Abstract
Recently, ethicists have posited that consideration of epigenetic mechanisms presents novel challenges to concepts of justice and equality of opportunity, such as elevating the importance of environments in bioethics and providing a counterpoint to gross genetic determinism. We argue that new findings in epigenetic sciences, including those regarding intergenerational health effects, do not necessitate reconceptualization of theories of justice or the environment. To the contrary, such claims reflect a flawed understanding of epigenetics and its relation to genetics that may unintentionally undermine appeals to social justice. We provide a brief summary of epigenetic sciences, focusing on phenomena central to the current ethical discourse. We identify three fallacious modes of reasoning arising from the emergent literature on the ethical and policy implications of epigenetics, including mischaracterization, undue extrapolation, and exceptionalism. We end by discussing how these issues may work against mobilizing health equity policies and present a more modest claim regarding the value of new epigenetic knowledge to health justice by setting this discourse within the context of known themes in biomedical ethics and health policy.
Introduction
The study of epigenetics, semi-stable biological features that play a role in controlling gene expression without changes in underlying DNA sequences, is a highly technical field. Initially dominated by experimental biologists, geneticists and statisticians, the field is now increasingly attracting social and population scientists and bioethicists. Epigenetics promises novel insights into the biologic function of organisms and their relationship to the environments they inhabit. Drawing on early empirical evidence, popular media has dubbed epigenetics the ‘ghost in your genes’, referring to the potential for the experiences of previous generations to impact gene expression in current and future ones (The Ghost in Your Genes’). Social and basic scientists alike have seized on this work as a powerful explanatory mechanism for the influence of social and economic circumstances on differential health attainment (Geronimus, 2013; Pickersgill et al., 2013; Meloni, 2015; Notterman and Mitchell, 2015) with potential implications for directing health policy (Olden et al., 2014; Park and Kobor, 2015; Wallack and Thornburg, 2016). Based on the promise of such mechanisms, some ethicists have commented on the novel challenges to public health law, health justice and equality of opportunity presented by epigenetics (Rothstein et al., 2009; Loi et al., 2013; Rothstein, 2013; Stapleton et al., 2013; Del Savio et al., 2015; Meloni, 2015), such as elevating the importance of environments in bioethics and providing a counterpoint to gross genetic determinism (Dupras et al., 2014). Others have discussed the complexities inherent in understanding the ethical ramifications of epigenetic science (Juengst etal., 2014; Meloni and Testa, 2014; Waggoner and Uller, 2015; Dupras and Ravitsky, 2016a).
As epigenetics moves into the mainstream of interdisciplinary social and biomedical science investigations, ethicists may increasingly find themselves commenting on the ethical implications of specific epigenetic findings. In this article, we discuss some potential pitfalls that may arise when one uncritically assumes that epigenetic findings pose novel problems regarding justice, equality of opportunity or health policy. We illustrate how a flawed understanding of epigenetic science, particularly with respect to its relation to genetic science, may lead to oversimplified or overstated claims of novelty. We further argue that new findings in epigenetic sciences, including those regarding intergenerational transmission of health effects, do not necessitate reconceptualization of theories of justice or the environment, paying particular attention to the case of fair equality of opportunity (FEO). Finally, we comment on how misrepresentations or overstatements may unintentionally undermine appeals to social justice.
We begin with a brief summary of epigenetic sciences, focusing on phenomena central to the current ethical discourse. We then identify three fallacious modes of reasoning that may arise when considering the ethical and policy implications of epigenetics. First, epigenetics may be mischaracterized as a singular mechanism for multigenerational transmission of human health risks. These mischaracterizations often recapitulate persistent misunderstandings of genetic science and ethics. Second, authors may unduly extrapolate from existing scientific findings to expansive claims about the ability to predict and intervene to prevent future illness. Third, there is a risk of epigenetic exceptionalism, suggesting that novel findings in epigenetic science necessarily present unique challenges to existing ethical frameworks, create novel moral obligations and rhetorical claims or demand specific interventions. We follow by discussing how these conceptual ‘ghosts’ of mischaracterization, extrapolation and exceptionalism may run contrary to efforts to protect vulnerable populations or improve health equity. We conclude by presenting a more modest claim regarding the value of new epigenetic knowledge to health justice, by setting this discourse within the context of extant themes in ethics and health policy.
Introduction to Epigenetics
Epigenetics, in its broadest construction, involves biological elements that persist in cells of living organisms (hereafter, ‘epigenetic elements’) and how these elements control the expression of DNA to produce biological function (hereafter, ‘epigenetic function’). Several types of biological elements or processes fall under the aegis of ‘epigenetics’ or ‘epigenetic mechanisms’, including DNA methylation, histone modification and microRNAs (miRNAs), each with unique dynamics. Importantly however, ethical analyses have tended to focus on the potential ramifications of several common features: first, epigenetic elements exist outside of the DNA sequence and are modifiable by environmental factors. Second, once established, these elements are ‘heritable’, that is retained relatively reliably across cell divisions throughout an individual’s life (Meloni and Testa, 2014; Szyf, 2015). Third, these elements are involved with numerous biologic functions. These three elements of epigenetic function present promising mechanisms for how environmental factors, social inequalities and experiences are ‘embedded’ (Hertzman and Boyce, 2010) or ‘embodied’ (Kuzawa and Sweet, 2009).
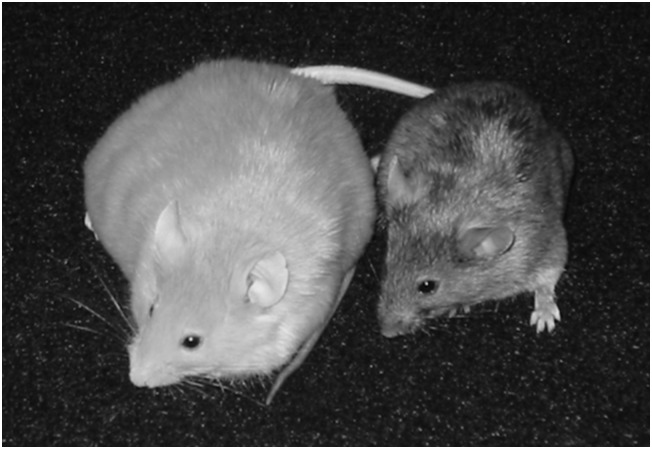
The archetypal example of the promise of epigenetic modification is the agouti mouse experiments by Jirtle and Waterland (Waterland and Jirtle, 2003). Agouti mice, bred to be predisposed to major human health conditions, have higher risks of obesity, cancer and diabetes. After feeding agouti mothers a diet supplemented with micronutrients including folic acid, the researchers found their offspring to be slimmer and healthier than offspring of mothers not fed such a diet (Figure 1). Researchers attributed this finding to the effects of supplementation on epigenetic features—specifically, methylation of an imprinted, transposable element in the agouti gene—in otherwise genetically identical mice (Waterland and Jirtle, 2003). This example illustrated to many the potential for clearly defined intrauterine environmental exposures to profoundly affect the long-term health and appearance of offspring without changes to the genetic sequence. Popular (Watters, 2006) and academic writers alike have since used the agouti mouse experiments, along with accompanying images, as shorthand for the far-reaching promise of epigenetic science for health interventions and social and environmental policy (Rothstein et al., 2009; Meloni and Testa, 2014; Meloni, 2015).
Figure 1.
Typical ‘high-risk’ agouti mouse on the left, ‘low-risk’ mouse whose mother was fed a methyl donor-supplemented diet on the right. ‘DNA is not Destiny: The New Science of Epigenetics’. Discover Magazine. 22 November 2006.
Mischaracterization
Considerations of the impact of epigenetic findings on ethics and policy may lead to a mischaracterization of both epigenetic and genetic science, with important ramifications for resulting ethical and normative arguments. The mischaracterization of the significance of heritability and determinism in epigenetic and genetic sciences particularly illustrates this point.
Heritability
One important claim, based on the observation that epigenetic changes are ‘heritable’, regards the novel situations that arise when biological features in parents and grandparents are inherited by children and grandchildren. For example, Dupras et al. argued that ‘the intergenerational heritability of epigenetic modifications should lead to ethical concerns about the impact that present exposure to environmental disruptors will have on the integrity of the epigenome of descendants, and thus the health of future generations’ (Dupras et al., 2014). The danger here is the potential interpretation of ‘heritable’ and ‘heritability’, which have specific scientific and statistical definitions, to be synonymous with the lay concept of ‘inheritance’, or the transmission of a trait from one generation to another. The immediate consequence of this is an erroneous identification of epigenetics as a specific mode of intergenerational transmission of health or disease risk. In genetic terminology, ‘heritable’ refers to the mitotic (or meiotic) stability of epigenetic elements such as methylated DNA or miRNAs, that is the conservation of such elements across cell divisions and possibly throughout the life of an individual. These elements may be altered by environmental exposures during gestation, or by exposures occurring in previous generations, but like the genome, they describe physiologic facts separate from their environmentally malleable connotations. For instance, the clearance and reinstatement of DNA methylation in early gestation play a role in cellular differentiation and the natural development of embryos (Messerschmidt et al., 2014). Thus, ‘heritability’ of epigenetic marks in a limited sense merely describes the natural process by which organisms develop, without any new implication of environmental influence or intergenerational transmission, though it leaves these possibilities open.
The ethics literature has tended to focus on inheritance, or the potential for environmental exposures in previous generations to affect the health of current or future ones, as a central novelty of epigenetic mechanisms, and a major contrast with genetic mechanisms (Loi et al., 2013; Del Savio et al., 2015)—the so-called ‘ghost in the genes’. Yet inheritance is not necessary for epigenetic mechanisms to operate. Rather, they interact with environmental exposures (Qiu et al., 2015) throughout the life course and can even change stochastically (Stapleton et al., 2013; Dupras and Ravitsky, 2016a) without necessitating inheritance from previous generations. Moreover, epigenetic mechanisms are directly tied to genetic variability (Voisin et al., 2015, Galanter, et al. 2017), undermining any simple distinction between epigenetics and genetics.
In summary, the wide umbrella of epigenetics research encompasses much more than prospects of new modes of inheritance, which while promising, are currently the least developed area of investigation (Nagy et al., 2015). Consequently, the conflation of ‘heritable’ (a fact of epigenetic mechanisms) with ‘inheritance’ (a specific ramification of epigenetics with a growing but still inchoate evidence base) may distort the implications of epigenetic mechanisms beyond current, and possibly future, bodies of evidence.
Determinism
Because of its apparent emphasis on the role of the environment, epigenetics is frequently characterized as distinctly anti-essentialist, and contrasted with putatively deterministic genetics. Yet this dichotomy rests on the dual oversimplification of both epigenetic and genetic sciences (Waggoner and Uller, 2015).
Genetic determinism—the belief that human physiological and behavioral characteristics are determined exclusively by genes, with no environmental influences—remains widespread in popular discourse about genetics (Dar-Nimrod and Heine, 2011). However, the influence of genetic factors on health and disease variation between individuals has always been understood within the context of broader environmental factors (Davey Smith, 2012), without excluding the possibility of intergenerational transmission of characteristics through shared environment. For example, the statistical definition of ‘heritability’ in genetic science is the proportion of the trait attributable to genetic factors in a population, understood to be context-specific and related to the variability of both genetic and environmental factors in that population (Visscher et al., 2008; Turkheimer, 2011a). Such values are often calculated without any quantification of DNA or genetic biomarkers per se. Nonetheless, genetic ‘heritability’ is often misinterpreted as an exact quantification of how likely an outcome or phenotype is to be inherited from parents due to the transmission of certain genes or gene variants. In other words, the study of genetics has long recognized the importance of shared environments alongside the biological function of genes, gene expression and the transmission of DNA within and between generations of organisms.
The common mischaracterization of genetic science as invariably deterministic can lead to claims that epigenetics uniquely allows us to understand the influence of environmental factors on biological function, disease processes and health across generations (Waggoner and Uller, 2015). However, epigenetics is not required to reshape ethical frameworks or public policies to account for both genes and environment; conventional understandings of genetics are enough (Lewontin, 2001).
Ironically, a parallel oversimplification of epigenetics may also have pernicious consequences, obscuring some of the more compelling evidence for multigenerational (Szyf, 2015) or transgenerational (Pembrey et al., 2014) (respectively, exposures affecting multiple generations simultaneously or consecutively) influences of epigenetic factors on health. In many cases, perinatal or early childhood exposures are presented as every bit as deterministic of life chances and future health as individual genes—a form of ‘epigenetic determinism’ that threatens a more robust understanding of the role of both genetics and human agency (Dar-Nimrod and Heine, 2011; Turkheimer, 2011a,b; Juengst et al., 2014). Indeed, in a recent examination of scientific and popular portrayals of epigenetic science, Waggoner and Uller find that ‘in contrast to the received view of epigenetics as anti-deterministic or anti-essentialist, epigenetic research is often couched in language as deterministic as genetics research’ and that ‘the epigenetic approach is firmly embedded in traditional notions of genetic control’(p. 178). From this perspective, the mischaracterization of epigenetics as a novel, anti-deterministic understanding of interactions between organisms, genes and environment may in fact underwrite the belief that an ultimate, complete characterization of epigenetic function can fully determine health and/or behavior.
An oversimplified epigenetic determinism may also result in overstated concerns regarding privacy, similar to concerns about privacy of genetic information (Rothstein et al., 2009; Rothstein, 2013). If epigenetic markers are understood to be as uniquely and powerfully predictive of disease as DNA sequences are believed to be, there may be similar calls for limits to ‘access and utilization of sensitive medical records’ to ‘maximize the privacy and reduce the threat of discrimination against adult citizens’ (Loi et al., 2013: 148). Similarly, Meloni notes concerns regarding ‘a new class of sensitive information’ (Meloni, 2015: 131). Fears of discrimination based upon the unique deterministic quality of genetic information threatened the creation of barriers to research and medical practice (Rothstein et al., 2009; Rothstein, 2013). Epigenetic determinism may lead to a similar dynamic regarding epigenetic information (Joly et al., 2015), including the use of epigenetic biomarkers as ‘health monitoring markers’ (Loi et al., 2013: 174) or as grounds for litigation against parents (Wiener, 2011).
Extrapolation
Considerations of the impact of epigenetic findings on ethics and policy may lead to extrapolation in various forms: from animal models to human interactions; from individual studies of specific mechanisms in specific populations to broad claims about environmental influences; and from demonstrations of possible mechanisms influencing health to broad classes of interventions.
Animal Models
It is tempting to assume that, because intergenerational transmission of health and disease susceptibility through epigenetic means is outside the control of affected individuals, demonstrations of epigenetic influences require public or collective action on causes of health differences. However, the current empirical evidence on multigenerational or transgenerational epigenetic effects (Skinner et al., 2011; Pembrey et al., 2014; Szyf, 2015) complicates this assumption in two ways.
First, most of the evidence for what is commonly understood as ‘inheritance’, that is multigenerational epigenetic transmission from grandparental exposures or earlier, comes from animal model experiments with specific environmental exposures, such as endocrine-disrupting chemicals (Skinner et al., 2011), folic acid or grooming behavior. It is well known that extrapolating from animal models to humans should only be done with great care (Geronimus, 2013); indeed, a well-developed ethics literature already exists on the dangers inherent in such extrapolation with regard to pharmaceuticals and other medical interventions (Shanks et al., 2009; Ioannidis, 2012; Green, 2015; Kimmelman and Henderson, 2015). Caution is particularly warranted when extrapolating from specific chemical or behavioral interventions in animals to complex social policies in humans.
Second, the evidence from human studies thus far has often featured occult, unexpected and somewhat conflicting associations that are sex- and timing-specific, such as changes in paternal grandmother food supply and cardiovascular mortality in females (Pembrey, et al., 2014). Such associations are compelling precisely because they are poorly explained by cultural or social inheritance and better explained by putative processes such as germ-line epigenetic modification. Consequently, such findings speak against the likelihood that broadly applied social policies could address epigenetics-related transmission phenomena. Indeed, so far the most mature application of epigenetic knowledge and interventions in humans is in the realm of cancer drugs and reversible epigenetics (Rodríguez-Paredes and Esteller, 2011), far afield from potential interventions regarding social equity or intergenerational health transmission.
Attributing Responsibility and Remedy
It is similarly tempting to assume that, given epigenetic findings about the role of the environment in intergenerational transmission, one can discount the role of individual agency in mitigating environmental exposures and instead emphasize social or political interventions (Dupras and Ravitsky, 2016a). For example, Dupras, etal. (2014) link molecular epigenetics to a communitarian view of bioethics, in contrast with an individualistic perspective supported by molecular genetics (p. 333). They argue that ‘molecular epigenetics is conceptually related to communitarian conceptualizations of bioethics that argue for more complex visions of individual and population health’ because of the focus on the ‘important influence of the environment [on the body]’ and ‘limits of individual responsibility, and thus the need for collection action and community responsibility’ (p. 333).
However, it does not necessarily follow that identifying, for example, the epigenetic effects of air pollution on individual biology requires a collective investment in air pollution reduction rather than personal investments, for example in air filtration masks or automobiles with ‘biodefense’ capabilities (Oremus, 2016). In fact, identification of epigenetic mechanisms that are influenced by environmental exposures (social, biological, chemical or otherwise) never imply who should be held responsible for any particular causal mechanism nor what the appropriate remedy might be. While social policy is certainly a potential solution, the identification of epigenetic pathways for the environment’s effect on individual health and disease does not necessarily require policy-level interventions.
Note that this form of extrapolation is also common in social epidemiology, with authors assuming that correlations between socioeconomic characteristics and health outcomes require specific policy interventions (Skalická et al., 2009; Harper and Strumpf, 2012). Normative reasoning about the necessity to address upstream societal versus individual, potentially more proximate, causes is based on ethical reasoning and not a consequence of facts determined by empirical investigation.
It is also worth noting that epigenetic markers may by contrast usher in a new era of personalized medicine vis a vis individualized ‘health monitoring markers’, and indeed there are now several private entities offering direct-to-consumer epigenetic (DNA methylation) tests under the aegis of ‘nutrigenomics’. As epigenetic research shifts attention toward to the dynamic, biochemical processes that lead from health to disease within individuals (Dupras and Ravitsky, 2016b), population-level interventions may be even harder to justify. Accordingly, what promise epigenetics has shown for intervention, as far as existing research is concerned, has been limited to biomedical, downstream interventions, notably in cancer diagnosis and treatment (Rodríguez-Paredes and Esteller, 2011), rather than upstream, environmental interventions targeting intergenerational social justice (Loi et al., 2013; Del Savio et al., 2015).
Using Epigenetics to Bolster Existing Ethical Claims
While epigenetic findings do not themselves provide new justifications for social policies, they might provide an improved evidence for existing ethical concerns. Under luck egalitarian (Loi et al., 2013) and Rawlsian (e.g. Daniel’s theory of justice in health) frameworks (Stapleton et al., 2013), some ethicists have contended that knowledge of epigenetic mechanisms contributes novel opportunities to identify, treat or otherwise provide remedy to those who have diminished health or opportunity for health due to controllable (Stapleton et al., 2013), environmentally caused epigenetic ‘damage’ (Loi et al., 2013). Leaving aside the issue of treatment, this claim is complicated by the difficulty of definitively characterizing epigenetic changes as unequivocally harmful (Dupras and Ravitsky, 2016a), and the difficulty of separating unjust epigenetic variations from the social or environmental processes that produced them.
Universality of Harm
The epigenetic mechanisms of intergenerational health transmission of greatest interest to ethicists arose from a synthesis of evolutionary and developmental biology, most notably through Developmental Origins of Health and Disease (DOHaD) theory. Briefly, one paradigm within DOHaD postulates that variations in programming of epigenetic marks in utero are evolved responses to environmental cues that attempt to predict future environments and thereby improve survivability through procreative years (Hanson and Gluckman, 2014). In the primordial example, experience of macronutrient deficiency in utero directs the fetus to develop an energy-conserving ‘thrifty phenotype’ through putative epigenetic programming which allows for greater survival chances despite a nutrient-poor environment. In modern societies, however, high-caloric food is readily available, making such a phenomenon maladaptive, leading to obesity, diabetes and early mortality (Wells, 2007). Ethicists have interpret this ‘mismatch hypothesis’ to imply that adverse health outcomes due to epigenetic variations may be context-specific (Loi et al., 2013; Stapleton et al., 2013), and therefore the normative identification of ‘epigenetically transmitted forms of disadvantage’ (Loi et al., 2013) must be qualified. However, the implications of such a theory, if true, have not been fully considered. If epigenetic programming improves individual adaption to their own environmental contexts, prescribing universal policies may cause unintended harms (Dupras and Ravitsky, 2016a). For example, proponents of the Adaptive Calibration Model of stress response development suggest normatively ‘poor parenting’ behaviors may be conditionally or locally beneficial for offspring, allowing them to be better prepared for future environments (Del Giudice et al., 2011).
Epigenetic Harm Versus Epigenetic Consequence of Social Harm
Epigenetic mechanisms do not in themselves necessarily produce disadvantage; they always work in concert with extant social and economic disadvantages. As such, the injustice of a particular epigenetic variations is always perfectly circumscribed by an existing mechanism of disadvantage, which includes both a prior recognition of a disadvantaged group and an undesirable outcome. As Stapleton, et al. note:
‘…the distribution of epigenetic patterns that are universally accepted to be disadvantageous, that disproportionately affect already disadvantages social groups, would be considered inequitable. However, epigenomics is a highly context-sensitive phenomenon. Alterations may not be iniquitous to health until certain environmental conditions are experienced. In this latter example, epigenetic alterations not universally accepted to be adverse but still presenting a health risk under particular conditions, when disproportionately associated with disadvantaged social groups, would be inequitable if the distribution of these conditions mirrored the distribution of epigenetic alterations’. (p. 141. Emphasis added.)
Finally, even if particular epigenetic signatures can be mapped precisely to a common, unjust environmental cause, interventions do not necessarily need to be targeted at the environmental cause itself. Indeed, researchers and lay observers alike are frequently most excited by the potential for pharmacological interventions to alleviate inherited disadvantage. In this respect, epigenetics is consonant with current tendencies toward ‘molecularization and biomedicalization’ (Dupras and Ravitsky, 2016b). We contend further that this is a direct consequence of the technologies and research questions that remain central to the investigation of epigenetic mechanisms, which favor specificity in identifying variation within individuals rather than in groups. Unlike Dupras and Ravitsky, however, we do not believe that new knowledge of specific epigenetic mechanisms can ever serve as sui generis evidence for moral or ethical obligations, the latter always requiring reasoning based on broader characteristics of causal elements or their effects, and not on the details of the molecular pathways. Thus, while it is possible that epigenetic research may help to identify health effects in groups of individuals with differential exposure (or susceptibility) to an environmental factor, it offers no additional guidance on whether the proper remedy ought to be individual or communal. For example, even if we accept that is ‘unfair to blame the poor for being malnourished or living in toxic environments, factors that, through epigenetics, can negatively affect their own as well as their children’s health’, nothing about the knowledge of the mechanism itself suggests that we must ‘lobby decision-makers, governments and industry to remedy environmental problems’ (Dupras et al., 2014).
Exceptionalism
Considerations of the impact of epigenetic findings on ethics and policy often ascribe considerable power to the field of epigenetics, often resulting in a form of exceptionalism, that is claims that epigenetics as a scientific field, or particular studies regarding epigenetic mechanisms, present unique challenges to existing ethical frameworks, create novel moral obligations and rhetorical claims or demand particular interventions.
Epigenetics as an Exceptional Field
We have already discussed one form of exceptionalism, namely, the assumption that epigenetics provides an unusually broad understanding of the interaction between environments, genes and multiple generations of organisms. As we argued above, this exceptionalism results from misreading both genetic and epigenetic science, and is thus unsupportable: epigenetics is at best an extension or re-orientation of ideas already quite common in genetic science already. In fact, one need not look to (epi)genetics for sophisticated understandings of these phenomena. Human evidence for perinatal and early childhood effects have been accumulating for some time prior to the advent of epigenetics, from maternal smoking or alcohol consumption to parental maltreatment.
Rhetorical Exceptionalism
Another form of exceptionalism is the assumption that epigenetic evidence will be unusually powerful in persuading individuals of the necessity for social justice. Thus, it is sometimes claimed that understanding the epigenetic basis for inherited health and disease susceptibilities will both convince skeptics of the social production of health inequalities to change their minds, and will provide ‘important policy tools’ [Loi] to address such inequalities. For example, Loi et al. (2013) argue that ‘epigenetic [risk markers] might counterbalance currently skeptical views held by the public of environmental risk…might aid in identifying the population more likely to be affected by the environmental risk…[and] more precise epigenetic markers may help convincing [sic] the public of the importance of these factors’. However, there is no evidence that epigenetics is rhetorically or logically superior to other forms of evidence regarding the social determinants of health, or that better messaging of personal genetic risk will improve health (Hollands et al., 2016).
Moreover, if the facts of epigenetic mechanisms merely recapitulate existing dynamics of health transmission and justice considerations as we have outlined above, particularly with respect to the relationships between epigenetics and individual behavior, it is difficult to conceive how resistant minds would be persuaded. There is already copious evidence for the impact of social, economic and environmental factors on the health of current and future generations (WHO, 2008). Resistance to acting on these factors does not result from a lack of evidence, but rather from disagreement over its significance for policy decisions, or the lack of specific studies demonstrating policy effects (Harper and Strumpf, 2012). The discovery of new epigenetic mechanisms may clarify our understanding of how these factors operate but will lend little in the way of changing how people interpret its significance or address the lack of direct evidence of policy effect. It would be difficult to conceive how epigenetic facts would convince an individual to choose a ‘social’ solution when one was not inclined to do so previously. While epigenetic research may indeed help identify ‘means to short circuit the processes—both social and biological—whereby membership in a racialized, gendered, and economically stratified society may lead to health inequalities’ (Geronimus, 2013), the interventions that are eventually indicated and adopted based on this knowledge may fall well short of the expectations of social justice-minded researchers and theorists.
Mobilizing epigenetics as a motivating force for social justice also carries certain inherent dangers. Tying claims to the necessity of social justice to specific empirical claims opens the door for opponents to challenge the normative arguments for social justice by attacking the empirical claims on which they are based. In the realm of evidence-based policy making, Smith has argued that apparent debates over evidence in public health are in reality debates over the ideas that are thought to underlie that evidence, and that evidence is often mobilized in an effort to build support for ideas even in the absence of consensus over the meaning of such evidence (Smith, 2013). There is no guarantee that epigenetic evidence will necessarily support, for example, the necessity of justice-promoting environmental or social policies. Moreover, privileging empirical findings over normative arguments may rob those arguments of their normative force. Epigenetic evidence can be presented in support of multifarious interventions, but cannot adjudicate among them.
Necessity of an Appeal to Epigenetics
Our final point raises the issue of whether an appeal to epigenetic mechanisms is even necessary to make an ethical case for action. Take our earlier example of epigenetic programming of the thrifty phenotype in response to poor nutritional conditions. If the unjustness of epigenetic variations is strictly contingent on the association with unjust distributions of health conditions based on acknowledged disadvantaged groups, epigenetic variations provide no novel understanding of natural variations of health. On the other hand, an environmental condition may be relatively benign for the general population but detrimental to a previously recognized disadvantaged group, in which case the injustice again is defined by group membership and not epigenetic marks, per se. Moreover, it would be difficult to conceive of the distribution of an environmental factor, for example the availability of calorie-dense foods, to be deemed unjust solely on the principle of epigenetic characteristics and not the vulnerable population. This is demonstrated in various theories regarding the intergenerational transmission of disadvantage, including Well’s concept of the ‘metabolic ghetto’, where oppressive colonialist environments produce cardiometabolic disease in women that are reinforced over generations through the health of the matrilineal line to produce disproportionate chronic disease in historically oppressed populations (Wells, 2010). Within such a theory, the identified insult (historical oppression), population (women of the oppressed group) and observed outcomes (disproportionate cardiometabolic disease) define the injustice, without relying on the putative epigenetic mechanisms.
The challenge of defining unjust harms that are uniquely epigenetic in nature, given the preliminary knowledge regarding social determinants of epigenetic variation, can be further illustrated by the common analogy to the genetic condition phenylketonuria (PKU). PKU, the first widely adopted newborn screen for a genetic condition, is characterized by an inability to process dietary phenylalanine caused by variants within the phenylalanine hydroxylase (PAH) gene. If undetected and untreated in newborns, this reduction in PAH activity eventually leads to developmental delays, seizures, behavioral and psychiatric disorders, as toxic concentrations of phenylalanine accumulate in the body despite no outward signs in the first few months of life. However, given near universal screening in wealthy nations and the ability to adopt phenylalanine-restricted diets, individuals identified with PKU may expect to live healthy lives similar to anyone else without PKU. The variants associated with PKU are more common among Northern Europeans and Native Americans, among others (NIH, 2000). PKU shares many features with the epigenetic paradigm of interest to ethicists, including: transmission from parents prior to any agency by the affected individual, the initial lack of outwardly visible disease or dysfunction, interactions with environmental factors to produce harm, a biological explanatory mechanism for suboptimal function and greater likelihood among a recognized disadvantaged group (Native Americans). Applying Stapleton’s definition of inequitable distribution to this case: an injustice can only exist for the Native American group and only if there were disproportionate outcomes, for example if PKU screening, monitoring or special diets were not available to members of this group. The delineation of inequity is established by a recognized disadvantaged group and by unequal treatment, irrespective of the particular source of biological variation. Moreover, the role of the life course environment itself is a relevant analogy: like the ubiquitous availability of calorie-dense foods, the source of the inequity is contingent both on the biologic variation present at birth and a recognized social group. Otherwise, the ubiquity of the environmental factor makes it irrelevant to justice issues.
Additionally, the two features that potentially distinguish epigenetic inheritance from this motivating example, environmental sources of initial biological variability and potential malleability of the condition, also do not contribute novel ethical issues. First, there are a wide range of well-known perinatal phenomena related to maternal exposures, including those with probable or likely epigenetic mechanisms including maternal folate deficiency and neural tube defects, maternal smoking and offspring birth weight, and diethylstilbestrol (DES) exposure and female cancers that are accompanied by various ethical treatments. Generally, these exposures are imagined to be within the scope of maternal control, and the consequent disproportionate burden on mothers is a major challenge to the prospect of using epigenetic knowledge to motivate appeals to social justice (Warin et al., 2011; Warin et al., 2012; Hessler, 2013; Richardson et al., 2014).
The Case of FEO
Perhaps the most compelling case for epigenetic sciences providing an exceptional challenge to health justice is the claim that epigenetic mechanisms demonstrate that (some) traits present at birth are not ‘natural’ or randomly distributed, thus troubling a premise of Rawlsian FEO (Loi, et al., 2013; Kollar and Loi, 2015).
In their article ‘Prenatal Equality of Opportunity’, Kollar and Loi (2015) persuasively argue that defining ‘natural endowments’ as ‘biological properties possessed at birth’ has troubling implications for Rawls’ principle of opportunity. Under this definition, social inequalities that influence the distribution of natural endowments—including intentional selection by advantaged parents, and differences in environmental exposures correlated with social advantage—would fully comply with FEO. Moreover, the persistence of these mechanisms would likely lead to the intensification rather than diminution of social inequalities, while fully complying with FEO. They thus recommend the following revision of FEO: ‘FEO is satisfied if, and only if, there are no inequalities explained by one’s class background; where class background is understood as a property of the family which one is born into and remains until the age of maturity’(p. 42).
We fully agree with Kollar and Loi’s arguments, and note only that they neither require nor necessarily follow from epigenetic findings. Any form of selection of traits in the population due to parental advantage, including maternal conditions that lead to lower (or higher) birth weight, but also fetal selection or germ-line engineering, would serve to undermine the concept of randomly distributed ‘natural’ endowments. Fetal programming is neither necessary nor sufficient to reformulate natural endowments, and natural endowments need not equate to fetal epigenetic programming.
Conclusions
Lest we be reproached for engaging in our own bit of exceptionalist discourse, we note here that many of the problems that we identify are hardly unique to the literature on ethics and epigenetics. Bioethics as a field has long concerned itself with the implications of novel health-related knowledge and technologies, as evidenced by the establishment in 1990 of the National Human Genome Research Institute’s Ethical, Legal and Social Implications (ELSI) Research Program as an integral part of the Human Genome Project (HGP). We do not in any way object to the ELSI-like study of the possible ethical implications of novel research and technology. Rather, we caution against the temptation to claim that new research findings or technologies might unilaterally necessitate changes in ethical theory or policy recommendations. Most would agree that in 1990, many claims to the deterministic impact of the HGP on ethical theory would have been premature and misguided; we believe that analogous claims regarding epigenetics are similarly premature and misguided.
Biomedical Knowledge Is Neither Necessary Nor Sufficient to Inform Moral Claims
The critiques presented here should not be seen as a counterpoint to Meloni’s assertion that ‘recognition of exaggerated claims and controversial issues is not a sufficient reason to shy away from the potential of epigenetic research’ (Meloni and Testa, 2014: 129). Instead, we argue that a more accurate understanding of the lineage and current limitations of the research suggests that the potential of epigenetic research is more likely to inspire novelty in biomedical interventions than in moral and ethical obligations. Recently, Dupras and Ravitsky have similarly suggested that bioethicists should be cautious of making sweeping claims about the specific relevance the field of epigenetics will have on notions of moral or ethical responsibility due to the complexities of epigenetic function within individuals and populations and the current limits on scientific knowledge (Dupras and Ravitsky, 2016b). However, we carry this argument further to suggest that no new discoveries of epigenetic mechanisms will serve as ipso facto justifications of ‘epigenetic responsibility’.
Paralleling a general critique of biomedicine, new knowledge of epigenetic mechanisms themselves is not sufficient or necessary to make claims about the duty to act on social inequities (Krieger, 1994; Meloni, 2015). As Meloni notes, ‘as usual in the history of how biological arguments are imported into the public sphere, there is no one-to-one relationship between scientific theories and social values’ (p. 143, emphasis in original). To the contrary, new mechanistic knowledge will likely be most convincing when it focuses on specific cellular biological mechanisms related to exposures experienced by individuals, whether the source of those exposures is ‘social’. Epigenetics may thus become ‘the basis for reproducing and consolidating structural differences in society (class, gender, and race)’, and for reifying the biological inferiority or incapacity of the poor (p. 142). Whether epigenetic knowledge is used to motivate individual-level or social-level interventions will depend upon the specific cultural and political context in which they emerge. As has been argued, in most Western societies, this context will largely favor individual interventions.
Potential Contributions of Epigenetic Knowledge to Health Justice
It is important to reiterate here that epigenetic research of course holds promise in identifying and clarifying the different ways in which environments, broadly construed, directly interact with human biology, both within and across generations. Furthermore, epigenetic research may contribute to the development of biological ‘signatures’ that may be mapped to the embedding of social injustices in individuals. Such information may provide rhetorical weight and specificity regarding existing social injustices. With respect to health and social policies, the ability of the field of epigenetics to encapsulate ideas of embodied social disadvantage (Kuzawa and Sweet, 2009; Pickersgill et al., 2013), and not the particular discoveries made therein, may be the strongest motivators for policy (Smith, 2013). In such instances, the role of epigenetics is to recapitulate existing claims rather than generate new ones. Similarly, research into transgenerational epigenetic inheritance has generated new interest in the importance of the sperm epigenome and, by extension, paternal factors such as obesity that may influence offspring health (Schagdarsurengin and Steger, 2016). This may begin to shift the balance of parental responsibility and culpability, where the vast majority of developmental research has thus far focused solely on women and their wombs (Warin et al., 2012; Geronimus, 2013). Here again, however, the case for attributing responsibility arises independently from facts of epigenetics: Are potential fathers or governments responsible for promoting healthy weights?
Our critique is in part motivated by a particular interest in claims of the policy relevance of the discovery of specific epigenetic mechanisms. We see our arguments as a complement to Meloni’s claims regarding the translation of scientific findings to public policy (Meloni, 2015: 142–143). In line with Smith’s theoretical work on evidence-based policy (Smith, 2013), we suggest the ideas drawn from and framing empirical research on epigenetics, rather than the findings per se, will be most important to policy making. Correspondingly, we advocate a more deliberate consideration of the ideas relating to social justice that might guide the interpretation and mobilization of epigenetic knowledge. Making unqualified claims of epigenetic research’s natural relevance to issues of social justice and public health ethics may harken back to ideas of genetic determinism and exceptionalism, detracting from important articulations of social justice claims and creating a chilling effect on research. Moreover, while it is important to recognize that epigenetics may reinvigorate existing discussions of intergenerational transmission (Loi et al., 2013; Del Savio et al., 2015), depending solely upon technological innovation to make justice claims brings with it its own set of dangers. At first glance, epigenetics may provide a compelling narrative of the contribution of environments to individual health, but it may just as easily support deterministic arguments (Waggoner and Uller, 2015) and magnify beliefs regarding personal responsibilities and behaviors (Dupras and Ravitsky, 2016b).
While we have used genetic analogues as a closely related illustration of the challenges and potential consequences of using scientific findings to make moral claims, we conclude by restating (Krieger, 1994; Geronimus, 2013) a broader concern with the premise that refining the technical understanding of biological mechanisms can justify policies or practices to eliminate social disparities in health and disease, without invoking independently articulated values, moral, ethics or preferences. Epigenetics joins other scientific advances, including not only genetics but also the study of the stress response, allostatic load and HPA axis function, in bearing the hopes that scientific research can produce social justice. Yet subsuming ethical considerations under the scientific ‘truth’ of biologic mechanisms may unintentionally wind up reinforcing both the epistemological superiority of the biomedical model (Dupras and Ravitsky, 2016b) and the pharmaceutical, clinical and behavioral interventions targeting the cellular and biochemical processes they describe. Such interventions are likely far afield from what theorists envisioned to be the promise of discovering the ‘ghosts in the genes’ as well as those promising a new era of social justice.
Funding
This work was supported by a Canadian Institutes of Health Research Operating Grant #115214, ‘Ethics, Social Determinants of Health, and Health Equity: Integrating Theory and Practice’, though no direct funding was received or set aside for the writing of this article.
References
- BBC. (November 2005). The Ghost In Your Genes. Retrieved from http://www.bbc.co.uk/sn/tvradio/programmes/horizon/ghostgenes.shtml. Accessed 31 July, 2017.
- Dar-Nimrod I., Heine S. J. (2011). Genetic Essentialism: On the Deceptive Determinism of DNA. Psychological Bulletin, 137, 800–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey Smith G. (2012). Epigenesis for Epidemiologists: Does Evo-Devo have Implications for Population Health Research and Practice? International Journal of Epidemiology, 41, 236–247. [DOI] [PubMed] [Google Scholar]
- Del Giudice M., Ellis B. J., Shirtcliff E. A. (2011). The Adaptive Calibration Model of Stress Responsivity. Neuroscience and Biobehavioral Reviews, 35, 1562–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Savio L., Loi M., Stupka E. (2015). Epigenetics and Future Generations. Bioethics, 29, 580–587. [DOI] [PubMed] [Google Scholar]
- Dupras C., Ravitsky V. (2016a). The Ambiguous Nature of Epigenetic Responsibility. Journal of Medical Ethics, 42, 534–541. [DOI] [PubMed] [Google Scholar]
- Dupras C., Ravitsky V. (2016b). Epigenetics in the Neoliberal “Regime of Truth”. Hastings Center Report, 46, 26–35. [DOI] [PubMed] [Google Scholar]
- Dupras C., Ravitsky V., Williams-Jones B. (2014). Epigenetics and the Environment in Bioethics. Bioethics, 28, 327–334. [DOI] [PubMed] [Google Scholar]
- Galanter J. M., Gignoux C. R., Oh S. S., Torgerson D., Pino-Yanes M., Thakur N., Eng C., Hu D., Huntsman S., Farber H. J., Avila P. C., Brigino-Buenaventura E., LeNoir M. A., Meade K., Serebrisky D., Rodríguez-Cintrón W., Kumar R., Rodríguez-Santana J. R., Seibold M. A., Borrell L. N., Burchard E. G., Zaitlen N. (2017). Differential Methylation Between Ethnic Sub-Groups Reflects the Effect of Genetic Ancestry and Environmental Exposures. eLife, 6, e20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus A. T. (2013). Deep Integration: Letting the Epigenome Out of the Bottle Without Losing Sight of the Structural Origins of Population Health. American Journal of Public Health, 103, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. B. (2015). Can Animal Data Translate to Innovations Necessary for a New Era of Patient-Centred and Individualised Healthcare? Bias in Preclinical Animal Research. BMC Medical Ethics, 16, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. A., Gluckman P. D. (2014). Early Developmental Conditioning of Later Health and Disease: Physiology or Pathophysiology? Physiological Reviews, 94, 1027–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper S., Strumpf E. C. (2012). Commentary: Social Epidemiology: Questionable Answers and Answerable Questions. Epidemiology, 23, 795–798. [DOI] [PubMed] [Google Scholar]
- Hertzman C., Boyce T. (2010). How Experience Gets Under the Skin to Create Gradients in Developmental Health. Annual Review of Public Health, 31, 329–347. [DOI] [PubMed] [Google Scholar]
- Hessler K. (2013). Epigenetic Inheritance and the Moral Responsibilities of Mothers. The Virtual Mentor: VM, 15, 767–770. [DOI] [PubMed] [Google Scholar]
- Hollands G. J., French D. P., Griffin S. J., Prevost A. T., Sutton S., King S., Marteau T. M. (2016). The Impact of Communicating Genetic Risks of Disease on Risk-Reducing Health Behaviour: Systematic Review with Meta-Analysis. BMJ, 352, i1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis J. P. (2012). Extrapolating from Animals to Humans. Science Translational Medicine, 4, 151ps15. [DOI] [PubMed] [Google Scholar]
- Joly Y., Dyke S. O. M., Cheung W. A., Rothstein M. A., Pastinen T. (2015). Risk of Re-Identification of Epigenetic Methylation Data: A More Nuanced Response is Needed. Clinical Epigenetics, 7, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juengst E. T., Fishman J. R., McGowan M. L., Settersten R. A. (2014). Serving Epigenetics Before its Time. Trends in Genetics, 30, 427–429. [DOI] [PubMed] [Google Scholar]
- Kimmelman J., Henderson V. (2015). Assessing Risk/Benefit for Trials Using Preclinical Evidence: A Proposal. Journal of Medical Ethics, 42, 50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollar, E. and Loi, M. (2015). Prenatal Equality ofOpportunity. Journal of Applied Philosophy, 32, 35–49. [Google Scholar]
- Krieger N. (1994). Epidemiology and the Web of Causation: Has Anyone Seen the Spider? Social Science and Medicine, 39, 887–903. [DOI] [PubMed] [Google Scholar]
- Kuzawa C. W., Sweet E. (2009). Epigenetics and the Embodiment of Race: Developmental Origins of US Racial Disparities in Cardiovascular Health. American Journal of Human Biology, 21, 2–15. [DOI] [PubMed] [Google Scholar]
- Lewontin R. C. (2001). The Triple Helix: Gene, Organism, and Environment. Cambridge, Mass: Harvard University Press. [Google Scholar]
- Loi M., Del Savio L., Stupka E. (2013). Social Epigenetics and Equality of Opportunity. Public Health Ethics, 6, 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni M. (2015). Epigenetics for the Social Sciences: Justice, Embodiment, and Inheritance in the Postgenomic Age. New Genetics and Society, 34, 125–151. [Google Scholar]
- Meloni M., Testa G. (2014). Scrutinizing the Epigenetics Revolution. Biosocieties, 9, 431–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmidt D. M., Knowles B. B., Solter D. (2014). DNA Methylation Dynamics During Epigenetic Reprogramming in the Germline and Preimplantation Embryos. Genes and Development, 28, 812–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy C., Turecki G., Turecki G. (2015). Transgenerational Epigenetic Inheritance: An Open Discussion. Epigenomics, 7, 781–790. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health (NIH). (2000). Phenylketonuria: Screening and Management. NIH Consensus Statement 2000 October 16–18; 17(3), 1–27. Retrieved from https://consensus.nih.gov/2000/2000phenylketonuria113html.htm. Accessed July 31, 2017.
- Notterman D. A., Mitchell C. (2015). Epigenetics and Understanding the Impact of Social Determinants of Health. Pediatric Clinics of North America, 62, 1227–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olden K., Lin Y. S., Gruber D., Sonawane B. (2014). Epigenome: Biosensor of Cumulative Exposure to Chemical and Nonchemical Stressors Related to Environmental Justice. American Journal of Public Health, 104, 1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremus, W. (May 3, 2016). Future Tense: Tesla's “Bioweapon Defense Mode” Sounds Like a Gimmick. It’s Actually Ingenious. Retrieved from http://www.slate.com/blogs/future_tense/2016/05/03/tesla_model_x_s_bioweapon_defense_mode_is_really_about_china_air_pollution.html. Accessed 31 July 2017.
- Park M., Kobor M. S. (2015). The Potential of Social Epigenetics for Child Health Policy. Canadian Public Policy. Analyse De Politiques, 41, S89–S96. [Google Scholar]
- Pembrey M., Saffery R., Bygren L. O.; Network in Epigenetic Epidemiology. (2014). Human Transgenerational Responses to Early-Life Experience: Potential Impact on Development, Health and Biomedical Research. Journal of Medical Genetics, 51, 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickersgill M., Niewoehner J., Mueller R., Martin P., Cunningham-Burley S. (2013). Mapping the New Molecular Landscape: Social Dimensions of Epigenetics. New Genetics and Society, 32, 429–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W., Wan E., Morrow J., Cho M. H., Crapo J. D., Silverman E. K., DeMeo D. L. (2015). The Impact of Genetic Variation and Cigarette Smoke on DNA Methylation in Current and Former Smokers from the COPDGene Study. Epigenetics, 10, 1064–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S. S., Daniels C. R., Gillman M. W., Golden J., Kukla R., Kuzawa C., Rich-Edwards J. (2014). Society: Don't Blame the Mothers. Nature, 512, 131–132. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Paredes M., Esteller M. (2011). A Combined Epigenetic Therapy Equals the Efficacy of Conventional Chemotherapy in Refractory Advanced Non-Small Cell Lung Cancer. Cancer Discovery, 1, 557–559. [DOI] [PubMed] [Google Scholar]
- Rothstein M. A. (2013). Epigenetic Exceptionalism. The Journal of Law, Medicine and Ethics, 41, 733–736. [DOI] [PubMed] [Google Scholar]
- Rothstein M. A., Cai Y., Marchant G. E. (2009). The Ghost in Our Genes: Legal and Ethical Implications of Epigenetics. Health Matrix, 19, 1–62. [PMC free article] [PubMed] [Google Scholar]
- Schagdarsurengin U., Steger K. (2016). Epigenetics in Male Reproduction: Effect of Paternal Diet on Sperm Quality and Offspring Health. Nature Reviews. Urology, 13, 584–595. [DOI] [PubMed] [Google Scholar]
- Shanks N., Greek R., Greek J. (2009). Are Animal Models Predictive for Humans? Philosophy, Ethics, and Humanities in Medicine, 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalická V., van Lenthe F., Bambra C., Krokstad S., Mackenbach J. (2009). Material, Psychosocial, Behavioural and Biomedical Factors in the Explanation of Relative Socio-Economic Inequalities in Mortality: Evidence from the HUNT Study. International Journal of Epidemiology, 38, 1272–1284. [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Manikkam M., Guerrero-Bosagna C. (2011). Epigenetic Transgenerational Actions of Endocrine Disruptors. Reproductive Toxicology, 31, 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. E. (2013). Beyond Evidence-Based Policy in Public Health: The Interplay of Ideas. Basingstoke, Hampshire, UK: Palgrave Macmillan. [Google Scholar]
- Stapleton G., Schroder-Back P., Townend D. (2013). Equity in Public Health: An Epigenetic Perspective. Public Health Genomics, 16, 135–144. [DOI] [PubMed] [Google Scholar]
- Szyf M. (2015). Nongenetic Inheritance and Transgenerational Epigenetics. Trends in Molecular Medicine, 21, 134–144. [DOI] [PubMed] [Google Scholar]
- Turkheimer E. (2011a). Commentary: Variation and Causation in the Environment and Genome. International Journal of Epidemiology, 40, 598–601. [DOI] [PubMed] [Google Scholar]
- Turkheimer E. (2011b). Genetics and Human Agency: Comment on Dar-Nimrod and Heine (2011). Psychological Bulletin, 137, 825–828. [DOI] [PubMed] [Google Scholar]
- Visscher P. M., Hill W. G., Wray N. R. (2008). Heritability in the Genomics Era–Concepts and Misconceptions. Nature Reviews. Genetics, 9, 255–266. [DOI] [PubMed] [Google Scholar]
- Voisin S., Almén M. S., Zheleznyakova G. Y., Lundberg L., Zarei S., Castillo S., Eriksson F. E., Nilsson E. K., Blüher M., Böttcher Y., Kovacs P., Klovins J., Rask-Andersen M., Schiöth H. B. (2015). Many Obesity-Associated SNPs Strongly Associate with DNA Methylation Changes at Proximal Promoters and Enhancers. Genome Medicine, 7, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner M. R., Uller T. (2015). Epigenetic Determinism in Science and Society. New Genetics and Society, 34, 177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallack L., Thornburg K. (2016). Developmental Origins, Epigenetics, and Equity: Moving Upstream. Maternal and Child Health Journal, 20, 935–940. [DOI] [PubMed] [Google Scholar]
- Warin M., Moore V., Zivkovic T., Davies M. (2011). Telescoping the Origins of Obesity to Women's Bodies: How Gender Inequalities are Being Squeezed Out of Barker's Hypothesis. Annals of Human Biology, 38, 453–460. [DOI] [PubMed] [Google Scholar]
- Warin M., Zivkovic T., Moore V., Davies M. (2012). Mothers as Smoking Guns: Fetal Overnutrition and the Reproduction of Obesity. Feminism and Psychology, 22, 360–375. [Google Scholar]
- Waterland R. A., Jirtle R. L. (2003). Transposable Elements: Targets for Early Nutritional Effects on Epigenetic Gene Regulation. Molecular and Cellular Biology, 23, 5293–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters E. (2006). DNA is not Destiny: The New Science of Epigenetics. Discover Magazine. 22 November 2006.
- Wells J. C. (2007). The Thrifty Phenotype as an Adaptive Maternal Effect. Biological Reviews, 82, 143–172. [DOI] [PubMed] [Google Scholar]
- Wells J. C. (2010). Maternal Capital and the Metabolic Ghetto: An Evolutionary Perspective on the Transgenerational Basis of Health Inequalities. American Journal of Human Biology, 22, 1–17. [DOI] [PubMed] [Google Scholar]
- Wiener C. J. (2011). Transgenerational Tort Liability for Epigenetic Disease. DePaul Journal Health Care Law, 13, 319. [Google Scholar]
- WHO. (2008). Closing the Gap in a Generation: Health Equity Through Action on the Social Determinants of Health: Commission on Social Determinants of Health Final Report. Geneva, Switzerland: World Health Organization, Commission on Social Determinants of Health.