Abstract
Background
There are 6 species of venomous snakes in Taiwan. Two of them, Deinagkistrodon acutus (D. acutus) and Daboia siamensis (D. siamensis), can cause significant coagulopathy. However, a significant proportion of patients with snakebites cannot identify the correct snake species after envenomation, which hampers the application of antivenom. Hence, the differential diagnosis between the two snakebites by clinical presentations is important. This study aims to compare their clinical and laboratory features for the purpose of differential diagnosis between the two snakebites.
Methods
We retrospectively reviewed the medical records of patients who arrived at the emergency department due to D. acutus or D. siamensis envenomation, between 2003 and 2016, in one medical center in eastern Taiwan. Since these snakebites are rare, we also included 3 cases reported from another hospital in central Taiwan.
Results
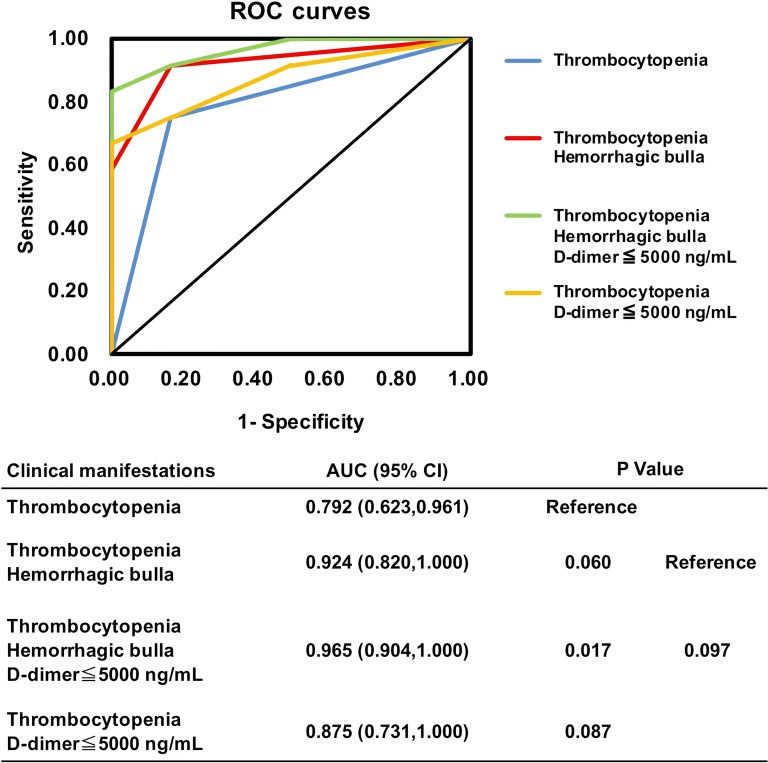
In total, 15 patients bitten by D. acutus and 12 patients by D. siamensis were analyzed. Hemorrhagic bulla formation and the need for surgical intervention only presented for D. acutus envenomation cases (Both 53.3% vs. 0.0%, P = 0.003). As to laboratory features, lower platelet counts (20.0 × 103/μL [interquartile range, 14–66 × 103/μL] vs. 149.0 × 103/μL [102.3–274.3 × 103/μL], P = 0.001), lower D-dimer level (1423.4 μg/L [713.4–4212.3 μg/L] vs. 12,500.0 μg/L [2351.4–200,000 μg/L], P = 0.008), higher proportion of patients with moderate-to-severe thrombocytopenia (platelet count < 100 × 103/μL) (80% vs. 16.7%, odds ratio (OR) = 20.0, 95% CI, 2.77–144.31; P = 0.002), and lower proportion of patients with extremely high D-dimer (> 5000 ng/mL) (16.7% vs. 66.7%, adjusted OR = 0.1 (95% CI, 0.01–0.69; P = 0.036) were found among cases of D. acutus envenomation compared to D. siamensis envenomation. The combination of hemorrhagic bulla, thrombocytopenia, and a lack of extremely high D-dimer had good discriminatory power (area under the curve (AUC) = 0.965; 95% CI, 0.904–1.00) for distinguishing D. acutus from D. siamensis envenomation.
Conclusions
The presentation of moderate to severe thrombocytopenia (platelet count < 100 × 103/μL) and hemorrhagic bulla formation may indicate D. acutus envenomation. However, the envenomed patient with extremely high D-dimer levels may indicate a D. siamensis envenomation. These findings may help diagnose and select the right antivenom in patients with unknown snakebites who present significant coagulopathy.
Keywords: Coagulopathy, Deinagkistrodon acutus, Daboia siamensis, Snakebite, Thrombocytopenia
Background
Snake envenomation is a serious and important public health issue worldwide, including in Taiwan [1, 2]. Taiwan is a natural habitat for more than 40 snake species, including 6 types of venomous snakes with clinical importance, namely: Protobothrops mucrosquamatus (Taiwan habu), Trimeresurus stejnegeri (Taiwan bamboo viper), Deinagkistrodon acutus (D. acutus), Daboia siamensis (D. siamensis), Bungarus multicinctus (banded krait), and Naja atra (Taiwan cobra) [3, 4]. Among the abovementioned venomous species, the first 4 belong to the Viperidae family, which possess hemotoxic venom that can cause varying degrees of bleeding tendency in humans. In general, most patients with Taiwan habu or Taiwan bamboo viper envenomation present with local hemotoxic effects; however, the manifestation of systemic coagulopathy is rare and mild [5]. A previous study by Chen et al. had reported only 6% of Taiwan habu and 0% of Taiwan bamboo viper envenomation presenting coagulopathy and less than 1% of Taiwan habu presenting severe coagulopathy [6]. However, in D. acutus and D. siamensis envenomation, systemic coagulopathies including thrombocytopenia, prolonged prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen consumption and D-dimer production are common [7–9].
D. acutus, also called the hundred pacer, is the largest snake of the crotalinae subfamily in Taiwan [4]. This species can inject a large amount of venom at each envenomation, with the venom containing several hemotoxins including pro-coagulation proteins, such as thrombin-like enzyme (TLE), as well as anticoagulation proteins, such as factor IX/X inhibitor and platelet aggregation inhibitor [10–13]. D. siamensis, which belongs to the viperinae subfamily, has venom composed of mixed hemotoxins, including pro-coagulation proteins, such as factor V, IX, and X activator, protease inhibitors, and phospholipase A2 [13–15]. The main habitats of D. acutus and D. siamensis are very similar and both are distributed from the eastern to southern regions of Taiwan [3]. Victims of these two snakebite types are rare and only account for 2.4% (D. acutus) and 2.9% (D. siamensis) of the total venomous snakebites in eastern Taiwan [16]. In addition to Taiwan, these two snakes are also concurrently distributed in other Southeastern Asian countries, such as Laos and Vietnam and southern China [17].
Currently, the definitive treatment for these types of snakebites is horse-derived antivenom, specific for D. acutus and D. siamensis. However, previous studies have shown that about 30% of patients with venomous snakebites were unable to identify the correct snake species [3, 6]. This leads to difficulty in administering the correct antivenom, especially in those patients with significant coagulopathy. Although the concurrent use of two specific antivenoms may be practiced clinically, the high cost in generating antivenom, low inventory, and its side effects, such as serum sickness, should also be considered [18–21]. The correct clinical differential diagnosis between these two types of snakebites is paramount.
Unlike four other types of venomous snakebites were well investigated in Taiwan, there is still a lack of data to distinguish clinical features between D. acutus and D. siamensis envenomation. The aim of this study was to investigate the clinical and laboratory differences between D. acutus and D. siamensis envenomation, which can help emergency physicians make correct clinical diagnosis, especially in those patients with systemic coagulopathy but unknown snake envenomation.
Methods
Study population
We conducted a retrospective study of patient data on D. acutus and D. siamensis envenomation, who were admitted to the Hualien Tzu Chi Medical Center, the only medical center in eastern Taiwan, between 2003 and 2016. The patient data collection methodology was previously described [5, 16]. Briefly, patient medical records were collected for those admitted with snakebites, using the computerized chart system and International Classification of Diseases, 9th Revision, Clinical Modification codes 989.5, E905.0, E905.9, E906.2, and E906.5. For D. acutus and D. siamensis envenomation, classification of the snake species was based on patient identification from a photograph taken by cell phone, or bringing the snake to the emergency department (ED). We only included patients with venomous snakebites who received specific antivenoms for D. acutus or D. siamensis and excluded those without receiving antivenom for suspicion of dry bite. Patients who could not confirm the correct snake species and who received more than one type of antivenom were also excluded. Three authors independently reviewed the clinical records of the included patients to confirm that each patient had a relevant history, typical manifestation, and consistent antivenom administration.
Due to the rare incidence of D. acutus and D. siamensis envenomation, we also searched case reports of D. acutus or D. siamensis envenomation in Taiwan registered during the most recent 10 years in the literature. However, only Cheng et al. had published 3 cases of D. acutus envenomation from Taichung Veterans General Hospital in 2017 [7]. After contacting the author, we obtained the de-identifiable patients’ original data and included the 3 patients in the study.
Demographic data and definition of variables
Patients’ age, gender, site of snakebite, comorbidities, envenomation details, clinical presentation, laboratory results, treatment, initial antivenom therapy timing, and total antivenom dose were analyzed. The laboratory analysis included initial patient data obtained upon arriving at the ED, including hematology, biochemistry, and coagulation profiles. We defined leukocytosis as a white blood cell count (WBC) of > 11.0 × 103/μL; moderate-to-severe thrombocytopenia as a platelet count of < 100 × 103/μL [22]; non-coagulation in prothrombin time (PT) and activated partial thromboplastin time (aPTT) as either PT or aPTT beyond laboratory upper limits; fibrinogen consumption as fibrinogen levels of < 1.0 g/L; extremely high D-dimer levels > 5000 ng/mL [23]; acute renal impairment as creatinine levels > 1.4 mg/dL [8]; and venom-induced consumption coagulopathy as a disseminated intravascular coagulation (DIC) score ≥ 5 points [24, 25]. All the reference standards had been checked for consistency during the study period. If the laboratory value was beyond the laboratory upper or lower limit, it was recorded as the upper or lower limit, respectively. If the patient’s initial laboratory tests were not performed in the ED, this was recorded as a missing value in the database. All patient records and information were de-identified and anonymized before the analysis. The institutional review board of the Hualien Tzu Chi Medical Center approved the study protocol (IRB106–128-B).
Statistical analyses
The normality of the quantitative variables’ distribution was tested by the Kolmogorov-Smirnov test (P > 0.10). Comparison of continuous variables between the two types of snakebites was conducted using the Mann-Whitney U-test or Student’s t test, depending on variable distribution. The chi-square test or Fisher’s exact test was applied for categorical variables as appropriate. Normally distributed data are expressed as mean ± standard deviation (SD), while non-parametric data were expressed as the median [25th–75th interquartile range]. All statistical tests were two-tailed while a P value of < 0.05 was considered statistically significant. Odds ratios (ORs) were calculated using logistic regression analysis. In addition, receiver operating characteristic (ROC) curves for different combination of the significant variables were calculated to determine which clinical manifestations can distinguish these two types of snakebite. All data were analyzed through the software SPSS, version 12.0 (IBM Corp.; Armonk, NY, USA).
Results
Respective totals of 15 and 12 patients with D. acutus and D. siamensis envenomation were analyzed. Among them, 2 patients of each envenomation type identified the species by bringing the snake to the ED; the remaining patients identified the species by a photograph taken on a cell phone or by examining the standard pictures of Taiwanese venomous snakes provided by the Centers for Disease Control, R.O.C. (Taiwan).
Demographic, clinical, and laboratory features
Comparisons of patient demographic, clinical, and laboratory characteristics are listed in Tables 1 and 2. As to clinical features, there was no significant difference between the two types of snakebite in age, gender, bite location or elapsed time until hospital arrival (Table 1). Eight of 15 patients (53.3%) with D. acutus envenomation received surgical intervention (debridement, fasciotomy, or skin graft) due to suspicion of compartment syndrome by clinical symptoms (4 patients), or tissue infection or necrosis according to local findings (4 patients), but no D. siamensis envenomation patient underwent surgery (P = 0.003; Table 1). Among the 8 surgical patients with D. acutus envenomation, mixed types of bacteria were detected in the surgical wounds of 5 patients (62.5%). Morganella morganii and enterococcus faecalis were the leading isolated pathogens (Table 3). In relation to local signs, hemorrhagic bulla formation was presented by more than half of the D. acutus envenomation patients, but by none of those with D. siamensis envenomation (53.3% vs. 0.0%, P = 0.003; Table 1).
Table 1.
Comparison of clinical and laboratory characteristics between patients with Deinagkistrodon acutus and Daboia siamensis envenomation
| Deinagkistrodon acutus (n = 15) | Daboia siamensis (n = 12) | P value | |
|---|---|---|---|
| Age | 47.7 ± 13.81 | 55.0 ± 10.06 | 0.140 |
| Male gender | 14 (93.3) | 9 (75.0) | 0.294 |
| Season | |||
| Summer & fall | 14 (93.3) | 6 (50.0) | 0.024 |
| Winter and spring | 1 (6.7) | 6 (50.0) | |
| Bite area | |||
| Upper limbs | 10 (66.7) | 10 (83.3) | 0.408 |
| Lower limbs | 5 (33.3) | 2 (16.7) | |
| Operation | 8 (53.3) | 0 (0) | 0.003 |
| Local signs | |||
| Swelling | 15 (100) | 12 (100) | 1.000 |
| Ecchymosis | 9 (60) | 4 (33.3) | 0.252 |
| Hemorrhagic bulla | 8 (53.3) | 0 (0) | 0.003 |
| Time to arrive hospital (hr) | 2.0 (1–7.5) | 7.5 (1–12) | 0.373 |
| Total dose of antivenom (vial) | 6.0 (4–10) | 4.0 (4–6) | 0.067 |
| Duration of hospitalization (day) | 5.0 (2–27) | 3.0 (2–7) | 0.183 |
| ED laboratory data: | |||
| WBC (× 103/μL) | 10.3 ± 2.98 | 12.1 ± 3.90 | 0.187 |
| Hb (g/dL) | 14.1 ± 2.77 | 14.0 ± 1.39 | 0.889 |
| PLT (× 103/μL) | 20.0 (14–66) | 149.0 (102.3–274.3) | 0.001 |
| PT (sec) | 100.0 (100–100) | 75.0 (11–100) | 0.025 |
| aPTT (sec) | 150.0 (25–191) (n = 14) | 29.0 (25–160) | 0.118 |
| Fibrinogen (g/L) | 0.5 (0.25–0.93) (n = 13) | 0.5 (0.25–0.95) | 0.689 |
| D-dimer (μg/L) | 1423.4 (713.4–4212.3) (n = 12) | 12,500.0 (2351.4–200,000.0) | 0.008 |
| DIC score | 7.0 (5–7) | 5.5 (4–6) | 0.041 |
| AST (IU/L) | 29.0 (22–35) | 36.0 (24–101) (n = 11) | 0.330 |
| ALT (IU/L) | 22.0 (18.0–44.5) (n = 13) | 25.0 (20–38) | 0.852 |
| BUN (mg/dL) | 14.0 (10.8–19.5) (n = 14) | 17.0 (12.5–30.8) | 0.193 |
| CRE (mg/dL) | 0.9 (0.78–1.33) (n = 14) | 1.2 (0.93–2.38) | 0.118 |
| CK (IU/L) | 242.0 (213.5–748.0) (n = 13) | 487.0 (257–1444.5) (n = 9) | 0.186 |
Values shown are n (%) or mean ± SD or median (interquartile range)
ALT alanine aminotransferase, aPTT activated partial thromboplastin time, AST aspartate aminotransferase, BUN blood urea nitrogen, CK creatine kinase, CRE creatinine. DIC disseminated intravascular coagulation, Hb hemoglobin, PLT platelet count, PT prothrombin time, WBC white blood cell count
Table 2.
Laboratory characteristics of patients with Deinagkistrodon acutus and Daboia siamensis envenomation
| Deinagkistrodon acutus (n = 15) | Daboia siamensis (n = 12) | P value | OR (95% CI) | |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Leukocytosis (WBC > 11.0 × 103/μL) | 8 (53.3) | 8 (66.7) | 0.696 | 0.57 (0.12–2.75) |
| Moderate-to-severe thrombocytopenia (PLT < 100 × 103 /μL) | 12 (80.0) | 2 (16.7) | 0.002 | 20.00 (2.77–144.31) |
| Noncoagulation in aPTT | 11 (73.3) | 4 (33.3) | 0.057 | 5.50 (1.05–28.88) |
| Noncoagulation in PT | 13 (86.7) | 7 (58.3) | 0.185 | 4.64 (0.71–30.42) |
| Fibrinogen consumption (< 1 g/L) | 10 (76.9) | 9 (75.0) | 1.000 | 1.11 (0.18–6.97) |
| Extremely high D-dimer (> 5000 ng/mL) | 2 (16.7) | 8 (66.7) | 0.036 | 0.10 (0.01–0.69) |
| DIC score≧5 | 13 (86.7) | 7 (58.3) | 0.185 | 4.64 (0.71–30.42) |
| Acute renal impairment (CRE > 1.4 mg/dL) | 3 (20.0) | 5 (41.7) | 0.398 | 0.35 (0.06–1.93) |
Values shown are n (%). N/A not applicable
aPTT activated partial thromboplastin time, AST aspartate aminotransferase, BUN blood urea nitrogen, CI confidence interval, CK creatine kinase, CRE creatinine, DIC disseminated intravascular coagulation, OR odds ratio, PLT platelet count, PT prothrombin time, WBC white blood cell count
Table 3.
Bacterial isolates identified from snakebite wounds of patients with Deinagkistrodon acutus envenomation who underwent surgery
| Pathogen | n |
|---|---|
| Aerobic gram-positive bacteria | |
| Enterococcus faecalis | 3 |
| Staphylococcus aureus | 2 |
| Aerobic gram-negative bacteria | |
| Morganella morganii | 3 |
| Pseudomonas aeruginosa | 2 |
| Citrobacter freundii | 1 |
| Anaerobic bacteria | |
| Bacteroides fragilis | 2 |
More than one type of bacteria were isolated from snakebite wound in 3 patients who underwent surgery due to Deinagkistrodon acutus envenomation
As to laboratory findings, both D. acutus and D. siamensis envenomation showed a certain degree of coagulopathy including thrombocytopenia, PT and aPTT prolongation, fibrinogen consumption, and elevated D-dimer levels (Table 1). However, significantly lower platelet (P = 0.001) and D-dimer levels (P = 0.008), but higher PT (P = 0.025) and DIC scores (P = 0.036) were found in patients with D. acutus envenomation (Table 1). A significantly higher proportion of patients with D. acutus envenomation presented with moderate-to-severe thrombocytopenia (P = 0.002; OR = 20.0, 95% confidence interval [CI], 2.77–144.31) compared to patients with D. siamensis envenomation. However, a significantly lower proportion of patients with D. acutus envenomation presented with extremely high D-dimer levels compared to patients with D. siamensis envenomation (P = 0.036; OR = 0.1, 95% CI, 0.01–0.69) (Table 2). The two groups did not differ significantly in WBCs, hemoglobin, fibrinogen, liver or renal function tests, or in creatine kinase levels (Tables 1 and 2).
Features distinguishing between Deinagkistrodon acutus and Daboia siamensis envenomation
We next measured and compared the discriminatory power of different combinations of clinical and laboratory features in distinguishing between D. acutus and D. siamensis envenomation by analyzing ROC curves (Fig. 1). The results showed that the combination of thrombocytopenia, hemorrhagic bulla formation, and D-dimer levels ≤5000 ng/mL had the best discriminatory power. The AUC of this combined model was significantly higher than thrombocytopenia-alone (AUC = 0.965 [95% CI, 0.904–1.00] vs. 0.792 [95% CI, 0.623–0.961], P = 0.017). Moreover, the presentation of both thrombocytopenia and hemorrhagic bulla was also a more apt predictor for D. acutus envenomation (AUC = 0.924 [95% CI, 0.820–1.00]; P = 0.06, when compared to thrombocytopenia-alone; P = 0.097, when compared to the combined model of thrombocytopenia, hemorrhagic bulla and low D-dimer levels) (Fig. 1).
Fig. 1.
Receiver-operating characteristic (ROC) curves for the different combinations of clinical manifestations in distinguishing D. acutus envenomation from D. siamensis envenomation. Thrombocytopenia means platelet count < 100 × 103/uL
Discussion
In this retrospective study, we discovered that the presentation of hemorrhagic bulla formation and the type of surgical intervention are clinical features indicating D. acutus envenomation. The laboratory findings of moderate-to-severe thrombocytopenia may indicate D. acutus envenomation while cases of patients with extremely high D-dimer levels most likely resulted from D. siamensis envenomation. Combining the clinical manifestations of thrombocytopenia, hemorrhagic bulla formation and D-dimer levels can help us to distinguish between these two snakebite types.
Different from the other two venomous snakes of the Viperidae family, Taiwan habu and Taiwan bamboo viper, severe systemic coagulopathy with a DIC score ≧ 5 can be found in most instances of D. acutus (86.7%) and D. siamensis (58.3%) envenomation (Table 2). This proportion is far higher than that of the Taiwan habu (< 1%) and Taiwan bamboo viper (0%) envenomation in previous observation [6]. Moreover, noncoagulation in PT and aPTT, and severe fibrinogen consumption was also found in a significant proportion of patients with D. acutus and D. siamensis envenomation (Table 2). These findings were also uncommon in Taiwan habu or Taiwan bamboo viper envenomation [5, 6]. Thus, the above manifestation of coagulopathy may be a suitable indicator to discriminate D. acutus and D. siamensis from Taiwan habu and Taiwan bamboo viper envenomation.
Among the significant differences in clinical manifestation between D. acutus and D. siamensis envenomation found in our study are abnormalities in the coagulation profiles. Both D. acutus and D. siamensis venoms are composed of several hemotoxins with varying degrees of procoagulant and anticoagulant effects, which act on different steps of the clotting pathway and consume different clotting factors.
D. acutus venom clinically presents as anticoagulant toxins, platelet aggregation inhibitors, hemorrhagins, and TLEs [10, 12, 26, 27]. The anticoagulant toxins of D. acutus directly inhibit coagulation factors V and IX/X, prothrombin and tissue factors, resulting in immediate and marked prolongation of the coagulation time after envenomation [10, 28, 29]. TLEs can break down fibrinogen, but unlike real thrombin, which can activate factor XIII to perform fibrin cross-linking and stabilize fibrin clots, TLE does not form fibrin clots and produce fibrin degradation products (D-dimer) [30–33]. However, in D. siamensis venom the main components are phospholipase A2 and pro-coagulation proteins, which include factor V, IX, and X activators, and are very potent [14, 15, 30–32, 34]. The activators can persistently activate the coagulation pathway, and, finally, consume massive downstream coagulation factors, resulting in clotting factor deficiency, hypofibrinogenemia, fibrinolysis, and greatly elevated D-dimer levels [8, 35]. Because the main etiology leading to coagulopathy in D. siamensis envenomation is consumptive coagulopathy, the prolongation of coagulation time is time-dependent; severe prolongation of PT and aPTT may occur subsequently after consuming the clotting factors. The abovementioned mechanism may explain the extremely high D-dimer levels in our D. siamensis envenomation patients, but relatively lower D-dimer levels in D. acutus envenomation, as well as the finding that more D. acutus envenomation patients presented with non-coagulation in PT and aPTT.
Another difference found between D. acutus and D. siamensis envenomation is local wound complication. More than half of D. acutus envenomation patients developed extensive hemorrhagic bulla formations, requiring surgical intervention due to suspected compartment syndrome or tissue infection and necrosis. However, none of the D. siamensis envenomation patients presented any significant local tissue injury and none required surgical intervention. We further found that D. acutus envenomation patients who had undergone surgery showed evidence of wound infection. The isolated bacteria from the surgical wounds are usually a mixed spectrum, including aerobic Gram-positive and -negative, and anaerobic bacteria. This finding indicates that wound infection may partly contribute to complication in cases of D. acutus envenomations. The use of broad-spectrum antibiotics to cover mixed bacterial infection may be necessary in D. acutus envenomation. In addition, being the most venomous snake of the crotalinae subfamily in Taiwan, D. acutus can inject 3, 5, and 15 times the amount of venom per envenomation than that of Protobothrops mucrosquamatus, D. siamensis and Trimeresurus stejnegeri, respectively [13]. Local hemotoxic venom can affect coagulation, destroy endothelial cells and tissue, increase vascular permeability, and cause extensive vascular damage, which may explain the extensive wound complications observed in D. acutus envenomations [36]. Conversely, the venom amount in each injection of D. siamensis bite was much less than that of D. acutus [13]. Although, similar toxic proteins exist in the venom of D. siamensis, relatively weaker local tissue effects were associated with D. siamensis envenomation in this study; similar findings in relation to D. siamensis in Taiwan have been reported [8, 9].
In our study, moderate-to-severe thrombocytopenia was the most significant feature that could discriminate between D. acutus and D. siamensis envenomation. Both envenomations are likely to develop thrombocytopenia, but D. acutus causes more severe thrombocytopenia. Previous studies have demonstrated that D. acutus venom contains components that target platelets [25, 37–40]. However, the mechanism of severe thrombocytopenia in D. acutus envenomation has scarcely been investigated in in vitro. Nonetheless, previous studies in rat models and humans both demonstrate this result [7, 15]. In human cases of D. acutus envenomation, severe thrombocytopenia was found in patients within 4 h after a snakebite [7]. In rat experiments, severe thrombocytopenia was found within 10 min after injecting agkicetin-C, a potent antagonist of platelet glycoprotein Ib-IX-V, purified from D. acutus venom [39]. In addition to the possible direct venom effects that result in platelet consumption, sequestration of platelets by extensive tissue and vascular injury, and the severe wound infections found in D. acutus envenomation may all contribute to severe thrombocytopenia in humans with D. acutus envenomation.
However, thrombocytopenia in D. siamensis is thought to be related to thrombin-induced platelet aggregation and activation [34]. The pro-coagulation proteins found in D. siamensis venom produce massive fibrin clots and consume platelets to form systemic microthrombi [9, 41]. Although severe thrombocytopenia may also occur in D. siamensis envenomation, it usually takes more than 12 h after the snakebite for thrombocytopenia to occur in these patients [8].
In order to apply our findings to the clinical practice of EDs, we focused our analysis on simply defined abnormalities of coagulation profiles, such as extremely high D-dimer levels and noncoagulation in PT or aPTT, but we did not measure the optimal cutoff point via the ROC curve from individual laboratory data. Moreover, when considering that a single clinical feature may not be acceptable to accurately differentiate between these two types of snakebites, we combined different clinical and laboratory features to optimize the ROC curve. The combined model using thrombocytopenia, hemorrhagic bulla formation, and a lack of extremely high D-dimer levels had the best discriminatory power in distinguishing D. acutus from D. siamensis envenomation (AUC = 0.965 [95% CI, 0.904–1.000]). Combining the two features of thrombocytopenia and hemorrhagic bulla formation is also an acceptable diagnostic marker in distinguishing these two types of snakebites (AUC = 0.924 [95% CI, 0.820–1.000]).
There are several limitations in our study. First, this is a 13-year retrospective study and all patient data were collected from patient charts or electronic medical records. Non-uniform descriptions of signs or symptoms recorded by different physicians may influence and induce some bias. Second, although this is the largest native study regarding D. acutus and D. siamensis envenomation over the last 20 years in Taiwan, the sample size was still small due to the rarity of both types of snakebites. Third, due to the lack of a definitive guideline to manage these two snakebites in Taiwan, different treatment strategies in clinical practice might influence clinical outcomes and result in the missing values of some laboratory tests. Fourth, although we tried our best to discriminate snake species according to patient’s identification, clinical symptoms or defined criteria, there is still probable misidentification owing to non-visible real snake in ED. A prospective study conducted to validate our findings should be considered. In addition, the time-dependent change in coagulation profiles, the quantification of specific clotting factors, such as factor X, and development of the severity grading system should be considered in further studies.
Conclusions
Among the 6 most common venomous snakes in Taiwan, life-threatening coagulopathy is frequently attributed to either D. acutus or D. siamensis envenomation. In the clinical differential diagnosis between these two types of snakebites, the presence of hemorrhagic bulla and moderate-to-severe thrombocytopenia are clinical features uniquely associated with D. acutus envenomation. However, extremely high D-dimer levels are indicative of D. siamensis envenomation.
Acknowledgements
We thank Miss Wan-Ting Huang and the Department of Medical Research of Ditmanson Medical Foundation, Chia-Yi Christian Hospital for assistance with the statistical analysis.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- APTT
Activated partial thromboplastin time
- AUC
Area under curve
- D. acutus
Deinagkistrodon acutus
- D. siamensis
Daboia siamensis
- DIC
Disseminated intravascular coagulation
- ED
Emergency department
- ORs
Odds ratios
- PT
Prothrombin time (PT)
- ROC
Receiver operating characteristic
- TLE
Thrombin-like enzyme
- WBC
White blood cell count
Authors’ contributions
The first two authors, HUS and SWH interpreted the clinical findings and drafted the manuscript. The third authors, YCM provide detailed data collection from Taichung Veteran General Hospital and provided professional opinion. The fourth and sixth authors, MWL and PFL provided data collection from Hualien Tzu Chi Medical center and professional opinion. The fifth author KHL provide professional opinion and help revised the manuscript. The correspondent author MJT designed this study, interpreted the clinical findings and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The institutional review board of the Hualien Tzu Chi Medical Center approved the study protocol (IRB106–128-B).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hung-Yuan Su, Email: hys927@hotmail.com.
Shih-Wei Huang, Email: tony11809@hotmail.com.
Yan-Chiao Mao, Email: doc1385e@gmail.com.
Ming-Wen Liu, Email: arice0215@gmial.com.
Kuo-Hsin Lee, Email: peter1005@gmail.com.
Pei-Fang Lai, Email: lpf2826@gmail.com.
Ming-Jen Tsai, Phone: 886-5-2765041 1984, Email: tshi33@gmail.com.
References
- 1.Chippaux JP, Akaffou MH, Allali BK, Dosso M, Massougbodji A, Barraviera B. The 6(th) international conference on envenomation by snakebites and scorpion stings in Africa: a crucial step for the management of envenomation. J Venom Anim Toxins incl Trop Dis. 2016;22:11. doi: 10.1186/s40409-016-0062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chippaux JP. Snakebite envenomation turns again into a neglected tropical disease! J Venom Anim Toxins incl Trop Dis. 2017;23:38. doi: 10.1186/s40409-017-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung DZ. Taiwan's venomous snakebite: epidemiological, evolution and geographic differences. Trans R Soc Trop Med Hyg. 2004;98(2):96–101. doi: 10.1016/S0035-9203(03)00013-0. [DOI] [PubMed] [Google Scholar]
- 4.Mao YC, Hung DZ. Epidemiology of snake envenomation in Taiwan. In: Gopalakrishnakone P, Faiz A, Fernando R, Gnanathasan CA, Habib AG, Yang CC, editors. Clinical toxinology in Asia Pacific and Africa. Dordrecht: Springer; 2015. pp. 3–22. [Google Scholar]
- 5.Su HY, Li YH, Tang CN, Su CI, Tsai MJ. Can surgery in patient with Protobothrops mucrosquamatus envenomation be predicted in emergency department? Hong Kong J Emerg Med. 2016;23(4):210–219. doi: 10.1177/102490791602300402. [DOI] [Google Scholar]
- 6.Chen YW, Chen MH, Chen YC, Hung DZ, Chen CK, Yen DH, et al. Differences in clinical profiles of patients with Protobothrops mucrosquamatus and Viridovipera stejnegeri envenoming in Taiwan. Am J Trop Med Hyg. 2009;80(1):28–32. doi: 10.4269/ajtmh.2009.80.28. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CL, Mao YC, Liu PY, Chiang LC, Liao SC, Yang CC. Deinagkistrodon acutus envenomation: a report of three cases. J Venom Anim Toxins incl Trop Dis. 2017;23:20. doi: 10.1186/s40409-017-0111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung DZ, Wu ML, Deng JF, Lin-Shiau SY. Russell's viper snakebite in Taiwan: differences from other Asian countries. Toxicon. 2002;40(9):1291–1298. doi: 10.1016/S0041-0101(02)00137-X. [DOI] [PubMed] [Google Scholar]
- 9.Hung DZ, Yu YJ, Hsu CL, Lin TJ. Antivenom treatment and renal dysfunction in Russell's viper snakebite in Taiwan: a case series. Trans R Soc Trop Med Hyg. 2006;100(5):489–494. doi: 10.1016/j.trstmh.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Ouyang C, Teng CM. In vivo effects of the purified thrombin-like and anticoagulant principles of Agkistrodon acutus (hundred pace snake) venom. Toxicon. 1978;16(6):583–593. doi: 10.1016/0041-0101(78)90186-1. [DOI] [PubMed] [Google Scholar]
- 11.Cox AC. Coagulation factor X inhibitor from hundred-pace snake (Deinagkistrodon acutus) venom. Toxicon. 1993;31(11):1445–1457. doi: 10.1016/0041-0101(93)90210-A. [DOI] [PubMed] [Google Scholar]
- 12.Xu X, Wang C, Liu J, Lu Z. Purification and characterization of hemorrhagic components from Agkistrodon acutus (hundred pace snake) venom. Toxicon. 1981;19(5):633–644. doi: 10.1016/0041-0101(81)90101-X. [DOI] [PubMed] [Google Scholar]
- 13.Liau MY, Huang RJ. Toxoids and antivenoms of venomous snakes in Taiwan. J Toxicol. 1997;16(3):163–175. [Google Scholar]
- 14.Schiffman S, Theodor I, Rapaport SI. Separation from Russell's viper venom of one fraction reacting with factor X and another reacting with factor V. Biochemistry. 1969;8(4):1397–1405. doi: 10.1021/bi00832a014. [DOI] [PubMed] [Google Scholar]
- 15.Kasturi S, Gowda TV. Purification and characterization of a major phospholipase A2 from Russell's viper (Vipera russelli) venom. Toxicon. 1989;27(2):229–237. doi: 10.1016/0041-0101(89)90136-0. [DOI] [PubMed] [Google Scholar]
- 16.Su HY, Wang MJ, Li YH, Tang CN, Tsai MJ. Can surgical need in patients with Naja atra (Taiwan or Chinese cobra) envenomation be predicted in the emergency department? Hong Kong Med J. 2016;22(5):435–444. doi: 10.12809/hkmj154739. [DOI] [PubMed] [Google Scholar]
- 17.McDiarmid RW, Campbell JA. Toure’ TA. Snake species of the world: a taxonomic and geographical reference, vol. 1. Washington, DC: The Herpetologists’ League; 1999. [Google Scholar]
- 18.Gold BS, Dart RC, Barish RA. Bites of venomous snakes. N Engl J Med. 2002;347(5):347–356. doi: 10.1056/NEJMra013477. [DOI] [PubMed] [Google Scholar]
- 19.de Silva HA, Ryan NM, de Silva HJ. Adverse reactions to snake antivenom, and their prevention and treatment. Br J Clin Pharmacol. 2015;81(3):446–452. doi: 10.1111/bcp.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamza M, Idris MA, Maiyaki MB, Lamorde M, Chippanux JP, Warrell DA, et al. Cost-effectiveness of antivenoms for snakebite envenoming in 16 countries in West Africa. PLoS Negl Trop Dis. 2016;10(3):e0004568. doi: 10.1371/journal.pntd.0004568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morais V. Antivenom therapy: efficacy of premedication for the prevention of adverse reactions. J Venom Anim Toxins incl Trop Dis. 2018;24:7. doi: 10.1186/s40409-018-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williamson DR, Albert M, Heels-Ansdell D, Arnold DM, Lauzier F, Zarychanski R, et al. Thrombocytopenia in critically ill patients receiving thromboprophylaxis: frequency, risk factors, and outcomes. Chest. 2013;144(4):1207–1215. doi: 10.1378/chest.13-0121. [DOI] [PubMed] [Google Scholar]
- 23.Schutte T, Thijs A, Smulders YM. Never ignore extremely elevated D-dimer levels: they are specific for serious illness. Neth J Med. 2016;74(10):443–448. [PubMed] [Google Scholar]
- 24.Isbister GK. Snakebite doesn't cause disseminated intravascular coagulation: coagulopathy and thrombotic microangiopathy in snake envenoming. Semin Thromb Hemost. 2010;36(4):444–451. doi: 10.1055/s-0030-1254053. [DOI] [PubMed] [Google Scholar]
- 25.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145(1):24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang C, Huang TF. Platelet aggregation inhibitors from Agkistrodon acutus snake venom. Toxicon. 1986;24(11–12):1099–1106. doi: 10.1016/0041-0101(86)90136-4. [DOI] [PubMed] [Google Scholar]
- 27.Xu X, Wang XS, Xi XT, Liu J, Huang JT, Lu ZX. Purification and partial characterization of hyaluronidase from five pace snake (Agkistrodon acutus) venom. Toxicon. 1982;20(6):973–981. doi: 10.1016/0041-0101(82)90099-X. [DOI] [PubMed] [Google Scholar]
- 28.Ouyang C, Teng CM. The effect of the purified anticoagulant principle of Agkistrodon acutus venom on blood coagulation. Toxicon. 1973;11(3):287–292. doi: 10.1016/0041-0101(73)90057-3. [DOI] [PubMed] [Google Scholar]
- 29.Kini RM. Anticoagulant proteins from snake venoms: structure, function and mechanism. Biochem J. 2006;397(Pt 3):377–387. doi: 10.1042/BJ20060302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markland FS. Snake venoms and the hemostatic system. Toxicon. 1998;36(12):1749–1800. doi: 10.1016/S0041-0101(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 31.Isbister GK. Procoagulant snake toxins: laboratory studies, diagnosis, and understanding snakebite coagulopathy. Semin Thromb Hemost. 2009;35(1):93–103. doi: 10.1055/s-0029-1214152. [DOI] [PubMed] [Google Scholar]
- 32.Maduwage K, Isbister GK. Current treatment for venom-induced consumption coagulopathy resulting from snakebite. PLoS Negl Trop Dis. 2014;8(10):e3220. doi: 10.1371/journal.pntd.0003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sajevic T, Leonardi A, Krizaj I. Haemostatically active proteins in snake venoms. Toxicon. 2011;57(5):627–645. doi: 10.1016/j.toxicon.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Than T, Hutton RA, Myint-Lwin K-E-H, Soe-Soe T-N-S, et al. Haemostatic disturbances in patients bitten by Russell's viper (Vipera russelli siamensis) in Burma. Br J Haematol. 1988;69(4):513–520. doi: 10.1111/j.1365-2141.1988.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 35.Warrell DA. Snake venoms in science and clinical medicine. 1. Russell's viper: biology, venom and treatment of bites. Trans R Soc Trop Med Hyg. 1989;83(6):732–740. doi: 10.1016/0035-9203(89)90311-8. [DOI] [PubMed] [Google Scholar]
- 36.Anz AW, Schweppe M, Halvorson J, Bushnell B, Sternberg M, Andrew Koman L. Management of venomous snakebite injury to the extremities. J Am Acad Orthop Surg. 2010;18(12):749–759. doi: 10.5435/00124635-201012000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Chen RH, Chen YC. Isolation of an acidic phospholipase A2 from the venom of Agkistrodon acutus (five pace snake) and its effect on platelet aggregation. Toxicon. 1989;27(6):675–682. doi: 10.1016/0041-0101(89)90018-4. [DOI] [PubMed] [Google Scholar]
- 38.Kamiguti AS. Platelets as targets of snake venom metalloproteinases. Toxicon. 2005;45(8):1041–1019. doi: 10.1016/j.toxicon.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 39.Xu G, Ulrichts H, Vauterin S, De Meyer SF, Deckmyn H, Teng M, et al. How does agkicetin-C bind on platelet glycoprotein Ibalpha and achieve its platelet effects? Toxicon. 2005;45(5):561–570. doi: 10.1016/j.toxicon.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Wang WJ. Purification and functional characterization of AAV1, a novel P-III metalloproteinase, from Formosan Agkistrodon acutus venom. Biochimie. 2007;89(1):105–115. doi: 10.1016/j.biochi.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Wu RC, Chou PT, Chen LK. Aspirin plus tirofiban inhibit the thrombosis induced by Russell's viper venom. Thromb J. 2016;14(Suppl 1):38. doi: 10.1186/s12959-016-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.