, Yoshimura T, Cantley JL, Majumdar SK, Guebre-Egziabher F, Kursawe R, Vatner DF, Fat I, Kahn M, Erion DM, Zhang XM, Zhang D, Manchem VP, Bhanot S, Gerhard GS, Petersen KF, Cline GW, Samuel VT, Shulman GI. Role of patatin-like phospholipase domain-containing 3 on lipid-induced hepatic steatosis and insulin resistance in rats. Hepatology. 2013;57(5):1763–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279(31):32345–32353. [DOI] [PubMed] [Google Scholar]
- 21. McNulty PH, Sinusas AJ, Shi CQ, Dione D, Young LH, Cline GC, Shulman GI. Glucose metabolism distal to a critical coronary stenosis in a canine model of low-flow myocardial ischemia. J Clin Invest. 1996;98(1):62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berry R, Church CD, Gericke MT, Jeffery E, Colman L, Rodeheffer MS. Imaging of adipose tissue. In: Ormond AM, ed. Methods in Enzymology. Vol 537. New York: Academic Press; 2014:47–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. [computer program]. 2006. [DOI] [PMC free article] [PubMed]
- 24. Kumashiro N, Beddow SA, Vatner DF, Majumdar SK, Cantley JL, Guebre-Egziabher F, Fat I, Guigni B, Jurczak MJ, Birkenfeld AL, Kahn M, Perler BK, Puchowicz MA, Manchem VP, Bhanot S, Still CD, Gerhard GS, Petersen KF, Cline GW, Shulman GI, Samuel VT. Targeting pyruvate carboxylase reduces gluconeogenesis and adiposity and improves insulin resistance. Diabetes. 2013;62(7):2183–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bederman IR, Foy S, Chandramouli V, Alexander JC, Previs SF. Triglyceride synthesis in epididymal adipose tissue: contribution of glucose and non-glucose carbon sources. J Biol Chem. 2009;284(10):6101–6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. RRID:AB_777191.
- 28. RRID:AB_10836185.
- 29. RRID:AB_2283532.
- 30. RRID:AB_2107514.
- 31. RRID:AB_2315049.
- 32. RRID:AB_329827.
- 33. RRID:AB_10167668.
- 34. RRID:AB_2068895.
- 35. RRID:AB_331659.
- 36. RRID:AB_632382.
- 37. Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–336. [DOI] [PubMed] [Google Scholar]
- 38. Vandanmagsar B, Youm Y-H, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim S-Y, Kim HI, Kim T-H, Im SS, Park SK, Lee IK, Kim KS, Ahn YH. SREBP-1c mediates the insulin-dependent hepatic glucokinase expression. J Biol Chem. 2004;279(29):30823–30829. [DOI] [PubMed] [Google Scholar]
- 40. Zhang B, Louet J-F, Dasgupta S, O’Malley B OR24-5: SRC-2 has dual function in hepatic glucose metabolism. Presented at: The Endocrine Society’s 94th Annual Meeting; June 2012, 2012; Houston, TX. [Google Scholar]
- 41. Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, Heidenreich KA, Sajan MP, Farese RV, Stolz DB, Tso P, Koo SH, Montminy M, Unterman TG. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem. 2006;281(15):10105–10117. [DOI] [PubMed] [Google Scholar]
- 42. Ramnanan CJ, Edgerton DS, Rivera N, Irimia-Dominguez J, Farmer B, Neal DW, Lautz M, Donahue EP, Meyer CM, Roach PJ, Cherrington AD. Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo. Diabetes. 2010;59(6):1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nye CK, Hanson RW, Kalhan SC. Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat. J Biol Chem. 2008;283(41):27565–27574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Altomonte J, Richter A, Harbaran S, Suriawinata J, Nakae J, Thung SN, Meseck M, Accili D, Dong H. Inhibition of Foxo1 function is associated with improved fasting glycemia in diabetic mice. Am J Physiol Endocrinol Metab. 2003;285(4):E718–E728. [DOI] [PubMed] [Google Scholar]
- 45. Arizmendi C, Liu S, Croniger C, Poli V, Friedman JE. The transcription factor CCAAT/enhancer-binding protein β regulates gluconeogenesis and phosphoenolpyruvate carboxykinase (GTP) gene transcription during diabetes. J Biol Chem. 1999;274(19):13033–13040. [DOI] [PubMed] [Google Scholar]
- 46. Hanson RW, Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem. 1997;66(1):581–611. [DOI] [PubMed] [Google Scholar]
- 47. Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413(6852):179–183. [DOI] [PubMed] [Google Scholar]
- 48. Valera A, Pujol A, Pelegrin M, Bosch F. Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci USA. 1994;91(19):9151–9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reshef L, Olswang Y, Cassuto H, Blum B, Croniger CM, Kalhan SC, Tilghman SM, Hanson RW. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem. 2003;278(33):30413–30416. [DOI] [PubMed] [Google Scholar]
- 50. Millward CA, Desantis D, Hsieh CW, Heaney JD, Pisano S, Olswang Y, Reshef L, Beidelschies M, Puchowicz M, Croniger CM. Phosphoenolpyruvate carboxykinase (Pck1) helps regulate the triglyceride/fatty acid cycle and development of insulin resistance in mice. J Lipid Res. 2010;51(6):1452–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Devine JH, Eubank DW, Clouthier DE, Tontonoz P, Spiegelman BM, Hammer RE, Beale EG. Adipose expression of the phosphoenolpyruvate carboxykinase promoter requires peroxisome proliferator-activated receptor gamma and 9-cis-retinoic acid receptor binding to an adipocyte-specific enhancer in vivo. J Biol Chem. 1999;274(19):13604–13612. [DOI] [PubMed] [Google Scholar]
- 52. Park EA, Roesler WJ, Liu J, Klemm DJ, Gurney AL, Thatcher JD, Shuman J, Friedman A, Hanson RW. The role of the CCAAT/enhancer-binding protein in the transcriptional regulation of the gene for phosphoenolpyruvate carboxykinase (GTP). Mol Cell Biol. 1990;10(12):6264–6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Burgess SC, Hausler N, Merritt M, Jeffrey FMH, Storey C, Milde A, Koshy S, Lindner J, Magnuson MA, Malloy CR, Sherry AD. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J Biol Chem. 2004;279(47):48941–48949. [DOI] [PubMed] [Google Scholar]
- 54. Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011;14(4):537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Méndez-Lucas A, Duarte JAG, Sunny NE, Satapati S, He T, Fu X, Bermúdez J, Burgess SC, Perales JC. PEPCK-M expression in mouse liver potentiates, not replaces, PEPCK-C mediated gluconeogenesis. J Hepatol. 2013;59(1):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Petersen MC, Madiraju AK, Gassaway BM, Marcel M, Nasiri AR, Butrico G, Marcucci MJ, Zhang D, Abulizi A, Zhang XM, Philbrick W, Hubbard SR, Jurczak MJ, Samuel VT, Rinehart J, Shulman GI. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J Clin Invest. 2016;126(11):4361–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117(3):739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. She P, Burgess SC, Shiota M, Flakoll P, Donahue EP, Malloy CR, Sherry AD, Magnuson MA. Mechanisms by which liver-specific PEPCK knockout mice preserve euglycemia during starvation. Diabetes. 2003;52(7):1649–1654. [DOI] [PubMed] [Google Scholar]
- 59. Tappy L, Dussoix P, Iynedjian P, Henry S, Schneiter P, Zahnd G, Jéquier E, Philippe J. Abnormal regulation of hepatic glucose output in maturity-onset diabetes of the young caused by a specific mutation of the glucokinase gene. Diabetes. 1997;46(2):204–208. [DOI] [PubMed] [Google Scholar]
- 60. Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274(1):305–315. [DOI] [PubMed] [Google Scholar]
- 61. Coate KC, Kraft G, Moore MC, Smith MS, Ramnanan C, Irimia JM, Roach PJ, Farmer B, Neal DW, Williams P, Cherrington AD. Hepatic glucose uptake and disposition during short-term high-fat vs. high-fructose feeding. Am J Physiol Endocrinol Metab. 2014;307:E151–E160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nuttall FQ, Gilboe DP, Gannon MC, Niewoehner CB, Tan AW. Regulation of glycogen synthesis in the liver. Am J Med. 1988; 85(5, 5A)77–85. [DOI] [PubMed] [Google Scholar]
- 63. von Wilamowitz-Moellendorff A, Hunter RW, García-Rocha M, Kang L, López-Soldado I, Lantier L, Patel K, Peggie MW, Martínez-Pons C, Voss M, Calbó J, Cohen PT, Wasserman DH, Guinovart JJ, Sakamoto K. Glucose-6-phosphate-mediated activation of liver glycogen synthase plays a key role in hepatic glycogen synthesis. Diabetes. 2013;62(12):4070–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Aiston S, Trinh KY, Lange AJ, Newgard CB, Agius L. Glucose-6-phosphatase overexpression lowers glucose 6-phosphate and inhibits glycogen synthesis and glycolysis in hepatocytes without affecting glucokinase translocation: evidence against feedback inhibition of glucokinase. J Biol Chem. 1999;274(35):24559–24566. [DOI] [PubMed] [Google Scholar]
- 65. Torres TP, Catlin RL, Chan R, Fujimoto Y, Sasaki N, Printz RL, Newgard CB, Shiota M. Restoration of hepatic glucokinase expression corrects hepatic glucose flux and normalizes plasma glucose in Zucker diabetic fatty rats. Diabetes. 2009;58(1):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Agius L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem J. 2008;414(1):1–18. [DOI] [PubMed] [Google Scholar]
- 67. Sun Y, Liu S, Ferguson S, Wang L, Klepcyk P, Yun JS, Friedman JE. Phosphoenolpyruvate carboxykinase overexpression selectively attenuates insulin signaling and hepatic insulin sensitivity in transgenic mice. J Biol Chem. 2002;277(26):23301–23307. [DOI] [PubMed] [Google Scholar]
- 68. Kim JY, Hwang JY, Lee DY, Song EH, Park KJ, Kim GH, Jeong EA, Lee YJ, Go MJ, Kim DJ, Lee SS, Kim BJ, Song J, Roh GS, Gao B, Kim WH. Chronic ethanol consumption inhibits glucokinase transcriptional activity by Atf3 and triggers metabolic syndrome in vivo. J Biol Chem. 2014;289(39):27065–27079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tsai WW, Matsumura S, Liu W, Phillips NG, Sonntag T, Hao E, Lee S, Hai T, Montminy M. ATF3 mediates inhibitory effects of ethanol on hepatic gluconeogenesis. Proc Natl Acad Sci USA. 2015;112(9):2699–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen BP, Wolfgang CD, Hai T. Analysis of ATF3, a transcription factor induced by physiological stresses and modulated by gadd153/Chop10. Mol Cell Biol. 1996;16(3):1157–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pan YX, Chen H, Thiaville MM, Kilberg MS. Activation of the ATF3 gene through a coordinated amino acid-sensing response programme that controls transcriptional regulation of responsive genes following amino acid limitation. Biochem J. 2007;401(1):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Allen-Jennings AE, Hartman MG, Kociba GJ, Hai T. The roles of ATF3 in glucose homeostasis: a transgenic mouse model with liver dysfunction and defects in endocrine pancreas. J Biol Chem. 2001;276(31):29507–29514. [DOI] [PubMed] [Google Scholar]
- 73. Allen-Jennings AE, Hartman MG, Kociba GJ, Hai T. The roles of ATF3 in liver dysfunction and the regulation of phosphoenolpyruvate carboxykinase gene expression. J Biol Chem. 2002;277(22):20020–20025. [DOI] [PubMed] [Google Scholar]
- 74. Kim JY, Park KJ, Hwang JY, Kim GH, Lee D, Lee YJ, Song EH, Yoo MG, Kim BJ, Suh YH, Roh GS, Gao B, Kim W, Kim WH. Activating transcription factor 3 is a target molecule linking hepatic steatosis to impaired glucose homeostasis. J Hepatol. 2017;67(2):349–359. [DOI] [PubMed] [Google Scholar]