Abstract
In this study, we sought to investigate the potential application of γδ T cell-derived extracellular vesicles (γδTDEs) as drug delivery system (DDS) for miR-138 in the treatment of oral squamous cell carcinoma (OSCC). Our data showed that overexpression of miR-138 in γδ T cells obtained miR-138-rich γδTDEs accompanying increased expansion and cytotoxicity of γδ T cells. γδTDEs inherited the cytotoxic profile of γδ T cells and could efficiently deliver miR-138 to OSCC cells, resulting in synergetic inhibition on OSCC both in vitro and in vivo. The pre-immunization by miR-138-rich γδTDEs inhibited the growth of OSCC tumors in immunocompetent C3H mice, but not in nude mice, suggesting an immunomodulatory role by miR-13-rich γδTDEs. γδTDEs and miR-138 additively increased the proliferation, interferon-γ (IFN-γ) production, and cytotoxicity of CD8+ T cells against OSCC cells. Only delivered by γδTDEs can miR-138 efficiently target programmed cell death 1 (PD-1) and CTLA-4 in CD8+ T cells. We conclude that γδTDEs delivering miR-138 could achieve synergetic therapeutic effects on OSCC, which is benefited from the individual direct anti-tumoral effects on OSCC and immunostimulatory effects on T cells by both γδTDEs and miR-138; γδTDEs could serve as an efficient DDS for microRNAs (miRNAs) in the treatment of cancer.
Keywords: extracellular vesicle, γδ T cell, miRNA, drug delivery system, oral squamous cell carcinoma
Introduction
Oral squamous cell carcinoma (OSCC), which includes epithelial neoplasms of the oral cavity and oropharynx, is a serious and growing problem in many parts of the world. The annual estimated incidence is approximately 275,000 worldwide.1 Despite numerous advances in the diagnosis and treatment of oral cancer, the 5-year survival rates for cancers of the tongue, oral cavity, and oropharynx are only approximately 50%.1 The 5-year survival rate of patients with advanced disease (stages III/IV) is approximately 20%.2 Current treatment modalities for locally advanced diseases, such as surgery, radiotherapy, and chemotherapy, cause significant dysfunctions and toxicities, which emphasizes the necessity for new treatment options.3
γδ T cells represent a minor lymphocyte population that constitute 0.5%–16% of total CD3+ cells in the peripheral blood, while predominating in the intestine and skin.4 The majority of human γδ T cells express T cell receptor (TCR) Vγ9 and Vδ2 chains that can be activated in a major histocompatibility complex (MHC)-independent manner.5 When expanded in vitro, γδ T cells isolated from patients with melanoma, glioblastoma, neuroblastoma, breast, lung, ovarian, colon, and pancreatic cancer efficiently killed tumor cell lines and/or primary cancer samples.6 In addition, activated γδ T cells display phenotypic characteristics of professional antigen-presenting cells (APCs) and induce CD4+ and CD8+ T cell proliferation and cytotoxicity.7 This direct cytotoxicity against cancer cells and antigen-presenting characteristics make γδ T cells good candidates for effective tumor immunotherapy.8 However, clinical trials exploiting γδ T cells in several cancer types have been conducted over the past decade, with a good safety profile but somewhat conflicting results in most solid tumors.9 Migration and homing properties are important aspects of γδ T cell physiology to consider for cancer immunotherapy.6 Thus, developing novel strategies to improve the therapeutic effect of human γδ T cell-based adoptive immunotherapy for solid tumors is drastically needed.4
Extracellular vesicles (EVs) are usually named according to their mode of biogenesis and include exosomes that originate from intracellular multivesicular bodies, microvesicles shed from the plasma membrane, and apoptotic bodies that are released by apoptotic cells.10 Although initially considered to be products of a pathway used to release unwanted material from cells, EVs are now believed to perform a variety of extracellular functions that involve interactions with the cellular microenvironment, such as morphogen signaling, immunological mediation, cell recruitment, and horizontal transfer of genetic material.11, 12 EVs contain a wide range of functional proteins, mRNAs, and microRNAs (miRNAs)13 that allow these structures to operate as signaling platforms for short-range or long-range delivery of information to other cells.14 As a result, the potential use of EVs as a drug delivery system (DDS) has gained considerable scientific interest.15, 16 EVs may have multiple advantages over currently available drug delivery vehicles, such as their ability to overcome natural barriers, their intrinsic cell-targeting properties, and stability in the circulation.15 However, therapeutic applications of EV-based drug delivery are restricted by a lack of ideal EV donor, limited scale of EV production, and inefficient drug cargo.
Because EVs derived from T lymphocytes,17 dendritic cells (DCs),18 and natural killer (NK) cells19 exhibit characteristics and functions from their parent cells, it is reasonable to assume that γδ T cell-derived EVs (γδTDEs) could inherit the direct cytotoxicity and antigen-presenting role of γδ T cells. A large-scale expansion protocol of γδ T cells has been established,20 making it achievable to obtain large-scale EV production. These characteristics make γδ T cells potential candidate donors for EV-based drug delivery. The last question is what should be embedded and delivered by γδTDEs.
miR-138 has been suggested to function as a tumor suppressor by targeting a set of genes that are relevant to cell migration, epithelial-to-mesenchymal transition (EMT), cell-cycle progression, DNA damage repair, senescence, and differentiation.21 Recently, Wei et al.22 demonstrated that miR-138 could target CTLA-4 and programmed cell death 1 (PD-1) in CD4+ T cells, resulting in marked glioma tumor regression in immune-competent mice. The direct anti-tumoral and indirect immunoenhancing properties of miR-138 make it a potential candidate for cancer therapy. Due to the multiple potential advantages of γδTDEs as a DDS and dual anti-tumoral functions by miR-138, we tested the hypothesis that γδTDEs with miR-138 cargo may be an effective therapeutic strategy for OSCC through direct anti-tumoral effects and indirect immunoenhancing effects.
Results
Expanded γδ T Cells Produce Typical EVs
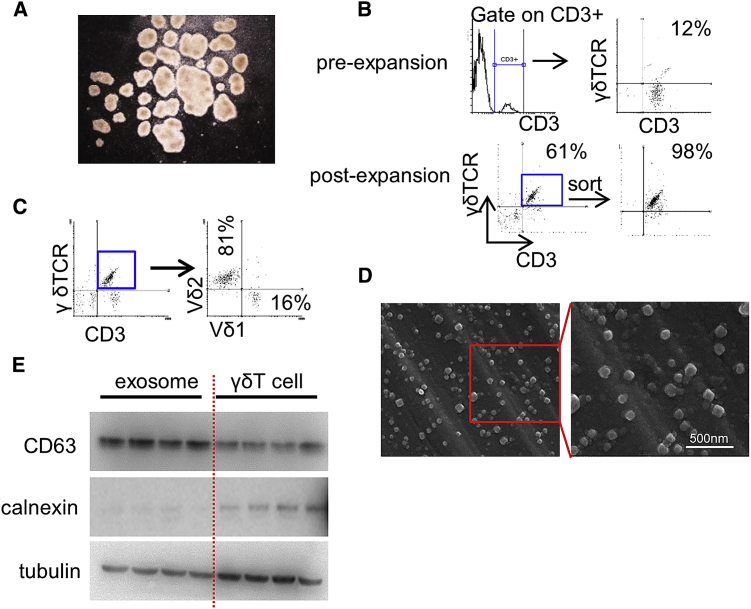
In order to obtain γδTDEs, we initially expanded γδ T cells ex vivo using a well-established zoledronate-dependent protocol.20 During the expansion, typical clusters were formed (Figure 1A), suggesting that γδ T cells were efficiently stimulated. At the initiation of culture, γδ T cells accounted for 3%–15% (median 8%) of CD3+ cells while increasing to 58%–76% (median 62%) of total population at day 7 before sorting (Figure 1B). After selection and successful induction, the frequency of γδ T cells reached up to more than 98% of cultured cells at day 14 (Figure 1B). The expanded γδ T cells are mainly Vδ2 with a percentage ranging from 76% to 96% (median 86%; Figure 1C). The absolute cell number of expanded γδ T cells increased 360- to 420-fold compared with those before expansion. On day 14, γδTDEs were purified from the supernatant of γδ T cells and then examined by scanning electron microscope, which showed typical rounded particles ranging from 50 to 200 nm in diameter (Figure 1D). The EV marker CD63 was confirmed to express in γδTDEs by western blot (Figure 1E).
Figure 1.
Expanded γδ T Cells Produce Typical EVs
(A) Representative image of γδ T cells cultured in vitro. (B) Representative flow cytometry of γδ T cells before expansion and post-expansion. Experiments were performed in triplicate. (C) Representative flow cytometric analysis of Vδ1and Vδ2 expression in CD3+γδTCR+ cells. Experiments were performed in triplicate. (D) Electron micrograph of γδTDEs, revealing the typical morphology and size (50–200 nm). Scale bar: 500 nm. (E) Western blot showing the presence of CD63, weakness of tubulin, and negative of calnexin in γδTDEs.
Lentiviral miR-138 Increase Proliferation and Cytotoxicity of γδ T Cells without Influence on EV Production
After purification on day 7, γδ T cells were infected with lenti-miR-138 virus and further selected and expanded. The levels of miR-138 were significantly increased by lentiviral miR-138 compared with scramble control in γδ T cells derived from both human PBMCs and mouse spleens (Figure 2A). miR-138 overexpression remarkably induced the expansion of γδ T cells determined by the total cell number (Figure 2B) and the kinetics of absolute number at the indicated time points (Figure 2C). Moreover, miR-138 overexpression increased the accumulation in S and G2/M phases, and decreased the amount of G1 phase cells (Figure S1A). We analyzed the miR-138 target genes by TargetScan, which revealed 300 target genes of miR-138. The expression profile of these target genes in γδ T cells and head and neck squamous cell carcinoma (HNSCC) cells was analyzed using two publically available high-throughput datasets, GSE27291 and GSE84557, respectively. We show that 217 out of 300 genes were expressed in γδ T cells and/or HNSCC cells. Among these 217 genes, 72 genes were highly expressed in γδ T cells, whereas 64 genes were increased in HNSCC cells (fold change [FC] > 2, false discovery rate [FDR] < 0.01). Gene enrichment analyses demonstrated that cell cycle is among the top enriched biological processes in HNSCC cells, but not in γδ T cells (Figures S1B and S1C).
Figure 2.
Lentiviral miR-138 Increase Proliferation and Cytotoxicity of γδ T Cells without Influence on EV Production
(A) miR-138 levels in γδ T cells were measured by qRT-PCR. Data represent at least three experiments done in triplicate. (B) The absolute cell number of γδ T cells after lentiviral miR-138 transfection and subsequent expansion. (C) Absolute number of γδ T cells at the indicated time points. Data represent at least three experiments done in triplicate. (D) The cytotoxicity of γδ T cells against cancer cells was measured by an LDH cytotoxicity assay with an E:T ratio of 10:1. Data represent at least three experiments done in triplicate. (E) Quantification of EVs derived from scramble miRNA or miR-138-transfected γδ T cells. Data represent at least three experiments done in triplicate. (F) miR-138 levels in γδTDEs were measured by qRT-PCR. Data represent at least three experiments done in triplicate. *p < 0.05.
To evaluate the influence of miR-138 overexpression on the cytotoxicity of γδ T cells against cancer cells, we performed a lactate dehydrogenase (LDH) assay with an effect:target (E:T) ratio of 10:1. As demonstrated in Figure 2D, miR-138 overexpression significantly induced the cytotoxicity of human and mouse γδ T cells against Cal-27 and SCC-VII cells, respectively, compared with scramble control. We then evaluated the EV amount by quantifying the concentration of exosomal proteins. The miR-138 overexpression in γδ T cells did not influence the amount of EVs expressed as μg/107 cells (Figure 2E). Furthermore, the overexpression by miR-138 lentivirus resulted in a mean 19.6-fold and 13.6-fold increase of miR-138 levels in human and mouse γδTDEs, respectively (Figure 2F). These results suggest that overexpression of miR-138 with lentivirus in ex-vivo-expanded γδ T cells would obtain miR-138-rich γδTDEs accompanying increased expansion and cytotoxicity of γδ T cells.
miR-138-Rich γδTDEs Directly Inhibit the Growth of Tumor
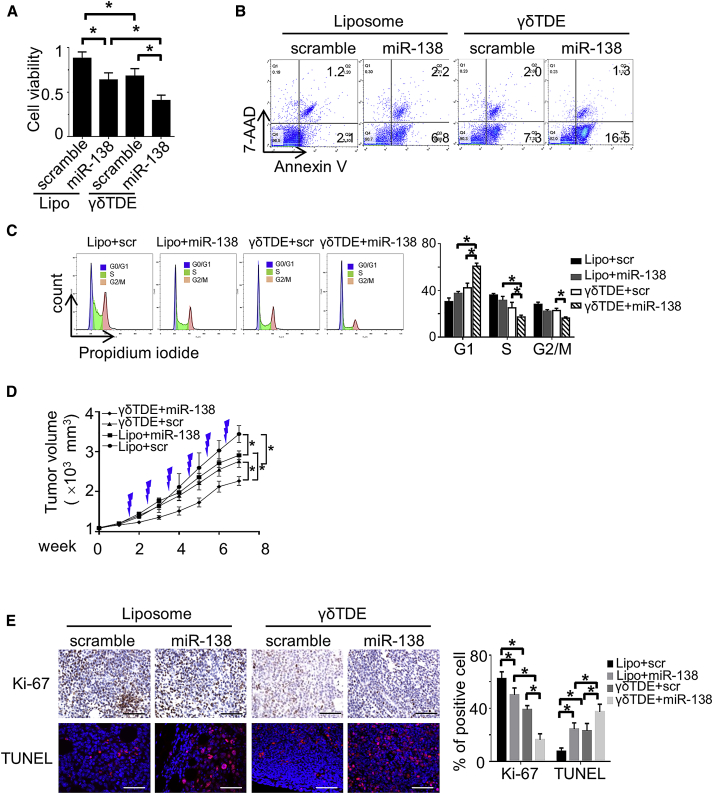
To investigate the role of miR-138-rich γδTDEs on tumor viability, Cal-27 cells were treated by miR-138 or scramble control delivered by liposome and γδTDEs, respectively. The cell viability of treated Cal-27 cells was measured by CCK-8 assay. We showed that miR-138 and γδTDEs, individually, could significantly inhibit the viability of Cal-27 cells. Moreover, delivery of miR-138 by γδTDEs had significantly decreased cell viability compared with individual effects of miR-138 and γδTDEs (Figure 3A). An apoptosis assay performed with fluorescein isothiocyanate (FITC)-Annexin V/7-aminoactinomycin D (7-AAD) confirmed that miR-138-rich γδTDEs induced more apoptosis of cancer cells than miR-138 and γδTDEs independently (Figure 3B). Cell-cycle distribution of OSCC cells treated by γδTDEs was further performed. Compared with liposome-transfected miR-138 and scramble-cargo γδTDEs, miR-138-rich γδTDE significantly decreased the accumulation of Cal-27 cells in S and G2/M phases, and increased the amount of G1 phase cells (Figure 3C).
Figure 3.
miR-138-Rich γδTDEs Directly Inhibit the Growth of Tumor
(A) The viability of OSCC cells treated by liposome and γδTDEs carrying either scramble miRNA or miR-138 were measured by CCK-8 assay. Data represent at least three experiments done in triplicate with 10 biological replicates. (B) Representative flow-cytometry-based apoptosis assay of OSCC cells treated by liposome and γδTDEs carrying either scramble miRNA or miR-138. Experiments were performed in triplicate. (C) Cell cycle of OSCC cells treated by liposome and γδTDEs carrying either scramble miRNA or miR-138 was analyzed by propidium iodide staining and flow cytometry. Data represent at least three experiments done in triplicate. (D) Cal-27 xenograft bearing nude mice received injection of liposome and γδTDEs carrying either scramble miRNA or miR-138, respectively. Growth curve of xenograft tumors was monitored. n = 6 in each group. (E) Representative image of immunostaining of Ki-67 (upper panel) and TUNEL assay (lower panel) in xenograft tumors. Scale bars: 100 μm. *p < 0.05.
We next sought to investigate the in vivo effects of γδTDEs on tumor growth. To rule out the potential interference of immune effects of γδTDEs, the immunodeficient nude mice were applied to establish xenografts with Cal-27 cells. Liposome and γδTDEs (10 μg) were injected to the xenografts twice a week for a total of 6 weeks. In parallel with ex-vivo results, liposome-transfected miR-138 and scramble miRNA γδTDEs could reduce the growth of xenograft tumors compared with liposome + scramble miRNA. Mice that received miR-138-rich γδTDEs treatment kept a much slower growth than other groups during the whole period (Figure 3D). The xenograft tumors were then harvested for histological analyses. Frozen sections were observed under fluorescence microscope for the GFP-positive cells that represent successful exogenous miRNA delivery. A higher frequency of GFP+ cells was observed in mice that had γδTDEs as delivery vesicle for miR-138 (Figure S2A). However, liposome and γδTDE delivery had equal miR-138 distribution in spleen, brain, lung, kidney, and liver (Figure S2B). The proliferation of tumor cells was detected by IHC staining of Ki-67, and the apoptosis was measured by TUNEL assay. Mice that received miR-138-rich γδTDE treatment had remarkably decreased Ki-67 staining (Figure 3E, left upper panel) and increased TUNEL staining (Figure 3E, left lower panel) compared with those that received either liposome-transfected miR-138 or scramble-cargo γδTDEs.
These results suggest that both miR-138 and γδTDEs, individually, have direct anti-tumor effects, and that therapeutic outcome of OSCC may benefit from delivering miR-138 by γδTDEs.
γδTDEs Inherit the Cytotoxic Profiles of γδ T Cells
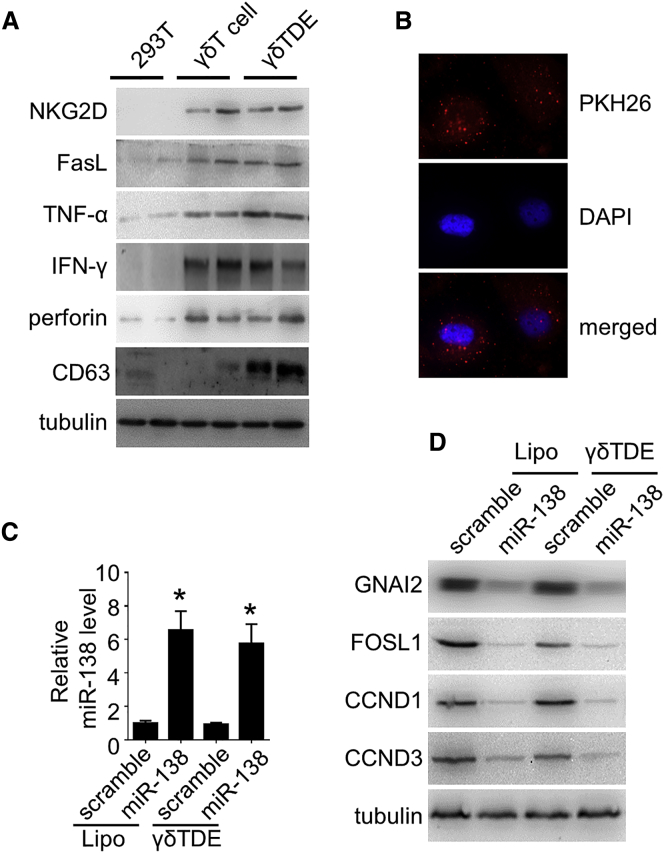
Because γδTDEs, independently, could inhibit the growth of tumor cells without carrying miR-138, we measured the expression of cytotoxic markers of γδ T cells in γδTDEs by western blot. Our data showed positive expression of NKG2D, Fas ligand (FasL), tumor necrosis factor alpha (TNF-α), interferon-γ (IFN-γ), and perforin in γδ T cells, as well as in γδTDEs, but not in 293T control cells (Figure 4A). γδTDEs were labeled with fluorescent PKH26 and then co-incubated with OSCC cells. The PKH26-labeled γδTDEs were visualized to be internalized by Cal-27 cells after 2-hour incubation measured by a fluorescence microscope (Figure 4B). We then measured the miR-138 expression in the recipient OSCC cells treated by liposome and γδTDEs, respectively. The qRT-PCR revealed that both liposome and γδTDEs could efficiently deliver miR-138 to the Cal-27 cells with 6.6-fold and 5.8-fold increase, respectively (Figure 4C). We next investigated whether miR-138 regulates its target genes in recipient cells. miR-138 delivered by liposome and γδTDEs significantly decreased the expression of selected miR-138 targets, GNAI2, FOSL1, CCND1, and CCND3, determined by western blot (Figure 4D). These represented targets of miR-138 are involved in the regulation of cell proliferation and cell cycle. These results suggest that γδTDEs, inheriting the cytotoxic profile of γδ T cells, could efficiently deliver miR-138 to cancer cells to serve as a cancer suppressor.
Figure 4.
γδTDEs Inherit the Cytotoxic Profiles of γδ T Cells
(A) Cytotoxic markers of γδ T cells were measured by western blot with 293T cells serving as control. (B) Representative fluorescence microscopy image showing the internalization of PKH26-labeled (red) γδTDEs by OSCC cells. (C) miR-138 levels in OSCC cells treated by liposomes or γδTDEs were measured by qRT-PCR. Data represent at least three experiments done in triplicate. *p < 0.05. (D) The representative targets of miR-138 in OSCC cells were measured by western blot.
miR-138-Rich γδTDEs Stimulate Anti-tumor Immunity
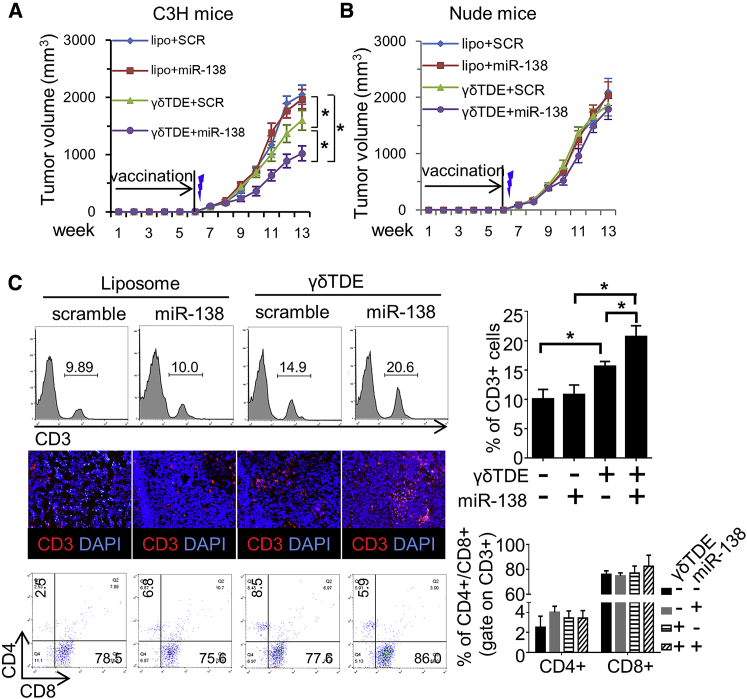
Activated γδ T cells have been suggested to display phenotypic characteristics of APCs and to induce the cytotoxicity of CD8+ T lymphocytes.7, 23 No study, to the best of our knowledge, has reported whether γδTDEs could inherit the antigen-presenting function of γδ T cells. We therefore sought to investigate whether miR-138-rich γδTDEs can modulate the anti-tumor immunity. Immunodeficient nude mice and immune-competent C3H mice received intravenous (i.v.) injection of either liposome or γδTDEs with or without miR-138 cargo, respectively. Twenty-four hours after the sixth immunization, 1 × 107 SCC-VII cells were subcutaneously injected, and tumor growth was monitored weekly. The xenograft tumors were then harvested for histological analyses. Fluorescence microscope showed that no remarkable GFP+ cell (exogenous miRNA-transfected cell) was observed in tumors harvested from mice that received either liposome or γδTDE vaccination (Figure S2C). Compared with liposome, γδTDEs (even with scramble miRNA) could significantly inhibit the growth of tumor in immunocompetent C3H mice. Although delivery by liposome had no influence on the tumor growth, miR-138 embedded by γδTDEs further decreased the growth of tumor compared with γδTDEs alone (Figure 5A). In nude mice, however, tumor growth was not different between any vaccinating strategies (Figure 5B). These results suggest that the effect of γδTDEs and their miR-138 cargo on tumor growth suppression was immunity dependent.
Figure 5.
miR-138-Rich γδTDEs Stimulate Anti-tumor Immunity
C3H mice (A) and nude mice (B) received weekly i.v. injection of either liposome or γδTDEs for 6 weeks. Twenty-four hours after the sixth immunization, 1 × 107 SCC-VII cells were subcutaneously injected, and tumor growth was monitored weekly. n = 6 in each group. Thunderbolt indicates subcutaneous (s.c.) injection of cancer cells. (C) Representative flow cytometry analysis (left upper panel) and immune-florescence staining (left middle panel) showing the infiltration of CD3+ lymphocytes in the tumor on C3H mice. Flow cytometry analysis (left bottom panel) of CD4+ and CD8+ T cells in the CD3+ population. Quantitative analysis of flow cytometry data of CD3+ T cells (right upper panel) and CD4+/CD8+ T cells (right lower panel). *p < 0.05.
The tumors in C3H mice were harvested and analyzed for the infiltration of CD3+ T lymphocytes. The flow cytometry analysis showed that liposome has no effect on the infiltration of CD3+ T cells no matter whether scramble or miR-138 was packaged in. Compared with liposome, γδTDEs significantly increased the recruitment of CD3+ T cells. Intriguingly, miR-138 delivered by γδTDEs further increased CD3+ T cell recruitment (Figure 5C). The immunofluorescence staining confirmed the increased infiltration of CD3+ T cells by miR-138 and γδTDEs. The percentage of CD4+ and CD8+ in the total CD3+ T cells was not different between groups. These results suggest that miR-138 and γδTDEs collaborate in the anti-tumor immunity regulation.
miR-138-Rich γδTDEs Regulate Anti-tumor Immunity by CD8+ T Cells
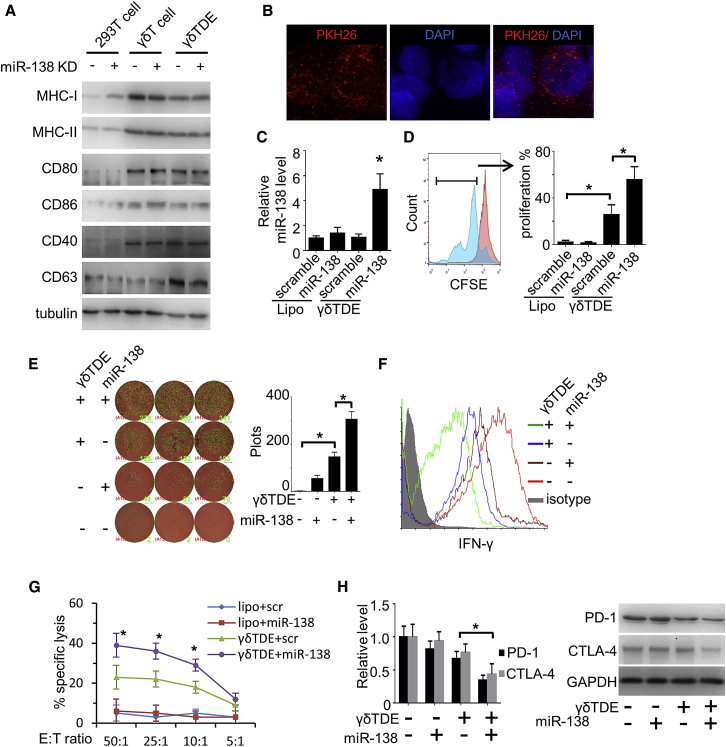
We measured the expression of antigen-presenting MHC class II molecules, as well as T cell co-stimulation and adhesion molecules, on γδ T cells and γδTDEs using western blot with 293T cells serving as a negative control. MHC class II molecular CD80, CD86, and CD40 were found to express on both γδ T cells and γδTDEs, but not 293T cells. MHC class I weakly expressed on 293T cells and was remarkably upregulated on both γδ T cells and γδTDEs. In addition, none of the co-stimulatory markers investigated were remarkably affected by miR-138 KD in either γδ T cells or γδTDEs (Figure 6A). These results suggest that γδTDEs inherit the antigen-presentation characteristics by their parent γδ T cells. The PKH26-labeled γδTDEs were labeled with fluorescent PKH26 and then co-incubated with CD8+ T cells. The PKH26-labeled γδTDEs were internalized by CD8+ T cells after 24-hour incubation measured by a fluorescence microscope (Figure 6B). The miR-138 expression in the recipient CD8+ T cells treated by liposome and γδTDEs was measured by qRT-PCR. γδTDEs, but not liposome, efficiently delivered miR-138 and significantly increased the miR-138 expression in the recipient CD8+ T cells (Figure 6C). To study the role of γδTDEs and miR-138 on CD8+ T cell proliferation, we stained CD8+ T cells by carboxyfluorescein succinimidyl ester (CFSE) and co-incubated with either γδTDEs or liposome for 6 days. γδTDEs significantly increased the proliferation of CD8+ T cells, which was further remarkably increased by their miR-138 cargo. Liposome, no matter whether scramble miRNA or miR-138 was carried, has no effect on CD8+ T cell proliferation (Figure 6D). Consistently, the IFN-γ production by CD8+ T cells was measured by ELISpot assay, which showed that γδTDEs significantly induced the IFN-γ production. γδTDEs with miR-138 cargo had significantly increased IFN-γ levels compared with scramble miRNA cargo (Figure 6E). Flow cytometry analysis further validated the increased IFN-γ expression by CD8+ T cells treated with γδTDEs with miR-138 cargo (Figure 6F). We then performed a cytotoxicity assay to assess the cytotoxic activity of γδTDE-treated CD8+ T cells against OSCC cells. As shown in Figure 6F, γδTDEs with scramble miRNA could induce a significant increase of CD8+ T cell cytotoxicity, which was further increased by γδTDEs carrying miR-138 (Figure 6G). Because immune checkpoint molecules PD-1 and CTLA-4 have been reported to be miR-138 targets, we measured PD-1 and CTLA-4 expression in CD8+ T cells by qRT-PCR and western blot, respectively. γδTDEs with miR-138 cargo significantly decreased the expression of PD-1 and CTLA-4 at both mRNA (Figure 6G, left panel) and protein levels (Figure 6H, right panel). These results suggest that both miR-138 and γδTDEs, individually, have immunostimulatory effects on CD8+ T cells, and that a combination of miR-138 and γδTDEs could achieve an additive effect, making miR-138-rich γδTDE a candidate for OSCC therapy.
Figure 6.
miR-138-Rich γδTDEs Regulate Anti-tumor Immunity by CD8+ T Cells
(A) Antigen-presenting markers of γδ T cells were measured by western blot with 293T cells serving as control. (B) Representative fluorescence microscopy image showing the internalization of PKH26-labeled (red) γδTDEs by CD8+ T cells. (C) miR-138 levels in CD8+ T cells treated by liposomes or γδTDEs were measured by qRT-PCR. Data represent at least three experiments done in triplicate. (D) CD8+ T cells were pre-labeled with CFSE and then incubated with liposomes or γδTDEs at the presence of IL-2. CFSE+ cells were measured by flow cytometry. Data represent at least three experiments done in triplicate. (E) IFN-γ production by CD8+ T cells was measured by ELISpot assay. (F) The production of IFN-γ by CD8+ T cells was measured by flow cytometry. The CD8+ population was gated for IFN-γ analysis. Experiments were performed in triplicate. (G) The cytotoxicity of liposome or γδTDE-treated CD8+ T cells against OSCC cells was measured by an LDH cytotoxicity assay with E:T ratios of 50:1, 25:1, 10:1, and 5:1. Data represent at least three experiments done in triplicate. (H) PD-1 and CTLA-4 expression on the mRNA level (left panel) and protein level (right panel) were measured by qRT-PCR and western blot, respectively. Data represent at least three experiments done in triplicate. *p < 0.05.
Discussion
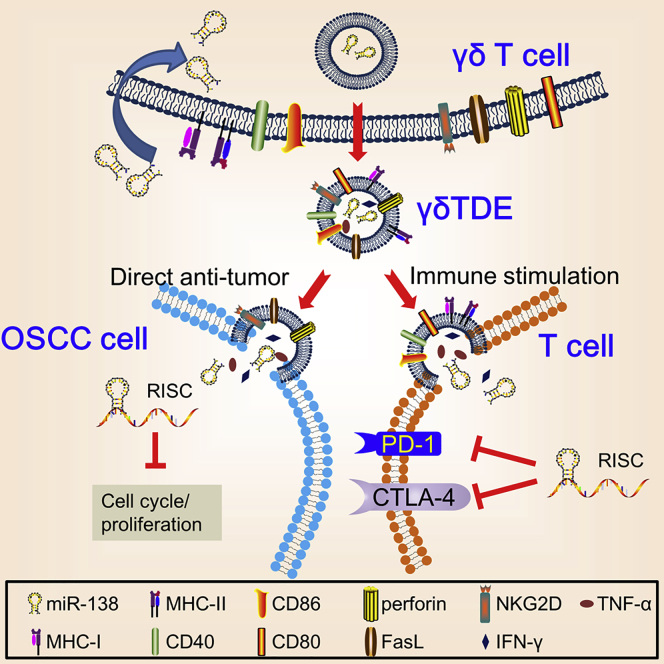
γδ T cells are suggested to have direct cytotoxicity against a wide range of cancer types in a manner similar to NK cells and can stimulate expansion of cytotoxic effector T cells by mimicking the functions of DCs.6, 7, 24 The dual functions of γδ T cells make them attractive candidates for cancer immunotherapy.25 EVs have emerged as potential tools for DDS due to the fact that they are naturally produced with expected organ tropism and low side effect.15, 26 However, to the best of our knowledge, no study has ever applied γδTDEs as DDS. In the present study, we demonstrated that γδTDEs, inheriting NK- and DC-like profiles of parent γδ T cells, could efficiently inhibit the growth of OSCC with miR-138 cargo, which can function as tumor suppressor and immune enhancer on the other hand (Figure 7). Our results suggest that an integration of γδTDEs and miR-138 could serve as a therapeutic candidate for OSCC.
Figure 7.
Schematic Cartoon Illustrates Dual Anti-tumoral Functions by miR-138-Rich γδTDEs
In the present study, we show that γδTDEs inherit the profiles of γδ T cells, having direct anti-tumor effects on OSCC and stimulation on anti-tumor immunity. This is the first study, to the best of our knowledge, to demonstrate the anti-tumor function of γδTDEs. It has been reported that EVs derived from NK cells not only express both typical NK markers (i.e., CD56) and killer proteins (i.e., FasL and perforin), but also exert antitumor and immune homeostatic activities.19, 27 Consistently, T cell-derived EVs contain TCR subunits, Src-like tyrosine kinases, and adhesion molecules.28 Moreover, DC-derived EVs express functional MHC class I, MHC class II, and T cell costimulatory molecules, which prime specific cytotoxic T lymphocytes against murine tumors.29 The profiles of γδTDEs described in our study further support the notion that EVs vary between cell types, reflecting the specific functions of their parent cells to a certain extent.17
Due to the intrinsic role of EVs in endogenous gene transfer in both biological and pathological settings, the nascent era of EV-based DDS has been rapidly growing. Perhaps the most tantalizing and ambitious applications for EVs as DDS are their potential to help realize the enormous opportunities for gene and biologic therapies in oncology, which has been a long-standing challenge because of the lack of appropriate carriers.30 Cancer-derived EVs have been described to function as natural carriers that can efficiently deliver CRISPR/Cas9 plasmids to cancer, resulting in the induction of apoptosis in ovarian cancer and enhancement of chemosensitivity to cisplatin.31 Several other preclinical studies have investigated the delivery efficiency by EVs derived from mesenchymal stem cells (MSCs),32 macrophages,33 NK cells,27 and erythrocytes34 in cancer treatment with encouraging results. Clinical trials that utilized DC-derived EVs to treat advanced melanoma35 and non-small cell lung cancer patients36 demonstrated partial immunological and clinical responses, but survival benefits were largely variable and limited. MSCs are multiple precursors with the ability to locate and migrate toward damaged and inflammatory microenvironments, such as solid tumors. This property makes them a candidate for therapeutic agent delivering.37 MSC-EVs have been demonstrated to recapitulate the therapeutic potential of MSCs on glioma through delivering exogenous miR-146b.38 However, MSC-EVs have also shown to have heterogeneous effector mechanisms as MSCs. For instance, MSC-EVs enhanced vascular endothelial growth factor (VEGF) expression in tumor cells by activating extracellular signal-regulated kinase 1/2 (ERK1/2) pathway and promoted tumor growth in vivo.39 In addition, NK cells release EVs expressing typical protein markers of NK cells and containing killer proteins (i.e., Fas ligand and perforin molecules).40 NK-EVs have been demonstrated to exert cytotoxic effects on different human tumor cells.27, 40 However, to the best of our knowledge, no study has evaluated the potential application of NK-EV as a DDS in the cancer treatment. According to our results, we suggest that γδTDEs may serve as an efficient DDS in cancer therapy attributing to several advantages: (1) γδTDEs inherit the NK-like profile of γδ T cells independently on MHC molecules, (2) γδTDEs could inherit APC-like function of γδ T cells, and (3) large-scale ex-vivo expansion protocol of γδ T cells has been established. Such advantages include the specificity of delivery to cancer cells and T lymphocytes with abilities to direct target cancer cell and indirect effects on anti-cancer immunity improvement.
Although EVs as DDS are still in their infant stage, liposomes have gained popularity for applications as drug carriers. Synthetic liposomes are easy to manufacture, allowing advantageous simplicity in isolation and high yield. However, the high toxicity, lack of targeting specificity, and rapid elimination from the circulation have hindered their wide application.41 Our results showed that liposomes failed to transfect miR-138 to T cells either in vivo or in vitro, whereas γδTDEs can efficiently deliver miR-138 to T cells, suggesting higher delivery efficiency by γδTDEs than liposomes in T cell targeting. Indeed, T cells are particular about the EVs to be incorporated, because it has been reported that T cells prefer a surface contact with tumor-derived EVs rather than internalizing them.42 Given that γδTDEs can efficiently be internalized by T cells, we suggest that γδTDEs are efficient DDSs to transport drugs and nuclear acids to T cells rather than tumor-derived EVs. But the efficiency between γδTDEs and EVs derived from other cell types, e.g., DCs, NK cells, and MSCs, needs to be explored further.
The clearance of EVs in the circulation by monocytes and macrophages might be a concern in the application of EVs as drug delivery vesicles. Compared with cell-based therapy, EVs may have a shorter lifetime in the circulation. The persistence of tumor-killing capacity of cell-based therapy depends on their own lifespan and further in vivo expansion, whereas the extent of cytokine release from cell-based therapy (e.g., chimeric antigen receptor-based T cell adoptive immunotherapy) and the status of in vivo expansion of these cells cannot be appropriately controlled, which is the potential source of adverse events, such as cytokine release syndrome and “on-target, off-tumor” response.43 The cell-free nature and biological properties of EVs make them an attractive replacement of cell-based therapy with advantages such as controllable cytokine release syndrome, well penetration within solid tumor, and easy modification.43 Moreover, Kamerkar et al.44 demonstrated an enhanced retention of EVs, compared with liposomes, in the circulation due to CD47-mediated protection of EVs from phagocytosis by monocytes and macrophages. These results suggest that EVs could serve as an attractive replacement of cell-based therapy with drug-delivering property. miR-138, as a tumor suppressor, has been consistently downregulated in OSCCs45, 46, 47 and many other cancer types as well.21, 48 The experimentally validated miR-138 target genes play essential roles in the initiation and progression of cancer, including cell migration (e.g., ZEB2, HIF-1α), EMT (e.g., TWIST2, EZH2), cell-cycle regulation (e.g., CCND1, CCND3, FOSL1), DNA damage and repair (e.g., H2AX, XRCC1), and senescence (e.g., Sirt1, TERT).21 By regulating these target genes, miR-138 can inhibit proliferation and invasion, induce apoptosis, and enhance chemosensitivity of many cancer types.48 More recently, miR-138 has been uncovered by its participation in immune regulation. Wei et al.22 demonstrated in vivo miR-138 treatment of GL261 gliomas in immune-competent mice resulted in remarked tumor regression through directly targeting immune checkpoints CTLA-4 and PD-1 in CD4+ T cells, suggesting miR-138 as a novel immunotherapeutic agent for glioma. Considering the multiple mechanisms by which miR-138 utilizes to inhibit cancer growth, we chose miR-138 as the “drug cargo” of γδTDEs and proved its dual effects on direct anti-tumor effects and indirect immunity enhancement in OSCC xenograft models.
Given that miR-138 targets genes involved in cell proliferation and cell cycle, the loading of miR-138 to γδ T cells has a potential inhibition on γδ T cell proliferation and γδTDE production. There is no evidence, to the best of our knowledge, to indicate any suppression by miR-138 on T cell function. In addition to the aforementioned role on CD4+ T cell immunoenhancement through targeting CTLA-4 and PD-1,22 miR-138 has been also suggested to regulate Th1/Th2 balance in CD4+ T cells through targeting RUNX3.49 In our present study, miR-138 overexpression in γδ T cells remarkably increased the expression of miR-138 in both γδ T cells and γδTDEs with moderate increased γδ T cells proliferation, but no influence on γδTDE production. In addition, gene enrichment analyses demonstrated that cell cycle is among the top enriched biological process in HNSCC cells, but not in γδ T cells, which might be the reason that miR-138 overexpression in OSCC cells caused proliferation inhibition rather than γδ T cells. These results suggest that miR-138 could be an ideal “bullet” loaded in γδ T cells for cancer therapy.
In conclusion, we showed in the present study that miR-138-rich γδTDEs achieved synergetic therapeutic effects on OSCC compared with miR-138 and γδTDEs alone, which is benefited from the individual direct anti-cancer effects on OSCC and immunostimulatory effects on T cells by both γδTDEs and miR-138. In addition, γδTDEs could serve as an efficient DDS for miRNAs in the treatment of cancer.
Materials and Methods
Human and animal studies have been approved by the Institutional Ethics Committee of Sichuan Cancer Hospital (approval no. KY-2017-017-01). Peripheral blood samples were obtained from healthy volunteers without any malignancy. Informed consent was obtained from all donors prior to blood collection.
γδ T Cell Culture
Human γδ T cells were expanded and cultured from human PBMCs as described previously.50 In brief, whole blood (7.5–8 mL) was collected in a BD vacutainer CPT cell preparation tube with sodium heparin (BD, Franklin Lakes, NJ, USA) and centrifuged to isolate PBMCs at the interphase. Murine γδ T cells were expanded from splenocytes of C3H mice. Human PBMCs or mouse splenocytes were then cultured in AIM V medium (Thermo Fisher Scientific, Waltham, MA, USA) with recombinant interleukin-2 (IL-2; R&D Systems, Minneapolis, MN, USA) and zoledronate (Aclasta, Novartis, Switzerland) to final concentrations of 1,000 IU/mL and 5 μM, respectively, at the presence of tumor conditioned medium. On day 7, γδ T cells were sorted using a MoFlo XDP cell sorter (Beckman Coulter, Brea, CA, USA).
Sorted γδ T cells were seeded in a 24-well plate and infected with LV-hsa-mir-138 (GENECHEM, Shanghai, China) with 5 μg/mL polybrene, and stable clones were selected and maintained in medium described above with 0.5 μg/mL puromycin.
EV Isolation, Quantification, Labeling, and RNA Extraction
Ten microliters of culture media was mixed with ExoQuick EV precipitation solution, and EV isolation was performed according to the manufacturer’s instructions (SBI System Biosciences, CA, USA) as described previously.51 The ExoQuick/biofluid mixture was centrifuged at 1,500 × g for 30 min, and the precipitated EVs were re-suspended in nuclease-free water. After re-suspension of precipitated EVs, the concentration of proteins contained in EVs was quantified using bicinchoninic acid assay (BCA; Pierce, Rockford, IL, USA); EV quantities are therefore expressed as micrograms of containing proteins as described by Roccaro et al.52
Purified EVs were labeled with the red fluorescent linker PKH26 (Sigma-Aldrich, St. Louis, MO, USA) as described previously.42 For in vitro EV treatment studies, equal amounts of EVs (micrograms of proteins) were added into the medium at a concentration of 10 μg/mL. To extract total RNA, an equal quantity of EV particles was lysed using RNeasy mini kit (QIAGEN, Germantown, MD, USA), then 5 pg of cel-miR-39 was added into the lysate as a spike-in control. Total RNA isolation was done according to the manufacturer’s protocol. EVs used for in vivo experiments were prepared with an ultracentrifugation protocol as described previously.42
qRT-PCR
Total RNA was reverse transcribed using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems). Quantification of miR-138 was performed with predesigned TaqMan microRNA assays on an ABI PRISM 7700 sequence detection system (Applied Biosystems, Foster City, CA, USA). For mRNA quantification, total RNA were reverse transcribed using first-strand cDNA synthesis Kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. PCR was performed using TaqMan universal PCR master mix (Applied Biosystems) on an ABI PRISM 7700 sequence detection system (Applied Biosystems). PCR conditions were 50°C for 2 min, followed by incubation at 95°C for 10 min, then 40 cycles of two-step PCR, including denaturing at 95°C for 15 s and annealing and extension at 60°C for 60 s. Reactions were run in triplicate, and the results were averaged. Relative expression was calculated using the ΔΔCt method after normalization to cel-miR-39 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), respectively, for miRNA and mRNA quantification. Primers and probes for PD-1 and CTLA-4 were described previously.53
LDH Release Assay
To determine the cytotoxicity of T cells, we performed the LDH assay with an LDH cytotoxicity assay kit (Thermo Fisher Scientific) following the manufacturer’s protocol. In brief, T cells (effect cells) and target cells (Cal-27 and SCC-VII) were co-cultured at the ratios of 50:1, 25:1, 10:1, and 5:1. The LDH released into the medium is transferred to a new plate and mixed with Reaction Mixture. After 30-min room temperature incubation, LDH activity was determined at 490 nm in a plate-reading spectrophotometer: % cytotoxicity = ([experimental value − effector cells spontaneous control − target cells spontaneous control]/[target cell maximum control − target cells spontaneous control]) × 100.
CCK-8 Assay
To evaluate the inhibitory effects of γδTDEs on OSCC cells, we cultured Cal-27 and SCC-VII cells (5,000 cells/well) in 96-well plates and incubated with 10 μg of γδTDEs for 24 hr. CCK-8 solution (10 μL/well; Beyotime, Shanghai, China) was added to the cells. After a 4-hr incubation, absorbance at 450 nm was measured using a microplate reader (Thermo Fisher Scientific).
Flow Cytometry
Single-cell suspensions were prepared from cultured cells or fresh tumor tissues. Red cells were removed using ammonium chloride lysis buffer when necessary. 1 × 106 cells were incubated at 4°C for 30 min with different combinations of fluorescent-conjugated antibodies for γδ T cells (anti-Vγ9 TCR-allophycocyanin [BioLegend, San Diego, CA, USA], anti-CD3-PECy7 [BioLegend], anti-Vδ2 TCR-peridinin chlorophyll [PerCP]-Cy5.5 [BioLegend], and anti-Vδ1 TCR-FITC [Thermo Fisher Scientific]), T cells (FITC-anti-CD4 [BD Biosciences, San Jose, CA, USA], PE-anti-CD8 [BD Biosciences], and PE/Cy7-anti-CD3 [BioLegend]). For intracellular staining of IFN-γ, cells were incubated with staphylococcal enterotoxin B for 6 hr in the presence of 19 brefeldin A as described by the manufacturer (BD Biosciences, San Jose, CA, USA). Cells were then stained with IFN-γ PerCP-Cy5.5 (BD Biosciences).
For γδTDE-mediated cell proliferation experiments, CD8+ T cells were stained with CFSE (4.5 mM) at 37°C for 20 min. The labeled cells were cultured in AIM V Medium with IL-2 and zoledronate at the presence of γδTDE derived from different conditions for 6 days. Cells were harvested, and CFSE was measured by a flow cytometer with 488 nm excitation and emission filters.
Apoptosis Assay
An FITC-Annexin V/7-AAD (Thermo Fisher Scientific) double-staining protocol was applied to measure the cytotoxic effects of γδTDEs against OSCC cells as described previously. Cal-27 and SCC-VII cells were treated by 10 μg of γδTDEs for 24 hr. OSCC cells were washed twice with PBS, centrifuged to remove the debris, and resuspended in binding buffer at a concentration of 1 × 106 cells/mL. Cells were stained with 5 μL of FITC-Annexin V and 5 μL of 7-AAD for 15 min at room temperature in the dark. After binding buffer (0.4 mL) being added, cells were analyzed using a BD FACSCanto II flow cytometer.
Cell Cycle
Cells were trypsinized, washed with PBS, and fixed in cold 70% ethanol for 30 min. After wash, cells were resuspended in 0.5 mL of PBS containing 0.25% Triton X-100 and incubated on ice for 15 min. Cells were then stained with propidium iodide (20 μg/mL) at the presence of 10 μg/mL RNase A at room temperature in the dark for 30 min. Flow cytometry was performed using a BD FACSCanto II flow cytometer. Cell cycle was analyzed in FlowJo software (FlowJo, Ashland, OR, USA).
Western Blot
Total protein was isolated from EVs and cultured cells with a radioimmunoprecipitation assay (RIPA) lysis and extraction buffer (Thermo Fisher Scientific), and protein concentrations were detected by a BCA protein assay kit (Pierce, Rockford, IL, USA). Thirty micrograms of proteins from each sample was separated on an 8% SDS-PAGE gel and electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Boston, MA, USA). Membranes were blocked with 2% BSA in TBS containing 0.1% Tween 20 at 37°C for 2 hr and then incubated for 2 hr with either CD63 (Santa Cruz, CA, USA), Calnexin (Cell Signaling Technology), NKG2D (R&D Systems), FasL (R&D Systems), TNF-α (NOVUS, Littleton, CO, USA), IFN-γ (NOVUS), perforin (Invitrogen), tubulin (NOVUS), MHC class I (Abnova, Beijing, China), MHC class II (NOVUS), CD80 (Bio-Rad Laboratories, Hercules, CA, USA), CD86 (R&D Systems), and CD40 (R&D Systems). Horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG was used as a secondary antibody (diluted 1:5,000 in TBST with 2% BSA and incubated for 1 hr). Bands were scanned using a densitometer (GS-700; Bio-Rad Laboratories), and quantification was performed using Quantity One 4.4.0 software.
Xenograft
The immunodeficient nude mice and immunocompetent C3H mice (females, 6–8 weeks of age) were obtained from Charles River (Beijing, China). Tumor cells were injected subcutaneously (1 × 107 cells/200 μL PBS/mouse) into the back of mice. In the EV injection experiments, 10 μg of EVs was i.v. injected into the tail vein of mice. The tumor size was monitored weekly by measuring diameters using vernier calipers and calculated as πls2/6, where l = the long side and s = the short side. Mice were euthanized at week 7.
ELISpot
An IFN-γ ELISpot kit was applied (BD, Franklin Lakes, NJ, USA) according to the manufacturer’s instructions. In brief, 1 × 104 T cells were seeded in BD ELISpot plate coated with anti-mouse IFN-γ and cultured with anti-mouse IL-2 and liposome/γδTDEs for 48 hr at 37°C. After incubation, plates were washed and incubated with biotinylated anti-mouse IFN-γ in detection antibody solution for 2 hr. After wash, 100 μL/well streptavidin-horseradish peroxidase (HRP) solutions were added to each well and incubated for 1 hr. 3-Amino-9-ethylcarbazole (AEC) substrate solution was then added and spots were counted with an AID ELISpot Reader system using ELISpot Reader v6.0. T cells alone in duplicate wells served as the background control.
Expression Analysis of miR-138 Target Genes in γδ T Cells and HNSCC Cells
There were 300 target genes of miR-138 predicted by TargetScan.54 Gene expression data (“cel” files of Affymetrix Human Genome U133 Plus 2.0 microarrays) of four γδ T cells (GEO: GSE27291) and 14 HNSCC cells (GEO: GSE84557) were downloaded from the GEO database. The gene expression profiles were processed by R package “affy.” Data normalization was performed using “RMA” method. “PMA” callings for probes were also detected. Probes characterized with “Present” at a frequency of <50% in both cell types were filtered, resulting in 217 miR-138 targeted genes. Differential expression of miR-138 targeted genes in γδ T cells and HNSCC cells was detected by t test. Multiple testing was adjusted by the Benjamini and Hochberg’s FDR methods.55 Genes with fold change ≥2 and FDR < 0.01 were considered as differentially expressed between the two cell types. The biological and functional annotations of the differentially expressed genes were analyzed by the online tool DAVID.56
Statistics
The comparisons of means among groups were analyzed by one-way ANOVA. All statistical analyses were performed using the SPSS package (version 13.0; SPSS, Chicago, IL, USA). A p value < 0.05 was considered statistically significant.
Author Contributions
Conception and Design: G.Z. and J.L.; Acquisition of Data: L.L., S.L., B.C., H.L., S.W., and S.H.; Analysis and Interpretation of Data: X.L. and J.J.; Writing and Review of the Manuscript: G.Z. and J.L.; Administrative, Technical, or Material Support: J.L.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the Department of Science and Technology of Sichuan Province (grants 2018JY0646, 2015SZ0053, and 2017JQ0040) and the National Natural Science Foundation of China (grants 81772900, 81672690, and 81872196).
Footnotes
Supplemental Information includes two figures and can be found with this article online at https://doi.org/10.1016/j.omtn.2018.11.009.
Contributor Information
Jinyi Lang, Email: langjy610@163.com.
Guiquan Zhu, Email: zgq@sichuancancer.org.
Supplemental Information
References
- 1.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Desiderio V., Papagerakis P., Tirino V., Zheng L., Matossian M., Prince M.E., Paino F., Mele L., Papaccio F., Montella R. Increased fucosylation has a pivotal role in invasive and metastatic properties of head and neck cancer stem cells. Oncotarget. 2015;6:71–84. doi: 10.18632/oncotarget.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Economopoulou P., Agelaki S., Perisanidis C., Giotakis E.I., Psyrri A. The promise of immunotherapy in head and neck squamous cell carcinoma. Ann. Oncol. 2016;27:1675–1685. doi: 10.1093/annonc/mdw226. [DOI] [PubMed] [Google Scholar]
- 4.Wu D., Wu P., Wu X., Ye J., Wang Z., Zhao S., Ni C., Hu G., Xu J., Han Y. Ex vivo expanded human circulating Vδ1 γδT cells exhibit favorable therapeutic potential for colon cancer. OncoImmunology. 2015;4:e992749. doi: 10.4161/2162402X.2014.992749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kondo M., Sakuta K., Noguchi A., Ariyoshi N., Sato K., Sato S., Sato K., Hosoi A., Nakajima J., Yoshida Y. Zoledronate facilitates large-scale ex vivo expansion of functional gammadelta T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy. 2008;10:842–856. doi: 10.1080/14653240802419328. [DOI] [PubMed] [Google Scholar]
- 6.Silva-Santos B., Serre K., Norell H. γδ T cells in cancer. Nat. Rev. Immunol. 2015;15:683–691. doi: 10.1038/nri3904. [DOI] [PubMed] [Google Scholar]
- 7.Brandes M., Willimann K., Moser B. Professional antigen-presentation function by human gammadelta T cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 8.Lo Presti E., Dieli F., Meraviglia S. Tumor-infiltrating γδ T lymphocytes: pathogenic role, clinical significance, and differential programing in the tumor microenvironment. Front. Immunol. 2014;5:607. doi: 10.3389/fimmu.2014.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rei M., Pennington D.J., Silva-Santos B. The emerging protumor role of γδ T lymphocytes: implications for cancer immunotherapy. Cancer Res. 2015;75:798–802. doi: 10.1158/0008-5472.CAN-14-3228. [DOI] [PubMed] [Google Scholar]
- 10.Menard J.A., Cerezo-Magaña M., Belting M. Functional role of extracellular vesicles and lipoproteins in the tumour microenvironment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018;373 doi: 10.1098/rstb.2016.0480. 20160480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peinado H., Lavotshkin S., Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin. Cancer Biol. 2011;21:139–146. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 12.O’Loughlin A.J., Woffindale C.A., Wood M.J. Exosomes and the emerging field of exosome-based gene therapy. Curr. Gene Ther. 2012;12:262–274. doi: 10.2174/156652312802083594. [DOI] [PubMed] [Google Scholar]
- 13.Mathivanan S., Ji H., Simpson R.J. Exosomes: extracellular organelles important in intercellular communication. J. Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Martins V.R., Dias M.S., Hainaut P. Tumor-cell-derived microvesicles as carriers of molecular information in cancer. Curr. Opin. Oncol. 2013;25:66–75. doi: 10.1097/CCO.0b013e32835b7c81. [DOI] [PubMed] [Google Scholar]
- 15.Vader P., Mol E.A., Pasterkamp G., Schiffelers R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016;106(Pt A):148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D., Lee H., Wang X., Rai A., Groot M., Jin Y. Exosome-mediated small RNA delivery: a novel therapeutic approach for inflammatory lung responses. Mol. Ther. 2018;26:2119–2130. doi: 10.1016/j.ymthe.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ventimiglia L.N., Alonso M.A. Biogenesis and function of T cell-derived exosomes. Front. Cell Dev. Biol. 2016;4:84. doi: 10.3389/fcell.2016.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitt J.M., André F., Amigorena S., Soria J.C., Eggermont A., Kroemer G., Zitvogel L. Dendritic cell-derived exosomes for cancer therapy. J. Clin. Invest. 2016;126:1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fais S. NK cell-released exosomes: natural nanobullets against tumors. OncoImmunology. 2013;2:e22337. doi: 10.4161/onci.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshikawa T., Takahara M., Tomiyama M., Nieda M., Maekawa R., Nakatsura T. Large-scale expansion of γδ T cells and peptide-specific cytotoxic T cells using zoledronate for adoptive immunotherapy. Int. J. Oncol. 2014;45:1847–1856. doi: 10.3892/ijo.2014.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin Y., Chen D., Cabay R.J., Wang A., Crowe D.L., Zhou X. Role of microRNA-138 as a potential tumor suppressor in head and neck squamous cell carcinoma. Int. Rev. Cell Mol. Biol. 2013;303:357–385. doi: 10.1016/B978-0-12-407697-6.00009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei J., Nduom E.K., Kong L.Y., Hashimoto Y., Xu S., Gabrusiewicz K., Ling X., Huang N., Qiao W., Zhou S. miR-138 exerts anti-glioma efficacy by targeting immune checkpoints. Neuro-oncol. 2016;18:639–648. doi: 10.1093/neuonc/nov292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altvater B., Pscherer S., Landmeier S., Kailayangiri S., Savoldo B., Juergens H., Rossig C. Activated human γδ T cells induce peptide-specific CD8+ T-cell responses to tumor-associated self-antigens. Cancer Immunol. Immunother. 2012;61:385–396. doi: 10.1007/s00262-011-1111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vantourout P., Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo Presti E., Pizzolato G., Gulotta E., Cocorullo G., Gulotta G., Dieli F., Meraviglia S. Current advances in γδ T cell-based tumor immunotherapy. Front. Immunol. 2017;8:1401. doi: 10.3389/fimmu.2017.01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tominaga N., Yoshioka Y., Ochiya T. A novel platform for cancer therapy using extracellular vesicles. Adv. Drug Deliv. Rev. 2015;95:50–55. doi: 10.1016/j.addr.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Zhu L., Kalimuthu S., Gangadaran P., Oh J.M., Lee H.W., Baek S.H., Jeong S.Y., Lee S.W., Lee J., Ahn B.C. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics. 2017;7:2732–2745. doi: 10.7150/thno.18752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanchard N., Lankar D., Faure F., Regnault A., Dumont C., Raposo G., Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J. Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 29.Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 30.Syn N.L., Wang L., Chow E.K., Lim C.T., Goh B.C. Exosomes in cancer nanomedicine and immunotherapy: prospects and challenges. Trends Biotechnol. 2017;35:665–676. doi: 10.1016/j.tibtech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Kim S.M., Yang Y., Oh S.J., Hong Y., Seo M., Jang M. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J. Control Release. 2017;266:8–16. doi: 10.1016/j.jconrel.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Bliss S.A., Sinha G., Sandiford O.A., Williams L.M., Engelberth D.J., Guiro K., Isenalumhe L.L., Greco S.J., Ayer S., Bryan M. Mesenchymal stem cell-derived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrow. Cancer Res. 2016;76:5832–5844. doi: 10.1158/0008-5472.CAN-16-1092. [DOI] [PubMed] [Google Scholar]
- 33.Kim M.S., Haney M.J., Zhao Y., Yuan D., Deygen I., Klyachko N.L., Kabanov A.V., Batrakova E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine (Lond.) 2018;14:195–204. doi: 10.1016/j.nano.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Usman W.M., Pham T.C., Kwok Y.Y., Vu L.T., Ma V., Peng B., Chan Y.S., Wei L., Chin S.M., Azad A. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 2018;9:2359. doi: 10.1038/s41467-018-04791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Escudier B., Dorval T., Chaput N., André F., Caby M.P., Novault S., Flament C., Leboulaire C., Borg C., Amigorena S. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J. Transl. Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Besse B., Charrier M., Lapierre V., Dansin E., Lantz O., Planchard D., Le Chevalier T., Livartoski A., Barlesi F., Laplanche A. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. OncoImmunology. 2015;5:e1071008. doi: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore C., Kosgodage U., Lange S., Inal J.M. The emerging role of exosome and microvesicle- (EMV-) based cancer therapeutics and immunotherapy. Int. J. Cancer. 2017;141:428–436. doi: 10.1002/ijc.30672. [DOI] [PubMed] [Google Scholar]
- 38.Katakowski M., Buller B., Zheng X., Lu Y., Rogers T., Osobamiro O., Shu W., Jiang F., Chopp M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335:201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu W., Huang L., Li Y., Zhang X., Gu J., Yan Y., Xu X., Wang M., Qian H., Xu W. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315:28–37. doi: 10.1016/j.canlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Lugini L., Cecchetti S., Huber V., Luciani F., Macchia G., Spadaro F., Paris L., Abalsamo L., Colone M., Molinari A. Immune surveillance properties of human NK cell-derived exosomes. J. Immunol. 2012;189:2833–2842. doi: 10.4049/jimmunol.1101988. [DOI] [PubMed] [Google Scholar]
- 41.Kibria G., Ramos E.K., Wan Y., Gius D.R., Liu H. Exosomes as a drug delivery system in cancer therapy: potential and challenges. Mol. Pharm. 2018;15:3625–3633. doi: 10.1021/acs.molpharmaceut.8b00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller L., Simms P., Hong C.S., Nishimura M.I., Jackson E.K., Watkins S.C., Whiteside T.L. Human tumor-derived exosomes (TEX) regulate Treg functions via cell surface signaling rather than uptake mechanisms. OncoImmunology. 2017;6:e1261243. doi: 10.1080/2162402X.2016.1261243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang X.J., Sun X.Y., Huang K.M., Zhang L., Yang Z.S., Zou D.D., Wang B., Warnock G.L., Dai L.J., Luo J. Therapeutic potential of CAR-T cell-derived exosomes: a cell-free modality for targeted cancer therapy. Oncotarget. 2015;6:44179–44190. doi: 10.18632/oncotarget.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A., Lee J.J., Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong T.S., Liu X.B., Wong B.Y., Ng R.W., Yuen A.P., Wei W.I. Mature miR-184 as potential oncogenic microRNA of squamous cell carcinoma of tongue. Clin. Cancer Res. 2008;14:2588–2592. doi: 10.1158/1078-0432.CCR-07-0666. [DOI] [PubMed] [Google Scholar]
- 46.Liu X., Chen Z., Yu J., Xia J., Zhou X. MicroRNA profiling and head and neck cancer. Comp. Funct. Genomics. 2009;2009:837514. doi: 10.1155/2009/837514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manikandan M., Deva Magendhra Rao A.K., Rajkumar K.S., Rajaraman R., Munirajan A.K. Altered levels of miR-21, miR-125b-2*, miR-138, miR-155, miR-184, and miR-205 in oral squamous cell carcinoma and association with clinicopathological characteristics. J. Oral Pathol. Med. 2015;44:792–800. doi: 10.1111/jop.12300. [DOI] [PubMed] [Google Scholar]
- 48.Sha H.H., Wang D.D., Chen D., Liu S.W., Wang Z., Yan D.L., Dong S.C., Feng J.F. miR-138: A promising therapeutic target for cancer. Tumour Biol. 2017;39 doi: 10.1177/1010428317697575. 1010428317697575. [DOI] [PubMed] [Google Scholar]
- 49.Fu D., Yu W., Li M., Wang H., Liu D., Song X., Li Z., Tian Z. MicroRNA-138 regulates the balance of Th1/Th2 via targeting RUNX3 in psoriasis. Immunol. Lett. 2015;166:55–62. doi: 10.1016/j.imlet.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 50.Kondo M., Izumi T., Fujieda N., Kondo A., Morishita T., Matsushita H., Kakimi K. Expansion of human peripheral blood γδ T cells using zoledronate. J. Vis. Exp. 2011;55:3182. doi: 10.3791/3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L., Li C., Wang S., Wang Z., Jiang J., Wang W., Li X., Chen J., Liu K., Li C., Zhu G. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res. 2016;76:1770–1780. doi: 10.1158/0008-5472.CAN-15-1625. [DOI] [PubMed] [Google Scholar]
- 52.Roccaro A.M., Sacco A., Maiso P., Azab A.K., Tai Y.T., Reagan M., Azab F., Flores L.M., Campigotto F., Weller E. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J. Clin. Invest. 2013;123:1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bolstad A.I., Eiken H.G., Rosenlund B., Alarcón-Riquelme M.E., Jonsson R. Increased salivary gland tissue expression of Fas, Fas ligand, cytotoxic T lymphocyte-associated antigen 4, and programmed cell death 1 in primary Sjögren’s syndrome. Arthritis Rheum. 2003;48:174–185. doi: 10.1002/art.10734. [DOI] [PubMed] [Google Scholar]
- 54.Lewis B.P., Burge C.B., Bartel D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 55.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 1995;57:289–300. [Google Scholar]
- 56.Huang da W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.