Abstract
This study investigated whether autophagy is activated after sepsis-induced acute kidney injury (AKI) and explored its biological role. Seventy-two normal C57 mice were randomly divided into sham operation group, cecal ligation and puncture (CLP) group and CLP+3-MA (autophagy inhibitor) group; 24 mice in each group. Mice in CLP and CLP+3-MA group were treated with cecal ligation to establish sepsis, while mice in sham operation group were treated with the same surgical operations, but not cecal ligation. Blood samples were collected from 12 mice of each group and the levels of serum creatinine (Cr) and blood urea nitrogen (BUN) were measured. The pathological changes were observed. The remaining 12 mice in each group were kept and the survival rate was recorded. Changes in the expressions of autophagy-related proteins were detected by reverse transcription-semi-quantitative PCR and western blotting. The results revealed that the levels of Cr and BUN in CLP and CLP+3-MA group were significantly higher than those in sham operation group (P<0.05), and the levels of Cr and BUN in CLP+3-MA group were higher than those in CLP group (P<0.05). The pathological score of renal injury in CLP+3-MA group was significantly higher than that of CLP group (P<0.01). The expression levels of Beclin1 and LC3-II/I were significantly increased in CLP group compared to sham operation group (P<0.01), while the expression of p62 was decreased (P<0.01). After 3-MA treatment the expression levels of Beclin1 and LC3-II/I were decreased, compared with CLP group, but accumulation of p62 occurred, and the degree of renal injury was increased. In conclusion, AKI induced by sepsis in mice can induce apoptosis and activate autophagy. The activation of autophagy aggravates the renal injury in mice, which in turn inhibits AKI after sepsis.
Keywords: sepsis, acute renal injury, autophagy, apoptosis
Introduction
Sepsis, as one of the serious complications of trauma, shock, infection and major surgery in patients with clinical critical disease, can often lead to septic shock and multiple organ dysfunction (1,2). Sepsis combined with acute kidney injury (AKI) is an important risk factor for the poor prognosis of patients with critical disease. Sepsis-induced AKI can promote and aggravate the damage of other organs, which in turn leads to death (3,4). Increasing number of recent studies have shown that sepsis-induced AKI is affected by multiple factors. In addition to hemodynamic changes, changes in renal cell apoptosis, endotoxin-induced complex inflammation, and immune network responses also play important roles in this process (5,6). However, the pathogenesis of sepsis-induced AKI is still unclear. Thomas (7) found that macrophage apoptosis is the main cause of immune function inhibition in sepsis. AKI can lead to increased levels of renal cell apoptosis, significantly increasing the mortality rate of patients. Sharma et al (8) also found that sepsis can activate autophagy, leading to a negative impact on the body. As a lysosome involved catabolic process, autophagy mainly acts on the recycle of intracellular macromolecules, removal of damaged organelles and clearance of intracellular pathogens, so as to maintain cell stability, ensure energy supply and protect cells. Autophagy can also assist antigen presenting cells to carry out antigen presentation. Autophagy is a linker between non-specific and specific immune system, and has important roles in infection control and immune steady-state maintenance. However, excessive activation brings damage to the body. Emergence of autophagy can affect the expression levels of autophagy-degrading substrate p62, autophagy constituent proteins LC3-II/I and autophagy-related protein Beclin1 (9–12). Autophagy activation and its role in AKI patients still have not been reported. Our study aimed to investigate the effects of autophagy on mice with sepsis-induced AKI so as to provide new insights into the pathogenesis and clinical treatment of AKI.
Materials and methods
Instruments and materials
Creatinine (Cr) assay kit and blood urea nitrogen (BUN) assay kit (R&D Systems, Inc., Minneapolis, MN, USA), 3-MA (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany); TRIzol kit and reverse transcription kit (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA); semi-quantitative PCR kit (Vazyme Biotech Co., Ltd., Nanjing, China); horseradish peroxidase-labeled anti-rabbit secondary polyclonal antibody (#7074), rabbit anti-mouse Beclin1, rabbit anti-p62, rabbit anti-mouse LC3-II, rabbit anti-LC3-I and rabbit anti-mouse GAPDH monoclonal antibodies (cat. nos. #3495, #39749, #3868, #4599, #2118; Cell Signaling Technology, Inc., Danvers, MA, USA); ECI luminous fluid and contrasting power (Invitrogen; Thermo Fisher Scientific, Inc.); pipettes (Eppendorf, Hamburg, Germany), PCR instrument (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA); UV imaging system (Biometra, Göttingen, Germany); electronic balance (BP121S, Sartorious, Göttingen, Germany); low temperature centrifuge (Thermo Fisher Scientific, Inc.); frozen slicer (Leica Microsystems GmbH, Wetzlar, Germany).
Experimental animals and grouping
Seventy-two male C57 mice, weighing 22–25 g, were purchased from Guangdong Medical Laboratory Animal Center [certificate no. SCXK (Guangdong) 2013-0015]. The animals were raised for 1 week following the circadian rhythm in quiet environment at 25°C with free access to water and food to adapt the feeding environment. The 72 C57 mice were randomly divided into three groups (24 mice in each group): CLP group (cercal ligation), sham operation group (same operation as sepsis group except cecum ligation), CLP+3-MA group (intraperitoneal injection of 3-MA saline solution at a dose of 10 mg/kg before cercal ligation). Peripheral blood of 12 mice of each group was taken at 12 and 24 h after modeling, and the kidneys were taken 24 h after operation (13). The remaining 12 mice in each group were used to observe behavioral changes and survival rates at the 4th and 7th day after surgery. The study was approved by the Ethics Committee of Xuzhou Municipal Hospital Affiliated to Xuzhou Medical University (Xuzhou, China).
Establishment of sepsis model
Mice in each group were fasted for 12 h before surgery to avoid obstructions of intestinal tract. The mice were anesthetized with isoflurane (RWD Life Science, Shenzhen, China). After anesthesia, hair of the mouse abdomen was removed by hair removal cream, and abdomen was opened to find the cecum. One-third of the cecum was ligated with 6-0 suture. Needle puncture with 18 gauge needle was performed and a little intestinal content was squeezed out. Then cecum was put back to the abdominal cavity without squeezing out intestinal contents to contaminate the surgical incision. Mice were injected with a small amount of saline to supplement body fluids. The incision was sutured and disinfected with iodophor (13). After waking up, the mice were reared in a cage with free access to food and water.
Sample collection and preparation
Peripheral blood was extracted from mice of each group at 12 and 24 h after surgery. Blood samples were centrifuged at 2,100 × g for 15 min at 4°C to collect the supernatant. The supernatant was stored in a refrigerator at −80°C. After blood extraction, the right kidney was rapidly collected and fixed in 10% formalin for pathological examination.
ELISA to measure serum concentrations of Cr and BUN
ELISA kit (Wuhan Boster Biological Technology Co., Ltd., Wuhan, China) was used to detect the serum levels of Cr and BUN in strict accordance with the manufacturer's instructions. After incubation, a microplate reader was used to measure OD values at 450 nm to calculate the serum concentrations of Cr and BUN in each group of mice.
Renal tissue injury scoring
Histopathological sections of the kidneys were observed under an optical microscope (Olympus Corp., Tokyo, Japan) to evaluate the loss of renal tissue in each group. Scoring was performed according to the following criteria: normal renal tissue, 0 point; area of damaged renal tubules in renal tissue <25%, 1 point; area of damaged renal tubules in renal tissue between 25 and 50%, 2 points; area of damaged renal tubules in renal tissue between 50 and 75%, 3 points; area of damaged renal tubules in renal tissue between 75 and 100%, 4 points. The levels of Cr and BUN in the serum of each group were detected by Cr and BUN assay kits according to the manufacturer's instructions.
Reverse transcription-semi-quantitative PCR to detect the expression of mRNAs
Total RNA was extracted from renal tissue using TRIzol kit, the OD values of the RNA samples were measured and the integrity of RNA samples was checked by agarose gel electrophoresis. Only RNA samples with a ratio of A260/A280 between 1.8 and 2.0 and three clear bands of 28S, 18S and 5S RNA (the signal of 28S is about twice higher than that of 18S) were used for subsequent studies. After cDNA synthesis through reverse transcription, the expression levels of Beclin1, p62, and LC3-II were detected by reverse transcription-semi-quantitative PCR using GAPDH as the endogenous control. PCR reaction conditions were: 95°C for 5 min, followed by 40 cycles of 95°C for 45 sec, 58°C for 30 sec and 72°C for 45 sec, and a final step of 72°C for 5 min. All primers used were synthesized by Tiangen Biotech Co., Ltd. (Beijing, China). Primer sequences are listed in Table I. PCR products were subjected to 15% agarose gel electrophoresis and the bands were observed using a UV imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Relative expression levels of mRNA in each group were represented by the ratio of Beclin1/GAPDH, p62/GAPDH and LC3-II/I/GAPDH.
Table I.
Sequences of primers used in PCR.
| Gene | Sequences |
|---|---|
| Beclin1 | F: 5′-GGCCTAGCTAAGCTTAGCTACG-3′ |
| R: 5′-CCGTTGTACGTACTAGCTACC-3′ | |
| p62 | F: 5′-CCGTGTGCATCGTAAACTGA −3′ |
| R: 5′-GGCGTTCGATCGATCAAGTC-3′ | |
| LC3-II | F: 5′-CCTGTGCATAGAATCAACT-3′ |
| R: 5′-CGTTGTCAGTCCAACTGAA-3′ | |
| GAPDH | F: 5′-GATGATTGGCATGGCTTT-3′ |
| R: 5′-CACCTTCCGTTCCAGTTT-3′ |
F, forward; R, reverse.
Western blotting to detect the expression levels of related proteins
Renal tissue was mixed with RIPA lysate (Beyotime Institute of Biotechnology, Shanghai, China) to make the homogenate, and lysis was stopped when no visible tissue was observed. After centrifugation (10,800 × g, 4°C) for 10 min, the supernatant was collected and BCA protein quantification kit (Invitrogen; Thermo Fisher Scientific, Inc.) was used to measure the protein concentration. Protein samples (20 µl) were subjected to 12% SDS-PAGE gel electrophoresis under 80 V for 120 min, followed by transmembrane to PVDF membrane under 100 V for 9 min. Membranes were blocked with TBST containing 5% skimmed milk at room temperature for 2 h, followed by incubation with Beclin1, p62, LC3-I and LC3-II monoclonal primary antibodies (1:1,000) at 4°C for 12 h. After that, the membranes were washed 3 times with TBST, 5 min for each time, followed by incubation with a secondary antibody (1:5,000) for 2 h. After that, the membranes were washed 3 times with TBST, 5 min for each time. The solutions A and B of ECL kit (EMD Millipore, Burlington, MA, USA) were added with a ratio of 1:1 to detect the signal. The results were scanned and ImageJ (National Institutes of Health, Bethesda, MD, USA) software was used to analyze gray values.
Statistical analysis
The data in this study are expressed as mean ± standard deviation. Data were processed using SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA). Comparisons between two groups were performed by t-test and comparisons among multiple groups were performed by variance analysis. Test for homogeneity of variances was performed. If the variance was homogeneous, Bonferronic method was used for comparison between two groups. If the variance was heterogeneous, multiple comparisons were performed using Dunnett's T3 method. Kaplan-Meier method was used for survival rate analysis and a log-rank test was performed for comparison. P<0.05 was considered to indicate a statistically significant difference.
Results
Changes in renal function in mice
Levels of Cr and BUN in the urine of each group were detected by Cr and BUN detection kit. As shown in Figs. 1 and 2, the levels of Cr and BUN in CLP and CLP+3-MA group were significantly higher than those in sham operation group (P<0.05), and the levels of Cr and BUN in CLP group were significantly lower than those in CLP+3-MA group (P<0.05).
Figure 1.
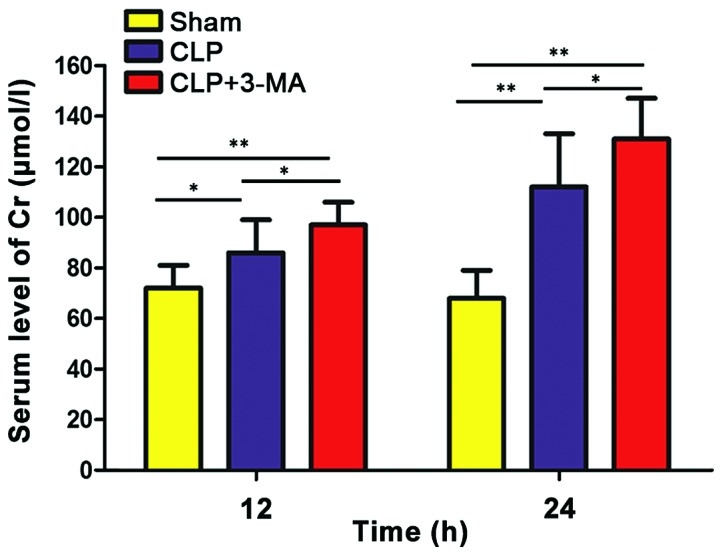
Serum levels of Cr in each group of mice. The levels of Cr in CLP and CLP+3-MA groups were significantly higher than that in sham operation group (**P<0.01, *P<0.05), and the levels of Cr in CLP+3-MA group were significantly higher than that in CLP group (*P<0.05), n=6. Cr, creatinine.
Figure 2.
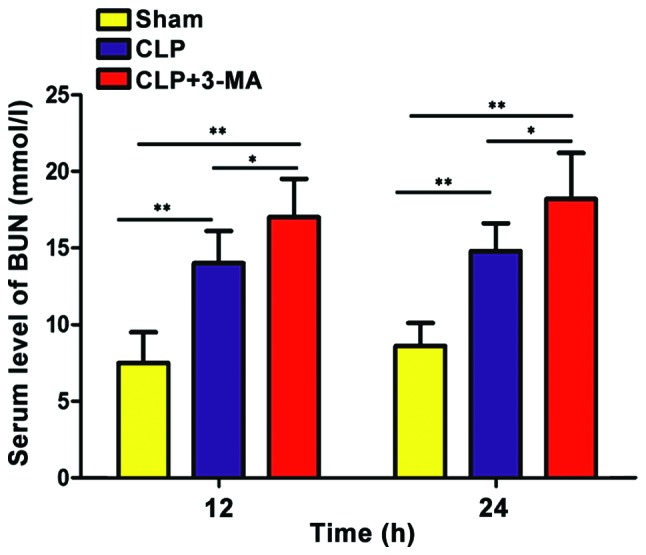
Serum levels of BUN in each group of mice. The levels of BUN in CLP and CLP+3-MA groups were significantly higher than that in sham operation group (**P<0.01), and the levels of BUN in CLP+3-MA group were significantly higher than that in CLP group (*P<0.05), n=6. BUN, blood urea nitrogen.
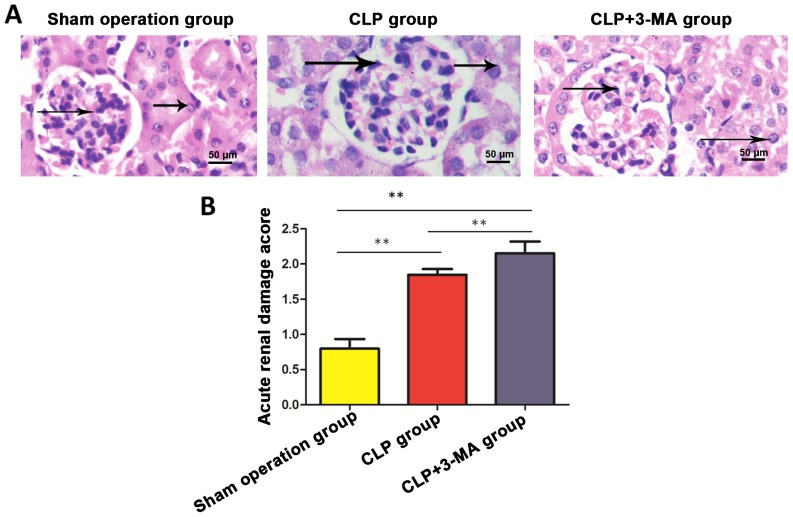
Pathological changes of renal tissue in mice
Pathological changes of renal tissue samples were observed under an optical microscope. No sepsis was observed in sham operation group, the tissue structure was clear and the structures of renal tubules and glomerulus were normal. Telangiectasia and severe congestion were observed in renal tissue sections of the CLP and CLP+3-MA group, obvious edema was observed in renal tubular epithelial cells, and granule-like degeneration or vacuolar degeneration was observed in the tissues (Fig. 3A). The tissues were scored using the same scoring criteria. The results showed that the scores of sepsis and CLP+3-MA group were significantly higher than that of sham operation group (P<0.01), and the score of CLP+3-MA group was significantly higher than that of the sepsis group (P<0.01) (Fig. 3B and Table II).
Figure 3.
H&E staining to detect renal damage in each group. (A) Compared with sham operation group, renal tissue glomerular capillary dilation, hyperemia, renal tubular epithelial cell edema, granulocyte degeneration or vacuolar degeneration, and lumen narrowing (arrows) were found in CLP and CLP+3-MA group. (B) Scores of CLP and CLP+3-MA group were significantly higher than that of sham operation group (**P<0.01); score of CLP+3-MA group was significantly higher than that of CLP group (**P<0.01), n=6. H&E, hematoxylin and eosin.
Table II.
Pathological changes of renal tissues in each group of mice.
| Groups | 12 h | 24 h |
|---|---|---|
| Sham operation | 0.68±0.19 | 0.71±0.16 |
| Sepsis | 1.78±0.26a | 1.86±0.19a |
| 3-MA | 1.95±0.21a,b | 2.06±0.27a,b |
P<0.01, compared with sham operation group
P<0.01, compared with sepsis group.
Mouse survival rate
The mice were reared in the same environment after surgery, and the survival rates at the 4th and 7th day after surgery were recorded. As shown in Fig. 4 and Table III, the survival rates of mice in CLP and CLP+3-MA group were significantly lower than that in sham operation group at the 4th and 7th day after surgery (P<0.05). The survival rate of mice in CLP+3-MA group was significantly higher than that in CLP group at the 4th and 7th day after surgery (P<0.01).
Figure 4.
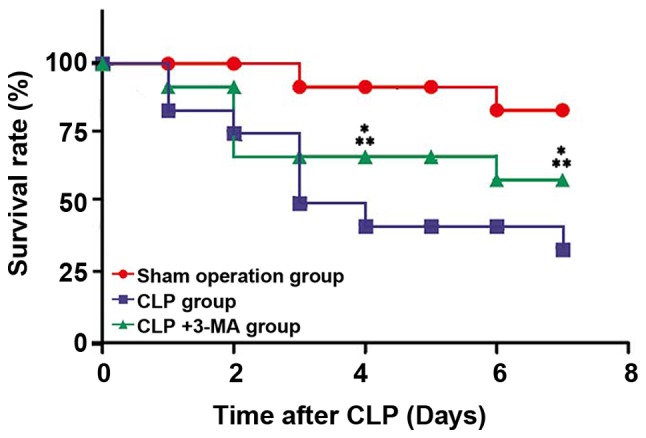
Survival rate of mice after modeling. The survival rate of mice in CLP+3-MA group was significantly higher than that in CLP group at the 4th and 7th day after surgery (**P<0.01). The survival rate of mice in CLP+3-MA group was significantly lower than that in sham operation group at the 4th and 7th day after surgery (*P<0.05), n=12.
Table III.
The survival rate of mice in each group.
| Groups | Survival rate of 4 days (%) | Survival rate of 7 days (%) |
|---|---|---|
| Sham operation | 91.6 | 87.5 |
| Sepsis | 41.6a | 37.5a |
| 3-MA | 66.7a,b | 58.3a,b |
P<0.01, compared with sham operation group
P<0.05, compared with sepsis group.
mRNA expression detected by reverse transcription-semi- quantitative PCR
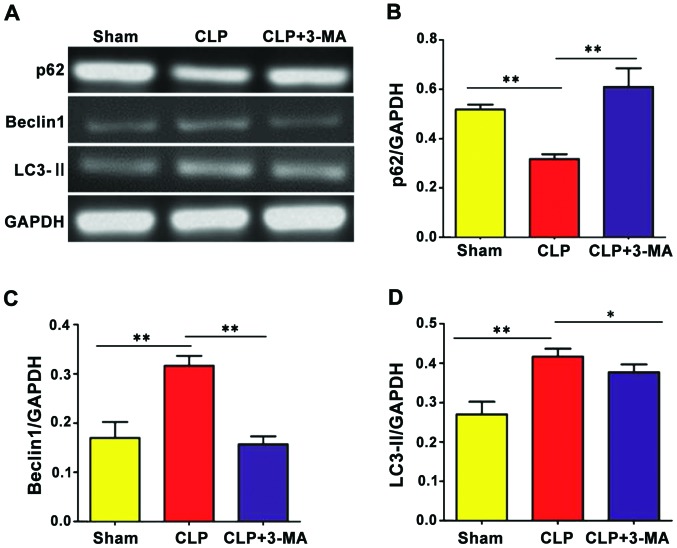
The expression of autophagy-related genes was detected by reverse transcription-semi-quantitative PCR. As shown in Fig. 5, the expression levels of autophagy-related genes Beclin1 and LC3-II were significantly increased in CLP group compared with the sham group (P<0.01), while the expression of p62 was significantly decreased (P<0.01). With 3-MA treatment, the expression levels of autophagy-related genes Beclin1 and LC3-II were decreased compared with CLP group (P<0.01 and P<0.05, respectively), but the expression level of p62 was increased (P<0.01).
Figure 5.
Reverse transcription-semi-quantitative PCR to detect the expression of autophagy-related genes. The expression levels of the autophagy-related genes (A and C) Beclin1 and (A and D) LC3-II were significantly increased in CLP group compared with those in the sham group (**P<0.01), while (A and B) the expression level of p62 was significantly decreased (**P<0.01). Compared with CLP group, the expression levels of (A and C) Beclin1 and (A and D) LC3-II were decreased in CLP+3-MA group (**P<0.01, *P<0.05), while (A and B) the expression level of p62 was increased (**P<0.01, *P<0.05), n=12.
Western blotting to detect the expression of related proteins
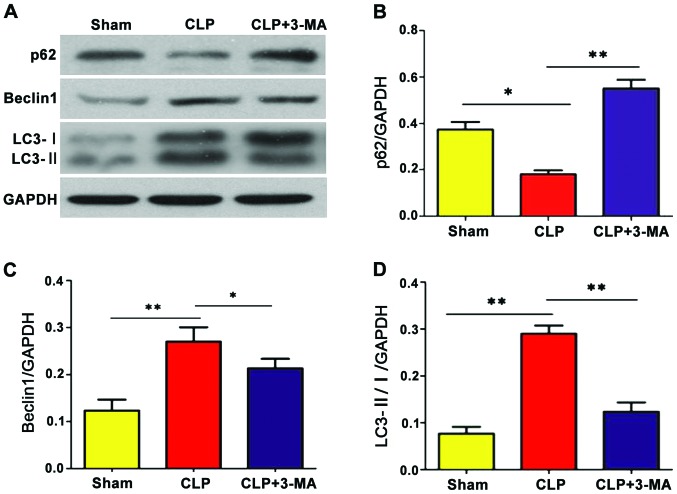
Western blotting was used to detect the expression of autophagy-related proteins. As shown in Fig. 6, the expression levels of autophagy-related proteins Beclin1 and LC3-II were significantly increased in CLP group compared with that in the sham group (P<0.01), while the expression level of p62 protein was significantly decreased (P<0.05). With 3-MA treatment, the expression of autophagy-related proteins Beclin1 and LC3-II was inhibited; compared with that in CLP group the expression levels were decreased (P<0.05 and P<0.01, respectively), but the expression level of p62 protein was increased in CLP+3-MA group (P<0.01).
Figure 6.
Western blotting to detect the expression of autophagy-related proteins. The expression levels of autophagy-related proteins (A and C) Beclin1 and (A and D) LC3-II/I were significantly increased in CLP group compared with the sham group (**P<0.01), while (A and B) the expression level of p62 protein was significantly decreased (*P<0.05). Compared with CLP group, the expression levels of (A and C) Beclin1 and (A and D) LC3-II/I protein were decreased in CLP+3-MA group (*P<0.05, **P<0.01), while (A and B) the expression level of p62 protein was increased (**P<0.01), n=12.
Discussion
Sepsis can lead to multiple organ failures. Pathophysiological changes of sepsis are complex, and the specific pathogenesis is still unclear, which in turn brings great obstacles to the clinical treatment (14). AKI is a common complication of severe sepsis and is an important factor for the death of patients (15). At present, the basic research on sepsis mainly includes in vivo and in vitro experiments. The use of animal models can effectively simulate the pathological process of sepsis, which provides reference for elucidating the pathogenesis of sepsis in human (16). In this study, cecal puncture was used to establish the mouse model of AKI induced by sepsis. The size of the selected puncture needle and the number of punctures can significantly affect the stability of the model. The position and number of the punctures in experiment were strictly in accordance with the standard to ensure the consistency of the experimental results. Infusion was performed after surgery to simulate the onset of clinical sepsis.
After model establishment, the degree of renal injury was evaluated by measuring serum Cr and BUN levels. Results showed that serum Cr and BUN levels in CLP group were significantly higher than those in Sham operation group, indicating the successfully established mouse sepsis model. 3-MA is a commonly used autophagy inhibitor that inhibits autophagy activity in vivo and is often used as a tool for studying the physiological significance of autophagy activity in the body (17). 3-MA treatment before surgery can reduce damage to renal function in mice with sepsis-induced AKI, indicating that the activation of autophagy plays a negative role in sepsis-induced AKI, and can be protective through the inhibition of autophagy. The expression of autophagy-related proteins was detected by reverse transcription-semi-quantitative PCR and western blotting. Results showed that the expression levels of autophagy-related proteins Beclin1 and LC3-II/I were significantly increased and the expression level of autophagy substrate p62 was decreased in CLP group compared to sham operating group, indicating that sepsis can activate the autophagy in the body. With 3-MA treatment, the expression levels of Beclin1 and LC3-II/I were decreased and the expression level of p62 was increased, compared to the CLP group, and thus the activation of autophagy in sepsis was further confirmed by this inhibitor. At the same time, compared with mice in CLP group, renal injury of CLP+3-MA group was less serious, and the survival rates of CLP+3-MA group at the 4th and 7th day after surgery were significantly higher than those of CLP group, indicating that the inhibition of autophagy can protect the body from AKI induced by sepsis. Autophagy in sepsis can be activated by a variety of cell stresses and the activated autophagy has protective effects on the body. Macdonald et al (18) found that macrophage apoptosis would occur after sepsis, leading to the dysfunction of the immune system. Macrophage apoptosis can also cause the production of inflammatory factors, leading to immunological suppression. Both autophagy and apoptosis present in sepsis-induced AKI. Autophagy and apoptosis share the same regulatory factors, and there is a dynamic balance between apoptosis and autophagy in the body (19,20). Our study still has some limitations. The activation of autophagy and apoptosis in sepsis-induced AKI was not deeply studied. The mechanism of the functions of autophagy and apoptosis was not elucidated. These will be the focus of future studies.
In summary, autophagy is activated in sepsis-induced AKI and autophagy activation brings damage to the body. The survival rate of patients with sepsis-induced AKI can be increased by inhibiting autophagy. This study provides a new direction for the clinical treatment of sepsis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Development Fund of Clinical Medicine Technology, Jiangsu University (JLY20160127), the Xuzhou Science and Technology Project (KC16SH020), the General Program of the National Natural Science Foundation of China (31571187) and the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (PPZY2015B161).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YW and YZ assisted with PCR and western blotting. LW and LM were responsible for the establishment of the sepsis model. GC and YLZ contributed to ELISA and renal tissue injury scoring. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Xuzhou Municipal Hospital Affiliated to Xuzhou Medical University (Xuzhou, China).
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
References
- 1.Endo A, Okamura M, Yoshikawa S, Otomo Y, Morio T. Multilateral functional alterations of human neutrophils in sepsis: From the point of diagnosis to the seventh day. Shock. 2017;48:629–637. doi: 10.1097/SHK.0000000000000883. [DOI] [PubMed] [Google Scholar]
- 2.Arulkumaran N, Sixma ML, Jentho E, Ceravola E, Bass PS, Kellum JA, Unwin RJ, Tam FWK, Singer M. Sequential analysis of a panel of biomarkers and pathologic findings in a resuscitated rat model of sepsis and recovery. Crit Care Med. 2017;45:e821–e830. doi: 10.1097/CCM.0000000000002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang N, Mao L, Yang L, Zou J, Liu K, Liu M, Zhang H, Xiao X, Wang K. Resveratrol protects against early polymicrobial sepsis-induced acute kidney injury through inhibiting endoplasmic reticulum stress-activated NF-κB pathway. Oncotarget. 2017;8:36449–36461. doi: 10.18632/oncotarget.16860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai ZY, Sheng ZX, Yao H. Pachymic acid ameliorates sepsis-induced acute kidney injury by suppressing inflammation and activating the Nrf2/HO-1 pathway in rats. Eur Rev Med Pharmacol Sci. 2017;21:1924–1931. [PubMed] [Google Scholar]
- 5.Martin-Loeches I, Muriel-Bombín A, Ferrer R, Artigas A, Sole-Violan J, Lorente L, Andaluz-Ojeda D, Prina-Mello A, Herrán-Monge R, Suberviola B, et al. GRECIA group, corp-author. The protective association of endogenous immunoglobulins against sepsis mortality is restricted to patients with moderate organ failure. Ann Intensive Care. 2017;7:44. doi: 10.1186/s13613-017-0268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wollborn J, Schlegel N, Schick MA. Phosphodiesterase 4 inhibition for treatment of endothelial barrier and microcirculation disorders in sepsis. Anaesthesist. 2017;66:347–352. doi: 10.1007/s00101-017-0305-5. (In German) [DOI] [PubMed] [Google Scholar]
- 7.Thomas H. Sepsis: Bile acids promote inflammation in cholestasis-associated sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:324–325. doi: 10.1038/nrgastro.2017.55. [DOI] [PubMed] [Google Scholar]
- 8.Sharma D, Farahbakhsh N, Shastri S, Sharma P. Biomarkers for diagnosis of neonatal sepsis: A literature review. J Matern Fetal Neonatal Med. 2018;31:1646–1659. doi: 10.1080/14767058.2017.1322060. [DOI] [PubMed] [Google Scholar]
- 9.Park SY, Shrestha S, Youn YJ, Kim JK, Kim SY, Kim HJ, Park SH, Ahn WG, Kim S, Lee MG, et al. Autophagy primes neutrophils for neutrophil extracellular trap formation during sepsis. Am J Respir Crit Care Med. 2017;196:577–589. doi: 10.1164/rccm.201603-0596OC. [DOI] [PubMed] [Google Scholar]
- 10.Crowell KT, Soybel DI, Lang CH. Inability to replete white adipose tissue during recovery phase of sepsis is associated with increased autophagy, apoptosis, and proteasome activity. Am J Physiol Regul Integr Comp Physiol. 2017;312:R388–R399. doi: 10.1152/ajpregu.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin L, Batra S, Jeyaseelan S. Deletion of Nlrp3 augments survival during polymicrobial sepsis by decreasing autophagy and enhancing phagocytosis. J Immunol. 2017;198:1253–1262. doi: 10.4049/jimmunol.1601745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi X, Yang F, Zheng YN, Zhang H, Wang XX, Shao GJ, Lai XL. Metabolomic approach for the identification of therapeutic targets of erythropoietin against sepsis in rat models. Eur Rev Med Pharmacol Sci. 2016;20:537–546. [PubMed] [Google Scholar]
- 13.Deng M, Huang L, Ning B, Wang N, Zhang Q, Zhu C, Fang Y. β-asarone improves learning and memory and reduces acetyl cholinesterase and Beta-amyloid 42 levels in APP/PS1 transgenic mice by regulating Beclin-1-dependent autophagy. Brain Res. 2016;1652:188–194. doi: 10.1016/j.brainres.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Salah FS, Ebbinghaus M, Muley VY, Zhou Z, Al-Saadi KR, Pacyna-Gengelbach M, O'Sullivan GA, Betz H, König R, Wang ZQ, et al. Tumor suppression in mice lacking GABARAP, an Atg8/LC3 family member implicated in autophagy, is associated with alterations in cytokine secretion and cell death. Cell Death Dis. 2016;7:e2205. doi: 10.1038/cddis.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng YH, Xiong B, Deng YY, Lai W, Zheng SY, Bian HN, Liu ZA, Huang ZF, Sun CW, Li HH, et al. Effects of allogeneic bone marrow mesenchymal stem cells on polarization of peritoneal macrophages in rats with sepsis. Zhonghua Shao Shang Za Zhi. 2017;33:217–223. doi: 10.3760/cma.j.issn.1009-2587.2017.04.006. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 16.Huan JN. Recognizing prevention and treatment of burn sepsis with the concept of holistic integrative medicine. Zhonghua Shao Shang Za Zhi. 2017;33:196–199. doi: 10.3760/cma.j.issn.1009-2587.2017.04.002. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 17.Greninger AL, Hess JR. Clostridium perfringens sepsis masquerading as a hemolytic transfusion reaction. Transfusion. 2017;57:1112. doi: 10.1111/trf.13863. [DOI] [PubMed] [Google Scholar]
- 18.Macdonald SPJ, Bosio E, Neil C, Arendts G, Burrows S, Smart L, Brown SGA, Fatovich DM. Resistin and NGAL are associated with inflammatory response, endothelial activation and clinical outcomes in sepsis. Inflamm Res. 2017;66:611–619. doi: 10.1007/s00011-017-1043-5. [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Guo J, Mu J, Tian L, Zhou D. Rapamycin protects sepsis-induced cognitive impairment in mouse hippocampus by enhancing autophagy. Cell Mol Neurobiol. 2017;37:1195–1205. doi: 10.1007/s10571-016-0449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan SX, Shi B, Lou XL, Liu JQ, Ma GG, Liang DY, Ma S. Ghrelin protects small intestinal epithelium against sepsis-induced injury by enhancing the autophagy of intestinal epithelial cells. Biomed Pharmacother. 2016;83:1315–1320. doi: 10.1016/j.biopha.2016.08.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.