Abstract
Lung cancer is one of the most common types of cancer with one of the highest incidence and mortality rates. Gefitinib is widely used for the treatment of non-small cell lung cancer (NSCLC). However, issues regarding drug resistance, toxicity and limited applicability have been associated with gefitinib. The aim of the present study was to investigate whether ginsenoside Rg3 enhances the anticancer activity of gefitinib in NSCLC cells. MTT assay demonstrated that ginsenoside Rg3 increased the cytotoxic effect of gefitinib in NSCLC cell lines in a dose- and time-dependent manner. In addition, flow cytometric analysis revealed that the combined treatment with gefitinib and ginsenoside Rg3 significantly increased apoptosis in NSCLC cell lines. Transwell migration assays demonstrated that the combined treatment with gefitinib and ginsenoside Rg3 significantly decreased NSCLC cell migration compared with gefitinib or ginsenoside Rg3 treatment alone. Furthermore, western blot analysis revealed that in NSCLC cell lines, the combined treatment with gefitinib and ginsenoside Rg3 increased protein expression levels of pro-apoptotic proteins Bax and cleaved-caspase-3, whilst the expression level of anti-apoptotic protein Bcl-2 decreased. In addition, western blot analysis revealed that, in NSCLC cell lines, the combined treatment with gefitinib and ginsenoside Rg3 decreased the protein expression levels of pro-migration factors SNAIL and SLUG, whilst the expression level of anti-migration protein E-cadherin increased. In conclusion, ginsenoside Rg3 may be able to enhance the anticancer activity of gefitinib, making NSCLC cells more sensitive to gefitinib.
Keywords: gefitinib, ginsenoside Rg3, lung cancer, viability, migration
Introduction
Lung cancer is one of the most common types of cancer with high incidence and mortality rates (1). Lung cancer accounts for 1.6 million mortalities each year and is one of the most common causes of cancer-associated mortality worldwide (2,3). Lung cancer is the fourth leading cause of mortality in China (2,3). Non-small cell lung cancer (NSCLC) is a subtype of lung cancer, which accounts for approximately 85% of all lung cancer cases (4). NSCLC can be classified into several subtypes which include, squamous cell carcinoma, adenocarcinoma, large-cell carcinoma and bronchioloalveolar carcinoma (5). The main treatment options for patients with NSCLC include surgery, radiotherapy and chemotherapy (4). Surgery is regarded as the most efficient treatment, however clinical application is limited to patients with advanced NSCLC, which account for 70% of all new lung cancer cases (6). The development of new therapeutic strategies based on chemotherapeutic agents is required to potentially improve clinical outcomes.
Targeted therapies for NSCLC have made promising progress (7,8). Gefitinib is a well-known targeted drug for the treatment with NSCLC, which acts as an epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) (9). Gefitinib competitively inhibits ATP binding at the ATP intracellular domain of EGFR, to prevent the autophosphorylation and activation of downstream signaling pathways, leading to the inhibition of tumor cell proliferation, metastasis, and angiogenesis (10). Gefitinib is widely used as the standard first-line treatment for patients with advanced NSCLC with active EGFR mutations (11,12). Although gefitinib can improve the progression-free survival and overall survival of patients with NSCLC, issues regarding drug resistance, toxicity and limited applicability need to be addressed (13).
Natural products, which include Chinese herbal medicine extracts, have gained increasing attention in tumor therapy due to high efficacy and low toxicity. Combined with chemotherapeutic agents or targeted therapies, natural products may enhance the antitumor efficacy whilst reducing side effects associated with traditional therapeutic strategies (14–16). Ginsenoside Rg3 is a steroidal saponin isolated from a traditional Chinese herbal medicine, Panax ginseng, with anticancer activity (17). Several studies revealed that ginsenoside Rg3 could enhance lung cancer sensitivity to chemotherapy (18,19). The aim of the current study was to investigate whether ginsenoside Rg3 enhances gefitinib efficiency in altering NSCLC cell proliferation, apoptosis, and migration by using two NSCLC cell lines with different sensitivities to gefitinib.
Materials and methods
Cell culture and reagents
NSCLC cell lines A549 and H1299 were purchased from Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI-1640 medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin and maintained at 37°C in a 5% CO2-humidified incubator. Gefitinib was purchased from Selleck Chemicals (cat. no. S1025; Houston, TX, USA) and dissolved in DMSO (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at a concentration of 100 mM. Ginsenoside Rg3 was purchased from Biopurify Phytochemicals Ltd. (Chengdu, China) and dissolved in DMSO at a concentration of 50 mg/ml.
Cell proliferation assay
Cell viability was measured using MTT assay (cat. no. C0009; Beyotime Institute of Biotechnology, Haimen, China). A549 or H1299 cells were seeded in 96-well plates at a density of 1×104 cells/well and cultured overnight. Following incubation, cells were treated with 0, 5, 10 or 20 µM gefitinib with or without ginsenoside Rg3 (12.5 or 25 µg/ml) at 37°C for 24, 48 or 72 h. After washing twice with PBS, MTT solution was added to each well to a final concentration of 0.5 mg/ml and further incubated for 4 h. The MTT solution was removed and 100 µl DMSO was added to each well to dissolve the formazan crystals. Cell viability was determined by measuring the absorbance at a wavelength of 570 nm using a microplate reader (Thermo Fisher Scientific, Inc.). The cell viability rates were normalized to the DMSO-treated control group.
Flow cytometric analysis of apoptosis
A549 or H1299 cells were seeded in six-well plates at a density of 5×105 cells/well and cultured overnight. Following incubation, cells were treated with 10 µM gefitinib with or without 12.5 µg/ml ginsenoside Rg3 at 37°C for 48 h. Following a 48-h incubation, cells were harvested, washed twice with PBS and subsequently stained using the Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Double Staining kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China), according to the manufacturer's protocol. Apoptotic cells were measured using a flow cytometer (BD Biosciences, Franklin Lakes, CA, USA) and the data were analyzed using FlowJo software (version 7.6.1; FlowJo LLC, Ashland, OR, USA).
Transwell migration assay
Cells were pre-treated with 10 µM gefitinib with or without 12.5 µg/ml ginsenoside Rg3 at 37°C for 24 h, cells were collected and re-suspended in culture medium without FBS at a density of 5×105 cells/ml. Using Transwell chambers with a pore size of 8 µm (Costar; Corning Inc., Corning, NY, USA), a total of 1×105 A549 or H1299 cells in RPMI-1640 medium without FBS were plated in the upper chamber, and 600 µl RPMI-1640 medium supplemented with 20% FBS was plated in the lower chamber. Following incubation for 24 h, the migratory cells were fixed with 4% paraformaldehyde at room temperature for 15 min, washed twice with PBS and stained with 0.05% crystal violet at room temperature for 10 min. The unmigrated cells were removed with cotton swabs and the stained migrated cells were counted using a light microscope (magnification, ×100).
Western blot analysis
A549 or H1299 cells in 2 ml RPMI-1640 medium were seeded in six-well plates at a density of 5×105 cells/well and cultured overnight. Subsequently, cells were treated with 10 µM gefitinib with or without 12.5 µg/ml ginsenoside Rg3 at 37°C for 48 h. Following treatment for 48 h, total protein was extracted from cells using radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology). Total protein was quantified using bicinchoninic acid assay (Beyotime Institute of Biotechnology) and 40 µg protein/lane was separated via SDS-PAGE on a 10% gel. The separated proteins were transferred onto 0.45 mm Immobilon-P polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked in 5% fat-free milk for 1 h at room temperature and incubated overnight at 4°C with primary antibodies against cleaved-caspase-3 (1:1,000; cat. no. 9661; CST Biological Reagents Co., Ltd., Shanghai, China), snail family transcriptional repressor 1 (SNAIL; 1:500; cat. no. sc-393172), snail family transcriptional repressor 2 (SLUG; 1:500; cat. no. sc-166476), Bcl-2-associated X (Bax; 1:200; cat. no. sc-49; all Santa Cruz Biotechnology, Inc., Dallas, TX, USA), E-cadherin (1:1,000; cat. no. ab15148), B-cell lymphoma 2 (Bcl-2; 1:1,000; cat. no. ab194583), and GAPDH (1:5,000; cat. no. ab181602: All Abcam, Cambridge, UK). Following the primary incubation, membranes were incubated with horseradish peroxidase-labelled secondary antibodies, including Peroxidase AffiniPrue Goat Anti-Rabbit IgG (1:3,000; cat. no. 111-035-003) and Peroxidase AffiniPrue Goat Anti-Mouse IgG (1:3,000; cat. no. 115-035-003; both Jackson ImmunoResearch Europe, Ltd., Newmarket, UK) for 1 h at room temperature. Protein bands were visualized using the enhanced chemiluminescence detection reagents (Pierce™ ECL Western Blotting Substrate; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol, and blots were analyzed using ChemiDoc™ XRS+ System (Bio-Rad Laboratories, Inc., Hercules, CA, USA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation. All statistical analyses were performed using GraphPad Prism software (version 5.0; Graphpad Software, Inc., La Jolla, CA, USA). Statistical analyses were performed using one-way analysis of variance followed by Bonferroni correction. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of ginsenoside Rg3 on the cytotoxic activity of gefitinib in NSCLC cell lines
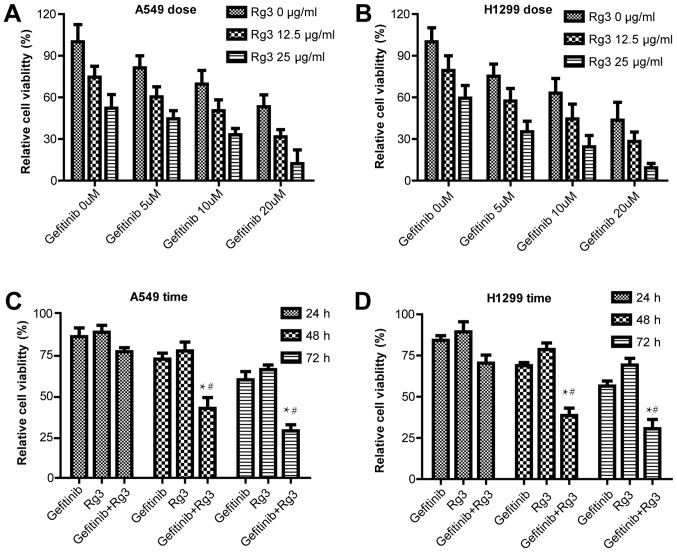
MTT assay was used to examine cell proliferation in A549 and H1299 cells treated with 0, 5, 10 or 20 µM gefitinib with or without 12.5 or 25 µg/ml ginsenoside Rg3 for 48 h. The current study demonstrated that in both NSCLC cell lines, gefitinib and ginsenoside Rg3 inhibited cell proliferation in a dose-dependent manner. The combined treatment with gefitinib and ginsenoside Rg3 further increased the cytotoxic effect compared with gefitinib or ginsenoside Rg3 treatment alone (Fig. 1A and B). In addition, A549 and H1299 cell viability was examined following treatment with 10 µM gefitinib with or without 12.5 µg/ml ginsenoside Rg3 for 24, 48 and 72 h. The current study demonstrated the combined treatment with gefitinib and ginsenoside Rg3 increased the cytotoxic effect compared with gefitinib or ginsenoside Rg3 treatment alone, and the difference was statistically significant at 48 and 72 h (Fig. 1C and D).
Figure 1.
Lung cancer cell viability following treatment with gefitinib with or without ginsenoside Rg3. MTT assay was used to examine the cell viability of (A) A549 and (B) H1299 cells treated with gefitinib (0, 5, 10 or 20 µM) with or without Rg3 (12.5 or 25 µg/ml) for 48 h. (C) Cell viability of A549 cells treated with 10 µM gefitinib and 12.5 µg/ml Rg3 for 24, 48 and 72 h. (D) Viability of H1299 cells treated with 10 µM gefitinib and 12.5 µg/ml Rg3 for 24, 48 and 72 h. Data are presented as the mean ± standard deviation. *P<0.05 vs. the gefitinib group and #P<0.05 vs. the Rg3 group. Rg3, ginsenoside Rg3; H1299 and A549, non-small cell lung cancer cell lines.
Effect of ginsenoside Rg3 on gefitinib-induced apoptosis in NSCLC cancer cell lines
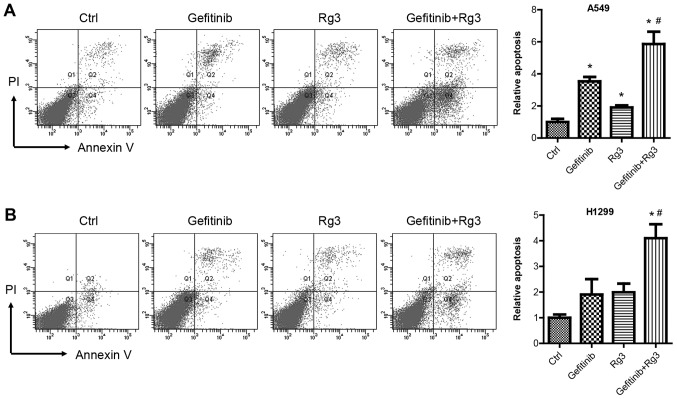
Flow cytometric analysis using Annexin V and PI double staining was used to examine cell apoptosis in A549 and H1299 cells treated with 10 µM gefitinib with or without 12.5 mg/ml ginsenoside Rg3 for 48 h. The current study demonstrated that gefitinib and Rg3 treatment significantly increased A549 cell apoptosis compared with the control group. In addition, the combined treatment with gefitinib + Rg3 significantly enhanced gefitinib-induced apoptosis in A549 cells (Fig. 2A). Similarly, gefitinib or ginsenoside Rg3 treatment alone increased H1299 cell apoptosis. The combined treatment with gefitinib + Rg3 significantly enhanced cell apoptosis compared with the control and gefitinib groups in H1299 cells (Fig. 2B).
Figure 2.
Lung cancer cell apoptosis following treatment with gefitinib with or without Rg3. Flow cytometry was used to examine apoptosis in (A) A549 and (B) H1299 cells treated with gefitinib (10 µM) with or without Rg3 (12.5 µg/ml) for 48 h. *P<0.05 vs. the control group and #P<0.05 vs. the gefitinib group. Rg3, ginsenoside Rg3; H1299 and A549, non-small cell lung cancer cell lines; Annexin V, Annexin V-fluorescein isothiocyanate; PI, propidium iodide.
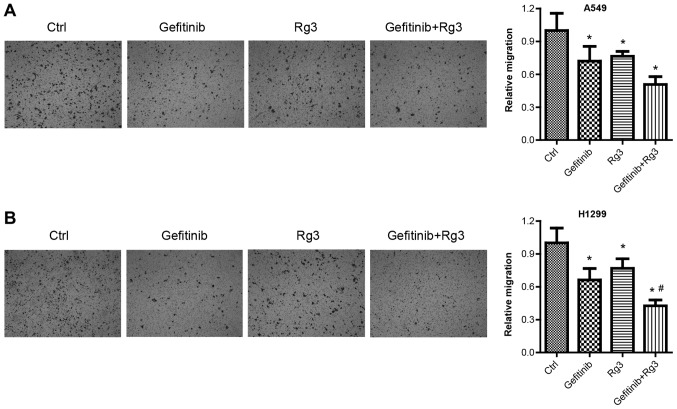
Ginsenoside Rg3 enhances the inhibitory effect of gefitinib on NSCLC cell migration
Cell migration assays were used to examine migration in A549 and H1299 cells treated with 10 µM gefitinib with or without 12.5 µg/ml ginsenoside Rg3 for 24 h. Cell migration assays were performed after a relatively short treatment time (24 h) to exclude the influence of drug-induced cell death. The results from the migration assays demonstrated that gefitinib, ginsenoside Rg3 and gefitinib + Rg3 treatment significantly decreased A549 cell migration compared with the control group. (Fig. 3A). Similarly, the migration assays demonstrated that gefitinib or ginsenoside Rg3 treatment alone significantly decreased H1299 cell migration compared with the control group. In addition, the combined treatment with gefitinib + Rg3 significantly increased H1299 cell migration compared with the control and gefitinib-treated group (Fig. 3B).
Figure 3.
Lung cancer cell migration following treatment with gefitinib with or without Rg3. Transwell migration assay was used to examine cell migration in (A) A549 and (B) H1299 cells treated with gefitinib (10 µM) with or without Rg3 (12.5 µg/ml) for 24 h. magnification, ×100. *P<0.05 vs. the control group and #P<0.05 vs. the gefitinib group. Rg3, ginsenoside Rg3; H1299 and A549, non-small cell lung cancer cell lines.
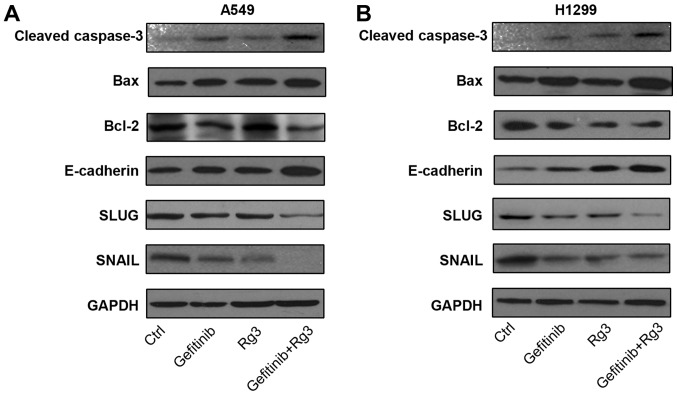
Ginsenoside Rg3 enhances the effects of gefitinib on the expression of migration- and apoptosis-associated proteins in NSCLC cell lines
To investigate the effects of treatment with gefitinib and ginsenoside Rg3 on apoptosis- and migration-associated pathways, the protein expression levels of apoptosis-associated proteins (Bax, cleaved-caspase-3 and Bcl-2) and migration-associated proteins (E-cadherin, SNAIL and SLUG) were determined by western blot analysis in A549 and H1299 cells treated with 10 µM gefitinib with or without 12.5 µg/ml ginsenoside Rg3 for 24 h. Cell death causes the degradation of cellular proteins, including pro-apoptotic proteins (20). Therefore, protein expression was examined after a relatively short treatment time (24 h) to exclude the influence of drug-induced cell death. The current study demonstrated that in both NSCLC cell lines, the combined treatment with gefitinib + Rg3 increased protein expression levels of pro-apoptotic proteins Bax and cleaved caspase-3, whilst the protein expression level of the anti-apoptotic protein Bcl-2 was decreased compared with the control group. Furthermore, the combined treatment with gefitinib and Rg3 increased the protein expression level of anti-migration protein E-cadherin, whilst the protein expression levels of two pro-migration factors SNAIL and SLUG were decreased compared with the control group (Fig. 4A and B). These results suggest that ginsenoside Rg3 may enhance gefitinib-induced apoptosis and inhibition of migration in NSCLC cell lines.
Figure 4.
Expression of migration and apoptosis-associated proteins in lung cancer cell lines following treatment with gefitinib with or without Rg3. Protein expression levels of caspase-3, Bax, Bcl-2, E-cadherin, SLUG, SNAIL and GAPDH were determined using western blot analysis in (A) A549 and (B) H1299 cells treated with gefitinib (10 µM) with or without Rg3 (12.5 µg/ml) for 24 h. Rg3, ginsenoside Rg3; H1299 and A549, non-small cell lung cancer cell lines. Bax, Bcl-2-associated X; Bcl-2, B-cell lymphoma 2; SLUG, snail family transcriptional repressor 2; SNAIL, snail family transcriptional repressor 1.
Discussion
The current study demonstrated that ginsenoside Rg3 enhances gefitinib-induced tumor cytotoxicity and apoptosis, as well as the inhibitory effect of gefitinib on cell migration, thereby sensitizing NSCLC cells to gefitinib. The 5-year survival rate for patients with NSCLC is approximately 15% (21,22). The discovery and use of EGFR-TKIs, including gefitinib, has improved prognosis in patients with advanced EGFR mutation-positive NSCLC (6,23,24). However, the majority of patients with NSCLC develop acquired gefitinib resistance within 9–16 months, as a result of secondary EGFR mutations (25,26). Second-generation EGFR-TKIs, including afatinib, were developed to overcome acquired resistance to first-generation inhibitors (27). Third-generation EGFR-TKIs can target the constitutive activation of EGFR mutations as well as resistant mutations (28,29). Issues regarding high cost, side effects and limited applicability of second- and third-generation EGFR-TKIs have prevented the widespread clinical application of these drugs (27,30). To overcome the limitations associated with EGFR-TKIs in the treatment with NSCLC, more efficient therapeutic strategies with fewer side effects are required. The current study demonstrated that ginsenoside Rg3 increased the cytotoxic activity of gefitinib in NSCLC cell. These results suggest that the combined treatment with gefitinib and ginsenoside Rg3 may be used for non-EGFR mutant cancer at a lower dose, whilst reducing any potential side effects associated with EGFR-TKIs.
Gefitinib is an effective treatment option for patients with advanced EGFR mutation-positive NSCLC (9). However numerous patients with NSCLC do not have gefitinib-sensitive mutations and therefore do not respond to treatment with gefitinib (13). In the present study, two NSCLC cell lines (H1299 and A549) with wild-type EGFR (31) and different sensitivities to gefitinib were selected and used to investigate the enhanced efficacy and sensitivity of gefitinib with ginsenoside Rg3. The current study demonstrated that both NSCLC cell lines could be re-sensitized to treatment with gefitinib when combined with ginsenoside Rg3. Furthermore, the different p53 states of A549 (p53-wildtype) and H1299 (p53-null) cells (31), suggests that p53 is unlikely to be involved in the potential underlying process.
Ginsenoside Rg3 is a natural product extracted from a traditional Chinese medicine, Panax ginseng (17). Several studies have suggested that ginsenoside Rg3 may serve roles in the complex process of tumor development, which includes proliferation, apoptosis, migration, angiogenesis and tumor immunogenicity (18,19,32–36). Unlike targeted drugs, ginsenoside Rg3 has multiple targets and exhibits anticancer activity through a number of mechanisms, which include targeting multiple tumor-associated signaling pathways as well as regulating intracellular reactive oxygen species (34–36). The potential mechanism of action of ginsenoside Rg3 is not dependent on EGFR mutations (37), which suggests that an enhanced therapeutic efficiency may be achieved through the combined treatment with gefitinib. Consistent with previous studies (34,35), the current study demonstrated that ginsenoside Rg3 inhibited cell proliferation, induced cell apoptosis and decreased NSCLC cell migration.
The current study demonstrated that ginsenoside Rg3 enhanced gefitinib-induced tumor cytotoxicity in NSCLC cells and the inhibitory effect of gefitinib on NSCLC cell migration. Western blot analysis demonstrated that the combined treatment with gefitinib and ginsenoside Rg3 enhanced protein expression levels of cell migration- and apoptosis-associated markers in NSCLC cell lines which suggests that ginsenoside Rg3 may enhance gefitinib efficacy in NSCLC. Several studies revealed that ginsenoside Rg3 reversed resistance to cisplatin in lung cancer (18,19,36). In the current study, ginsenoside Rg3 enhanced gefitinib efficiency in NSCLC cell proliferation, apoptosis, and migration. The mechanisms of action of ginsenoside Rg3 may be distinct from gefitinib (17), exerting a synchronous inhibitory effect on EGFR. However, under different dose combinations, gefitinib and ginsenoside Rg3 may exert a potential synergistic anticancer effect.
The current study has several limitations. Two NSCLC cell lines were used to investigate the antitumor effects of ginsenoside Rg3 and gefitinib, therefore NSCLC cell lines with different EGFR status should be used for further investigation. In addition, the underlying mechanism of ginsenoside Rg3 and gefitinib in NSCLC remains unknown and should be further investigated.
In conclusion, ginsenoside Rg3 may be able to enhance the anticancer activity of gefitinib. Ginsenoside Rg3 enhanced gefitinib-induced cytotoxicity, apoptosis and migration inhibition, making NSCLC cells more sensitive to gefitinib. These results indicated the potential clinical application of the combined treatment with gefitinib and ginsenoside Rg3 for patients with NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YD, WW and JT designed the experiments and analyzed the data. QS performed flow cytometric experiments. YD and WW wrote the manuscript. JT supervised the work.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wong MCS, Lao XQ, Ho KF, Goggins WB, Tse SLA. Incidence and mortality of lung cancer: Global trends and association with socioeconomic status. Sci Rep. 2017;7:14300. doi: 10.1038/s41598-017-02997-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. In J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, Li Y, Wang L, Liu Y, Yin P. Cause-specific mortality for 240 causes in China during 1990–2013: A systematic subnational analysis for the global burden of disease study 2013. Lancet. 2016;387:251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 4.Zappa C, Mousa SA. Non-small cell lung cancer: Current treatment and future advances. Transl Lung Cancer Res. 2016;5:288–300. doi: 10.21037/tlcr.2016.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: New biological insights and recent therapeutic advances. CA Cancer J Clin. 2011;61:91–112. doi: 10.3322/caac.20102. [DOI] [PubMed] [Google Scholar]
- 6.Prabhu VV, Devaraj N. Epidermal growth factor receptor tyrosine kinase: A potential target in treatment of non-small-cell lung carcinoma. J Environ Pathol Toxicol Oncol. 2017;36:151–158. doi: 10.1615/JEnvironPatholToxicolOncol.2017018341. [DOI] [PubMed] [Google Scholar]
- 7.Goss G, Tsai CM, Shepherd FA, Bazhenova L, Lee JS, Chang GC, Crino L, Satouchi M, Chu Q, Hida T, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17:1643–1652. doi: 10.1016/S1470-2045(16)30508-3. [DOI] [PubMed] [Google Scholar]
- 8.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 9.Ghafoor Q, Baijal S, Taniere P, O'Sullivan B, Evans M, Middleton G. Epidermal growth factor receptor (EGFR) kinase inhibitors and non-small cell lung cancer (NSCLC)-advances in molecular diagnostic techniques to facilitate targeted therapy. Pathol Oncol Res. 2018;24:723–731. doi: 10.1007/s12253-017-0377-1. [DOI] [PubMed] [Google Scholar]
- 10.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 11.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Wang Y, Niu K, Chen X, Xia L, Lu D, Kong R, Chen Z, Duan Y, Sun J. Clinical benefit from EGFR-TKI plus ginsenoside Rg3 in patients with advanced non-small cell lung cancer harboring EGFR active mutation. Oncotarget. 2016;7:70535–70545. doi: 10.18632/oncotarget.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li ZX, Qu LY, Wen H, Zhong HS, Xu K, Qiu XS, Wang EH. Mig-6 overcomes gefitinib resistance by inhibiting EGFR/ERK pathway in non-small cell lung cancer cell lines. Int J Clin Exp Pathol. 2014;7:7304–7311. [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng R, Jiang H, Li J, Liu X, Xu H. Polyphyllin II restores sensitization of the resistance of PC-9/ZD cells to gefitinib by a negative regulation of the PI3K/Akt/mTOR signaling pathway. Curr Cancer Drug Target. 2017;17:376–385. doi: 10.2174/1568009616666161213141608. [DOI] [PubMed] [Google Scholar]
- 15.Song S, Du L, Jiang H, Zhu X, Li J, Xu J. Paris saponin I sensitizes gastric cancer cell lines to cisplatin via cell cycle arrest and apoptosis. Med Sci Monit. 2016;22:3798–3803. doi: 10.12659/MSM.898232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang H, Zhao PJ, Su D, Feng J, Ma SL. Paris saponin I induces apoptosis via increasing the Bax/Bcl-2 ratio and caspase-3 expression in gefitinib-resistant non-small cell lung cancer in vitro and in vivo. Mol Med Rep. 2014;9:2265–2272. doi: 10.3892/mmr.2014.2108. [DOI] [PubMed] [Google Scholar]
- 17.Sun M, Ye Y, Xiao L, Duan X, Zhang Y, Zhang H. Anticancer effects of ginsenoside Rg3 (Review) Int J Mol Med. 2017;39:507–518. doi: 10.3892/ijmm.2017.2857. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Z, Yang Y, Yang Y, Zhang Y, Yue Z, Pan Z, Ren X. Ginsenoside Rg3 attenuates cisplatin resistance in lung cancer by downregulating PD-L1 and resuming immune. Biomed Pharmacother. 2017;96:378–383. doi: 10.1016/j.biopha.2017.09.129. [DOI] [PubMed] [Google Scholar]
- 19.Kim YJ, Choi WI, Jeon BN, Choi KC, Kim K, Kim TJ, Ham J, Jang HJ, Kang KS, Ko H. Stereospecific effects of ginsenoside 20-Rg3 inhibits TGF-beta1-induced epithelial-mesenchymal transition and suppresses lung cancer migration, invasion and anoikis resistance. Toxicology. 2014;322:23–33. doi: 10.1016/j.tox.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Socinski MA, Evans T, Gettinger S, Hensing TA, VanDam Sequist L, Ireland B, Stinchcombe TE. Treatment of stage IV non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(Suppl 5):e341S–e368S. doi: 10.1378/chest.12-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 23.Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005;23:2445–2459. doi: 10.1200/JCO.2005.11.890. [DOI] [PubMed] [Google Scholar]
- 24.Bareschino MA, Schettino C, Rossi A, Maione P, Sacco PC, Zeppa R, Gridelli C. Treatment of advanced non small cell lung cancer. J Thorac Dis. 2011;3:122–133. doi: 10.3978/j.issn.2072-1439.2010.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su KY, Chen HY, Li KC, Kuo ML, Yang JC, Chan WK, Ho BC, Chang GC, Shih JY, Yu SL, Yang PC. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol. 2012;30:433–440. doi: 10.1200/JCO.2011.38.3224. [DOI] [PubMed] [Google Scholar]
- 26.Xu C, Zhou Q, Wu YL. Can EGFR-TKIs be used in first line treatment for advanced non-small cell lung cancer based on selection according to clinical factors?-A literature-based meta-analysis. J Hematol Oncol. 2012;5:62. doi: 10.1186/1756-8722-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsh V. Next-Generation covalent irreversible kinase inhibitors in NSCLC: Focus on afatinib. BioDrugs. 2015;29:167–183. doi: 10.1007/s40259-015-0130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan CS, Gilligan D, Pacey S. Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer. Lancet Oncol. 2015;16:e447–e459. doi: 10.1016/S1470-2045(15)00246-6. [DOI] [PubMed] [Google Scholar]
- 29.Yver A. Osimertinib (AZD9291)-a science-driven, collaborative approach to rapid drug design and development. Ann Oncol. 2016;27:1165–1170. doi: 10.1093/annonc/mdw129. [DOI] [PubMed] [Google Scholar]
- 30.Engel J, Lategahn J, Rauh D. Hope and disappointment: Covalent inhibitors to overcome drug resistance in non-small cell lung cancer. ACS Med Chem Lett. 2015;7:2–5. doi: 10.1021/acsmedchemlett.5b00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rho JK, Choi YJ, Ryoo BY, Na II, Yang SH, Kim CH, Lee JC. p53 enhances gefitinib-induced growth inhibition and apoptosis by regulation of Fas in non-small cell lung cancer. Cancer Res. 2007;67:1163–1169. doi: 10.1158/0008-5472.CAN-06-2037. [DOI] [PubMed] [Google Scholar]
- 32.Kim JW, Jung SY, Kwon YH, Lee JH, Lee YM, Lee BY, Kwon SM. Ginsenoside Rg3 attenuates tumor angiogenesis via inhibiting bioactivities of endothelial progenitor cells. Cancer Biol Ther. 2012;13:504–515. doi: 10.4161/cbt.19599. [DOI] [PubMed] [Google Scholar]
- 33.Son KJ, Choi KR, Lee SJ, Lee H. Immunogenic cell death induced by ginsenoside Rg3: Significance in dendritic cell-based anti-tumor immunotherapy. Immune Netw. 2016;16:75–84. doi: 10.4110/in.2016.16.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun HY, Lee JH, Han YS, Yoon YM, Yun CW, Kim JH, Song YS, Lee SH. Pivotal roles of ginsenoside Rg3 in tumor apoptosis through regulation of reactive oxygen species. Anticancer Res. 2016;36:4647–4654. doi: 10.21873/anticanres.11015. [DOI] [PubMed] [Google Scholar]
- 35.Tian L, Shen D, Li X, Shan X, Wang X, Yan Q, Liu J. Ginsenoside Rg3 inhibits epithelial-mesenchymal transition (EMT) and invasion of lung cancer by down-regulating FUT4. Oncotarget. 2016;7:1619–1632. doi: 10.18632/oncotarget.6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Tian L, Khan MN, Zhang L, Chen Q, Zhao Y, Yan Q, Fu L, Liu J. Ginsenoside Rg3 sensitizes hypoxic lung cancer cells to cisplatin via blocking of NF-κB mediated epithelial-mesenchymal transition and stemness. Cancer Lett. 2018;415:73–85. doi: 10.1016/j.canlet.2017.11.037. [DOI] [PubMed] [Google Scholar]
- 37.Joo EJ, Chun J, Ha YW, Ko HJ, Xu MY, Kim YS. Novel roles of ginsenoside Rg3 in apoptosis through downregulation of epidermal growth factor receptor. Chem Biol Interact. 2015;233:25–34. doi: 10.1016/j.cbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.